Partial Miscibility and Concentration Distribution of Two-Phase Blends of Crosslinked NBR and PVC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Optical Microscopic Observation
2.4. DMA Measurement
2.5. TEM Observation
3. Results and Discussion
3.1. Phase Behavior
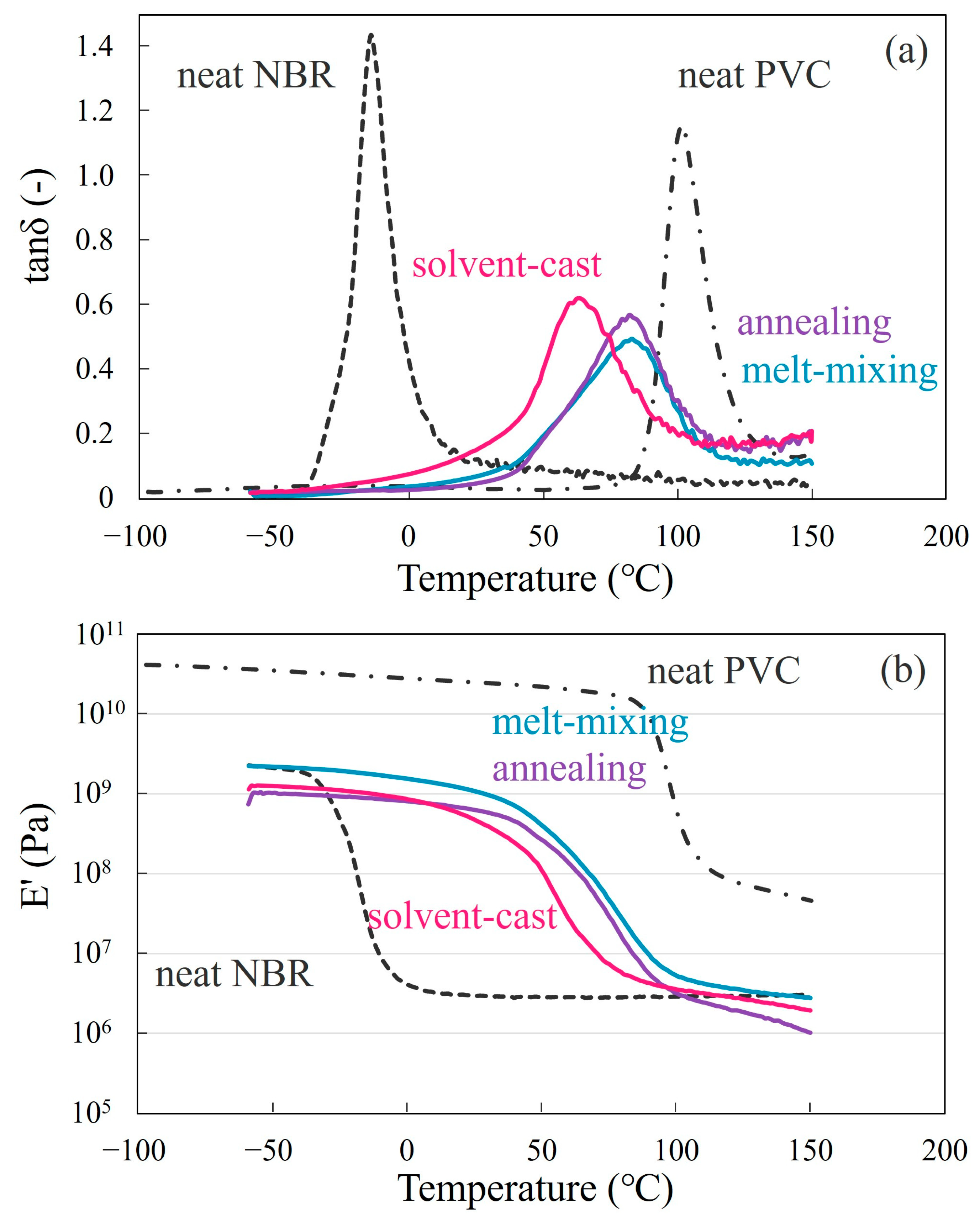
3.2. Partial Miscibility
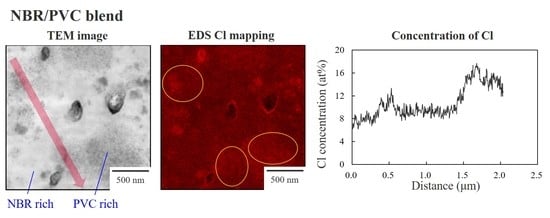
3.3. Two-Phase Morphology and Concentration Distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olabis, O. Polymer-Polymer Miscibility; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Paul, D.R. Polymer Blends Volume 1; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1. [Google Scholar]
- Utracki, L.A.; Wilkie, C.A. Polymer Blends Handbook; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; Volume 1. [Google Scholar]
- Thomas, S.; Grohens, Y.; Jyotishkumar, P. Characterization of Polymer Blends: Miscibility, Morphology and Interfaces; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Stockdale, M.K. Thermoplastic elastomers from NBR and polyvinyl chloride. J. Vinyl Technol. 1990, 12, 235–244. [Google Scholar] [CrossRef]
- Mousa, A.; Ishiaku, U.; Mohd Ishak, Z. Oil resistance of dynamically vulcanized poly (vinyl chloride)/nitrile butadiene rubber thermoplastic elastomers. Polym. Bull. 2005, 53, 203–212. [Google Scholar] [CrossRef]
- Gheno, S.; Passador, F.; Pessan, L. Investigation of the phase morphology of dynamically vulcanized PVC/NBR blends using atomic force microscopy. J. Appl. Polym. Sci. 2010, 117, 3211–3219. [Google Scholar] [CrossRef]
- Sen, A.; Mukherjee, G. Studies on the thermodynamic compatibility of blends of poly (vinyl chloride) and nitrile rubber. Polymer 1993, 34, 2386–2391. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, X.; Wu, L.; Li, Y.; Qi, Z.; Choy, C.; Wang, F. Effects of interfacial adhesion on the rubber toughening of poly (vinyl chloride) Part 1. Impact tests. Polymer 2001, 42, 737–746. [Google Scholar] [CrossRef]
- George, K.; Joseph, R.; Francis, D.J. Studies on NBR/PVC blends. J. Appl. Polym. Sci. 1986, 32, 2867–2873. [Google Scholar] [CrossRef]
- Zakrzewski, G. Investigation of the compatibility of butadiene—Acrylonitrile copolymers with poly (vinyl chloride). Polymer 1973, 14, 347–351. [Google Scholar] [CrossRef]
- Perera, M.S.; Ishiaku, U.; Ishak, Z.M. Characterisation of PVC/NBR and PVC/ENR50 binary blends and PVC/ENR50/NBR ternary blends by DMA and solid state NMR. Eur. Polym. J. 2001, 37, 167–178. [Google Scholar] [CrossRef]
- Pena, J.; Hidalgo, M.; Mijangos, C. Plastification of poly (vinyl chloride) by polymer blending. J. Appl. Polym. Sci. 2000, 75, 1303–1312. [Google Scholar] [CrossRef]
- Migahed, M.; Ishra, M.; El-Khodary, A.; Fahmy, T. Compatibility of polyacrylonitrile-butadiene with polyvinylchloride as explored by thermally stimulated depolarization current. Polym. Test. 1993, 12, 335–349. [Google Scholar] [CrossRef]
- Huang, J.C. Analysis of the thermodynamic compatibility of poly (vinyl chloride) and nitrile rubbers from inverse gas chromatography. J. Appl. Polym. Sci. 2003, 89, 1242–1249. [Google Scholar] [CrossRef]
