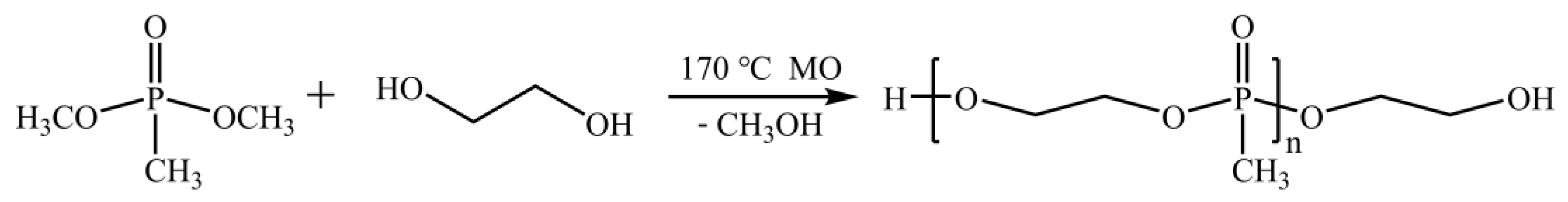
3.1. FT-IR Characterization of PPCP and Partial Part B Slurry
The chemical reaction mechanism between DMMP and ethylene glycol, used to generate PPCP, is shown in
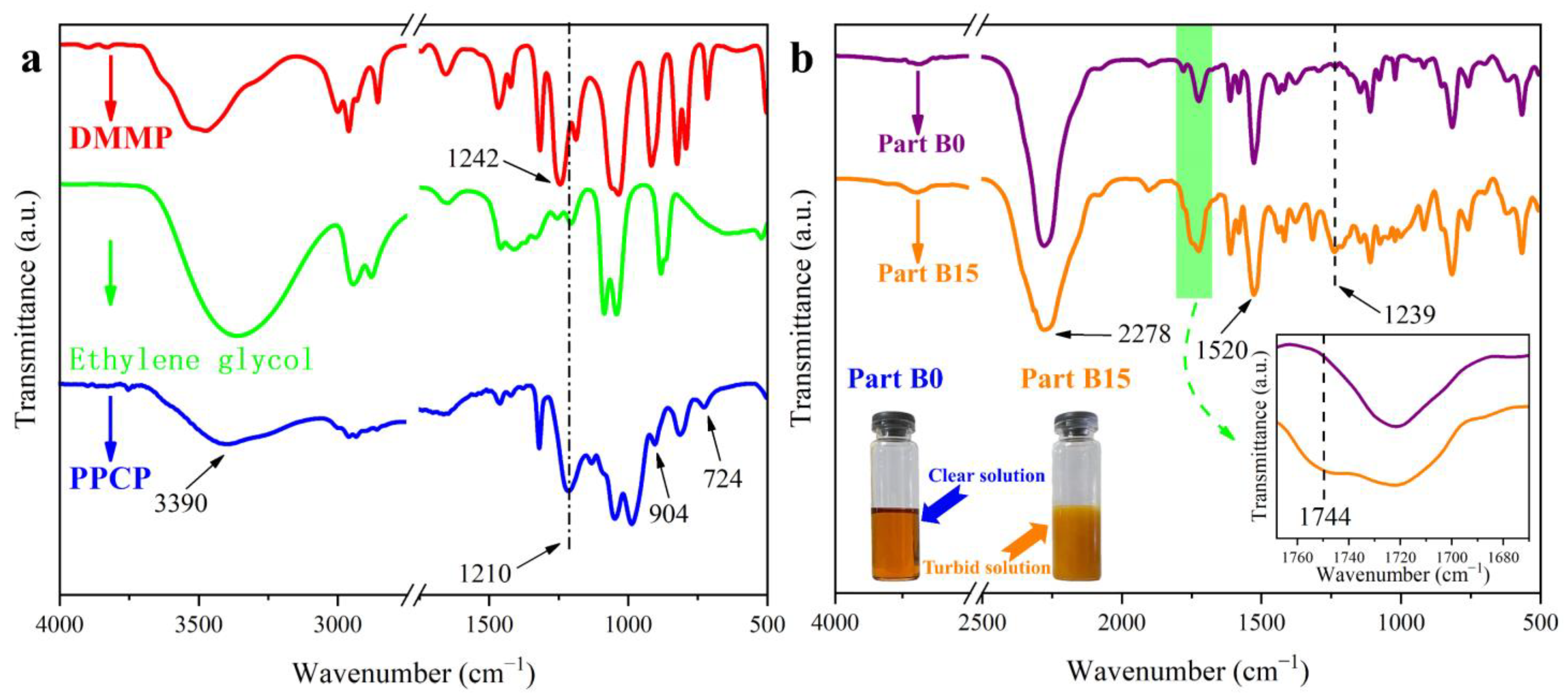
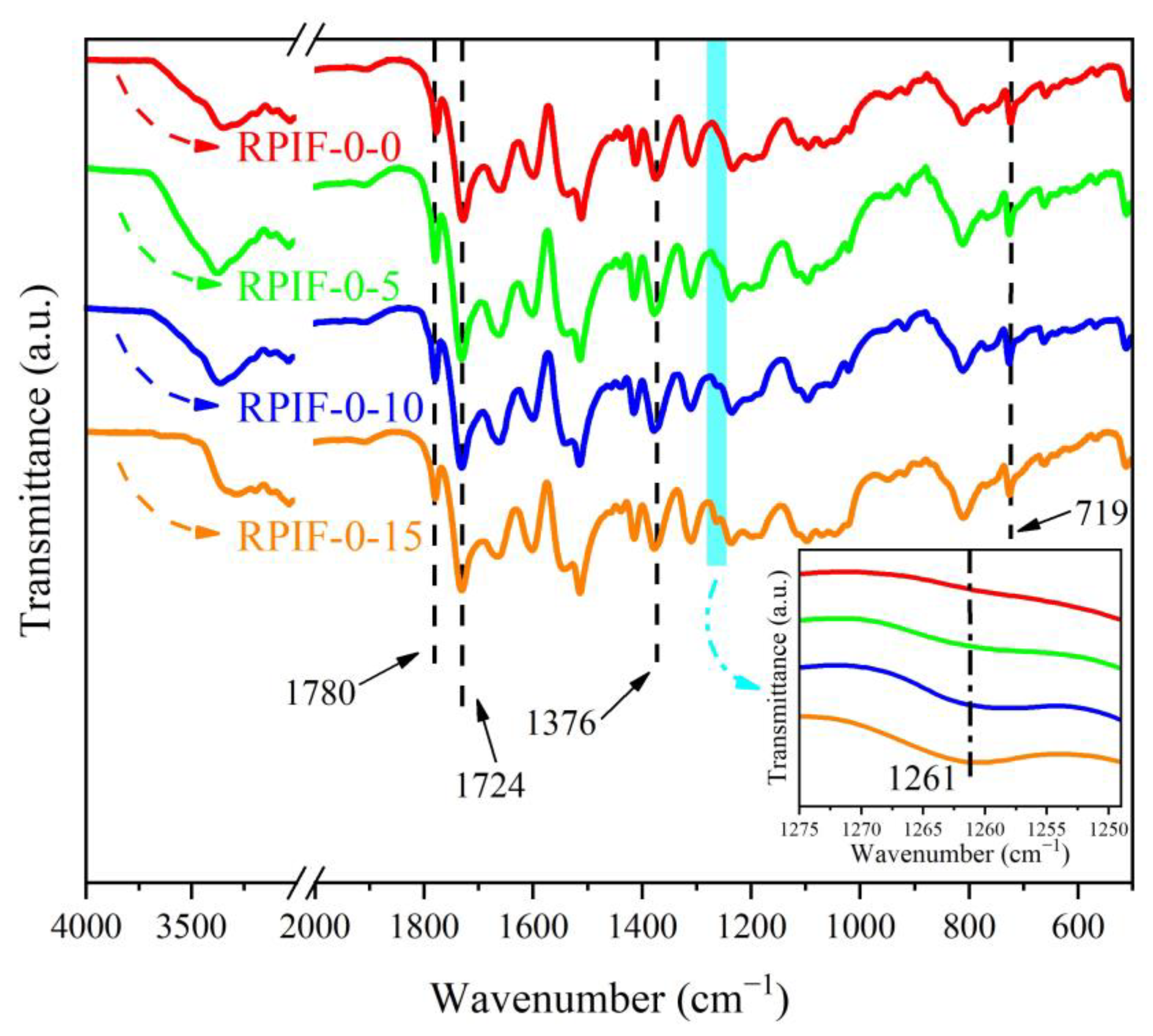
Scheme 1. In order to demonstrate the successful synthesis of PPCP and its chemical bonding reaction with PAPI, FT-IR curves of raw materials, PPCP, Part B0, and Part B15 are shown in
Figure 1.
