Storage Stability of 6FDA-DMB Polyamic Acid Solution Detected by Gel Permeation Chromatography Coupled with Multiple Detectors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of (6FDA-DMB) PAA Concentrated Solution
2.3. Storage Conditions of (6FDA-DMB) PAA Concentrated and Diluted Solution
2.4. GPC-RI-MALLS-VIS Characterization
3. Results and Discussion
3.1. Molecular Parameter Characterization of (6FDA-DMB) PAA
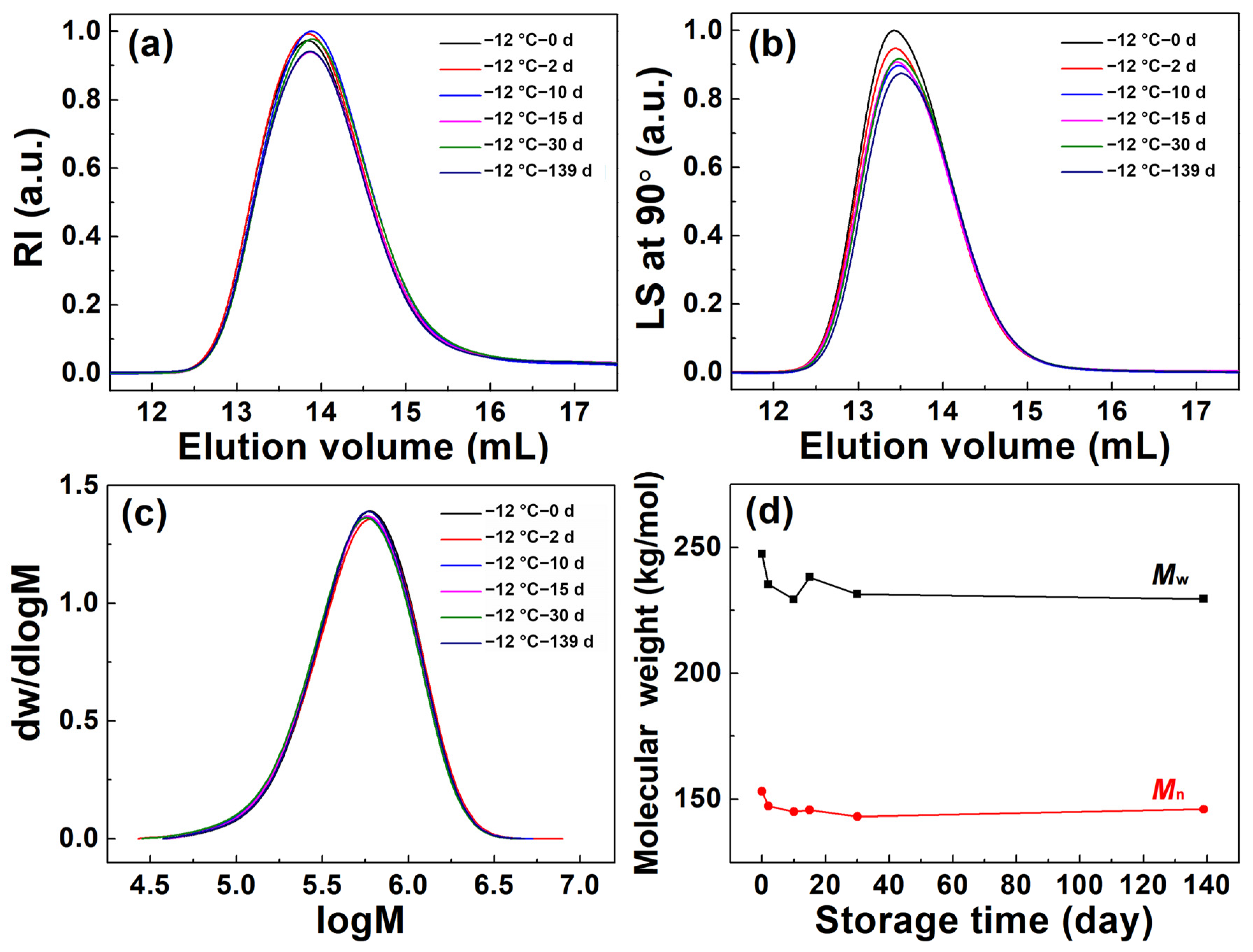
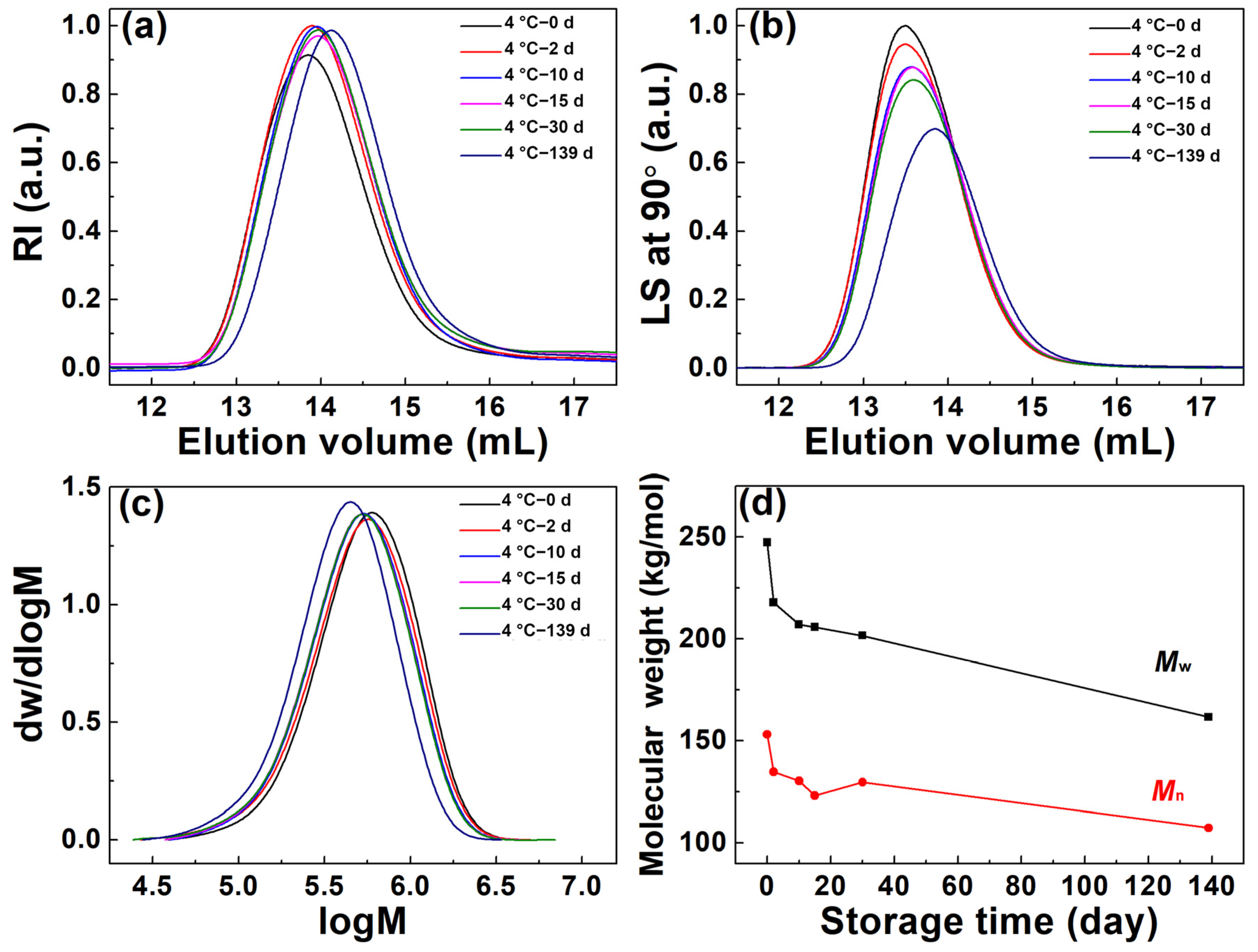
3.2. Molecular Parameter Changes of (6FDA-DMB) PAA in Concentrated Solution under Different Storage Conditions
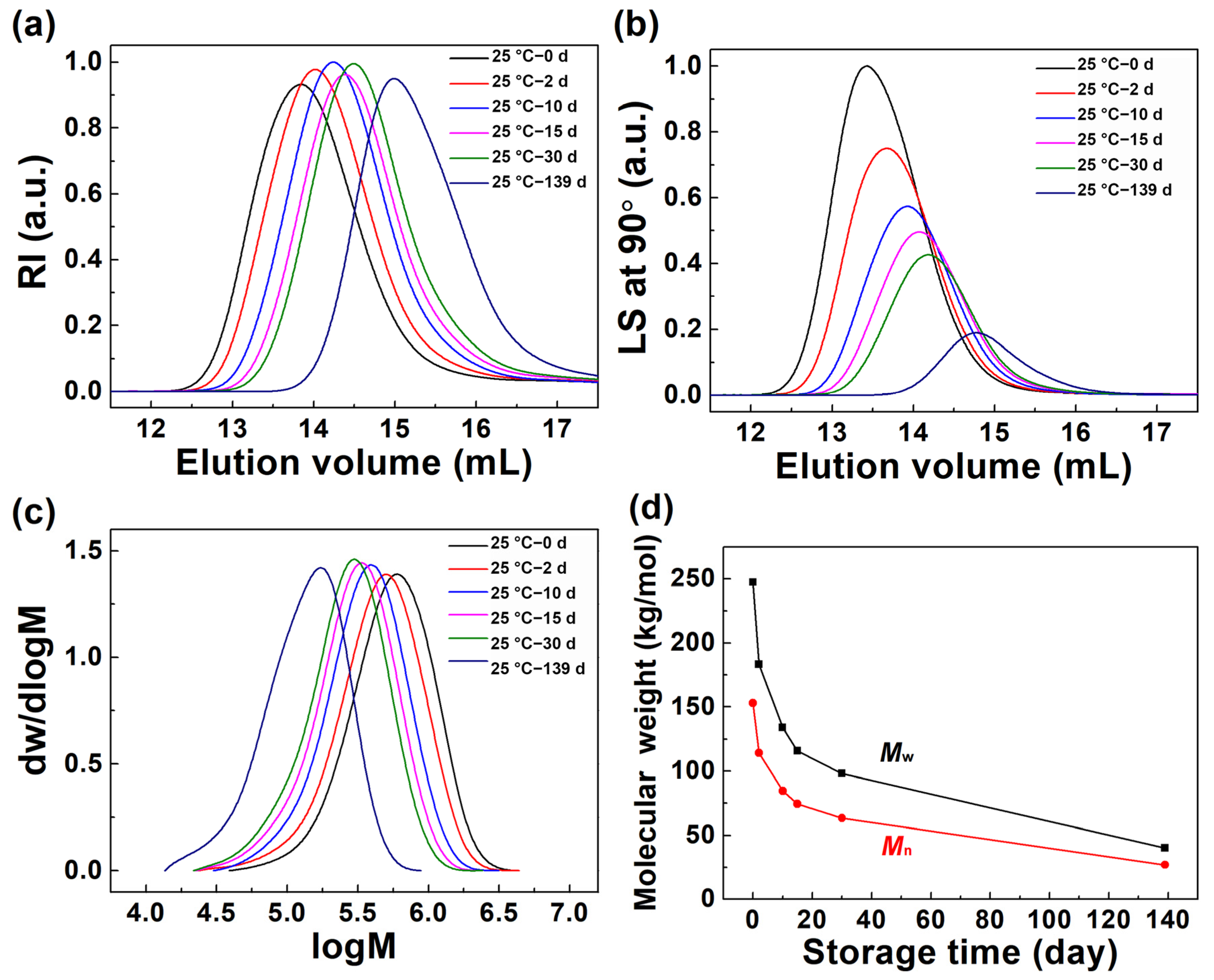
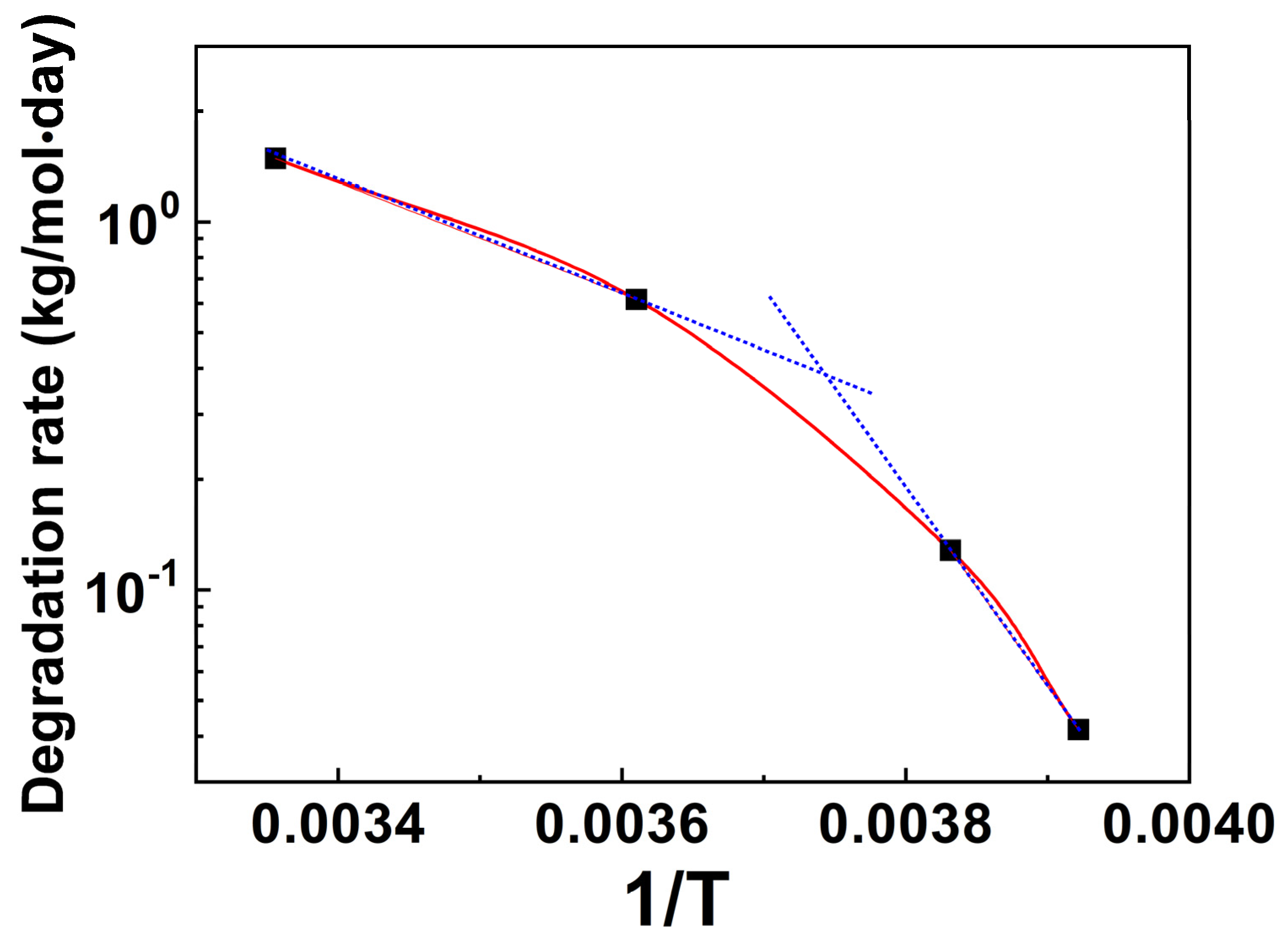
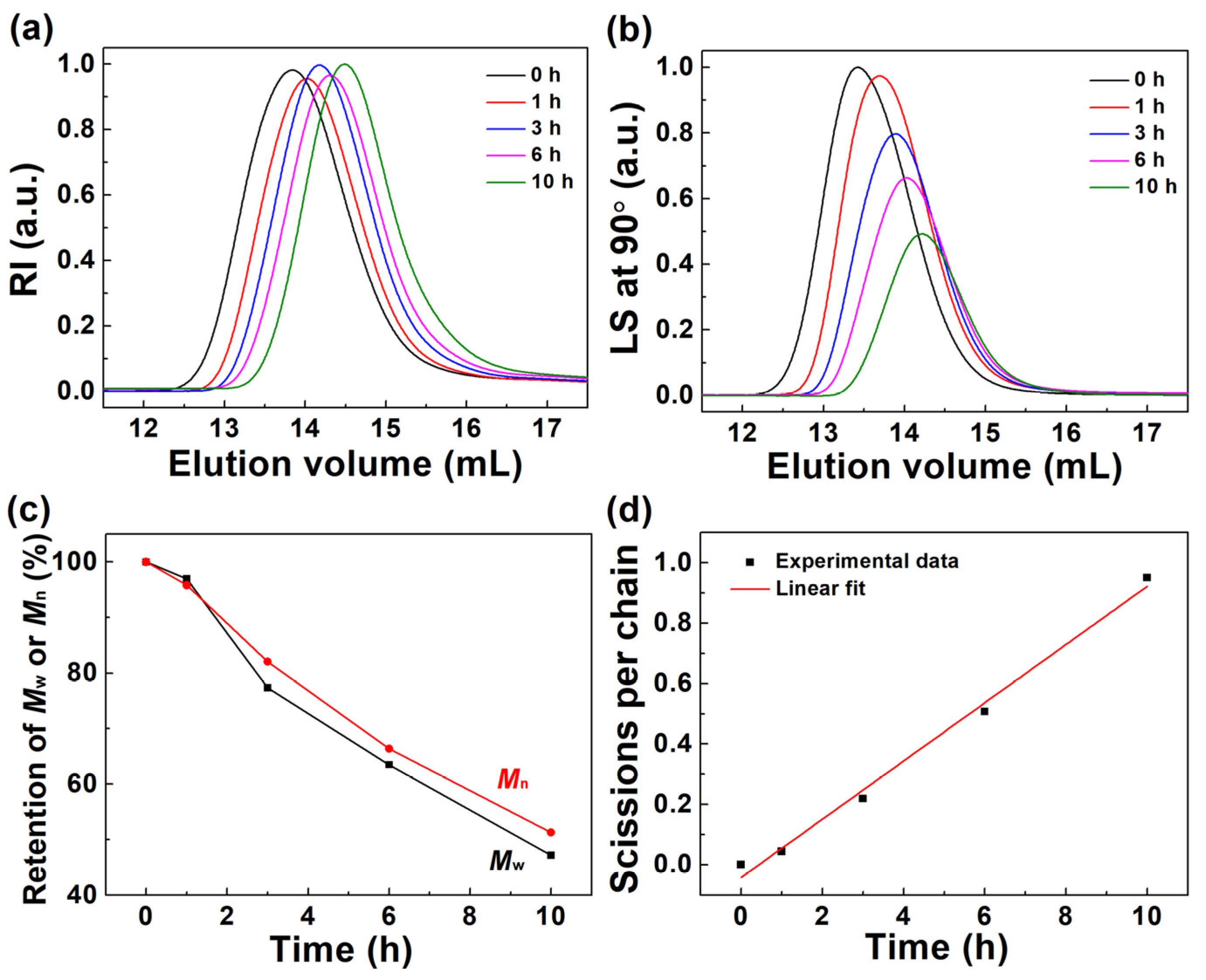
3.3. Molecular Parameter Change of (6FDA-DMB) PAA in Diluted Solution Stored at 25 °C for Different Times
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tao, R.; Zahertar, S.; Torun, H.; Liu, Y.R.; Wang, M.; Lu, Y.C.; Luo, J.T.; Vernon, J.; Binns, R.; He, Y.; et al. Flexible and integrated sensing platform of acoustic waves and metamaterials based on polyimide-coated woven carbon fibers. ACS Sens. 2020, 5, 2563–2569. