Influence of Polyols on the In Vitro Biodegradation and Bioactivity of 58S Bioactive Sol–Gel Coatings on AZ31B Magnesium Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. 58S Sol Preparation
2.3. Dip Coating Process
2.4. Characterisations of the 58S Sols and Coatings
2.4.1. Ellipsometry Characterization
2.4.2. X-ray Diffraction Studies
2.4.3. FT-IR Analysis
2.4.4. Contact Angle Measurements
2.4.5. Hydrogen Evolution Studies
2.4.6. pH Evaluation
2.4.7. Immersion Studies in Hank’s Solution
2.4.8. Electrochemical Characterization of the 58S-Coated Samples
3. Results and Discussions
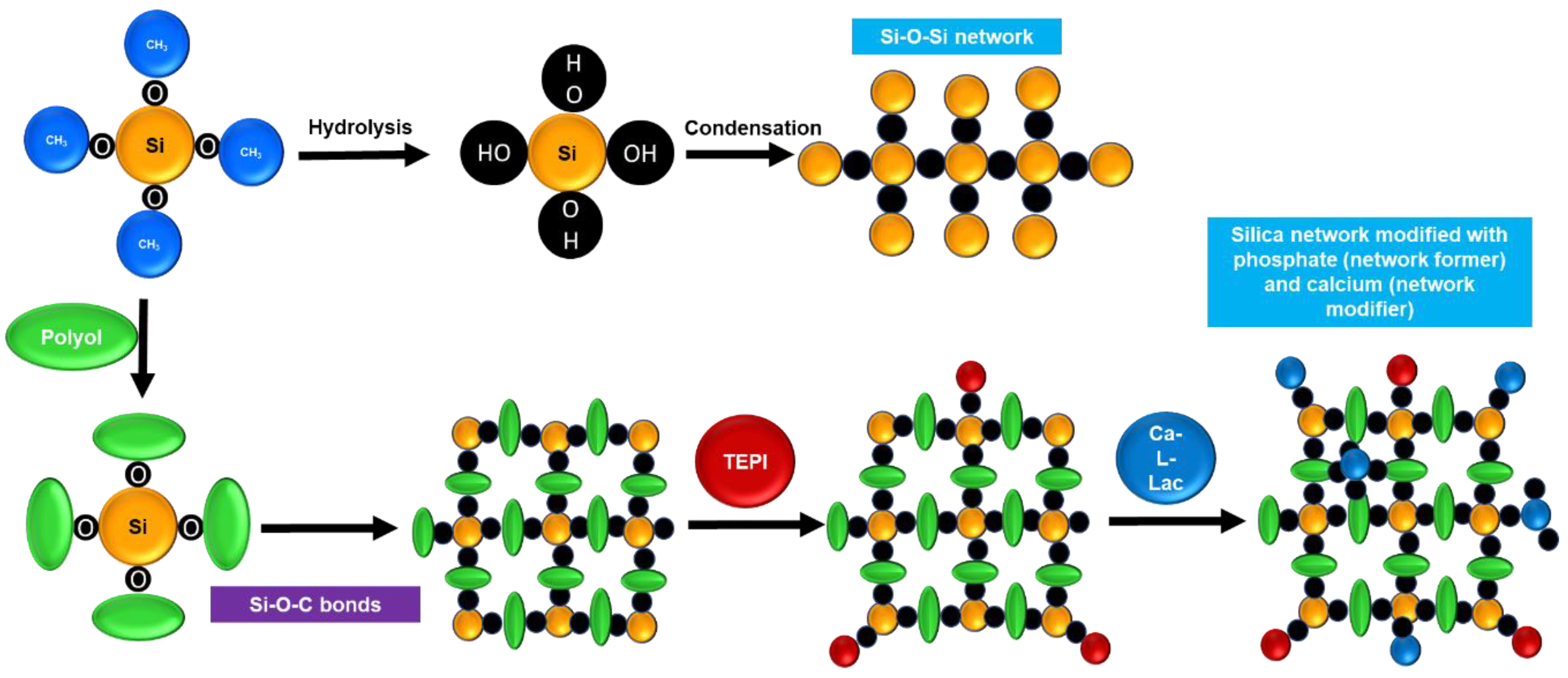
3.1. Characterization of 58S Sols without and with Different Polyols and Their Coating Deposition
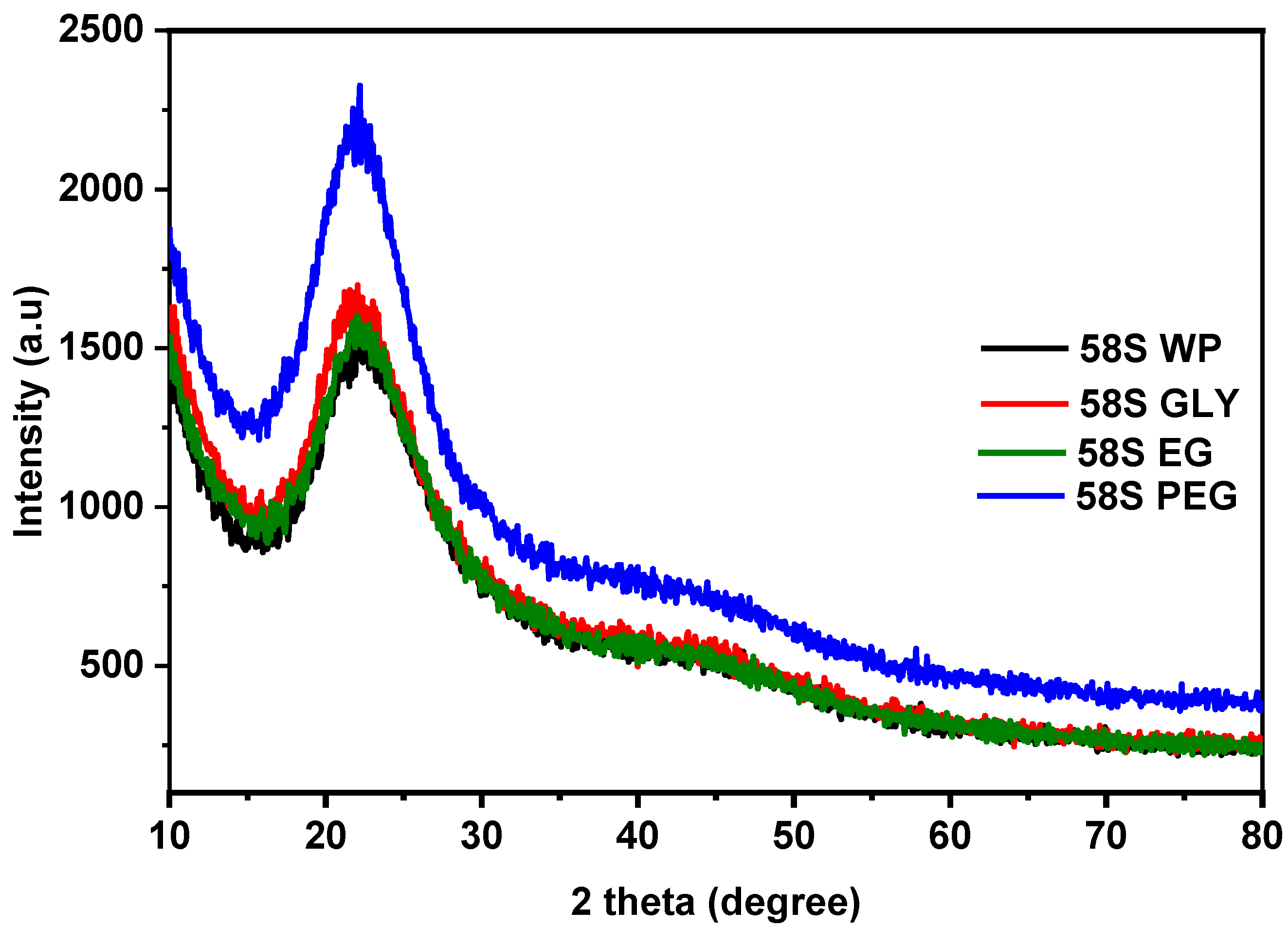
3.2. X-ray Diffraction Studies
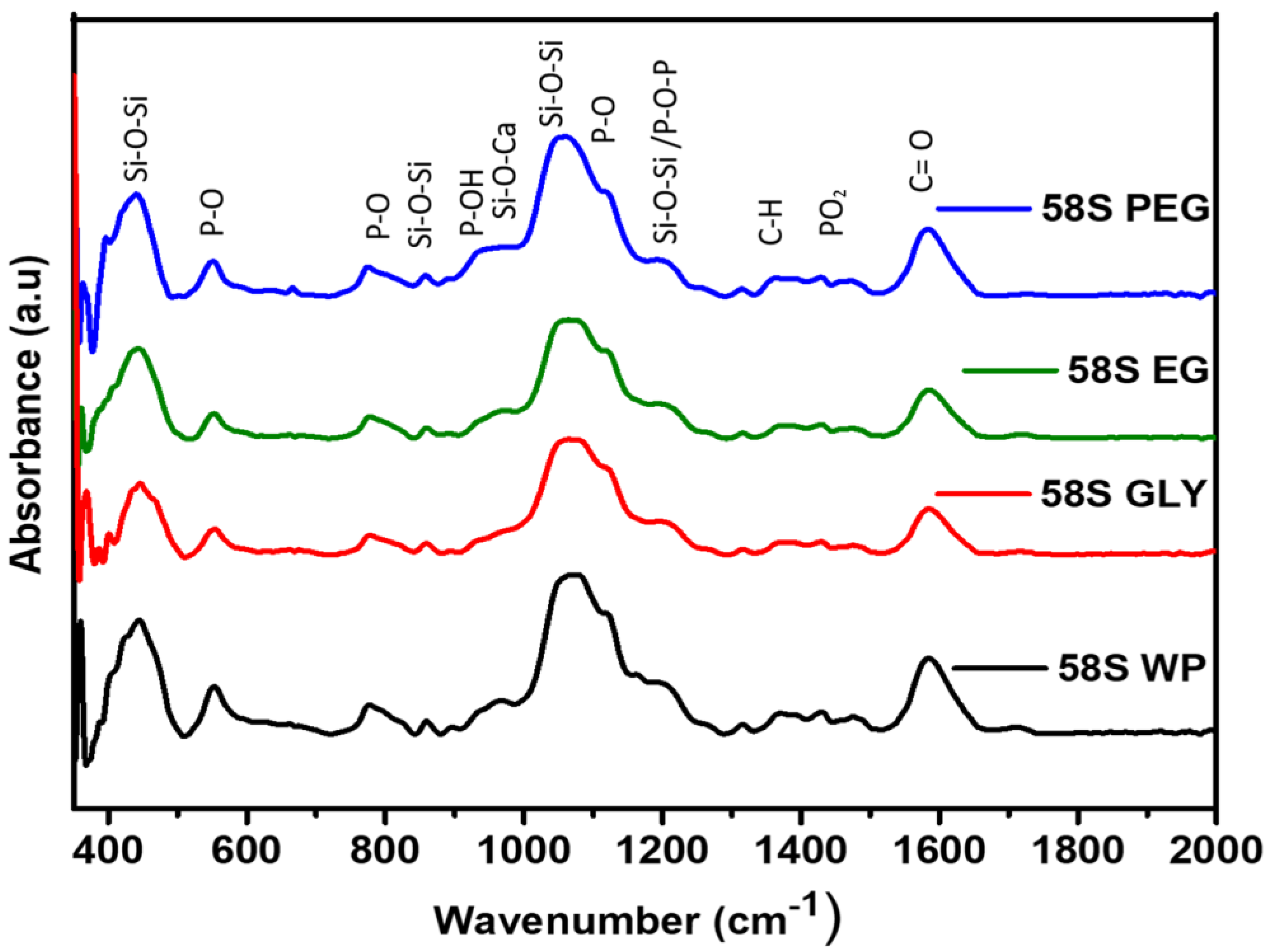
3.3. FT-IR Analysis
3.4. Contact Angle Measurements