As shown in
Figure 1a, owing to the formation of hydrogen bonds between the hydroxyl groups and P=O groups in PPCP, the stretching vibration absoption peak of P=O in PPCP was located at 1244 cm
−1, while the redshift value compared with DMMP was 34 cm
−1. Characteristic absorption peaks at 903 cm
−1 and 724 cm
−1 corresponded to the stretching vibration of P-O-C and P-C bonds, respectively [
41]. Meanwhile, the stretching vibration absorption peak of O-H can be obviously seen at 3390 cm
−1 in the curve of PPCP. These results preliminarily indicate that PPCP is successfully obtained.
Figure 1b shows the FT-IR curves of PAPI (Part B0) and Part B15. Compared with Part B0, a new peak appeared at 1744 cm
−1 in Part B15, which corresponds to the symmetrical stretching vibration absorption peak of the C=O bond in the carbamate group (-O-CO-NH-) structure owing to the reaction between PPCP and PAPI, as shown in
Scheme 3. In this study, a characteristic absorption peak at 1520 cm
−1, assigned to the benzene ring, also represents an internal standard peak [
8]. Compared with Part B0, the relative intensity of the isocyanate group’s absorption peak at 2278 cm
−1 in Part B15 is weakened. These results effectively indicate that PPCP can form chemical bonds with PAPI. Moreover, compared with the clarified Part B0, a digital photo shows that the state of Part B15 is turbid, further demonstrating the reaction between PPCP and PAPI.
3.5. Thermal Stability of RPIF
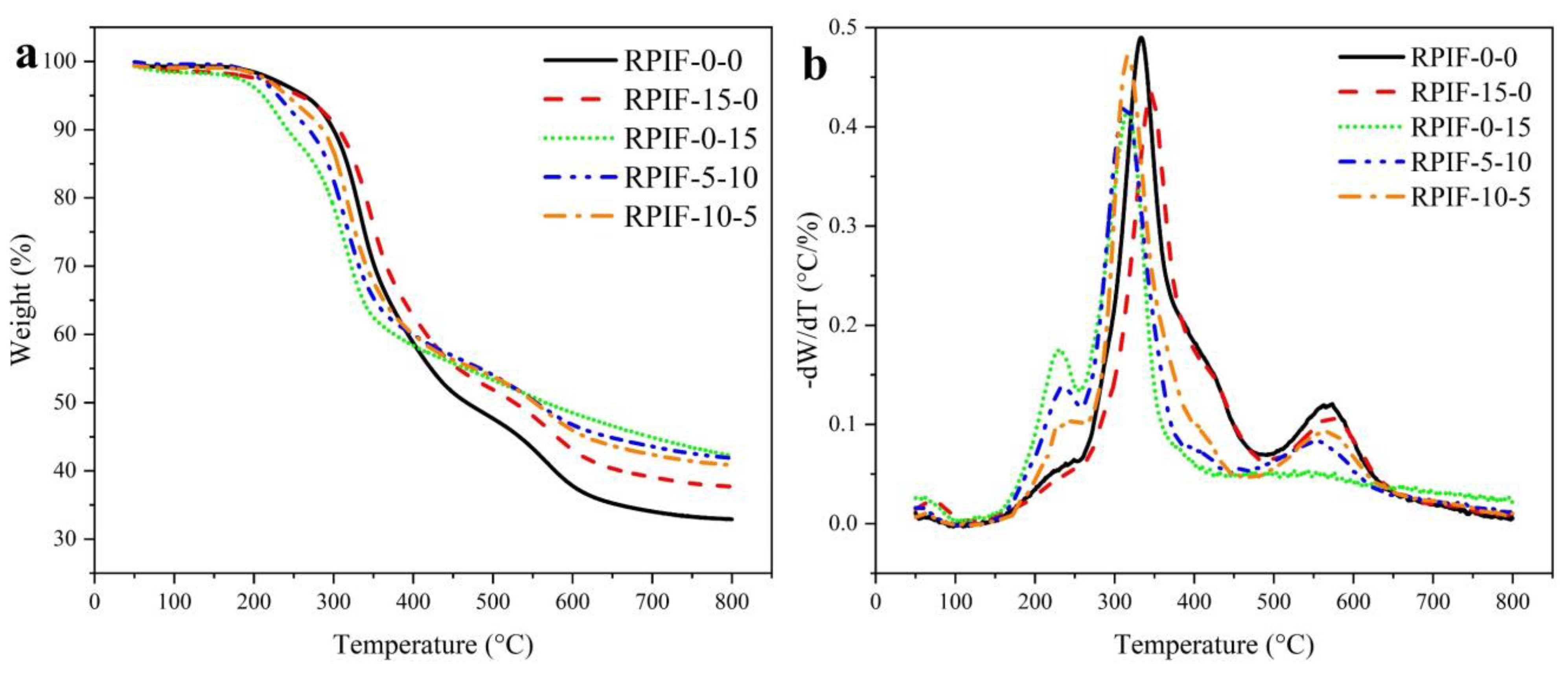
TGA and derivative thermogravimetry (DTG) are used to evaluate the thermal stability of RPIF. The TGA and DTG curves of RPIF-0-0 and RPIF possessing a total dosage of EG and PPCP at 15 wt. % are shown in
Figure 6. The decomposition temperature at 5% weight loss (T
5%) and residual weight at 800 °C (R
w) are listed in
Table S1. RPIF presents three decomposition regions in the ranges of 160~257 °C, 257~475 °C, and 475~640 °C, respectively [
8].
For RPIF-0-0, the small region from 160 to 257 °C refers to the further evaporation of residual and generated small molecules, and the T5% reaches 263 °C. However, the introduction of PPCP significantly improves weight loss in this region. The T5% of RPIF-0-15, which has the highest dosage of PPCP, is directly reduced to 211 °C. The second region corresponds to the decomposition of polyurea. From the DTG curves, it is obvious that the temperature at the maximum decomposition rate of the second region drops after PPCP is introduced, whereas the use of EG can push it to a higher value. The last region of 450~640 °C ascribes to the decomposition of PI. Moreover, a higher PPCP dosage leads to the slower decomposition of PI, and all RPIFs, including samples to which only PPCP is applied, show higher Rw than RPIF-0-0.
The above data indicate that the PPCP incorporated in RPIF can be pyrolyzed in the first region, and also releases PO radicals as previous reported, which leads to the increase in T
5% [
28]. To a certain extent, the introduction of PPCP reduces the thermal stability of RPIF. In addition, the produced phosphoric acid derivatives coming from the PPCP segments’ decomposition effectively promotes the degree of carbonization of polyurea and PI in the following decomposition region, as indicated by the increased R
w value. These actions can facilitate the formation of thicker and denser char layers. The generated char layers act as fireproof layers to inhibit contact between the fire and the internal matrix, playing an important part in the high flame retardancy of RPIF.