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shi, J.; Wang, T.; Zheng, S.; Lv, W.; Chen, X.; Yang, J.; Zeng, M.; Hu, N.; Su, Y.; et al. High-performance wearable sensor inspired by the neuron conduction mechanism through gold-induced sulfur vacancies. ACS Sens. 2022, 7, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.L.; Jiang, M.; Li, R.Y.; Qi, S.L.; Wu, D.Z. Structure and properties of polyimide fiber prepared from polyamic acid solution with high solid content and low viscosity. Mater. Lett. 2022, 312, 131628. [Google Scholar] [CrossRef]
- Unsal, E.; Cakmak, M. Real-time characterization of physical changes in polyimide film formation: From casting to imidization. Macromolecules 2013, 46, 8616–8627. [Google Scholar] [CrossRef]
- Bower, G.M.; Frost, L.W. Aromatic polyimides. J. Polym. Sci. Part A Gen. Pap. 1963, 1, 3135–3150. [Google Scholar] [CrossRef]
- Sroog, C.E.; Endrey, A.L.; Abramo, S.V.; Berr, C.E.; Edwards, W.M.; Olivier, K.L. Aromatic polypyromellitimides from aromatic polyamic acids. J. Polym. Sci. Part A Gen. Pap. 1965, 3, 1373–1390. [Google Scholar] [CrossRef]