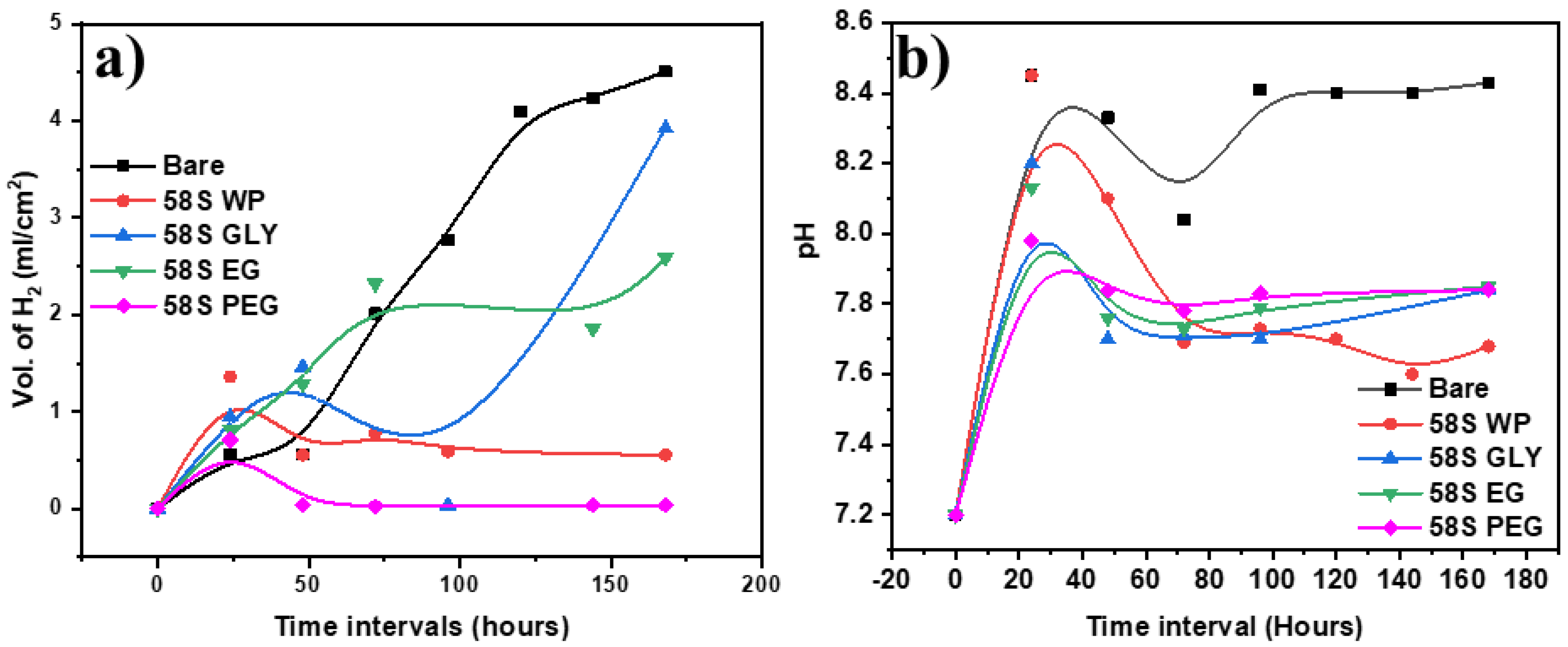
3.5. Hydrogen Evolution and pH Evaluation
3.6. Immersion Studies in Hank’s Solution
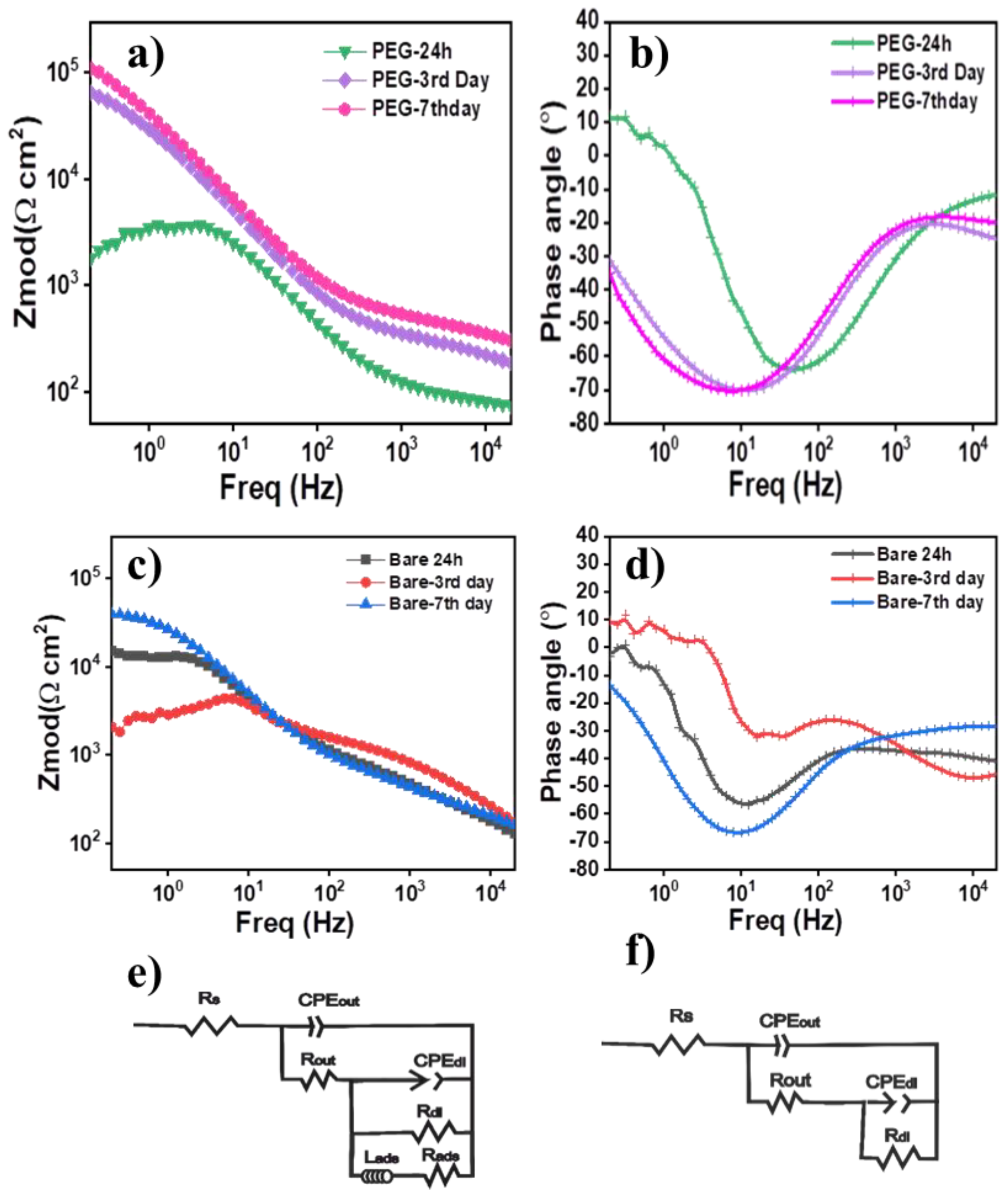
3.7. Degradation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.Imarcgroup.Com/Bio-Implants-Market8 (accessed on 15 February 2023).
