Chitosan-Bead-Encapsulated Polystyrene Sulfonate for Adsorption of Methylene Blue and Regeneration Studies: Batch and Continuous Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
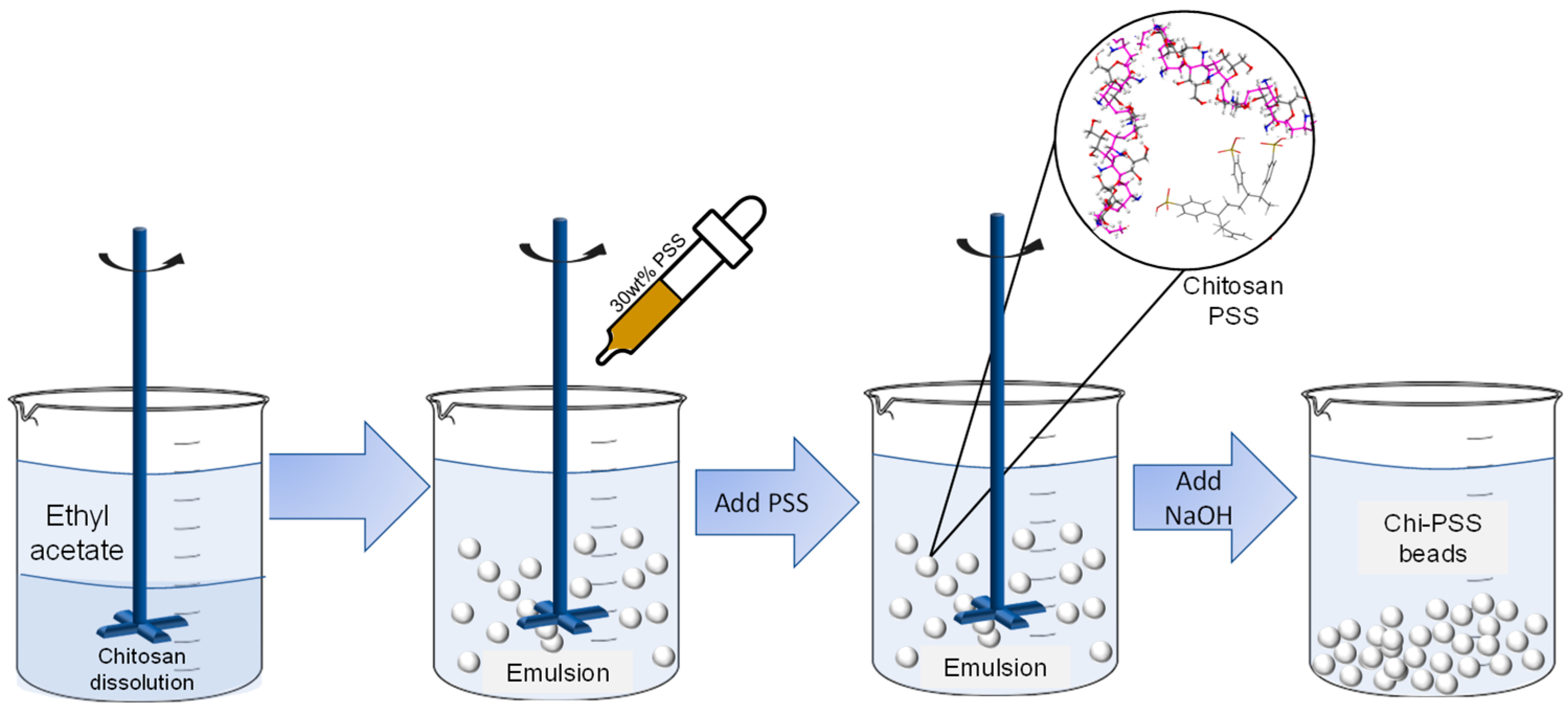
2.2. Preparation of Chitosan and Chitosan-PSS (Chi-PSS) Beads
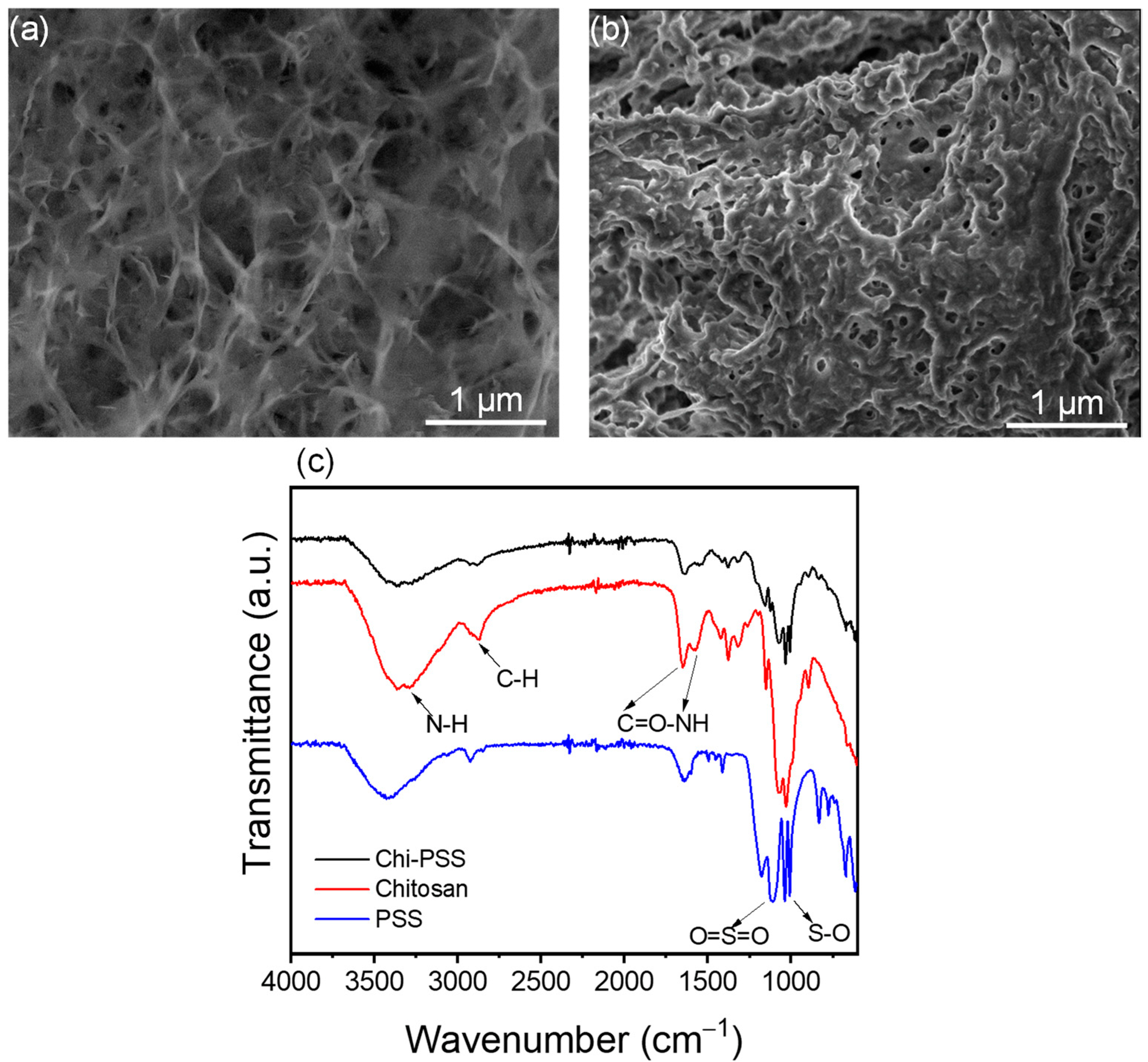
2.3. Characterisation of Chitosan and Chi-PSS Beads
2.4. Adsorption Isotherms, Kinetics, and Thermodynamics
3. Results
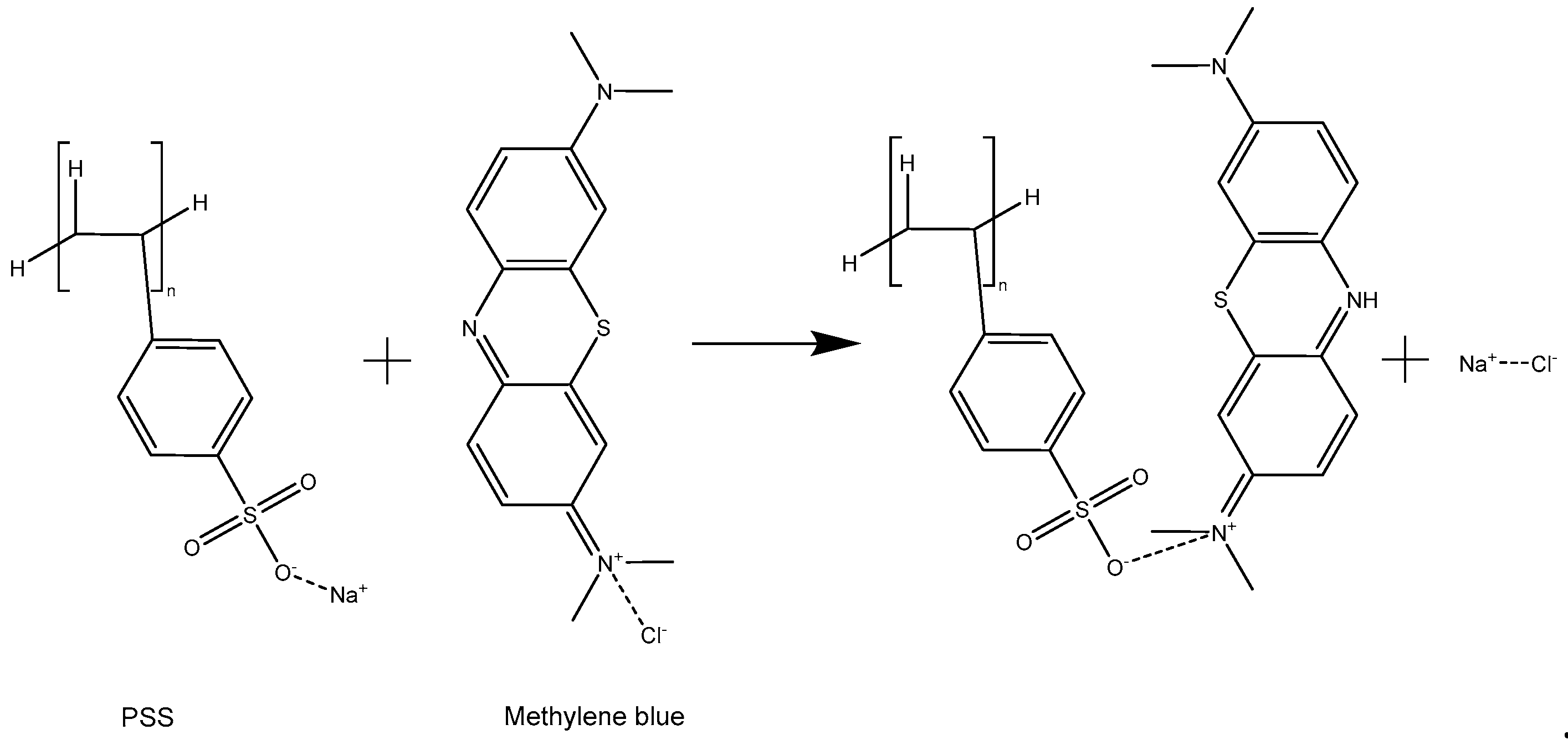
3.1. Characterization
3.2. Adsorption Kinetics
3.3. Adsorption Isotherm and Thermodynamic Analysis
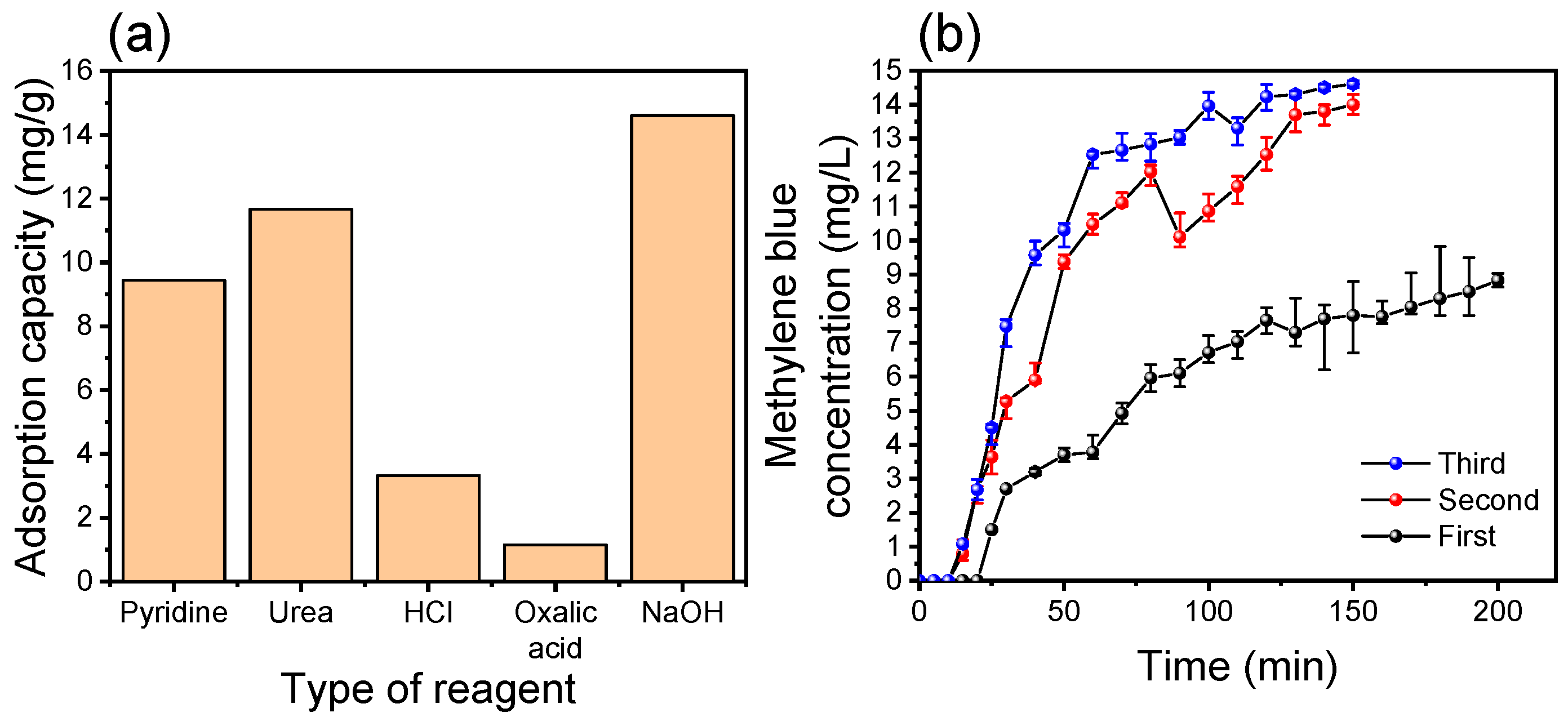
3.4. Adsorbent Regeneration Studies and Continuous Adsorption Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, W.S.; Lee, H.-J. Nanostructured Materials for Water Purification: Adsorption of Heavy Metal Ions and Organic Dyes. Polymers 2022, 14, 2183. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.N.; Tamboli, S.R.; Sutar, D.S.; Jadhav, S.R.; Marathe, J.V.; Shaikh, A.A.; Prajapati, A.A. Batch and continuous studies for adsorption of anionic dye onto waste tea residue: Kinetic, equilibrium, breakthrough and reusability studies. J. Clean. Prod. 2019, 252, 119778. [Google Scholar] [CrossRef]
- Zereshki, S.; Daraei, P.; Shokri, A. Application of edible paraffin oil for cationic dye removal from water using emulsion liquid membrane. J. Hazard. Mater. 2018, 356, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Karimifard, S.; Moghaddam, M.R.A. Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review. Sci. Total Environ. 2018, 640–641, 772–797. [Google Scholar] [CrossRef]