3.6. Combustion Behavior
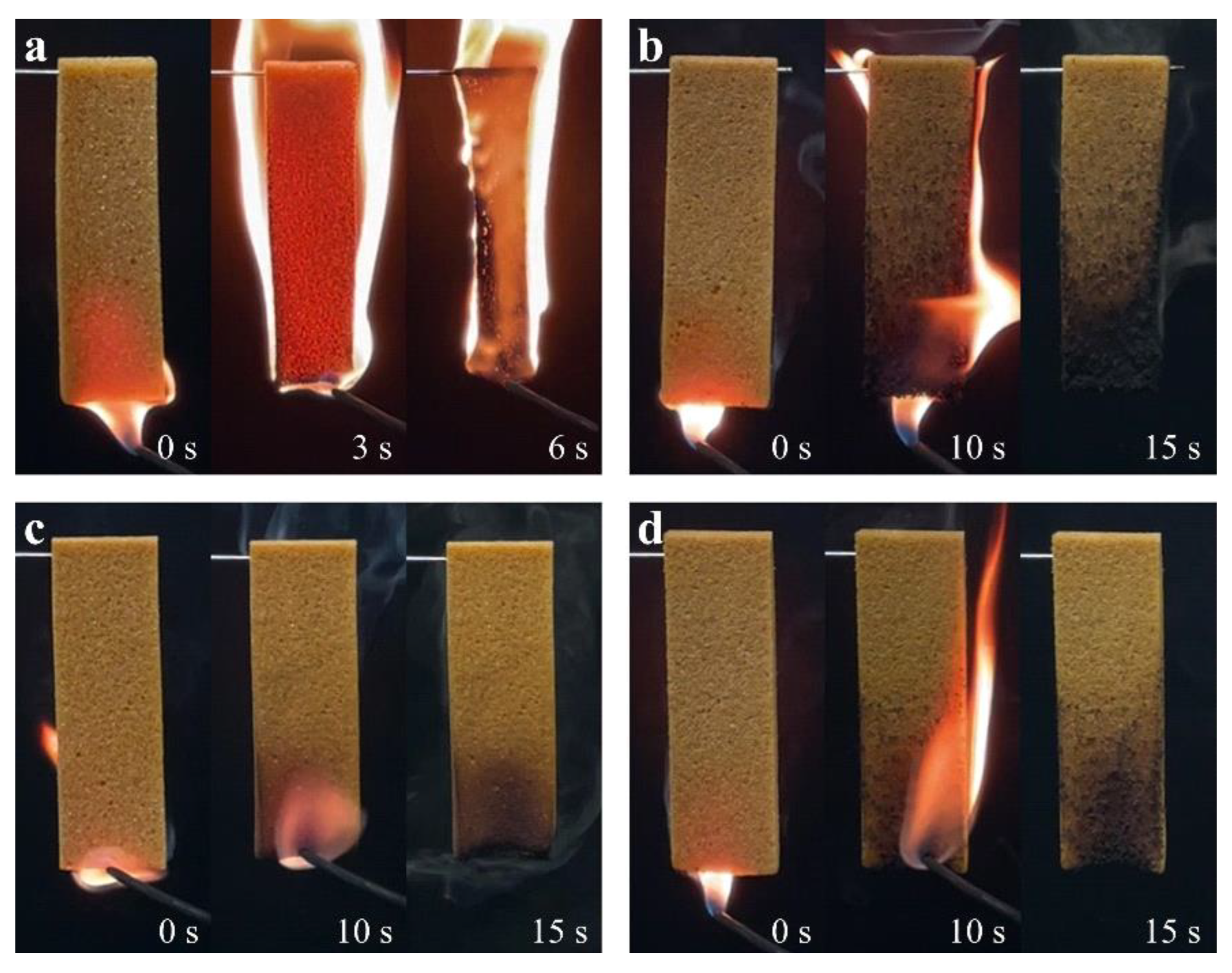
The actual ignition test is used to intuitively characterize the effects of PPCP and EG on the flame retardancy of RPIF. The photos are presented in
Figure 7. They show that RPIF-0-0 can be quickly ignited in less 3 s and entirely burned out before removing the igniter. However, no dripping and a better shape-retention ability are found when comparing with RPUF. It demonstrates that common RPIF achieves better use safety than RPUF. In addition, although RPIF-5-0, RPIF-0-5, and RPIF-5-5 are combusted for more than 10 s, they all can achieve self-extinguishing after removing the igniter, and still retain their original size. RPIF-0-5 and RPIF-5-5 are basically undamaged. These results indicate the positive effects of EG and PPCP on enhancing the flame retardancy and use safety of RPIF.
The CCT results can provide information close to the practical situation for flammability and smoke release performance [
8,
33]. The flammability parameters, including PHRR and THR at 155 s, are listed in
Table S2.
Figure S1 shows the heat release rate (HRR) and THR curves of RPIF with EG or PPCP introduced alone. The results show that EG and PPCP effectively reduce the HRR and PHRR of RPIF. So, the flame retardancy is effectively enhanced. However, EG is more effective in weakening the violent combustion behavior of RPIF than PPCP. It can thus better enhance the safety of RPIF when applied alone. A detailed analysis and comparison are presented in the
supporting information section.
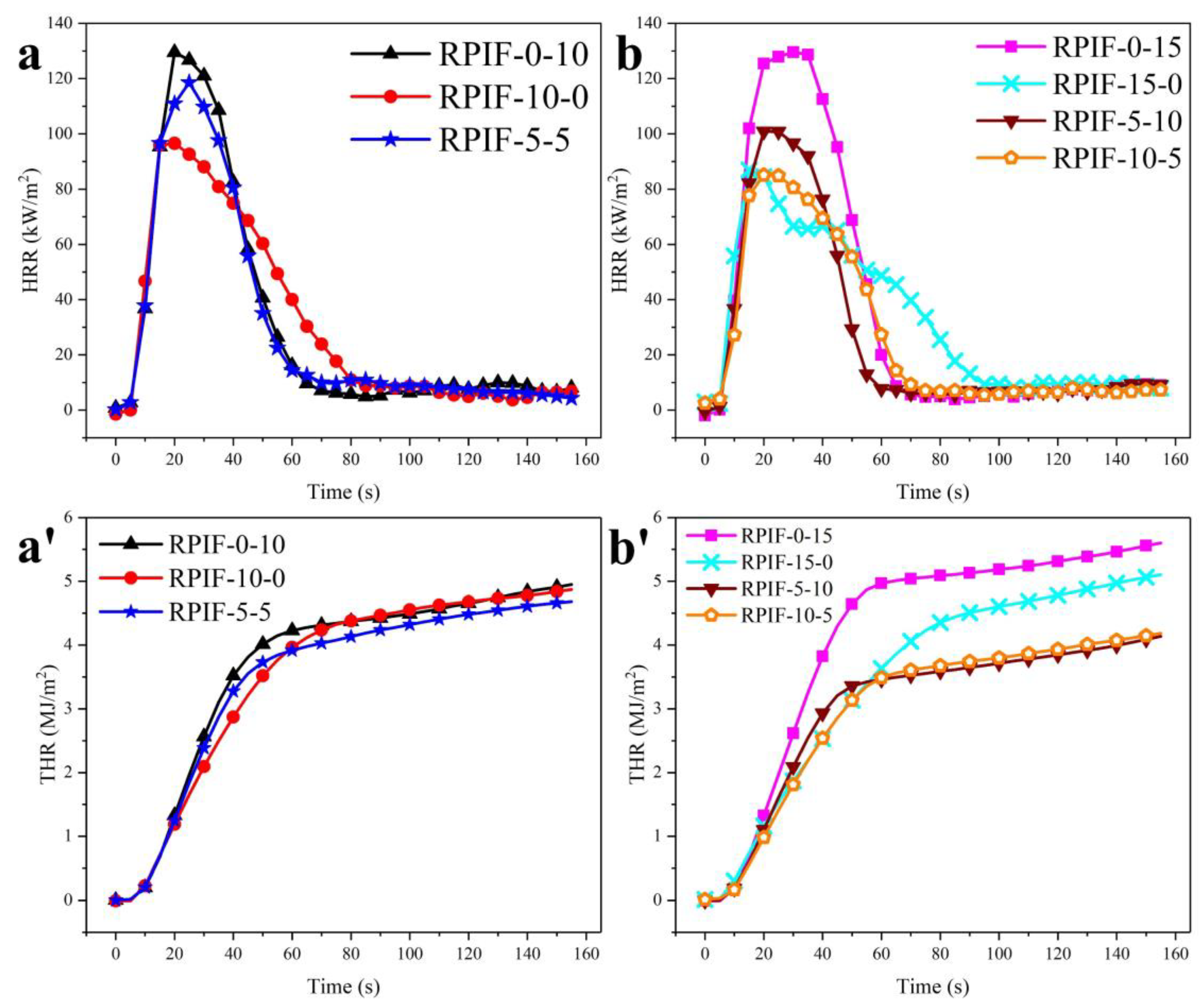
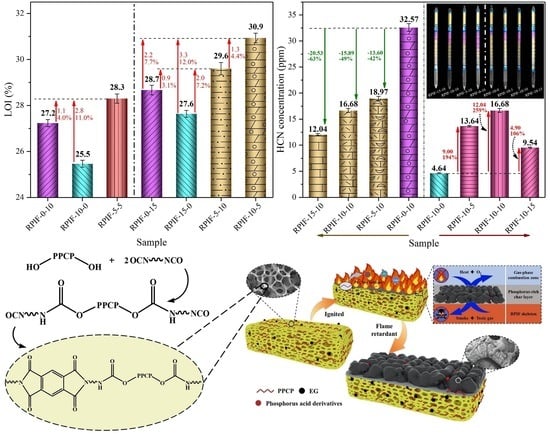
To study the synergistic effects of EG and PPCP on the flame retardancy, combustion behavior, and safety of RPIF, two series of RPIF have been selected. The first includes RPIF-0-10, RPIF-10-0, and RPIF-5-5, each with a total dosage of EG and PPCP of 10 wt. %. The second comprises RPIF-0-15, RPIF-15-0, RPIF-5-10, and RPIF-10-5, each with a total dosage of EG and PPCP of 15 wt. %.