- Varma, I.K.; Goel, R.N.; Varma, D.S. Stability of polyamic acids and polyimides. Angew. Makromol. Chem. 1977, 64, 101–113. [Google Scholar] [CrossRef]
- Miwa, T.; Numata, S. A mechanism describing polyamic acid-solution viscosity change on storage at high-temperature. Polymer 1989, 30, 893–896. [Google Scholar] [CrossRef]
- Kreuz, J.A. Hydrolyzes of polyamic-acid solutions. J. Polym. Sci. Part A Polym. Chem. 1990, 28, 3787–3793. [Google Scholar] [CrossRef]
- Tong, Y.J.; Liu, T.X.; Veeramani, S.; Chung, T.S. Bulk viscosity and its unstable behavior upon storage in polyimide precursor solutions. Ind. Eng. Chem. Res. 2002, 41, 4266–4272. [Google Scholar] [CrossRef]
- Krishnan, P.S.G.; Vora, R.H.; Chung, T.S.; Uchimura, S.I.; Sasaki, N. Studies on ionic salt of polyamic acid and related compounds. J. Polym. Res. 2004, 11, 299–308. [Google Scholar] [CrossRef]
- Cai, D.D.; Su, J.F.; Huang, M.; Liu, Y.H.; Wang, J.J.; Dai, L.X. Synthesis, characterization and hydrolytic stability of poly (amic acid) ammonium salt. Polym. Degrad. Stab. 2011, 96, 2174–2180. [Google Scholar] [CrossRef]