- Kumari, M.; Gupta, S.K. A novel process of adsorption cum enhanced coagulation-flocculation spiked with magnetic nanoadsorbents for the removal of aromatic and hydrophobic fraction of natural organic matter along with turbidity from drinking water. J. Clean. Prod. 2020, 244, 118899. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N.; Abu Hasan, H.; Othman, A.R.; Purwanti, I.F. Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, S.; Tian, Z.; Duan, G.; Pan, H.; Yue, Y.; Li, S.; Jian, S.; Yang, W.; Liu, K.; et al. MOFs meet wood: Reusable magnetic hydrophilic composites toward efficient water treatment with super-high dye adsorption capacity at high dye concentration. Chem. Eng. J. 2022, 446, 136851. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Zhao, X.; Chen, L.; Peng, S.; Ma, C.; Duan, G.; Liu, Z.; Wang, H.; Yuan, Y.; et al. A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater. E-Polymers 2022, 22, 399–410. [Google Scholar] [CrossRef]
- Jian, S.; Cheng, Y.; Ma, X.; Guo, H.; Hu, J.; Zhang, K.; Jiang, S.; Yang, W.; Duan, G. Excellent fluoride removal performance by electrospun La–Mn bimetal oxide nanofibers. New J. Chem. 2021, 46, 490–497. [Google Scholar] [CrossRef]
- Azmi, A.; Lau, K.S.; Chin, S.X.; Khiew, P.S.; Zakaria, S.; Chia, C.H. Zinc oxide-filled polyvinyl alcohol–cellulose nanofibril aerogel nanocomposites for catalytic decomposition of an organic dye in aqueous solution. Cellulose 2021, 28, 2241–2253. [Google Scholar] [CrossRef]