Figure 8 presents HRR and THR’s results. In the first series, the PHRR of RPIF-5-5 is lower than that of RPIF-0-10, but it is still higher than that of RPIF-10-0. However, the THR at 155 s of RPIF-5-5 is only 4.68 MJ/m
2, and the THR curve becomes lower than that of RPIF-0-10 and RPIF-10-0 from about 60 s. For the second series, interestingly, the PHRR and THR of RPIF-10-5 are all lower than those of RPIF-15-0 and RPIF-0-15, while THR at 155 s is only 4.18 MJ/m
2. Furthermore, from the beginning, the THR curve of RPIF-10-5 is consistently lower than those of the other RPIFs in this series.
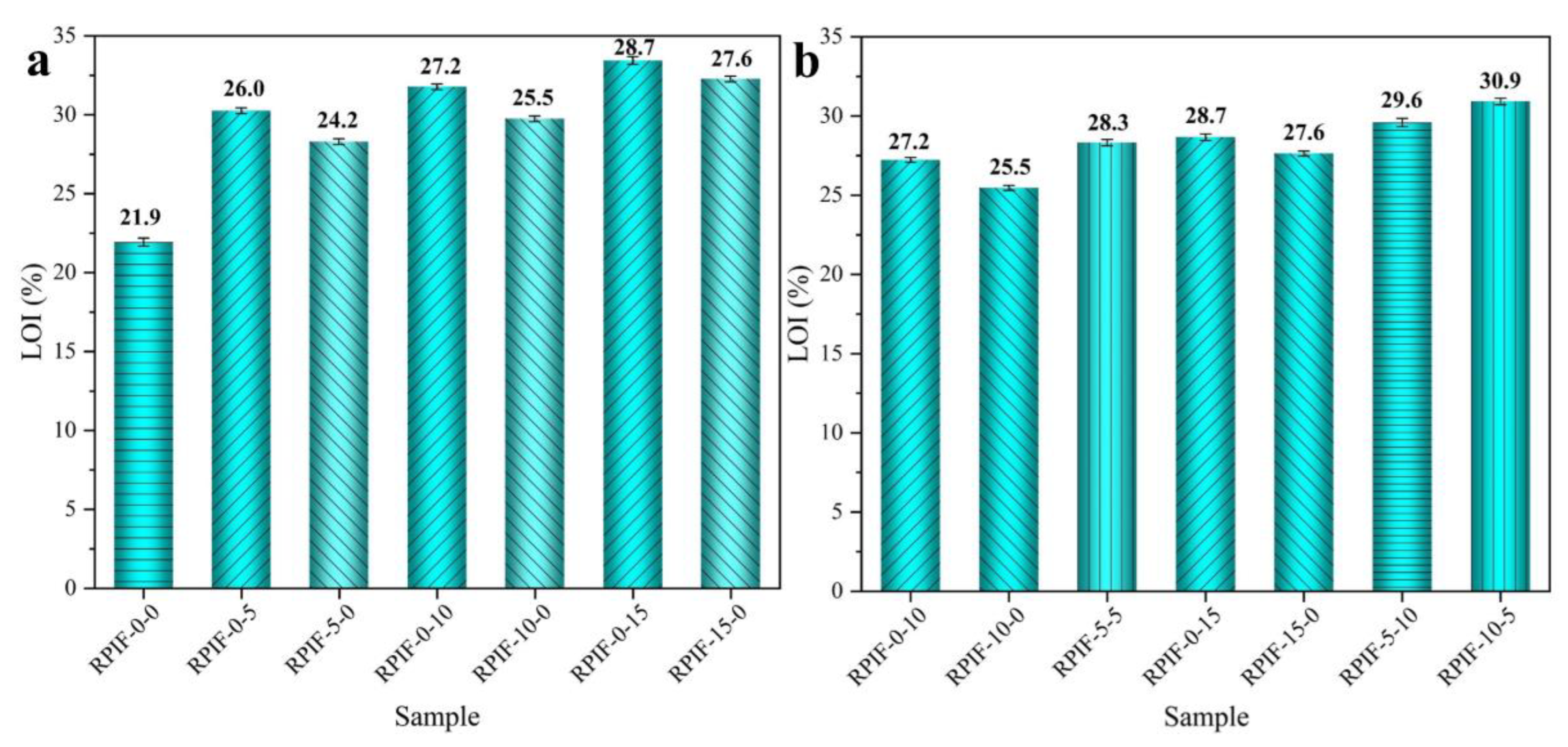
These results effectively demonstrate that the combined use of EG and PPCP can further improve the flame retardancy of RPIF. Furthermore, when comparing the PHRR and THR of RPIF-10-5 and RPIF-5-10, EG exhibits greater synergistic effects of inhibiting the heat releasing behavior of the RPIF combustion flame than PPCP. This, combined with the LOI results obtained in
Figure 5, show that, when the distribution ratio of EG to PPCP is set within the range of 1:2 to 2:1, they exhibit significant synergy for the flame retardancy of RPIF. It can be further stated that the most suitable positive synergy ratio of EG and PPCP for the RPIF system is about 2:1. On the other hand, the price of commercialized EG is lower than synthesized PPCP. Therefore, the combined introduction of EG with PPCP to RPIF can not only decrease the dosage of PPCP, but also further enhance the flame retardancy of RPIF.
From the comprehensive analysis of the LOI, HRR, and THR results, EG can be inferred to be an ideal synergistic flame retardant for PPCP in the RPIF system, while the combined use of EG and PPCP leads to better flame retardancy effects for RPIF than singular use. The source of positive synergy is the formation of a dense flame and a heat barrier carbon layer. During the combustion process, the worm-like graphite carbon formed by EG can provide a primitive unit of a large carbon source for the formation of a carbon layer. The decomposition of RPIF offers a carbon network skeleton to ensure the distribution of worm-like graphite carbon in the 3D interspace. Finally, the crosslinked phosphorus oxide and phosphorus carbon compounds formed by PPCP pyrolysis can effectively bond the worm-like loose porous char and carbon network skeleton, or themselves. The effective linkages prevent the formation of long cracks, so a dense and more stable carbon layer is fabricated to realize enhanced fire resistance and use safety of RPIF. Moreover, as an increasing amount of EG is used, a larger carbon source is supplied, which is more conducive to the formation of a carbon layer, so the most suitable positive synergy ratio of EG and PPCP for RPIF system is about 2:1 in this study.
3.7. Smoke Release and Toxic Fume Generation
Thick smoke particles and their toxic components are major factors causing casualties in fire [
33]. Smoke production release (SPR), peak of SPR (PSPR), carbon monoxide production (COP), total carbon monoxide production (TCOP) obtained by CCT, Ds, and HCN concentration in smoke are analyzed. The results of PSPR, TSP at 155 s, and Ds max of partial RPIF are listed in
Table S2.
The SPR and TSP curves of RPIF with only PPCP or EG introduced are shown in
Figure S2. The results clearly demonstrate the negative effect of PPCP on the smoke release performance of RPIF, and PSPR and TSP all show dramatic increases. The smoke toxicity and hazard to human health of RPIF during the combustion process are also enhanced, attributable to the gas-phase flame retardancy process and mechanism, as stated in the
supporting information. However, EG exhibits diametrically opposite effects to PPCP regarding the smoke release behavior of RPIF. EG shows an effective smoke-suppressant effect on RPIF owing to the adsorption of particulate matter generated during RPIF combustion by porous and worm-like graphitic carbon. Moreover, this research also finds that EG presents more excellent smoke suppression behavior than flame retardancy performance for RPIF. A detailed analysis and comparison are presented in the
supporting information.