- Li, J.-X.; Chan, C.-M. Effect of the size of the dispersed NBR phase in PVC/NBR blends on the stability of PVC to electron irradiation. Polymer 2001, 42, 6833–6839. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Nakajima, N. Monitoring of Homogenization and Analysis of Nanoscale Structure in a Butadiene− Acrylonitrile Copolymer/Poly (vinyl chloride) Blend. Macromolecules 1996, 29, 5446–5452. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Liu, S.; Gui, H.; Lai, J.; Liu, Y.; Gao, J.; Huang, F.; Song, Z.; Tan, B. Ultrafine full-vulcanized powdered rubbers/PVC compounds with higher toughness and higher heat resistance. Polymer 2005, 46, 10614–10617. [Google Scholar] [CrossRef]
- Kader, M.; Kim, W.; Kaang, S.; Nah, C. Morphology and dynamic mechanical properties of natural rubber/nitrile rubber blends containing trans-polyoctylene rubber as a compatibilizer. Polym. Int. 2005, 54, 120–129. [Google Scholar] [CrossRef]
- Samsudin, S.A.; Kelly, C.A.; Kukureka, S.N.; Jenkins, M.J. Development of partial miscibility in polycarbonate/polypropylene blends via annealing. J. Polym. Eng. 2017, 37, 707–714. [Google Scholar] [CrossRef]
- Takamatsu, K.; Suzuki, S.; Nishimura, Y.; Saito, H. Reduction of birefringence by dynamic asymmetry in miscible blends of dissimilar polycarbonates. Polymer 2021, 222, 123632. [Google Scholar] [CrossRef]
- Nair, T.M.; Kumaran, M.; Unnikrishnan, G.; Pillai, V. Dynamic mechanical analysis of ethylene–propylene–diene monomer rubber and styrene–butadiene rubber blends. J. Appl. Polym. Sci. 2009, 112, 72–81. [Google Scholar] [CrossRef]
- Ziquinatti, F.; Hugen, R.G.; Oliveira, C.M.; Coelho, L.A.; Pezzin, S.H. Miscibility and mechanical behavior of SAN/NBR blends. Macromol. Symp. 2005, 229, 276–280. [Google Scholar] [CrossRef]
- Nara, S.; Sagawa, H.; Saito, H.; Oyama, H.T. Synergetic toughening of poly (phenylene sulfide) by poly (phenylsulfone) and poly (ethylene-ran-methacrylate-ran-glycidyl methacrylate). J. Appl. Polym. Sci. 2021, 138, 49994. [Google Scholar] [CrossRef]
- Sun, H.; Yu, B.; Sheng, J. Phase Morphology and Miscibility of iPP/PcBR Blends. Polym. -Plast. Technol. Eng. 2010, 49, 414–417. [Google Scholar] [CrossRef]
- Wu, C.; Akiyama, S. Dynamic mechanical and adhesive properties of acrylate rubber/chlorinated polypropylene blends compatibilized with a hindered phenol compound. Polym. J. 2001, 33, 955–958. [Google Scholar] [CrossRef]
- Liu, C.; Fan, J.; Chen, Y. Design of regulable chlorobutyl rubber damping materials with high-damping value for a wide temperature range. Polym. Test. 2019, 79, 106003. [Google Scholar] [CrossRef]
- Lu, X.; Yang, Y.; Cheng, B.; Li, T. High damping epoxized natural rubber/diallyl adiallyl phthalate prepolymer (ENR/DAP-A) blends engineered by interphase crosslinking. J. Appl. Polym. Sci. 2018, 135, 46300. [Google Scholar] [CrossRef]