- Frost, L.W.; Kesse, I. Spontaneous degradation of aromatic polypromellitamic acids. J. Appl. Polym. Sci. 1964, 8, 1039–1051. [Google Scholar] [CrossRef]
- Walker, C.C. High-performance size exclusion chromatography of polyamic acid. J. Polym. Sci. Part A Polym. Chem. 1988, 26, 1649–1657. [Google Scholar] [CrossRef]
- Cotts, P.M.; Volksen, W. Solution characterization of polyamic acids and polyimides. ACS Symp. Ser. 1984, 242, 227–237. [Google Scholar]
- Kreuz, J.A.; Goff, D.L. A study of polyamic-acid interchange reactions. MRS Online Proc. Libr. 1991, 227, 11–21. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.Y.; Han, C.C. Understanding the viscosity decline of poly(amic acid) solution from the correlation point of view. Polymer 2020, 202, 122728. [Google Scholar] [CrossRef]
- Cotts, P.M. Polyelectrolyte effects in low-angle light-scattering from solutions of polyamic acid in organic-solvents. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 923–930. [Google Scholar] [CrossRef]
- Wallach, M.L. Aromatic poly(amic acids): Fundamental structural studies. J. Polym. Sci. Part B Polym. Phys. 1967, 5, 653–662. [Google Scholar] [CrossRef]
- Hong, M.; Liu, W.; Liu, Y.G.; Dai, X.M.; Kang, Y.; Li, R.; Bao, F.; Qiu, X.P.; Pan, Y.X.; Ji, X.L. Improved characterization on molecular weight of polyamic acids using gel permeation chromatography coupled with differential refractive index and multi-angle laser light scattering detectors. Polymer 2022, 260, 125370. [Google Scholar] [CrossRef]
- Berry, G.C. Thermodynamic and conformational properties of polystyrene.I. Light-scattering studies on dilute solutions of linear polystyrenes. J. Chem. Phys. 1966, 44, 4550–4564. [Google Scholar] [CrossRef]
- Bender, M.L.; Chow, Y.-L.; Chloupek, F. Intramolecular catalysis of hydrolytic reactions. Ii. The hydrolysis of phthalamic acid. J. Am. Chem. Soc. 1958, 80, 5380–5384. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]




| Sample | Mw (kg/mol) | Mn (kg/mol) | Mw/Mn | Rg,z (nm) | [η] (mL/g) | Recovery (%) |
|---|---|---|---|---|---|---|
| Original PAA | 247.3 | 153.1 | 1.62 | 29.6 | 161.4 | 91.5 |