- Lau, K.S.; Chin, S.X.; Khiew, P.S.; Zakaria, S.; Yin, M.L.J.; Key, K.H.M.; Chia, C.H. Enhanced adsorption of anionic phenol red using cationic polyethylenimine-incorporated chitosan beads. J. Porous Mater. 2022, 29, 609–619. [Google Scholar] [CrossRef]
- Yusoff, I.I.; Rohani, R.; Ng, L.Y.; Mohammad, A.W. Conductive polyelectrolyte multilayers PANI membranes synthesis for tunable filtration ranges. J. Mater. Sci. 2019, 54, 12988–13005. [Google Scholar] [CrossRef]
- Chakraborty, G.; Bhattarai, A.; De, R. Polyelectrolyte–Dye Interactions: An Overview. Polymers 2022, 14, 598. [Google Scholar] [CrossRef]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.; Wang, Q.; Wu, J.; Duan, G.; Xu, W.; Jian, S. Magnetically separable and recyclable Fe3O4@PDA covalent grafted by l-cysteine core-shell nanoparticles toward efficient removal of Pb2+. Vacuum 2021, 189, 110229. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Li, X.; Mei, C.; Zheng, J.; Shiju, E.; Duan, G.; Liu, K.; Jiang, S. Liquid Transport and Real-Time Dye Purification via Lotus Petiole-Inspired Long-Range-Ordered Anisotropic Cellulose Nanofibril Aerogels. ACS Nano 2021, 15, 20666–20677. [Google Scholar] [CrossRef]
- Bagheri, N.; Lakouraj, M.M.; Hasantabar, V.; Mohseni, M. Biodegradable macro-porous CMC-polyaniline hydrogel: Synthesis, characterization and study of microbial elimination and sorption capacity of dyes from waste water. J. Hazard. Mater. 2020, 403, 123631. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Mosallanezhad, A. Facile and green rout to prepare magnetic and chitosan-crosslinked κ-carrageenan bionanocomposites for removal of methylene blue. J. Water Process. Eng. 2016, 10, 143–155. [Google Scholar] [CrossRef]