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg Alloys for Orthopedic Implants—A Review. J. Magnes. Alloy. 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Mamidi, N.; Flores Otero, J.F. Metallic and Carbonaceous Nanoparticles for Dentistry Applications. Curr. Opin. Biomed. Eng. 2023, 25, 100436. [Google Scholar] [CrossRef]
- Mamidi, N.; García, R.G.; Martínez, J.D.H.; Briones, C.M.; Martínez Ramos, A.M.; Tamez, M.F.L.; del Valle, B.G.; Segura, F.J.M. Recent Advances in Designing Fibrous Biomaterials for the Domain of Biomedical, Clinical, and Environmental Applications. ACS Biomater. Sci. Eng. 2022, 8, 3690–3716. [Google Scholar] [CrossRef] [PubMed]
- García-Estrada, P.; García-Bon, M.A.; López-Naranjo, E.J.; Basaldúa-Pérez, D.N.; Santos, A.; Navarro-Partida, J. Polymeric Implants for the Treatment of Intraocular Eye Dieases: Trends in Biodegradable and Non-Biodegradable Materials. Pharmaceutics 2021, 13. [Google Scholar]
- Shekhawat, D.; Singh, A.; Bhardwaj, A.; Patnaik, A. A Short Review on Polymer, Metal and Ceramic Based Implant Materials A Short Review on Polymer, Metal and Ceramic Based Implant Materials. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Jaipur, India, 5–6 November 2020; Volume 1017, p. 012038. [Google Scholar]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of Biodegradable, Implantable Devices towards Clinical Translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Seuba, J.; Maire, E.; Adrien, J.; Meille, S.; Deville, S. Mechanical Properties of Unidirectional, Porous Polymer/Ceramic Composites for Biomedical Applications. Open Ceramics 2021, 8, 100195. [Google Scholar] [CrossRef]
- Naghavi, S.A.; Lin, C.; Sun, C.; Tamaddon, M.; Basiouny, M.; Garcia-Souto, P.; Taylor, S.; Hua, J.; Li, D.; Wang, L.; et al. Stress Shielding and Bone Resorption of Press-Fit Polyether–Ether–Ketone (PEEK) Hip Prosthesis: A Sawbone Model Study. Polymers 2022, 14, 4600. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, S.; Roy, M. Recent Developments in Magnesium Metal–Matrix Composites for Biomedical Applications: A Review. ACS Biomater Sci. Eng. 2020, 6, 4748–4773. [Google Scholar] [CrossRef]
- Chalisgaonkar, R. Insight in Applications, Manufacturing and Corrosion Behaviour of Magnesium and Its Alloys—A Review. Mater Today Proc. 2020, 26, 1060–1071. [Google Scholar] [CrossRef]
- Vennimalai Rajan, A.; Mathalai Sundaram, C.; Vembathu Rajesh, A. Mechanical and Morphological Investigation of Bio-Degradable Magnesium AZ31 Alloy for an Orthopedic Application. Mater Today Proc. 2020, 21, 272–277. [Google Scholar] [CrossRef]
- Zemková, M.; Minárik, P.; Jablonská, E.; Veselý, J.; Bohlen, J.; Kubásek, J.; Lipov, J.; Ruml, T.; Havlas, V.; Král, R. Concurrence of High Corrosion Resistance and Strength with Excellent Ductility in Ultrafine-Grained Mg-3Y Alloy. Materials 2022, 15, 7571. [Google Scholar] [CrossRef]
- Chandra, G.; Pandey, A. Biodegradable Bone Implants in Orthopedic Applications: A Review. Biocybern Biomed. Eng. 2020, 40, 596–610. [Google Scholar] [CrossRef]
- Shuai, C.; Li, S.; Peng, S.; Feng, P.; Lai, Y.; Gao, C. Biodegradable Metallic Bone Implants. Mater. Chem. Front. 2019, 3, 544–562. [Google Scholar] [CrossRef]
- Kulinich, S.; Kuchmizhak, A.; Honda, M.; Svetlichnyi, V.A.; Akimchenko, I.O.; Rutkowski, S.; Tran, T.-H.; Dubinenko, G.E.; Petrov, V.I.; Kozelskaya, A.I.; et al. Polyether Ether Ketone Coated with Ultra-Thin Films of Titanium Oxide and Zirconium Oxide Fabricated by DC Magnetron Sputtering for Biomedical Application. Materials 2022, 15, 8209. [Google Scholar] [CrossRef]
