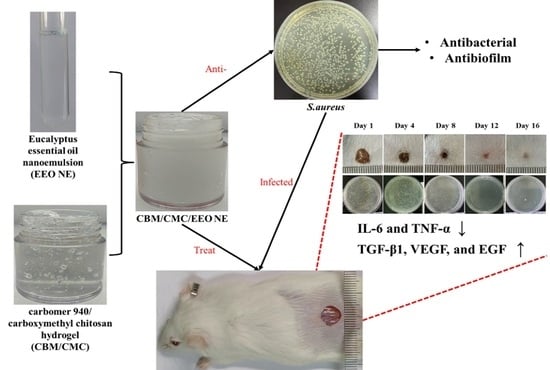
Essential Oil Nanoemulsion Hydrogel with Anti-Biofilm Activity for the Treatment of Infected Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of EEO NEs and EEO NE Hydrogels
2.3. Characterization of EEO NE and CBM/CMC/EEO NE
2.3.1. Basic Properties and the Particle Size Distribution of EEO NE
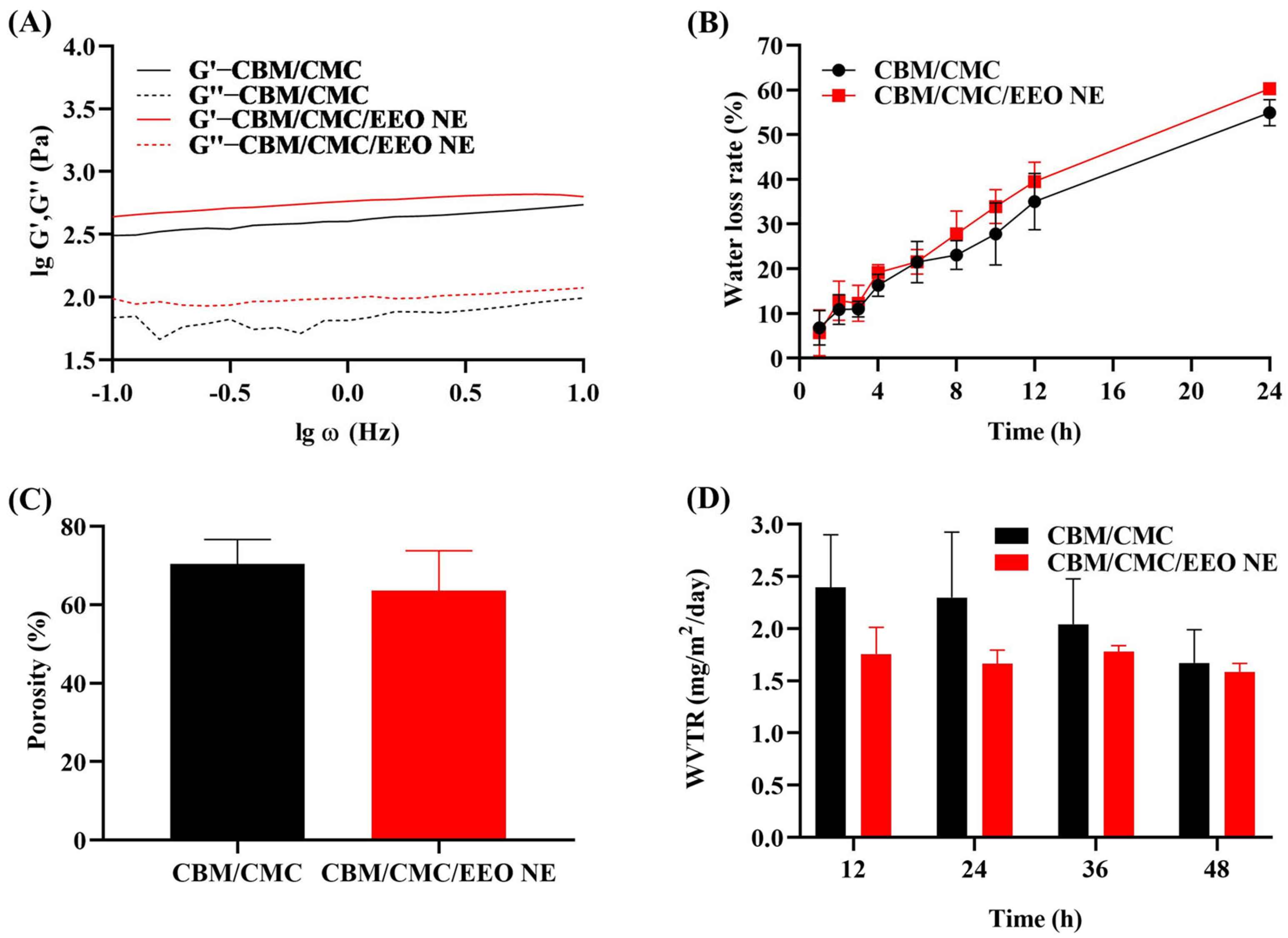
2.3.2. Rheological Measurements of CBM/CMC/EEO NE
2.3.3. Water Loss Rate of CBM/CMC/EEO NE
2.3.4. Porosity of CBM/CMC/EEO NE
2.3.5. Water Vapor Transmission Rate (WVTR) Test for CBM/CMC/EEO NE
2.4. In Vitro Antibacterial Activity
2.4.1. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of EEO NE
2.4.2. Inhibitory Activity Assay of EEO NE and CBM/CMC/EEO NE against Suspended S. aureus
2.4.3. Inhibitory Activity of EEO NE on the S. aureus Biofilm Formation
2.4.4. Determination of the Scavenging Activity of EEO NE on S. aureus Biofilms
2.5. In Vitro Biocompatibility Tests
2.5.1. In Vitro Cytotoxicity Assay
2.5.2. Blood Compatibility Test
2.6. In Vivo Wound Healing Evaluation
2.6.1. Infected Trauma Molding
2.6.2. Analysis of Wound Healing Rates
2.6.3. Trauma Tissue Sampling
2.6.4. Trauma Bacterial Load Testing
2.6.5. Histological Testing
2.6.6. Tissue Cytokine Assays
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of EEO NE and CBM/CMC/EEO NE
3.2. In Vitro Antibacterial Activity
3.3. In Vitro Biocompatibility
3.4. In Vivo Experiments
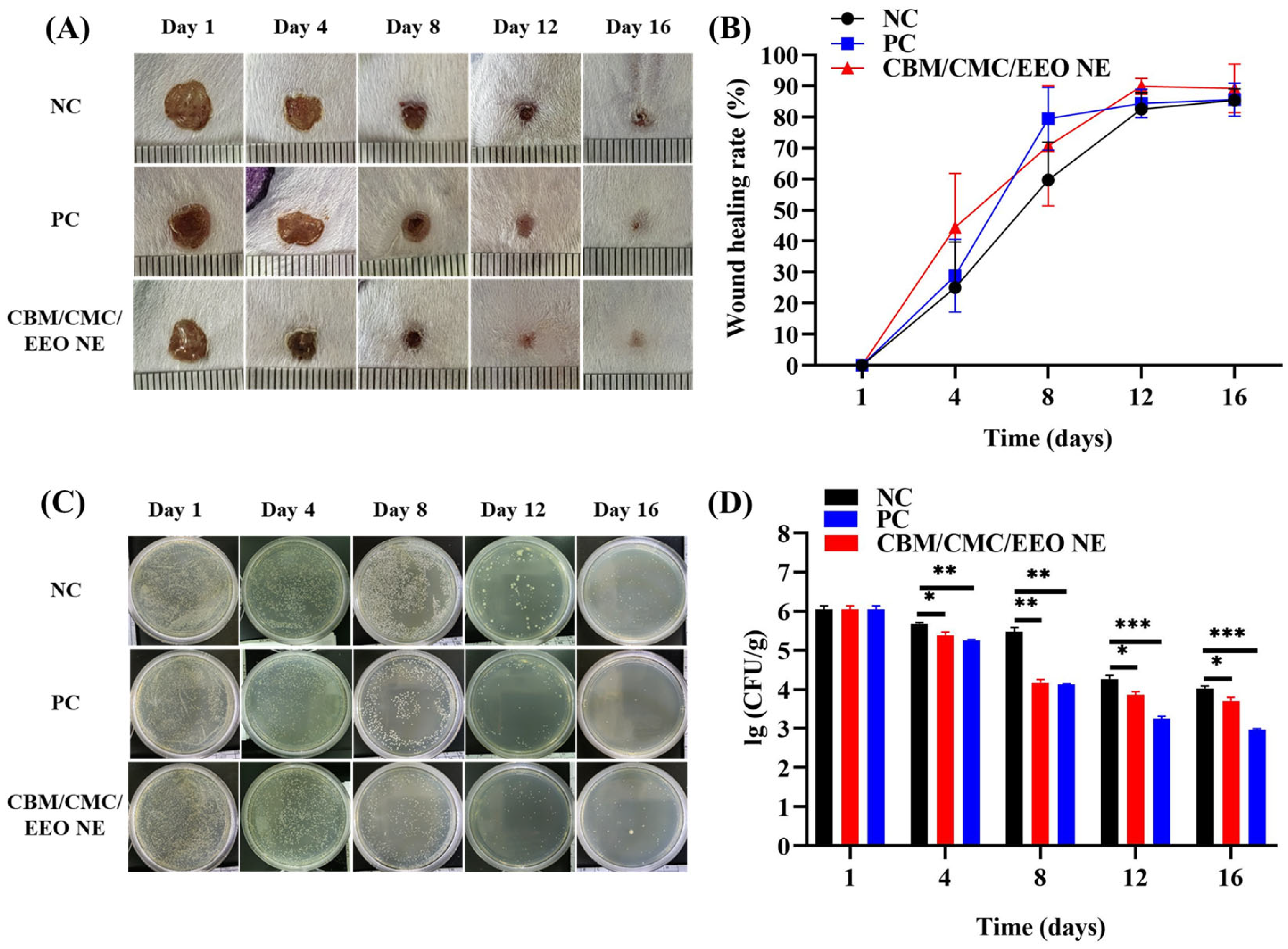
3.4.1. Analysis of Wound Healing
3.4.2. Trauma Bacterial Load Analysis
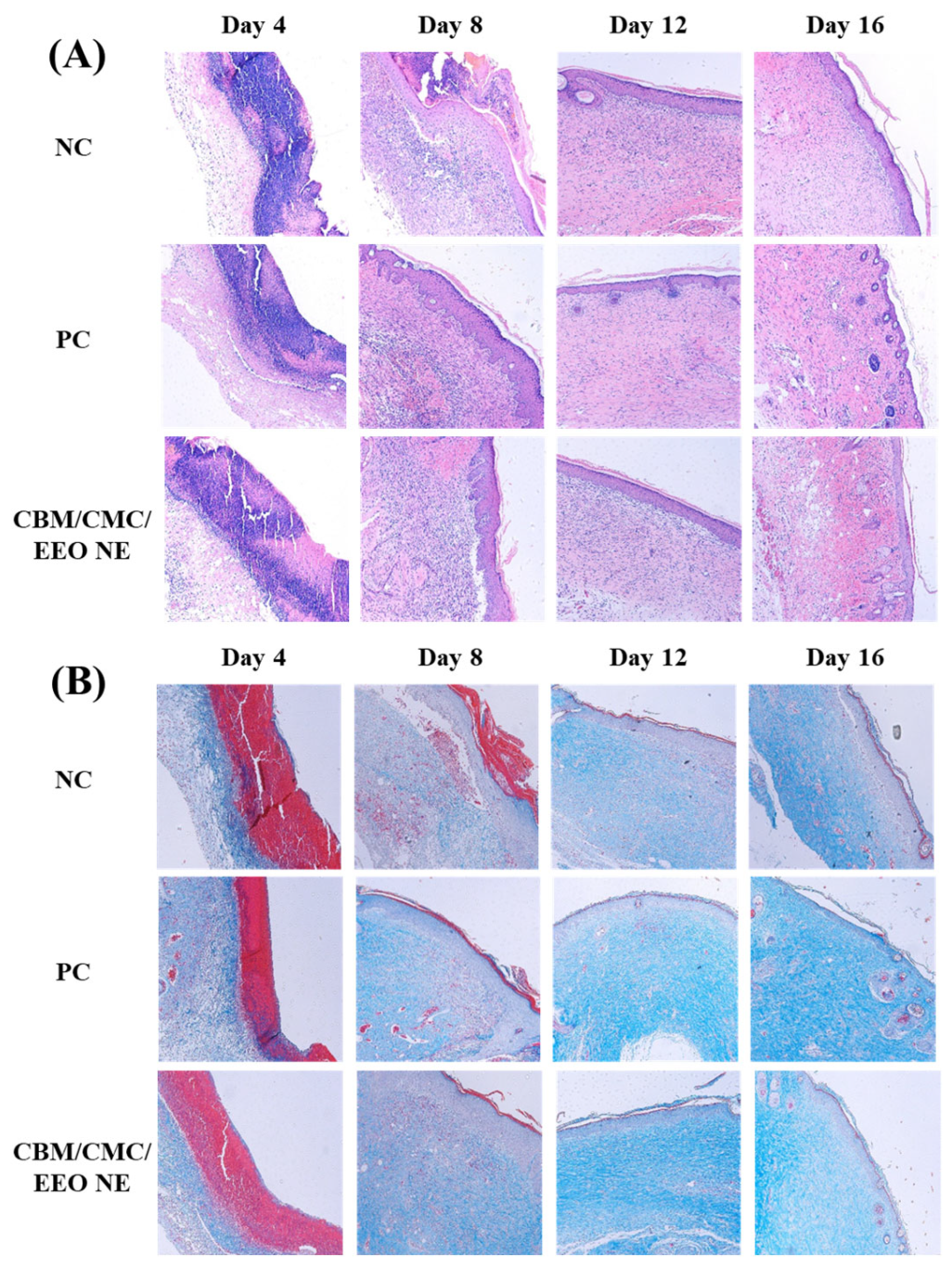
3.4.3. Histological Analysis of the Trauma Surface
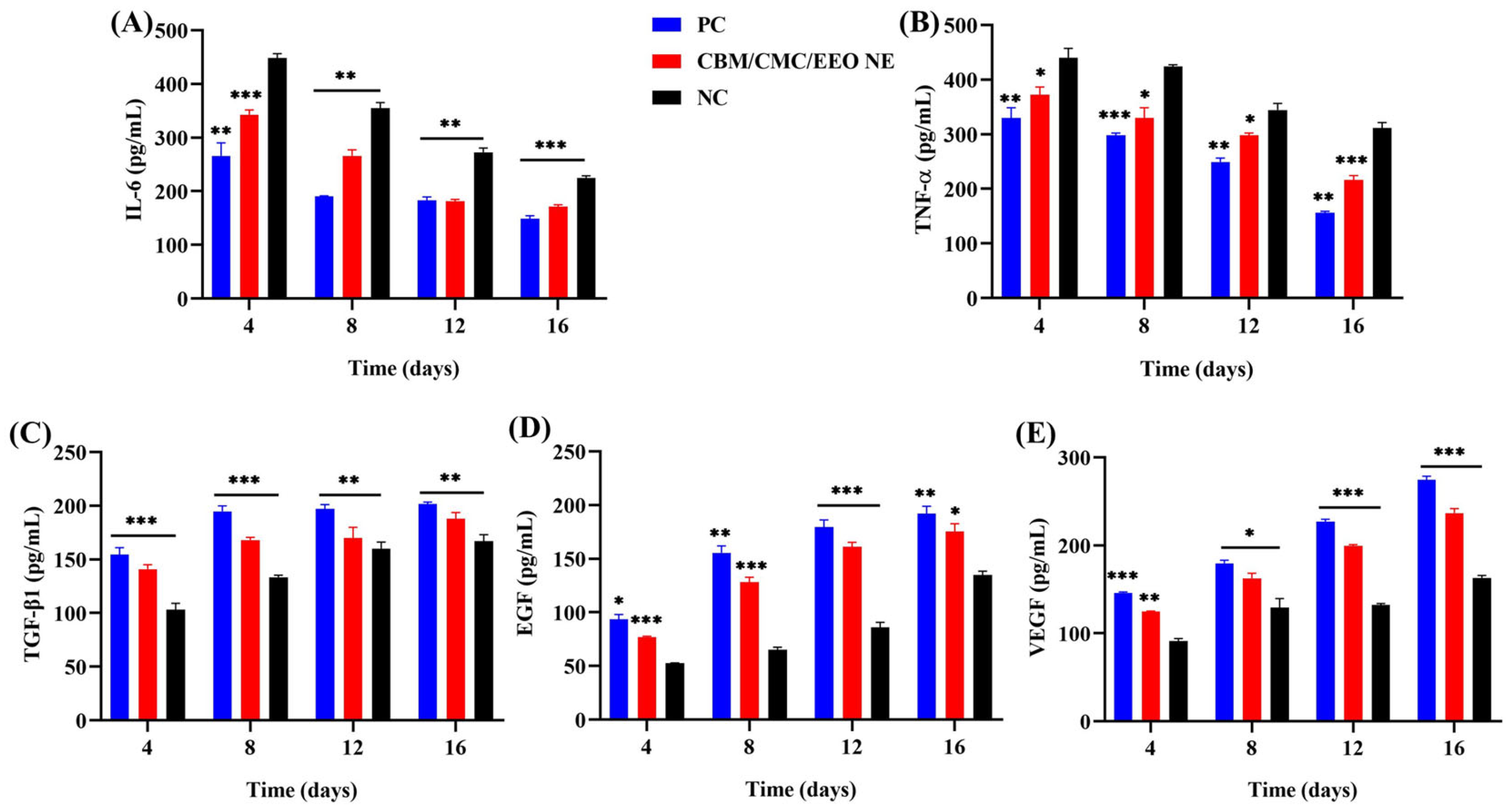
3.4.4. Trauma Tissue Cytokine Analysis