- Ruiz, C.; Vera, M.; Rivas, B.L.; Sánchez, S.; Urbano, B.F. Magnetic methacrylated gelatin-g-polyelectrolyte for methylene blue sorption. RSC Adv. 2020, 10, 43799–43810. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2018, 121, 889–904. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Chin, S.X.; Lau, K.S.; Zakaria, S.; Chia, C.H.; Wongchoosuk, C. Chitosan Fibers Loaded with Limonite as a Catalyst for the Decolorization of Methylene Blue via a Persulfate-Based Advanced Oxidation Process. Polymers 2022, 14, 5165. [Google Scholar] [CrossRef] [PubMed]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Shen, L.; Shan, D.; Liu, D.; Yu, G. Regeneration of Chitosan-Based Adsorbents for Eliminating Dyes from Aqueous Solutions. Sep. Purif. Rev. 2017, 48, 1–13. [Google Scholar] [CrossRef]
- Zheng, X.; Zheng, H.; Xiong, Z.; Zhao, R.; Liu, Y.; Zhao, C.; Zheng, C. Novel anionic polyacrylamide-modify-chitosan magnetic composite nanoparticles with excellent adsorption capacity for cationic dyes and pH-independent adsorption capability for metal ions. Chem. Eng. J. 2019, 392, 123706. [Google Scholar] [CrossRef]
- Zhao, J.; Xing, T.; Li, Q.; Chen, Y.; Yao, W.; Jin, S.; Chen, S. Preparation of chitosan and carboxymethylcellulose-based polyelectrolyte complex hydrogel via SD-A-SGT method and its adsorption of anionic and cationic dye. J. Appl. Polym. Sci. 2020, 137, 48980. [Google Scholar] [CrossRef]