- Jia, B.; Yang, H.; Han, Y.; Zhang, Z.; Qu, X.; Zhuang, Y.; Wu, Q.; Zheng, Y.; Dai, K. In Vitro and in Vivo Studies of Zn-Mn Biodegradable Metals Designed for Orthopedic Applications. Acta Biomater 2020, 108, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Xu, J.-K.; Hopkins, C.; Chow, D.H.-K.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Lin, G.; Huang, J. The Role of Rare Earth Elements in Biodegradable Metals: A Review. Acta Biomater 2021, 129, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Saijilafu; Wu, X.; Wu, K.; Chen, J.; Tan, L.; Witte, F.; Yang, H.; Mantovani, D.; Zhou, H.; et al. Biodegradable Mg-Based Alloys: Biological Implications and Restorative Opportunities. Int. Mater. Rev. 2022, 1–39. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Abazari, S.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Daroonparvar, M.; Berto, F. A Comprehensive Review on Surface Modifications of Biodegradable Magnesium-Based Implant Alloy: Polymer Coatings Opportunities and Challenges. Coatings 2021, 11, 747. [Google Scholar] [CrossRef]
- Barzegari, M.; Mei, D.; Lamaka, S.V.; Geris, L. Computational Modeling of Degradation Process of Biodegradable Magnesium Biomaterials. Corros. Sci. 2021, 190, 109674. [Google Scholar] [CrossRef]
- Hassan, H.W.; Grasso, V.; Korostynska, O.; Khan, H.; Jose, J.; Mirtaheri, P. An Overview of Assessment Tools for Determination of Biological Magnesium Implant Degradation. Med. Eng. Phys. 2021, 93, 49–58. [Google Scholar] [CrossRef]
- Hassan, H.W.; Rahmati, M.; Barrantes, A.; Haugen, H.J.; Mirtaheri, P. In Vitro Monitoring of Magnesium-Based Implants Degradation by Surface Analysis and Optical Spectroscopy. Int. J. Mol. Sci. 2022, 23, 6099. [Google Scholar] [CrossRef]
- Kumar, R.; Katyal, P. Effects of Alloying Elements on Performance of Biodegradable Magnesium Alloy. Mater. Today. Proc. 2022, 56, 2443–2450. [Google Scholar] [CrossRef]
- Chandra, G.; Pandey, A. Preparation Strategies for Mg-Alloys for Biodegradable Orthopaedic Implants and Other Biomedical Applications: A Review. IRBM 2022, 43, 229–249. [Google Scholar] [CrossRef]
- Zaffora, A.; di Franco, F.; Virtù, D.; Carfì Pavia, F.; Ghersi, G.; Virtanen, S.; Santamaria, M. Tuning of the Mg Alloy AZ31 Anodizing Process for Biodegradable Implants. ACS Appl. Mater. Interfaces 2021, 13, 12866–12876. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.-Z.; Qi, W.-C.; Zeng, R.-C.; Chen, X.-B.; Gu, C.-D.; Guan, S.-K.; Zheng, Y.-F. Advances in Coatings on Biodegradable Magnesium Alloys. J. Magnes. Alloy. 2020, 8, 42–65. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to Plasma Electrolytic Oxidation-an Overview of the Process and Applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Merino, E.; Durán, A.; Ceré, S.; Castro, Y. Hybrid Epoxy-Alkyl Sol–Gel Coatings Reinforced with SiO2 Nanoparticles for Corrosion Protection of Anodized AZ31B Mg Alloy. Gels 2022, 8, 242. [Google Scholar] [CrossRef]
- Merino, E.; Durán, A.; Castro, Y. Integrated Corrosion-Resistant System for AZ31B Mg Alloy via Plasma Electrolytic Oxidation (PEO) and Sol-Gel Processes. Nternational. J. Appl. Glass Sci. 2021, 12, 519–530. [Google Scholar] [CrossRef]
- Merino, E.; Durán, A.; Castro, Y. The Role of Silane Sol-Gel Coatings on the Corrosion Protection of Magnesium Alloys; American Chemical Society: Washington, DC, USA, 2020. [Google Scholar]
- Johari, N.A.; Alias, J.; Zanurin, A.; Mohamed, N.S.; Alang, N.A.; Zain, M.Z.M. Anti-Corrosive Coatings of Magnesium: A Review. Mater Today Proc. 2022, 48, 1842–1848. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, F.; Qiu, J.; Tan, J.; Zhang, X.; Chen, S.; Qian, S.; Liu, X. Regulating Corrosion Reactions to Enhance the Anti-Corrosion and Self-Healing Abilities of PEO Coating on Magnesium. Corros. Sci. 2021, 192, 109840. [Google Scholar] [CrossRef]
- Omar, S.A.; Ballarre, J.; Castro, Y.; Martinez Campos, E.; Schreiner, W.; Durán, A.; Cere, S.M. 58S and 68S Sol-Gel Glass-like Bioactive Coatings for Enhancing the Implant Performance of AZ91D Magnesium Alloy. Surf. Coat. Technol. 2020, 400, 126224. [Google Scholar] [CrossRef]
- Saji, V.S. Electrophoretic (EPD) Coatings for Magnesium Alloys. J. Ind. Eng. Chem. 2021, 103, 358–372. [Google Scholar] [CrossRef]
