Poly(ε-caprolactone)-poly(ethylene glycol) Tri-Block Copolymer as Quercetin Delivery System for Human Colorectal Carcinoma Cells: Synthesis, Characterization and In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Measurements
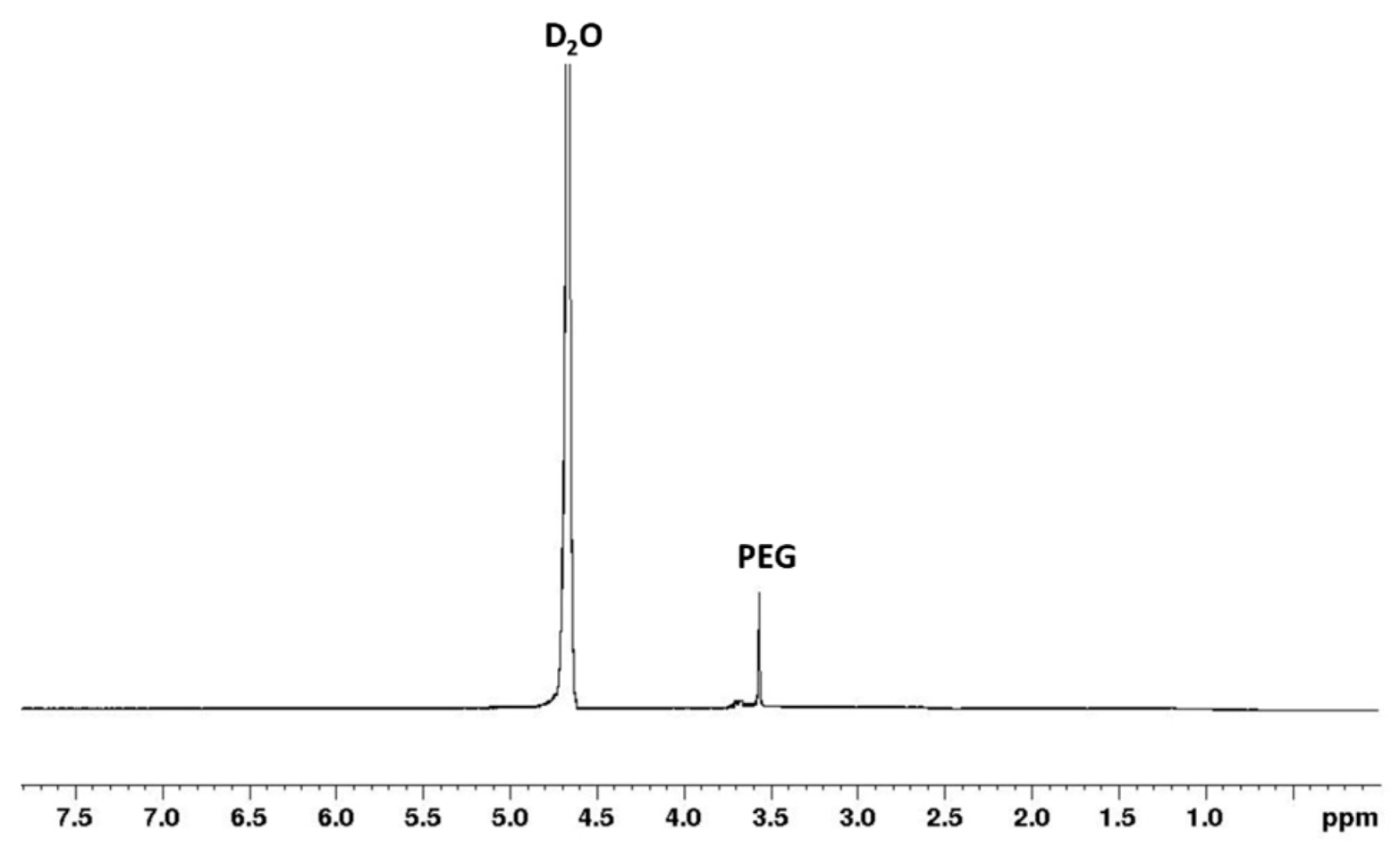
2.1.1. Nuclear Magnetic Resonance (NMR)
2.1.2. Gel Permeation Chromatography (GPC)
2.1.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.1.4. Dinamic Light Scattering (DLS)
2.2. Chemicals
2.3. Cell Cultures
2.4. Synthetic Procedures
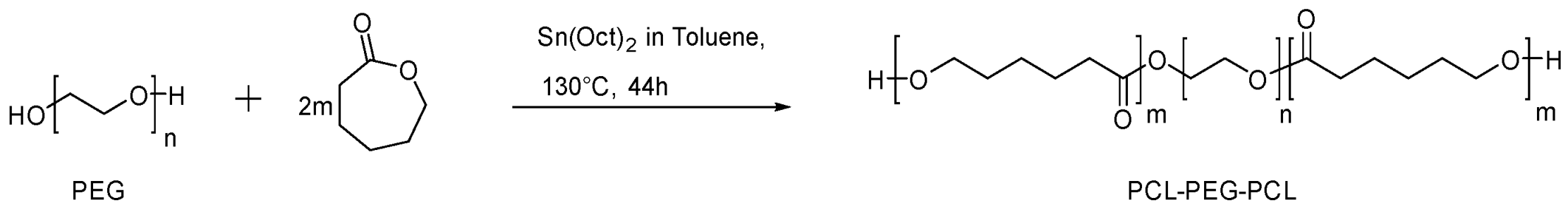
2.4.1. Synthesis of Triblock PCL-PEG-PCL Copolymer
2.4.2. Preparation of Triblock PCL-PEG-PCL Copolymer Nanoparticles (NP)
2.4.3. Preparation of Triblock PCL-PEG-PCL Copolymer Nanoparticles Loaded with Nile Red (NP+NR)
2.4.4. Preparation of Triblock PCL-PEG-PCL Copolymer Nanoparticles Loaded with Quercetin (NP+Q)
2.5. In Vitro Cells Studies
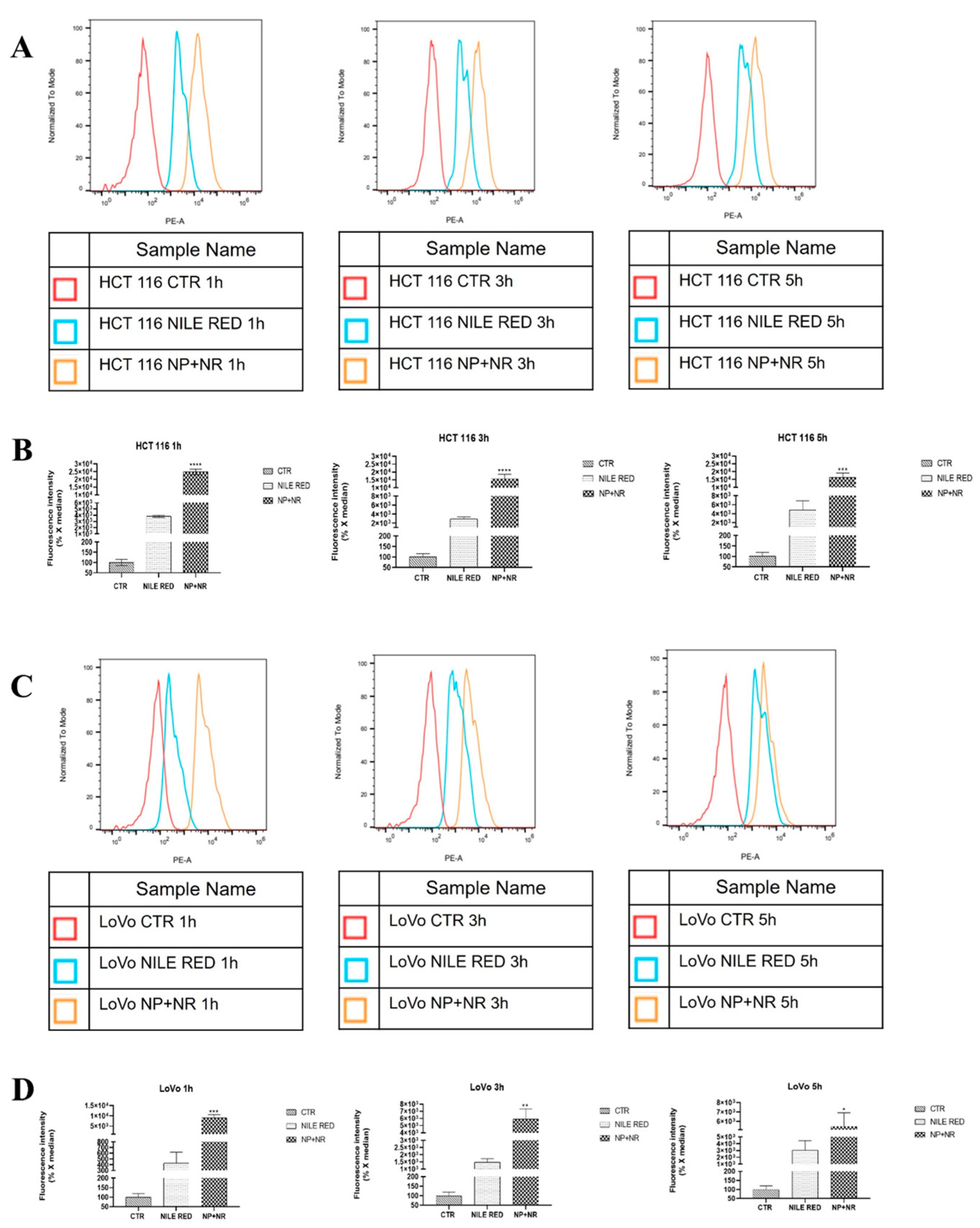
2.5.1. Analysis of Cell Uptake of Nile Red-Loaded Nanoparticles (NP+NR) by Fluorescence Microscopy and Fluorescence-Activated Cell Sorting (FACS)
2.5.2. Cell Proliferation Assay
2.5.3. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of PCL-PEG-PCL Copolymer
3.2. Self-Assembly of Triblock PCL-PEG-PCL Copolymers to Form Nanoparticles
3.3. Cellular Studies
3.3.1. Nanoparticle Uptake in HCT 116 and LoVo Cells
3.3.2. Effects of Quercetin-Loaded Nanoparticles (NP+Q) on Colon Cancer Cell Line HCT 116
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and Fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Miyata, T.; Namera, T.; Liu, Y.; Kawamura, A.; Yamaoka, T. Photoresponsive Behaviour of Zwitterionic Polymer Parti-cles with Photodimerizable Groups on Their Surfaces. J. Mater. Chem. B 2022, 10, 2637–2648. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Zheng, R.; Guo, J.; Cai, X.; Bin, L.; Lu, C.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Manganese Complexes and Manga-nese-Based Metal-Organic Frameworks as Contrast Agents in MRI and Chemotherapeutics Agents: Applications and Prospects. Colloids Surf. B Biointerfaces 2022, 213, 112432. [Google Scholar] [CrossRef]
- Rao, C.; Liao, D.; Pan, Y.; Zhong, Y.; Zhang, W.; Ouyang, Q.; Nezamzadeh-Ejhieh, A.; Liu, J. Novel formulations of metal-organic frameworks for controlled drug delivery. Expert Opin. Drug Deliv. 2022, 19, 1183–1202. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Bannerjee, S.; Bhati, L.; Pandey, S.; Pandey, P.; Sriwastawa, B. Drug Delivery Systems: An Updated Review. Int. J. Pharm. Investig. 2012, 2, 2. [Google Scholar] [CrossRef]
- Buishvili, L.L.; Khalvashi, E.K. The Theory of Nonstationary Dynamic Polarization of Nuclei. Radiophys. Quantum Electron. 1971, 14, 1143–1144. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H.; Yu, Y.; Chen, Y.; Wang, D.; Zhang, G.; Zhou, G.; Liu, J.; Sun, Z.; Sun, D.; et al. Biodegradable Self-Assembled Nanoparticles of Poly (d,l-Lactide-Co-Glycolide)/Hyaluronic Acid Block Copolymers for Target Delivery of Docetaxel to Breast Cancer. Biomaterials 2014, 35, 550–566. [Google Scholar] [CrossRef] [PubMed]
- Shuai, X.; Ai, H.; Nasongkla, N.; Kim, S.; Gao, J. Micellar Carriers Based on Block Copolymers of Poly(ε-Caprolactone) and Poly(Ethylene Glycol) for Doxorubicin Delivery. J. Control. Release 2004, 98, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(Ethylene Glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem.–Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Sisson, A.L.; Ekinci, D.; Lendlein, A. The Contemporary Role of ε-Caprolactone Chemistry to Create Advanced Polymer Architectures. Polymer 2013, 54, 4333–4350. [Google Scholar] [CrossRef]