- Wan, X.; Liu, Z.; Xie, L.; Qu, G.; Zhang, H.; Wang, B.; Li, Y.; Zhang, Y.-F.; Zhao, S. Efficiently ion-enhanced adsorption of anion dyes by acrolein crosslinked polyethylenimine/chitosan hydrogel with excellent recycling stability. Int. J. Biol. Macromol. 2022, 222, 2017–2027. [Google Scholar] [CrossRef]
- Yin, T.; Zhang, X.; Shao, S.; Xiang, T.; Zhou, S. Covalently crosslinked sodium alginate/poly(sodium p-styrenesulfonate) cryogels for selective removal of methylene blue. Carbohydr. Polym. 2023, 301, 120356. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Mostafa, M.K.; Peters, R.W. A prototype of textile wastewater treatment using coagulation and adsorption by Fe/Cu nanoparticles: Techno-economic and scaling-up studies. Nanomater. Nanotechnol. 2021, 11. [Google Scholar] [CrossRef]
- Patel, H. Batch and continuous fixed bed adsorption of heavy metals removal using activated charcoal from neem (Azadirachta indica) leaf powder. Sci. Rep. 2020, 10, 16895. [Google Scholar] [CrossRef]
- Marsiezade, N.; Javanbakht, V. Novel hollow beads of carboxymethyl cellulose/ZSM-5/ZIF-8 for dye removal from aqueous solution in batch and continuous fixed bed systems. Int. J. Biol. Macromol. 2020, 162, 1140–1152. [Google Scholar] [CrossRef]
- Chook, S.W.; Chia, C.H.; Kaco, H.; Zakaria, S.; Huang, N.M.; Neoh, H.M. Highly porous chitosan beads embedded with silver-graphene oxide nanocomposites for antibacterial application. Sains Malays. 2016, 45, 1663–1667. [Google Scholar]
- Sakr, A.K.; Aal, M.M.A.; El-Rahem, K.A.A.; Allam, E.M.; Dayem, S.M.A.; Elshehy, E.A.; Hanfi, M.Y.; Alqahtani, M.S.; Cheira, M.F. Characteristic Aspects of Uranium(VI) Adsorption Utilizing Nano-Silica/Chitosan from Wastewater Solution. Nanomaterials 2022, 12, 3866. [Google Scholar] [CrossRef]