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Alexis, A.; Carrer, D.-P.; Droggiti, D.-I.; Louis, K.; Pistiki, A.; Netea, M.G.; Kapessidou, Y.; Gimarellos-Bourboulis, E.J. Immune responses in relation to the type and time of thermal injury: An experimental study. Injury 2015, 46, 227–232. [Google Scholar] [CrossRef]
- Shu, G.; Xu, D.; Xie, S.; Chang, L.-J.; Liu, X.; Yang, J.; Li, Y.; Wang, X. The antioxidant, antibacterial, and infected wound healing effects of zno quantum dots-chitosan biocomposite. Appl. Surf. Sci. 2023, 611, 155727. [Google Scholar] [CrossRef]
- Chamania, S.; Hemvani, N.; Joshi, S. Burn wound infection: Current problem and unmet needs. Indian J. Burn 2012, 20, 18. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, S.; Wu, S.; Xu, M.; Gao, T.; Wu, Q.; Xu, H.; Liu, Y. Rational design of silver nps-incorporated quaternized chitin nanomicelle with combinational antibacterial capability for infected wound healing. Int. J. Biol. Macromol. 2023, 224, 1206–1216. [Google Scholar] [CrossRef]
- Deng, Q.; Sun, P.; Zhang, L.; Liu, Z.; Wang, H.; Ren, J.; Qu, X. Porphyrin mof dots–based, function-adaptive nanoplatform for enhanced penetration and photodynamic eradication of bacterial biofilms. Adv. Funct. Mater. 2019, 29, 1903018. [Google Scholar] [CrossRef]
- Benoit, D.S.W.; Sims, K.R., Jr.; Fraser, D. Nanoparticles for oral biofilm treatments. ACS Nano 2019, 13, 4869–4875. [Google Scholar] [CrossRef] [PubMed]
- Pletzer, D.; Coleman, S.R.; Hancock, R.E.W. Anti-biofilm peptides as a new weapon in antimicrobial warfare. Curr. Opin. Microbiol. 2016, 33, 35–40. [Google Scholar] [CrossRef]
- Mohammed, Y.H.E.; Manukumar, H.M.; Rakesh, K.P.; Karthik, C.S.; Mallu, P.; Qin, H.-L. Vision for medicine: Staphylococcus aureus biofilm war and unlocking key’s for anti-biofilm drug development. Microb. Pathog. 2018, 123, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment strategies for infected wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef]