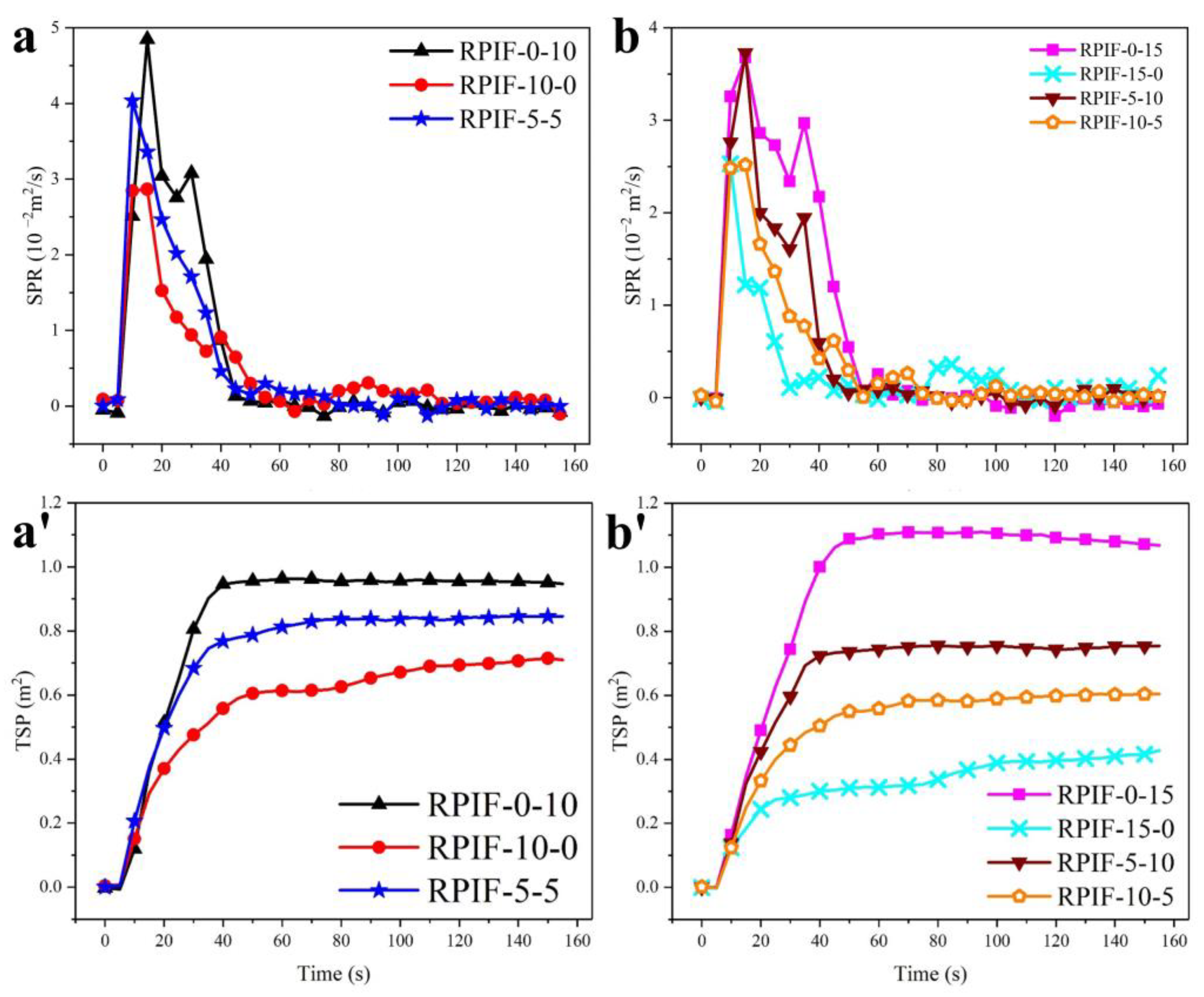
Figure 9 shows the actual smoke suppression effect of simultaneously employing EG and PPCP on RPIF. For RPIF with the sum of EG and PPCP at 10 wt. % (
Figure 9a,a′), the PSPR and TSP of RPIF-5-5 are 0.040 m
2/s and 0.84 m
2, respectively. These results are between those of RPIF-10-0 and RPIF-0-10. For RPIF with the sum of EG and PPCP at 15 wt. % (
Figure 9b,b′), the PSPR and TSP of RPIF-10-5 and RPIF-5-10 are also between those of RPIF-0-15 and RPIF-15-0. These results are logical given the exact opposite effects of EG and PPCP on the smoke release behavior of RPIF.
Fortunately, it is determined that the PSPR and TSP of RPIF-10-5 are all even lower than those of RPIF-10-0. The additional introduction of 5 wt. % PPCP is supposed to increase PSPR and TSP according to the above-mentioned results and those in the
supporting information. These promising results effectively show that the combination of EG and PPCP can synergistically reduce smoke release. Coincidentally, the PSPR and TSP of RPIF-5-10 are all even lower than those of RPIF-5-5. The synergistic effect of EG and PPCP on the smoke release behavior of RPIF is fully manifested. Owing to this synergistic effect, the incorporation or increased dosage of an additive, which should cause enhanced smoke generation, unexpectedly brings about a decrease in smoke release. The key factor is the strengthened char layer for pyrolysis and the bonding effect of the PPCP chain. The phosphoric acid derivatives generated from the pyrolysis of PPCP can adhere to the worm-like loose porous char layer formed by EG. Therefore, the loose char layer cannot be stripped out or blown out of the condensed phase. Furthermore, the greater the amount of PPCP that is incorporated, the stronger the reinforcement that will be produced, and more bonding between the worm-like loose porous char and carbon network skeleton will occur. Thus, a denser and more porous char layer is formed. Compared with the unanchored worm-like loose porous expanded EG, this condensed and fixed porous structure formed by EG and the crosslinked structure can achieve the greater absorption of smoke. Meanwhile, the condenser and condenser char layers block the path of smoke release and delay such release, which directly increases the amount of time for which smoke exists in the carbon layer. This in turn helps the expanded EG to absorb all of the smoke. Thus, an increasing amount of smoke is absorbed and filtered. Hence, although the increased PPCP dosage leads to an increase in TSP in the interior of RPIF during the combustion process, the synergistic effects of EG and PPCP can effectively decrease the TSP value due in the unique carbon layer structure. On the other hand, the formation of a dense and more stable char layer also effectively acts as a fire barrier, which restricts the spread of flame and heat to RPIF’s interior, so the combustion of resin can also be lowered.
In summary, for RPIF-10-0 and RPIF-5-5, the continuous increase in PPCP dosage effectively enhances the flame retardancy and use safety of RPIF. These results further demonstrate the reasonableness and correctness of using EG and PPCP combination in the RPIF system. These two additives play complementary and positive synergistic roles to enhance the safety of RPIF. They are an ideal match.
Ds is an important parameter used to evaluate the safety and smoke hazards of a polymer. There are clear requirements for the Ds value of materials in fire test procedures (FTP) to ensure their qualified security. So, Ds evaluation is important for polymeric foams.
Figure 10 shows Ds curves of RPIF with the total dosage of EG and PPCP of 15 wt. % without or with a flame. The maximum value of the Ds curve during whole test process over 1200 s is defined as Ds max. The Ds max values are listed in
Table S2. In this series of samples, under two different characterization conditions, all the results show that a higher proportion of added EG leads to a lower Ds curve and Ds max of RPIF. The Ds max values of RPIF-10-5 under different test conditions are all lower by about 33% than RPIF-5-10.
The Ds results are consistent with the regularity of TSP data in CCT. This further supports the importance of the combined use of EG and TCPP for ensuring use safety of RPIF. Meanwhile, the Ds max in the presence of a flame is lower than that without a flame. This is because the flame condition can promote the combustion degree and velocity. This is also in line with the classic combustion behavior. Moreover, the Ds max of all samples is lower than 45, which means that the safety of RPIF modified by EG and PPCP in this study meets the requirements of FTP rules for thermal insulation and sound absorption foamed materials. These results prove again that the introduced PPCP increases the generation of smoke particles, but the composited EG can effectively reduce the releasing of smoke particles and weaken the adverse effects on smoke release behavior caused by PPCP.
Meanwhile, in
Table S2 and
Figure 5, although the corresponding CCT results, including the PHRR, THR, PSPR, and TSP of RPIF-10-5 and RPIF-5-10, are all lower than that of RPIF-0-0, and the LOI are all higher than that of RPIF-0-0, only the Ds max values of RPIF-10-5 under different test conditions are still all lower than that of RPIF-0-0, while those of RPIF-5-10 under different test conditions are all higher than for RPIF-0-0. Thus, the comprehensive evaluations of the smoke release behavior and fire behavior tests all preliminarily show that RPIF-10-5 possesses the optimal use safety in this study, and the ratio of EG to PPCP of 2:1 is a suitable value for the RPIF system.
The generation of CO is a very important factor to assess smoke toxicity, toxic fume generation, and the use safety of a polymer during the burning process [
44,
45].