- Jarek, E.; Krasińska-Krawet, Z.; Kruk, T.; Lamch, Ł.; Ronka, S.; Wilk, K.; Warszyński, P. Adsorption Properties of Soft Hydrophobically Functionalized PSS/MA Polyelectrolytes. Colloids Interfaces 2021, 5, 3. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. A general kinetic model for adsorption: Theoretical analysis and modeling. J. Mol. Liq. 2019, 288, 111100. [Google Scholar] [CrossRef]
- Bujdák, J. Adsorption kinetics models in clay systems. The critical analysis of pseudo-second order mechanism. Appl. Clay Sci. 2020, 191, 105630. [Google Scholar] [CrossRef]
- Chandarana, H.; Kumar, P.S.; Seenuvasan, M.; Kumar, M.A. Kinetics, equilibrium and thermodynamic investigations of methylene blue dye removal using Casuarina equisetifolia pines. Chemosphere 2021, 285, 131480. [Google Scholar] [CrossRef]
- Idris, S.A.; Alotaibi, K.M.; Peshkur, T.A.; Anderson, P.; Morris, M.; Gibson, L.T. Adsorption kinetic study: Effect of adsorbent pore size distribution on the rate of Cr (VI) uptake. Microporous Mesoporous Mater. 2012, 165, 99–105. [Google Scholar] [CrossRef]
- Paluri, P.; Ahmad, K.A.; Durbha, K.S. Importance of estimation of optimum isotherm model parameters for adsorption of methylene blue onto biomass derived activated carbons: Comparison between linear and non-linear methods. Biomass Convers. Biorefinery 2020, 12, 4031–4048. [Google Scholar] [CrossRef]