- Percival, S.L.; Hill, K.E.; Malic, S.; Thomas, D.W.; Williams, D.W. Antimicrobial tolerance and the significance of persister cells in recalcitrant chronic wound biofilms. Wound Repair Regen. 2011, 19, 1–9. [Google Scholar] [CrossRef]
- Stokes, J.M.; Lopatkin, A.J.; Lobritz, M.A.; Collins, J.J. Bacterial metabolism and antibiotic efficacy. Cell Metab. 2019, 30, 251–259. [Google Scholar] [CrossRef]
- Ma, Y.; Zohaib Aslam, M.; Wu, M.; Nitin, N.; Sun, G. Strategies and perspectives of developing anti-biofilm materials for improved food safety. Food Res. Int. 2022, 159, 111543. [Google Scholar] [CrossRef]
- Huang, B.; Liu, X.; Li, Z.; Zheng, Y.; Wai Kwok Yeung, K.; Cui, Z.; Liang, Y.; Zhu, S.; Wu, S. Rapid bacteria capturing and killing by agnps/n-cd@zno hybrids strengthened photo-responsive xerogel for rapid healing of bacteria-infected wounds. Chem. Eng. J. 2021, 414, 128805. [Google Scholar] [CrossRef]
- Mackevica, A.; Olsson, M.E.; Hansen, S.F. Silver nanoparticle release from commercially available plastic food containers into food simulants. J. Nanoparticle Res. 2016, 18, 5. [Google Scholar] [CrossRef]
- León-Silva, S.; Fernández-Luqueño, F.; López-Valdez, F. Silver nanoparticles (agnp) in the environment: A review of potential risks on human and environmental health. Water Air Soil Pollut. 2016, 227, 306. [Google Scholar] [CrossRef]
- Becker, K.; Schroecksnadel, S.; Geisler, S.; Carriere, M.; Gostner, J.M.; Schennach, H.; Herlin, N.; Fuchs, D. Tio2 nanoparticles and bulk material stimulate human peripheral blood mononuclear cells. Food Chem. Toxicol. 2014, 65, 63–69. [Google Scholar] [CrossRef]