Figure 11 shows the peak value of the CO production curve (PCOP), as well as the COP and TCOP curves of RPIF with total dosage of EG and PPCP at 15 wt. %. Curves with the same colors and symbols as in
Figure 11a,b correspond to the same sample. It can be clearly observed that a higher EG proportion corresponds to lower PCOP and TCOP values under the same total dosage. These data indicate that EG can effectively inhibit the release of CO, while the introduction of PPCP directly promotes CO generation. It also demonstrates the opposite effect of PPCP and EG on toxic fume generation behavior. The PCOP and TCOP of RPIF-10-5 are 3.18 mg/s and 0.22 g, respectively, which also indicate an obvious reduction compared to RPIF-5-10. These results are consistent with those of smoke release behavior, as above-mentioned; they also match with the results obtained in
Section 3.6, which state that the most suitable positive synergy ratio of EG and PPCP for the RPIF system is about 2:1 in terms of flame retardancy performance. It further means that the increased proportion of EG in the composited additive can effectively enhance the flame retardancy, use safety, and low toxic smoke index. This result also indicates that although PPCP is bonded with matrix resin, its existence remains a hugely unfavorable factor for the controlling of the generation and releasing of toxic fumes and smoke. By extinguishing the flame and capturing the oxygen-containing free radicals of PO generated and released from the phosphorous flame-retardant chain segment, this effect induces the incomplete combustion of resin, and finally leads to an increase in CO generation [
30]. However, the common use of EG can effectively limit these drawbacks of PPCP to ensure the fire prevention behavior of RPIF. Thus, EG can be selected as an ideal partner for PPCP in terms of enhancing the use safety of RPIF.
The weakened effect of EG on COP, PCOP, and TCOP is also due to the following reasons, as previous stated. During the combustion process, EG can release steam and other non-flammable gases, so the concentration of gas in the combustion zone is effectively reduced [
38]. Most important is the fact that the generated porous structure of EG also plays an important role in the absorption of toxic fume.
HCN has been considered as the most toxic gas in the smoke produced by polymer combustion [
46]. The HCN concentration in RPIF combustion flue gas is portrayed in
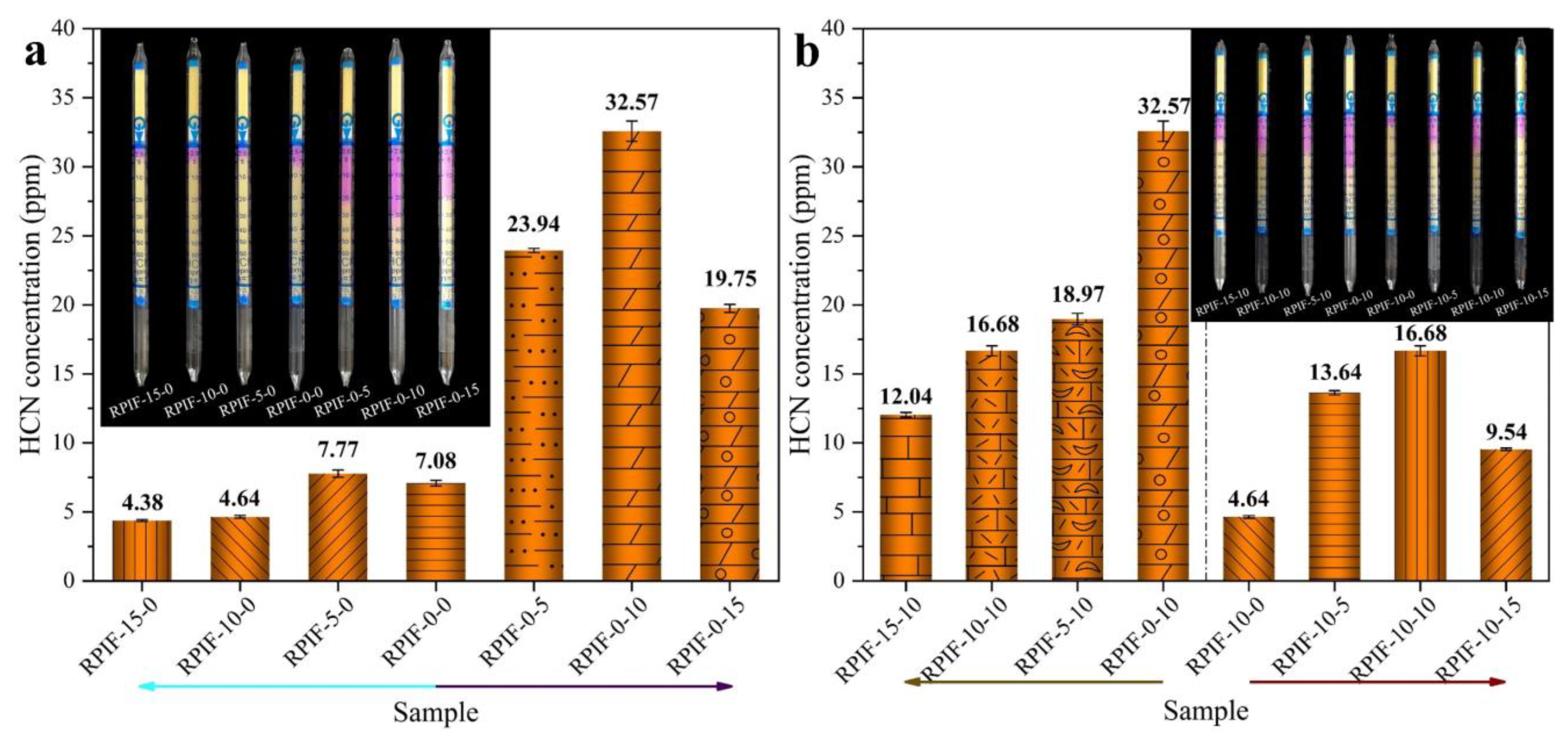
Figure 12.
Figure 12a presents the HCN concentrations of the RPIF-E-0 and RPIF-0-P series. The HCN concentrations of RPIF in the RPIF-E-0 series are all within a low range and at a relatively safe level. For RPIF-10-0 and RPIF-15-0, the HCN concentrations are only 4.64 ppm and 4.38 ppm, respectively. They correspond to reductions of 34% and 38% compared with RPIF-0-0, respectively. This is also due to the distinctive adsorption effect of the loose and porous expanded EG, and the enhanced flame retardancy of RPIF. However, for the RPIF with PPCP, the HCN concentrations are all higher than that of RPIF-0-0 in this study. For the RPIF-0-P series and RPIF-10-P (EG dosage fixed at 10 wt. %, PPCP dosage ranges from 0 to 15 wt. %) series, regardless of whether EG is commonly used, the HCN concentrations all present a significant increasing trend with increasing PPCP dosage. The falling mutation does not appear until the PPCP dosage reaches 15 wt. %. This change can be clearly proven by reference to the columns in
Figure 12. The value of RIF-0-10 even reaches 32.75 ppm, corresponding to an increase of 360% compared with RPIF-0-0. That is deemed a highly toxic state. That of RIF-10-10 also reaches 16.68 ppm and corresponds to an increase of 259% compared to RPIF-10-0. However, the continuous increase in PPCP dosage leads to a decrease in HCN concentration, and the values of RPIF-0-15 and RPIF-10-15 decline to 19.75 ppm and 9.54 ppm, respectively. The HCN concentration of RPIF-10-15 is only 2.46 ppm higher than that of RPIF-0-0, and they are basically at same toxicity level. However, the HCN concentrations of all RPIF applied with PPCP in this study are higher than that of RPIF-0-0, which reflects the negative effect of PPCP on the toxic fume generation of RPIF.
It has been suggested that structures containing an amide structure (-COR-NH-) are broken down to form HCN [
47,
48]. In this study, the introduced PPCP reacts with isocyanate to form urethane, which contains an amide structure; the increase in PPCP dosage directly and continuously enhances the contents of amide group in RPIF, so the HCN concentration will be dramatically increased. The decrease in HCN concentration after the PPCP dosage reaches 15 wt. % may be due to the high flame retardancy of RPIF-0-15 and RPIF-10-15. Because the test conditions in the NES-713 smoke toxicity test chamber correspond to a typical atmospheric environment, the oxygen concentration is also about 21%, so when the flame retardancy of RPIF reaches a certain level, it is difficult to ignite and decompose under normal flame conditions. Then, the original matrix resin and size will be maintained as in
Figure 7, so the HCN concentration is reduced.