- Deshmukh, K.; Kovářík, T.; Křenek, T.; Docheva, D.; Stich, T.; Pola, J. Recent Advances and Future Perspectives of Sol–Gel Derived Porous Bioactive Glasses: A Review. RSC Adv. 2020, 10, 33782–33835. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Salinas, A.J. Mesoporous Bioactive Glasses for Regenerative Medicine. Mater. Today Bio. 2021, 11, 100121. [Google Scholar] [CrossRef]
- Müller, V.; Jobbagy, M.; Djurado, E. Coupling Sol-Gel with Electrospray Deposition: Towards Nanotextured Bioactive Glass Coatings. J. Eur. Ceram. Soc. 2021, 41, 7288–7300. [Google Scholar] [CrossRef]
- Omar, S.; Pastore, J.; Bouchet, A.; Pellice, S.; Ballarín, V.; Cere, S.; Ballarre, J. SiO2-CaO-P2O5 (58S) Sol Gel Glass Applied onto Surgical Grade Stainless Steel by Spray Technique: Morphological Characterization by Digital Image Processing. Biomed. Glasses 2016, 2, 10–18. [Google Scholar] [CrossRef]
- Islam, S. Impact of PH on Structural and Sensing Characteristics of Cresol Red Encapsulated Polyethylene Glycol Assisted Silica Nanomatrix: Sol-Gel Method. Opt. Mater. (Amst) 2021, 121, 111546. [Google Scholar] [CrossRef]
- Ghamarpoor, R.; Jamshidi, M. Synthesis of Vinyl-Based Silica Nanoparticles by Sol–Gel Method and Their Influences on Network Microstructure and Dynamic Mechanical Properties of Nitrile Rubber Nanocomposites. Sci. Rep. 2022, 12, 15286. [Google Scholar] [CrossRef]
- Ravaine, D.; Seminel, A.; Vincens, M. A new family of organically modified silicates prepared from gels. J. Non-Cryst. Solids 1986, 82, 210–219. [Google Scholar] [CrossRef]
- Giz, A.S.; Aydelik-Ayazoglu, S.; Catalgil-Giz, H.; Bayraktar, H.; Alaca, B.E. Stress Relaxation and Humidity Dependence in Sodium Alginate-Glycerol Films. J. Mech. Behav. Biomed. Mater. 2019, 100, 103374. [Google Scholar] [CrossRef] [PubMed]
- Stefanescu, M.; Stoia, M.; Stefanescu, O. Thermal and FT-IR Study of the Hybrid Ethylene-Glycol-Silica Matrix. J. Solgel. Sci. Technol. 2007, 41, 71–78. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Ferrara, C.; Mustarelli, P. Silica-Polyethylene Glycol Hybrids Synthesized by Sol-Gel: Biocompatibility Improvement of Titanium Implants by Coating. Mater. Sci. Eng. C 2015, 55, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, H.B.; Andrade, J.D. Blood Compatibility of Polyethylene Oxide Surfaces. Prog. Polym. Sci. 1995, 20, 1043–1079. [Google Scholar] [CrossRef]
- Alcantar, N.A.; Aydil, E.S.; Israelachvili, J.N. Polyethylene Glycol-Coated Biocompatible Surfaces. J. Biomed. Mater. Res. 2000, 51, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Atrens, A.; StJohn, D. An Hydrogen Evolution Method for the Estimation of the Corrosion Rate of Magnesium Alloys. In Essential Readings in Magnesium Technology; Mathaudhu, S.N., Luo, A.A., Neelameggham, N.R., Nyberg, E.A., Sillekens, W.H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 565–572. ISBN 978-3-319-48099-2. [Google Scholar]
- Agustín-Sáenz, C.; Sánchez-García, J.Á.; Machado, M.; Brizuela, M.; Zubillaga, O.; Tercjak, A. Broadband Antireflective Coating Stack Based on Mesoporous Silica by Acid-Catalyzed Sol-Gel Method for Concentrated Photovoltaic Application. Sol. Energy Mater. Sol. Cells 2018, 186, 154–164. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, L.; Chen, X.; Liao, T.; Zheng, J. Preparation and Characterization of Bioactive Glass Tablets and Evaluation of Bioactivity and Cytotoxicity in Vitro. Bioact. Mater. 2018, 3, 315–321. [Google Scholar] [CrossRef]
- Bui, X.V.; Dang, T.H. Bioactive Glass 58S Prepared Using an Innovation Sol-Gel Process. Process. Appl. Ceram. 2019, 13, 98–103. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Ramakrishna, S. Surface Engineering of Biomaterials in Orthopedic and Dental Implants: Strategies to Improve Osteointegration, Bacteriostatic and Bactericidal Activities. Biotechnol. J. 2021, 16, 2000116. [Google Scholar] [CrossRef]