- Carvalho, A.P.A.d.; Conte Junior, C.A. Green strategies for active food packagings: A systematic review on active properties of graphene-based nanomaterials and biodegradable polymers. Trends Food Sci. Technol. 2020, 103, 130–143. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Uliana, A.A.; Tian, M.; Zhang, Y.; Zhang, Y.; Volodin, A.; Simoens, K.; Yuan, S.; Li, J.; et al. Mussel-inspired architecture of high-flux loose nanofiltration membrane functionalized with antibacterial reduced graphene oxide–copper nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 28990–29001. [Google Scholar] [CrossRef]
- Nandi, N.; Gayen, K.; Ghosh, S.; Bhunia, D.; Kirkham, S.; Sen, S.K.; Ghosh, S.; Hamley, I.W.; Banerjee, A. Amphiphilic peptide-based supramolecular, noncytotoxic, stimuli-responsive hydrogels with antibacterial activity. Biomacromolecules 2017, 18, 3621–3629. [Google Scholar] [CrossRef]
- Wu, T.; Huang, J.; Jiang, Y.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Formation of hydrogels based on chitosan/alginate for the delivery of lysozyme and their antibacterial activity. Food Chem. 2018, 240, 361–369. [Google Scholar] [CrossRef]
- Mohamed, A.; Taha, O.; El-Sherif, H.A.-O.; Connerton, P.L.; Hooton, S.P.T.; Bassim, N.D.; Connerton, I.F.; El-Shibiny, A. Bacteriophage ZCSE2 is a Potent Antimicrobial Against Salmonella enterica Serovars: Ultrastructure, Genomics and Efficacy. Viruses 2020, 12, 424. [Google Scholar] [CrossRef]
- Liu, H.-B.; Gao, W.-W.; Tangadanchu, V.K.R.; Zhou, C.-H.; Geng, R.-X. Novel aminopyrimidinyl benzimidazoles as potentially antimicrobial agents: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2018, 143, 66–84. [Google Scholar] [CrossRef]
- Silva, J.C.; Silva Pereira, R.L.; Sampaio de Freitas, T.; Rocha, J.E.; Macedo, N.S.; de Fatima Alves Nonato, C.; Linhares, M.L.; Tavares, D.S.A.; Bezerra de Cuhna, F.A.; Coutinho, H.D.M.; et al. Evaluation of antibacterial and toxicological activities of essential oil of ocimum gratissimum l. And its major constituent eugenol. Food Biosci. 2022, 50, 102128. [Google Scholar] [CrossRef]