| Storage Time (day) | Mw (kg/mol) | Mn (kg/mol) | Mw/Mn | Rg,z (nm) | [η] (mL/g) | Recovery (%) |
|---|---|---|---|---|---|---|
| 0 | 247.3 | 153.1 | 1.62 | 29.6 | 161.4 | 91.5 |
| 2 | 251.0 | 154.0 | 1.63 | 30.4 | 165.1 | 99.6 |
| 10 | 243.3 | 151.7 | 1.60 | 29.4 | 173.5 | 98.3 |
| 15 | 253.6 | 151.6 | 1.67 | 30.3 | 176.2 | 99.8 |
| 30 | 241.8 | 147.7 | 1.64 | 29.4 | 171.0 | 98.9 |
| 139 | 253.1 | 150.8 | 1.68 | 30.7 | 169.5 | 98.7 |
| Storage Time (day) | Mw (kg/mol) | Mn (kg/mol) | Mw/Mn | Rg,z (nm) | [η] (mL/g) | Recovery (%) |
|---|---|---|---|---|---|---|
| 0 | 247.3 | 153.1 | 1.62 | 29.6 | 161.4 | 91.5 |
| 2 | 235.3 | 147.2 | 1.60 | 29.3 | 160.5 | 99.7 |
| 10 | 229.3 | 144.9 | 1.58 | 28.6 | 168.9 | 95.0 |
| 15 | 238.2 | 145.6 | 1.64 | 29.6 | 176.2 | 96.0 |
| 30 | 231.4 | 143.1 | 1.62 | 28.9 | 169.3 | 98.6 |
| 139 | 229.5 | 145.9 | 1.57 | 28.6 | 159.8 | 93.9 |
| Storage Time (day) | Mw (kg/mol) | Mn (kg/mol) | Mw/Mn | Rg,z (nm) | [η] (mL/g) | Recovery (%) |
|---|---|---|---|---|---|---|
| 0 | 247.3 | 153.1 | 1.62 | 29.6 | 161.4 | 91.5 |
| 2 | 217.9 | 134.7 | 1.62 | 28.0 | 153.6 | 99.2 |