- Bläker, C.; Pasel, C.; Luckas, M.; Dreisbach, F.; Bathen, D. A study on the load-dependent enthalpy of adsorption and interactions in adsorption of C5 and C6 hydrocarbons on zeolites 13X and ZSM-5 and an activated carbon. Microporous Mesoporous Mater. 2020, 302, 110205. [Google Scholar] [CrossRef]
- Lima, E.C.; Gomes, A.A.; Tran, H.N. Comparison of the nonlinear and linear forms of the Van’t Hoff equation for calculation of adsorption thermodynamic parameters (∆S° and ∆H°). J. Mol. Liq. 2020, 311, 113315. [Google Scholar] [CrossRef]
- Tran, H.N. Improper Estimation of Thermodynamic Parameters in Adsorption Studies with Distribution Coefficient KD (qe/Ce) or Freundlich Constant (KF): Considerations from the Derivation of Dimensionless Thermodynamic Equilibrium Constant and Suggestions. Adsorpt. Sci. Technol. 2022, 2022, 1–23. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Simchi, A.; Far, H.S. Nanoporous composites of activated carbon-metal organic frameworks for organic dye adsorption: Synthesis, adsorption mechanism and kinetics studies. J. Ind. Eng. Chem. 2019, 81, 405–414. [Google Scholar] [CrossRef]
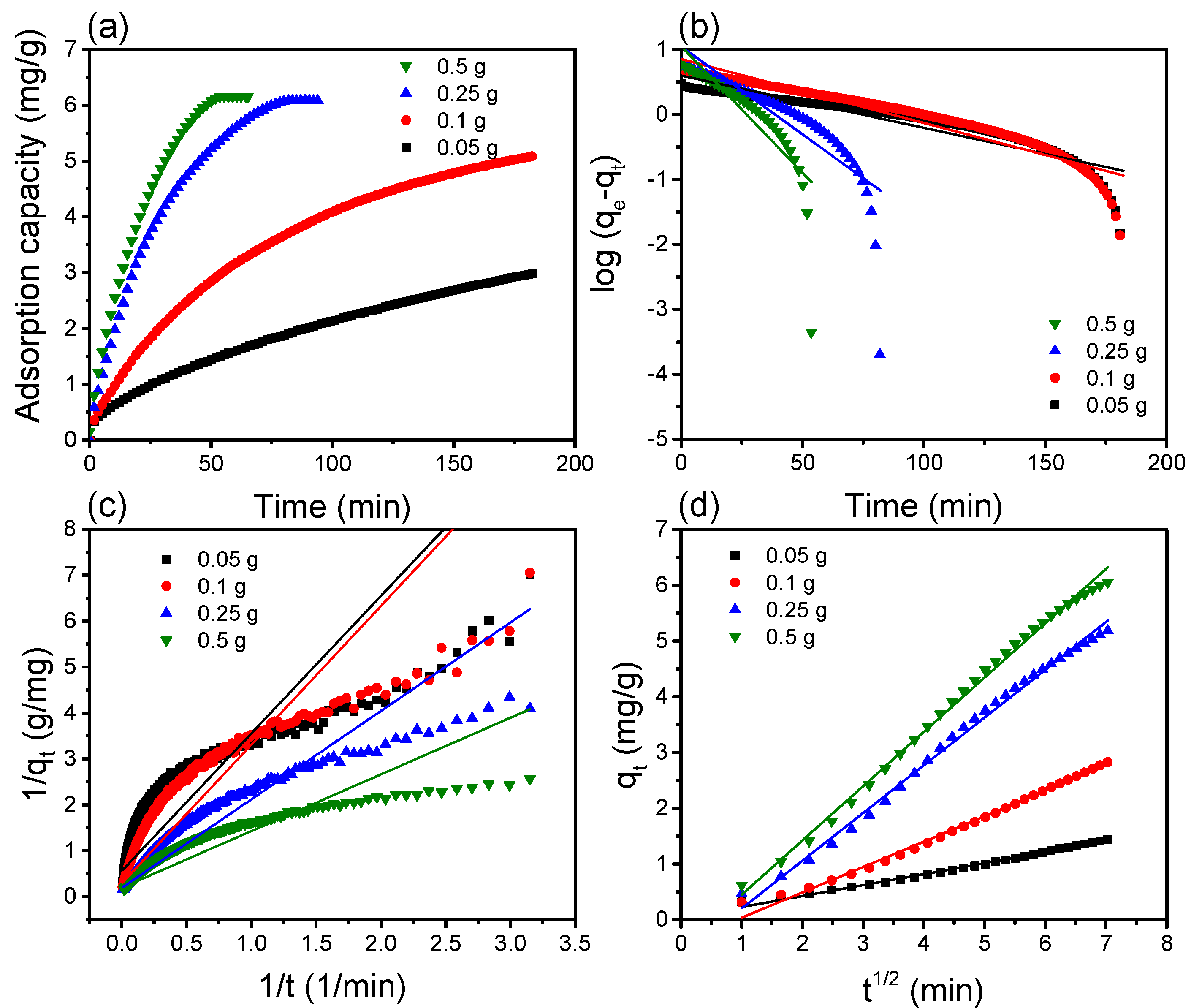

| Mass of Adsorbent (g) | Pseudo-First-Order | Pseudo-Second- Order | qe exp (mg/g) | Intraparticle Diffusion | |||||
|---|---|---|---|---|---|---|---|---|---|
| qe1 cal (mg/g) | k1 | r2 | qe2 cal (mg/g) | k2 | r2 | Kp1 | r2 | ||
| 0.05 | 3.95 | 0.019 | 0.84 | 1.78 | 0.250 | 0.61 | 2.99 | 0.196 | 0.99 |
| 0.1 | 7.09 | 0.023 | 0.88 | 3.42 | 0.179 | 0.77 | 5.09 | 0.456 | 0.99 |
| 0.25 | 11.03 | 0.062 | 0.77 | 5.12 | 0.229 | 0.88 | 6.08 | 0.857 | 0.99 |
| 0.5 | 11.18 | 0.090 | 0.78 | 5.12 | 0.358 | 0.85 | 6.16 | 0.975 | 0.99 |