- Noshad, M.; Alizadeh Behbahani, B.; Nikfarjam, Z. Chemical composition, antibacterial activity and antioxidant activity of citrus bergamia essential oil: Molecular docking simulations. Food Biosci. 2022, 50, 102123. [Google Scholar] [CrossRef]
- Banday, J.A.; Rather, Z.-u.-k.; Yatoo, G.N.; Hajam, M.A.; Bhat, S.A.; Santhanakrishnan, V.P.; Farozi, A.; Rather, M.A.; Rasool, S. Gas chromatographic-mass spectrometric analysis, antioxidant, antiproliferative and antibacterial activities of the essential oil of prangos pabularia. Microb. Pathog. 2022, 166, 105540. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Cai, K.; Zhang, B.; Tang, S.; Zhang, W.; Liu, W. Antibacterial polysaccharide-based hydrogel dressing containing plant essential oil for burn wound healing. Burn. Trauma 2021, 9, 22–35. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.; Pignatello, R.; Carbone, C. Essential oils: Pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Garcia, C.R.; Malik, M.H.; Biswas, S.; Tam, V.H.; Rumbaugh, K.P.; Li, W.; Liu, X. Nanoemulsion delivery systems for enhanced efficacy of antimicrobials and essential oils. Biomater. Sci. 2022, 10, 633–653. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, S.K.; Pandiyan, R.; Natesan, S.; Chindam, S.; Gouti, A.K.; Sugumaran, A. Fabrication of basil oil nanoemulsion loaded gellan gum hydrogel—Evaluation of its antibacterial and anti-biofilm potential. J. Drug Deliv. Sci. Technol. 2022, 68, 103129. [Google Scholar] [CrossRef]
- Rao, J.; Chen, B.; McClements, D.J. Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef]
- Liu, M.; Pan, Y.; Feng, M.; Guo, W.; Fan, X.; Feng, L.; Huang, J.; Cao, Y. Garlic essential oil in water nanoemulsion prepared by high-power ultrasound: Properties, stability and its antibacterial mechanism against mrsa isolated from pork. Ultrason. Sonochem. 2022, 90, 106201. [Google Scholar] [CrossRef]