| 10 | 207.0 | 130.4 | 1.59 | 27.7 | 156.0 | 97.9 |
| 15 | 205.8 | 123.0 | 1.67 | 27.3 | 158.4 | 98.8 |
| 30 | 201.6 | 129.6 | 1.56 | 27.1 | 151.9 | 99.3 |
| 139 | 161.6 | 107.3 | 1.51 | 23.4 | 127.0 | 95.4 |
| Storage Time (day) | Mw (kg/mol) | Mn (kg/mol) | Mw/Mn | Rg,z (nm) | [η] (mL/g) | Recovery (%) |
|---|---|---|---|---|---|---|
| 0 | 247.3 | 153.1 | 1.62 | 29.6 | 161.4 | 91.5 |
| 2 | 183.2 | 114.2 | 1.60 | 25.9 | 137.5 | 99.9 |
| 10 | 133.7 | 84.6 | 1.58 | 21.5 | 117.2 | 98.3 |
| 15 | 115.8 | 74.4 | 1.56 | 20.6 | 105.4 | 99.5 |
| 30 | 98.1 | 63.5 | 1.54 | 18.8 | 94.8 | 97.5 |
| 139 | 40.1 | 27.0 | 1.49 | 11.6 | 51.5 | 99.6 |
| Storage Time (h) | Mw (kg/mol) | Mn (kg/mol) | Mw/Mn | [η] (mL/g) | Recovery (%) |
|---|---|---|---|---|---|
| 0 | 247.3 | 153.1 | 1.62 | 161.4 | 91.5 |
| 1 | 239.8 | 146.7 | 1.64 | 116.6 | 94.3 |
| 3 | 191.3 | 125.6 | 1.52 | 102.5 | 91.7 |
| 6 | 157.0 | 101.6 | 1.55 | 91.7 | 91.1 |
| 10 | 116.7 | 78.5 | 1.49 | 73.6 | 91.