- Almassri, H.N.S.; Ma, Y.; Dan, Z.; Ting, Z.; Cheng, Y.; Wu, X. Implant Stability and Survival Rates of a Hydrophilic versus a Conventional Sandblasted, Acid-Etched Implant Surface: Systematic Review and Meta-Analysis. J. Am. Dent. Assoc. 2020, 151, 444–453. [Google Scholar] [CrossRef]
- Ng, W.F.; Chiu, K.Y.; Cheng, F.T. Effect of PH on the in Vitro Corrosion Rate of Magnesium Degradable Implant Material. Mater. Sci. Eng. C 2010, 30, 898–903. [Google Scholar] [CrossRef]
- Stammeier, J.A.; Purgstaller, B.; Hippler, D.; Mavromatis, V.; Dietzel, M. In-Situ Raman Spectroscopy of Amorphous Calcium Phosphate to Crystalline Hydroxyapatite Transformation. MethodsX 2018, 5, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Hussin, R.; Shawal Husin, M.; Nur Fazliana Abdul Halim, D.; Hamdan, S. Vibrational studies of crystalline phase strontium magnesium phosphates doped with Eu2O3. Solid State Sci. Technol. 2010, 18, 288–301. [Google Scholar]
- Li, A.; Shen, H.; Ren, H.; Wang, C.; Wu, D.; Martin, R.A.; Qiu, D. Bioactive Organic/Inorganic Hybrids with Improved Mechanical Performance. J. Mater. Chem. B 2015, 3, 1379–1390. [Google Scholar] [CrossRef]
- Durán, K.S.; Hernández, N.; Rueda, L.M.; Hernández-Barrios, C.A.; Coy, A.E.; Viejo, F. Design of Multilayer Hybrid Sol-Gel Coatings with Bifunctional Barrier-Bioactive Response on the Elektron 21 Magnesium Alloy for Biomedical Applications. J. Magnes. Alloy. 2021, 9, 2097–2112. [Google Scholar] [CrossRef]
- Su, Y.; Niu, L.; Lu, Y.; Lian, J.; Li, G. Preparation and Corrosion Behavior of Calcium Phosphate and Hydroxyapatite Conversion Coatings on AM60 Magnesium Alloy. J. Electrochem. Soc. 2013, 160, C536–C541. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, J.; Feng, J.; Cao, Y.; Deng, C.; Zhang, X. Dissolution and Mineralization Behaviors of HA Coatings. Biomaterials 2003, 24, 4741–4748. [Google Scholar] [CrossRef]
- Lombardi, M.; Gremillard, L.; Chevalier, J.; Lefebvre, L.; Cacciotti, I.; Bianco, A.; Montanaro, L. A Comparative Study between Melt-Derived and Sol-Gel Synthesized 45S5 Bioactive Glasses. Key Eng. Mater. 2013, 541, 15–30. [Google Scholar] [CrossRef]


| 58 S Sol | Aging Time (Days) | Coating Thickness (µm) |
|---|---|---|
| 58 WP | 4 | 1.53 ± 0.01 |
| 58 GLY | 4 | 1.65 ± 0.008 |
| 58 EG | 5 | 1.5 ± 0.01 |
| 58 PEG | 3 | 1.76 ± 0.01 |
| Sample | Rout Ω cm2 | Rdl Ω cm2 | CPEout Ω−1 cm−2 sαcoat | αcoat | RS Ω cm2 | Rads Ω cm2 | Lads H cm2 | RP Ω cm2 |
|---|---|---|---|---|---|---|---|---|
| 58S PEG 7th day | 527.4 ± 11.4 | 171,630.00 ± 3846 | 2.12 × 10−6 ± 1.27 × 10−7 | 0.58 | 40 | 172,157.4 | ||
| 58S PEG 3th day | 352.6 ± 5.95 | 102,730.00 ± 1303.4 | 3.88 × 10−6 ± 2.6 × 10−7 | 0.60 ± 5.6 × 10−3 | 40 | 103,055.6 | ||
| 58S PEG 24 h | 58.81 ± 15.8 | 3744 ± 145.7 | 1.61 × 10−6 ± 4.7 × 10−6 | 0.81 ± 0.22 | 45 | 2041 ± 177.8 | 1783 ± 146.8 | 58.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekar, A.R.; Merino, E.; Pakseresht, A.; Galusek, D.; Duran, A.; Castro, Y. Influence of Polyols on the In Vitro Biodegradation and Bioactivity of 58S Bioactive Sol–Gel Coatings on AZ31B Magnesium Alloys. Polymers 2023, 15, 1273. https://doi.org/10.3390/polym15051273
Chandrasekar AR, Merino E, Pakseresht A, Galusek D, Duran A, Castro Y. Influence of Polyols on the In Vitro Biodegradation and Bioactivity of 58S Bioactive Sol–Gel Coatings on AZ31B Magnesium Alloys. Polymers. 2023; 15(5):1273. https://doi.org/10.3390/polym15051273
Chicago/Turabian StyleChandrasekar, Ashok Raja, Emilia Merino, Amirhossein Pakseresht, Dusan Galusek, Alicia Duran, and Yolanda Castro. 2023. "Influence of Polyols on the In Vitro Biodegradation and Bioactivity of 58S Bioactive Sol–Gel Coatings on AZ31B Magnesium Alloys" Polymers 15, no. 5: 1273. https://doi.org/10.3390/polym15051273
APA StyleChandrasekar, A. R., Merino, E., Pakseresht, A., Galusek, D., Duran, A., & Castro, Y. (2023). Influence of Polyols on the In Vitro Biodegradation and Bioactivity of 58S Bioactive Sol–Gel Coatings on AZ31B Magnesium Alloys. Polymers, 15(5), 1273. https://doi.org/10.3390/polym15051273