- Marinković, J.; Bošković, M.; Tasić, G.; Vasilijević, B.; Marković, D.; Marković, T.; Nikolić, B. Cymbopogon martinii essential oil nanoemulsions: Physico-chemical characterization, antibacterial and antibiofilm potential against enterococcus faecalis. Ind. Crops Prod. 2022, 187, 115478. [Google Scholar] [CrossRef]
- Hou, K.; Xu, Y.; Cen, K.; Gao, C.; Feng, X.; Tang, X. Nanoemulsion of cinnamon essential oil co-emulsified with hydroxypropyl-β-cyclodextrin and tween-80: Antibacterial activity, stability and slow release performance. Food Biosci. 2021, 43, 101232. [Google Scholar] [CrossRef]
- Cecchini, M.E.; Paoloni, C.; Campra, N.; Picco, N.; Grosso, M.C.; Soriano Perez, M.L.; Alustiza, F.; Cariddi, N.; Bellingeri, R. Nanoemulsion of minthostachys verticillata essential oil. In vitro evaluation of its antibacterial activity. Heliyon 2021, 7, e05896. [Google Scholar] [CrossRef] [PubMed]
- Garzoli, S.; Petralito, S.; Ovidi, E.; Turchetti, G.; Laghezza Masci, V.; Tiezzi, A.; Trilli, J.; Cesa, S.; Casadei, M.A.; Giacomello, P.; et al. Lavandula x intermedia essential oil and hydrolate: Evaluation of chemical composition and antibacterial activity before and after formulation in nanoemulsion. Ind. Crops Prod. 2020, 145, 112068. [Google Scholar] [CrossRef]
- Razdan, K.; Garcia-Lara, J.; Sinha, V.R.; Singh, K.K. Pharmaceutical strategies for the treatment of bacterial biofilms in chronic wounds. Drug Discov. Today 2022, 27, 2137–2150. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Saravanan, A.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Naturally derived carbon dots in situ confined self-healing and breathable hydrogel monolith for anomalous diffusion-driven phytomedicine release. ACS Appl. Bio Mater. 2022, 5, 5617–5633. [Google Scholar] [CrossRef]