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.; Liu, W.; Gao, R.; Li, R.; Liu, Y.; Dai, X.; Kang, Y.; Qiu, X.; Pan, Y.; Ji, X. Storage Stability of 6FDA-DMB Polyamic Acid Solution Detected by Gel Permeation Chromatography Coupled with Multiple Detectors. Polymers 2023, 15, 1360. https://doi.org/10.3390/polym15061360
Hong M, Liu W, Gao R, Li R, Liu Y, Dai X, Kang Y, Qiu X, Pan Y, Ji X. Storage Stability of 6FDA-DMB Polyamic Acid Solution Detected by Gel Permeation Chromatography Coupled with Multiple Detectors. Polymers. 2023; 15(6):1360. https://doi.org/10.3390/polym15061360
Chicago/Turabian StyleHong, Mei, Wei Liu, Runxiang Gao, Rui Li, Yonggang Liu, Xuemin Dai, Yu Kang, Xuepeng Qiu, Yanxiong Pan, and Xiangling Ji. 2023. "Storage Stability of 6FDA-DMB Polyamic Acid Solution Detected by Gel Permeation Chromatography Coupled with Multiple Detectors" Polymers 15, no. 6: 1360. https://doi.org/10.3390/polym15061360
APA StyleHong, M., Liu, W., Gao, R., Li, R., Liu, Y., Dai, X., Kang, Y., Qiu, X., Pan, Y., & Ji, X. (2023). Storage Stability of 6FDA-DMB Polyamic Acid Solution Detected by Gel Permeation Chromatography Coupled with Multiple Detectors. Polymers, 15(6), 1360. https://doi.org/10.3390/polym15061360