Furthermore, from the HCN results of the RPIF-E-10 series (PPCP dosage is fixed at 10 wt. %, EG dosage ranges from 0 to 15 wt. %) compared with the RPIF-10-P and RPIF-0-P series, it is clearly shown that EG plays an effective and ideal partnering role with PPCP in reducing HCN concentration. For the RPIF-E-10 series, the combined use of only 5 wt. % EG can directly reduce the HCN concentration by about 42%, and the efficiency of the reduction reaches 2.72 ppm per 1 wt. % EG. When the EG dosage is continuously enhanced, the HCN concentration is further declined, and when that of RPIF-15-10 is only 12.04 ppm, the weakening efficiency of EG for HCN is still as high as 1.37 ppm per 1 wt. % EG. These all owe to the effective absorption action of the porous structure in expanded EG in relation to the generated HCN. So, the smoke toxicity is effectively ameliorated.
Compared with RPIF-10-P and RPIF-0-P, the fixed 10 wt. % EG dosage lowers the HCN concentration by 10.30 ppm, 15.89 ppm, and 10.21 ppm for RPIF-0-5, RPIF-0-10, and RPIF-0-15, respectively. Further calculations show that the combined use of only 10 wt. % EG can directly decrease the HCN production level to about half of all the RPIF in the original RPIF-0-P series, even though the PPCP dosage is gradually enhanced. Such a large reduction in HCN concentration illustrates the effective action of EG in decreasing the toxic gas production caused by the introduction of PPCP. So, the simultaneous incorporation and combination of EG with PPCP can effectively inhibit the serious toxic gas releasing problem caused by PPCP. This owes to the enhanced flame retardancy of RPIF, which can decrease the decomposition of the matrix resin when subjected to fire, as well as the effective absorption of toxic fumes by expanded EG.
According to the release results of toxic gas, including the CO and HCN studied in this paper, it can be inferred that EG is an excellent and ideal partner for PPCP when preparing RPIF with excellent use safety in the low-toxic gas generation field during the combustion process.
3.8. Discussions of the Synergistic Flame-Retardant Mechanism between EG and PPCP
As above-mentioned, under the synergistic effects of EG and PPCP, RPIF shows excellent use safety. In this section, the flame-retardant effect, smoke-suppressant effect, and toxic fume reduction mechanisms are further proven and discussed according to the composition and morphology of char residues left after CCT. Digital camera and SEM images of partial RPIF (total dosage of EG and PPCP at 15 wt. %) after CCT are shown in
Figure 13. It is obvious that the residue of RPIF-0-0 is thin and has wide cracks (
Figure 13(a-1),(a-2)). Meanwhile, there are many connected pores and holes in the interior of the residue (
Figure 13(a-3)), which allow the free passage of gas and smoke. No cracks are observed on the surface of the RPIF-0-15 residues (
Figure 13(b-1)), while the numbers of connected pores and holes in the interior also decrease significantly (
Figure 13(b-3)). A comparison of
Figure 13(a-2),(b-2) indicates that PPCP had little effect on residue thickness. The surface of the RPIF-15-0 residue (
Figure 13(c-1)) also barely has any cracks. Compared with RPIF-0-15 and RPIF-0-0, the char layer thickness of RPIF-15-0 residue (
Figure 13(c-2)) is enhanced owing to the expansion of EG. However,
Figure 13(c-3) shows that almost no linked skeleton structure can be found after combustion, and the expanded EG is only in a worm-like graphite state and loosely distributed. Although this worm-like loose porous char layer has an excellent barrier effect against a flame, the loosened state also leads to the slow passage of oxygen and heat, and RPIF-15-0 exhibits secondary combustion in the CCT and double peaks in the HRR curve under these two effects. However, when EG and PPCP are used in combination, the linked and dense char layer network (
Figure 13(d-3),(e-3)) composed of a residue skeleton and worm-like graphite can be observed in the microstructure of the char layer, owing to the bonding between the worm-like loose porous char and carbon network skeleton. The phosphoric acid derivatives formed by the thermal decomposition of the phosphorous flame-retardant chain segment in PPCP are deposited layer by layer, and crosslink with the porous expanded EG, so the compactness of the char layer is enhanced and the number of pores is reduced. These changes in structure can have great flame-retardant and smoke-suppressant effects. Moreover, the thickness of char layer is enhanced, so the isolation effect for flames is also improved. Thus, the combined use of EG and PPCP is an effective way to greatly enhance the use safety of RPIF.
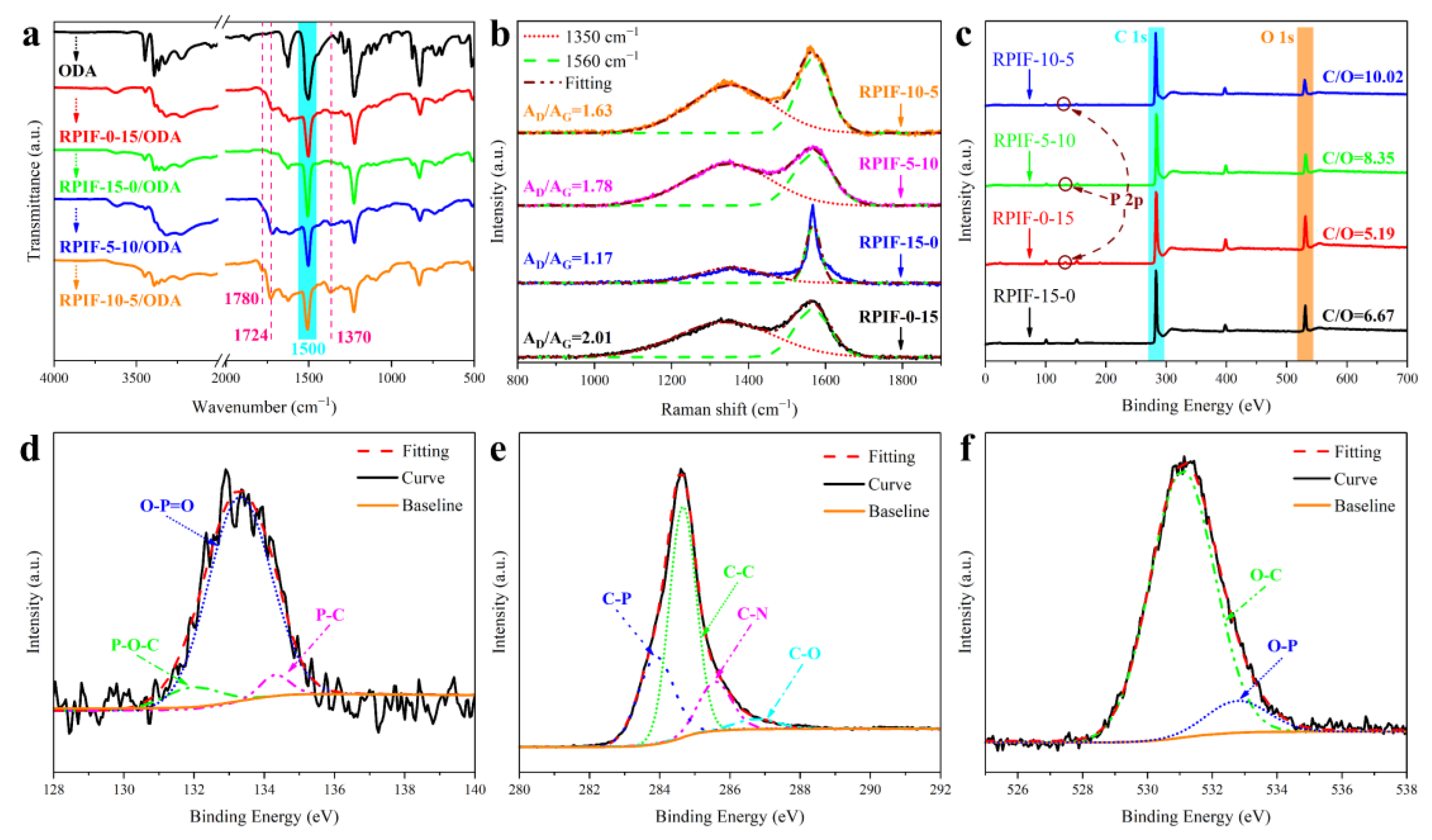
Because no characteristic absorption peak in the FT-IR curves of RPIF residues can be used as the internal standard peak, in order to analyze the composition of char residue and burning degree, the residue is mixed with 4,4’-oxydianiline (ODA) at a mass ratio of 4:1, and the FT-IR curves of the corresponding mixtures are shown in
Figure 14a. A characteristic absorption peak at 1500 cm
−1 owing to the benzene ring in ODA is taken as the internal standard peak [
8]. The results show that the relative intensities of PI characteristic absorption peaks at 1780 cm
−1, 1724 cm
−1, and 1370 cm
−1 in the RPIF-10-5 residue are obviously higher than those of other RPIF residues. This indicates that the char layer of the RPIF-10-5 residue has outstanding heat and oxygen insulation properties, and the matrix burns incompletely at a heat flux of 35 kW/m
2 [
8]. Thus, RPIF-10-5 shows low CCT results and high flame retardancy.