- Razack, S.A.; Lee, Y.; Shin, H.; Duraiarasan, S.; Chun, B.-S.; Kang, H.W. Cellulose nanofibrils reinforced chitosan-gelatin based hydrogel loaded with nanoemulsion of oregano essential oil for diabetic wound healing assisted by low level laser therapy. Int. J. Biol. Macromol. 2023, 226, 220–239. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, S.-A.; Park, M.; Jeon, E.J.; Kim, M.J.; Kim, J.M.; Kim, H.; Jung, S.; Kim, S.K. Ultrafast real-time pcr in photothermal microparticles. ACS Nano 2022, 16, 20533–20544. [Google Scholar] [CrossRef]
- Lin, W.; Kluzek, M.; Iuster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science 2020, 370, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Correa, J.J.; Gisbert-Garzarán, M.; Mediero, A.; Carias-Cálix, R.A.; Jiménez-Jiménez, C.; Esteban, J.; Vallet-Regi, M. Arabic gum plus colistin coated moxifloxacin-loaded nanoparticles for the treatment of bone infection caused by escherichia coli. Acta Biomater. 2022, 137, 218–237. [Google Scholar] [CrossRef]
- Chang, Y.; McClements, D.J. Optimization of orange oil nanoemulsion formation by isothermal low-energy methods: Influence of the oil phase, surfactant, and temperature. J. Agric. Food Chem. 2014, 62, 2306–2312. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, S.; Liu, Y.; Guo, F.; Miao, Q.; Huang, H. A composite hydrogel scaffold based on collagen and carboxymethyl chitosan for cartilage regeneration through one-step chemical crosslinking. Int. J. Biol. Macromol. 2023, 226, 706–715. [Google Scholar] [CrossRef]
- Song, Z.; Sun, H.; Yang, Y.; Jing, H.; Yang, L.; Tong, Y.; Wei, C.; Wang, Z.; Zou, Q.; Zeng, H. Enhanced efficacy and anti-biofilm activity of novel nanoemulsions against skin burn wound multi-drug resistant mrsa infections. Nanomedicine 2016, 12, 1543–1555. [Google Scholar] [CrossRef]