| Model | Parameters | |
|---|---|---|
| Langmuir isotherm | Q0 (mg g−1) | 42.21 |
| KL (L mg−1) | 6.36 | |
| r2 | 0.97 | |
| Freundlich isotherm | KF | 25.73 |
| n | 10.08 | |
| r2 | 0.85 | |
| Temkin isotherm | AT | 2733.35 |
| bT | 739.61 | |
| r2 | 0.88 | |
| Temperature (K) | ΔH (kJ/mol) | ΔS (J/mol) | ΔG (J/mol) |
|---|---|---|---|
| 298 | −103.66 | −295.38 | 15.57 |
| 318 | 9.87 | ||
| 338 | 3.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, K.S.; Azmi, N.A.S.; Chin, S.X.; Zakaria, S.; Chia, C.H. Chitosan-Bead-Encapsulated Polystyrene Sulfonate for Adsorption of Methylene Blue and Regeneration Studies: Batch and Continuous Approaches. Polymers 2023, 15, 1269. https://doi.org/10.3390/polym15051269
Lau KS, Azmi NAS, Chin SX, Zakaria S, Chia CH. Chitosan-Bead-Encapsulated Polystyrene Sulfonate for Adsorption of Methylene Blue and Regeneration Studies: Batch and Continuous Approaches. Polymers. 2023; 15(5):1269. https://doi.org/10.3390/polym15051269
Chicago/Turabian StyleLau, Kam Sheng, Nur Alia Sahira Azmi, Siew Xian Chin, Sarani Zakaria, and Chin Hua Chia. 2023. "Chitosan-Bead-Encapsulated Polystyrene Sulfonate for Adsorption of Methylene Blue and Regeneration Studies: Batch and Continuous Approaches" Polymers 15, no. 5: 1269. https://doi.org/10.3390/polym15051269
APA StyleLau, K. S., Azmi, N. A. S., Chin, S. X., Zakaria, S., & Chia, C. H. (2023). Chitosan-Bead-Encapsulated Polystyrene Sulfonate for Adsorption of Methylene Blue and Regeneration Studies: Batch and Continuous Approaches. Polymers, 15(5), 1269. https://doi.org/10.3390/polym15051269