The Raman spectra of residues all show strong characteristic peaks at 1350 cm
−1 and 1560 cm
−1; they belong to D and G bands, respectively (
Figure 14b). The lower the peak area ratio of the D and G bands (AD/AG), the higher the graphitization degree of the char layer [
41]. It is obviously observed that the specific value of the RPIF-15-0 residue is lower than that of the other RPIFs. Hence, the graphitization degree of RPIF-15-0 is the highest. This is caused by the worm-like graphite in the EG. Generally speaking, a higher graphitization degree corresponds to a higher fire-resistant behavior of the char layer; this result also demonstrates the enhanced flame retardancy of RPIF with increased EG dosage. However, SEM images of the RPIF-15-0 residue show that the residue formed by EG is loose and porous. This state can be easily altered upon disturbance by a flame, which reduces the flame-retardant effect. However, RPIF-10-5 residue not only has a higher degree of graphitization (AD/AG = 1.63) than RPIF-0-15 residue, but it also possesses a denser char layer structure than RPIF-15-0, so the flame retardancy of RPIF-10-5 is superior to that of other samples. Meanwhile, the RPIF-5-10 residue’s degree of graphitization is basically equivalent to that of RPIF-10-5, but obvious cracks (
Figure 13(d-1)) are found, and RPIF-10-5 has better safety, further confirming the importance of generating an ideal and dense char layer for RPIF to enhance flame retardancy.
Meanwhile, the XPS survey spectra of the RPIF residue are shown in
Figure 14c, and the elemental contents are listed in
Table S3. In
Figure 14c and
Table S3, the C/O ratio of RPIF-10-5 is obviously greater than those of the others, and that of RPIF-0-0 is only 3.24, which means that the RIF-10-5 residue possesses the highest proportion of carbon. The char layer with a higher carbon content will form the more effective fire protection layer and hinder the transmission of flame. So, RPIF-10-5 presents the highest flame retardancy. In theory, RPIF-15-0 possesses the highest EG dosage and the residue should have a C/O ratio. Following the comparison presented in this paper, it is speculated that the phosphorous flame-retardant chain segment may play another role. That is, the generation of phosphoric acid derivatives from the pyrolysis of PPCP may also be involved in catalytic dehydration and deoxygenation carbonization in the matrix resin, because the C/O ratio of the RPIF-0-15 residue is also higher than that of RPI-0-0.
Figure 14d–f show the fitting analysis of high-resolution P 2p, C 1s, and O 1s XPS spectra of the RPIF-10-5 residue, respectively. The high-resolution P 2p XPS spectra in
Figure 15d show three peaks at 132.1 eV, 133.2 eV, and 134.1 eV. They correspond to the P-O-C, O-P=O, and P-C structures, respectively [
41]. In addition, deconvolution peaks of C-P and O-P appear in the high-resolution C 1s and O 1s XPS spectra (
Figure 14e,f), respectively. The results obtained in this study indicate that the crosslinking phosphorus oxides and phosphorus carbon compounds generated by the thermal decomposition of PPCP are present in the char layer. They can play a chemical crosslinking role for residues and increase the integrity of the carbon layer. Finally, a barrier layer with a denser structure and higher carbon content is obtained, and exerts an excellent flame-retardant effect for RPIF.
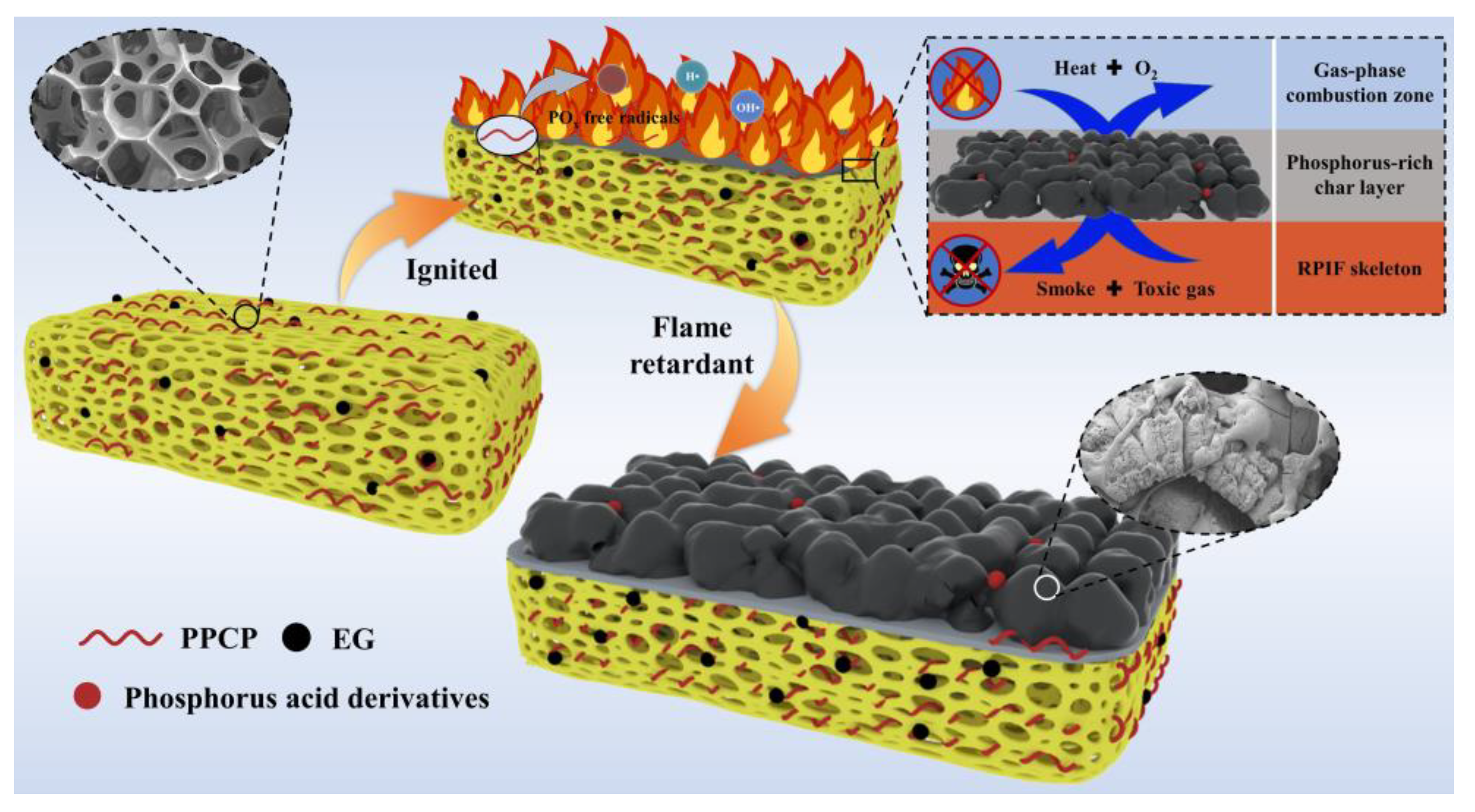
Based on the above analysis, the flame-retardant effect, smoke-suppressant, and toxic fume inhibition mechanism schematics are shown in
Figure 15. When the surface of the RPIF begins to ignite, EG rapidly expands and forms worm-like graphitic carbon, which can adsorb smoke particles and toxic fume, including HCN and CO. Meanwhile, the introduced PPCP starts to degrade before the matrix resin and releases PO radicals to catch H, HO, and hydrocarbyl radicals in the gas-phase combustion zone, so their combustion reaction with oxygen and combustion degree in the gas-phase are all weakened. These two factors directly enhance the flame retardancy and use safety of RPIF. Going a step further, the phosphoric acid derivatives in the condensed phase play a chemical bonding role; they work together with expanded EG to form a char layer with a higher graphitization degree, more complete surface structure, and a higher C/O ratio. Then, an ideal char layer with refractory and isolation functions is obtained by the combination of the introduced EG and PPCP. This directly decreases the combustion degree of RPIF and enhances its use safety, including flame retardancy and toxic fume generation behavior.