- Ramalingam, K.; Amaechi, B.T.; Ralph, R.H.; Lee, V.A. Antimicrobial activity of nanoemulsion on cariogenic planktonic and biofilm organisms. Arch. Oral Biol. 2012, 57, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Tótoli, E.G.; Salgado, H.R.N. Miniaturized turbidimetric assay: A green option for the analysis of besifloxacin in ophthalmic suspension. Talanta 2020, 209, 120532. [Google Scholar] [CrossRef]
- Choi, M.; Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Yoo, J.-W. Chitosan-based nitric oxide-releasing dressing for anti-biofilm and in vivo healing activities in mrsa biofilm-infected wounds. Int. J. Biol. Macromol. 2020, 142, 680–692. [Google Scholar] [CrossRef]
- Omer, R.A.; Hama, J.R.; Rashid, R.S.M. The effect of dextran molecular weight on the biodegradable hydrogel with oil, synthesized by the michael addition reaction. Adv. Polym. Technol. 2017, 36, 120–127. [Google Scholar] [CrossRef]
- Omer, R.A.; Hughes, A.; Hama, J.R.; Wang, W.; Tai, H. Hydrogels from dextran and soybean oil by uv photo-polymerization. J. Appl. Polym. Sci. 2015, 132, 41446. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, K.; Liu, Y.; Yue, Y.; Liu, Y.; Guo, F. Essential Oil Nanoemulsion Hydrogel with Anti-Biofilm Activity for the Treatment of Infected Wounds. Polymers 2023, 15, 1376. https://doi.org/10.3390/polym15061376
Cai K, Liu Y, Yue Y, Liu Y, Guo F. Essential Oil Nanoemulsion Hydrogel with Anti-Biofilm Activity for the Treatment of Infected Wounds. Polymers. 2023; 15(6):1376. https://doi.org/10.3390/polym15061376
Chicago/Turabian StyleCai, Kun, Yang Liu, Yan Yue, Yuancheng Liu, and Fengbiao Guo. 2023. "Essential Oil Nanoemulsion Hydrogel with Anti-Biofilm Activity for the Treatment of Infected Wounds" Polymers 15, no. 6: 1376. https://doi.org/10.3390/polym15061376
APA StyleCai, K., Liu, Y., Yue, Y., Liu, Y., & Guo, F. (2023). Essential Oil Nanoemulsion Hydrogel with Anti-Biofilm Activity for the Treatment of Infected Wounds. Polymers, 15(6), 1376. https://doi.org/10.3390/polym15061376