- Liu, B.; Gao, X.; Zhao, Y.; Dai, L.; Xie, Z.; Zhang, Z. 9, 10-Dihydro-9-oxa-10-phosphaphenanthrene 10-oxide-based oligosiloxane as a promising damping additive for methyl vinyl silicone rubber (VMQ). J. Mater. Sci. 2017, 52, 8603–8617. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.; Tian, M. Preparation of high damping elastomer with broad temperature and frequency ranges based on ternary rubber blends. Polym. Adv. Technol. 2014, 25, 21–28. [Google Scholar] [CrossRef]
- Bohn, L. Incompatibility and phase formation in solid polymer mixures and graft and block copolymers. Rubber Chem. Technol. 1968, 41, 495–513. [Google Scholar] [CrossRef]
- Matsuo, M.; Nozaki, C.; Jyo, Y. Fine structures and fracture processes in plastic/rubber two-phase polymer systems. I. Observation of fine structures under the electron microscope. Polym. Eng. Sci. 1969, 9, 197–205. [Google Scholar] [CrossRef]
- Fukumori, K.; Sato, N.; Kurauchi, T. Pulsed NMR-study of motional heterogeneity in acrylonitrile butadiene poly(vinyl chloride) blends. Rubber Chem. Technol. 1991, 64, 522–533. [Google Scholar] [CrossRef]
- Nakajima, N.; Liu, J. The effect of gel on the deformational behavior of polyvinylchloride (PVC) and nitrile rubber (NBR) blends. Rubber Chem. Technol. 1992, 65, 453–474. [Google Scholar] [CrossRef]
- Wang, W.; Yao, Q.; Song, J.; Yao, B.; Wang, H.; Li, Z. Direct visualization of the nanoscopic three-phase structure and stiffness of NBR/PVC blends by AFM nanomechanical mapping. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 662–669. [Google Scholar] [CrossRef]
- Tomura, H.; Saito, H.; Inoue, T. Light scattering analysis of upper critical solution temperature behavior in a poly (vinylidene fluoride)/poly (methyl methacrylate) blend. Macromolecules 1992, 25, 1611–1614. [Google Scholar] [CrossRef]
- Lee, C.H.; Saito, H.; Goizueta, G.; Inoue, T. An immiscibility loop in isotactic polypropylene/partially hydrogenated oligo (styrene-co-indene) blend. Macromolecules 1996, 29, 4274–4277. [Google Scholar] [CrossRef]
- Sugeno, K.; Kokubun, S.; Saito, H. Ucst type phase boundary and accelerated crystallization in ptt/pet blends. Polymers 2020, 12, 2730. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komori, Y.; Taniguchi, A.; Shibata, H.; Goto, S.; Saito, H. Partial Miscibility and Concentration Distribution of Two-Phase Blends of Crosslinked NBR and PVC. Polymers 2023, 15, 1383. https://doi.org/10.3390/polym15061383
Komori Y, Taniguchi A, Shibata H, Goto S, Saito H. Partial Miscibility and Concentration Distribution of Two-Phase Blends of Crosslinked NBR and PVC. Polymers. 2023; 15(6):1383. https://doi.org/10.3390/polym15061383
Chicago/Turabian StyleKomori, Yuka, Aoi Taniguchi, Haruhisa Shibata, Shinya Goto, and Hiromu Saito. 2023. "Partial Miscibility and Concentration Distribution of Two-Phase Blends of Crosslinked NBR and PVC" Polymers 15, no. 6: 1383. https://doi.org/10.3390/polym15061383
APA StyleKomori, Y., Taniguchi, A., Shibata, H., Goto, S., & Saito, H. (2023). Partial Miscibility and Concentration Distribution of Two-Phase Blends of Crosslinked NBR and PVC. Polymers, 15(6), 1383. https://doi.org/10.3390/polym15061383