- Pappalardo, D.; Mathisen, T.; Finne-Wistrand, A. Biocompatibility of Resorbable Polymers: A Historical Perspective and Framework for the Future. Biomacromolecules 2019, 20, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.W.; Gong, C.Y.; Gou, M.L.; Fu, S.Z.; Guo, Q.F.; Shi, S.; Luo, F.; Guo, G.; Qiu, L.Y.; Qian, Z.Y. Biodegradable Poly(ε-Caprolactone)-Poly(Ethylene Glycol) Copolymers as Drug Delivery System. Int. J. Pharm. 2009, 381, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sana, B.; Ferrentino, N.; Kohlan, T.B.; Liu, Y.; Pasiskevicius, V.; Finne-Wistrand, A.; Pappalardo, D. Coumarin End-Capped Poly(ε-Caprolactone)-Poly(Ethylene Glycol) Tri-Block Copolymer: Synthesis, Characterization and Light-Response Behavior. Eur. Polym. J. 2023, 183, 111760. [Google Scholar] [CrossRef]
- Youxin, L.; Kissel, T. Synthesis and Properties of Biodegradable ABA Triblock Copolymers Consisting of Poly(l-Lactic Acid) or Poly (l-Lactic-Co-Glycolic Acid) A-Blocks Attached to Central Poly (Oxyethylene ) B-Blocks. J. Control. Release 1993, 27, 247–257. [Google Scholar] [CrossRef]
- Gan, Z.; Zhang, J.; Jiang, B. Poly(ϵ-Caprolactone)/Poly(Ethylene Oxide) Diblock Copolymer II. Nonisothermal Crystallization and Melting Behavior. J. Appl. Polym. Sci. 1997, 63, 1793–1804. [Google Scholar] [CrossRef]
- Zamani, S.; Khoee, S. Preparation of Core-Shell Chitosan/PCL-PEG Triblock Copolymer Nanoparticles with ABA and BAB Morphologies: Effect of Intraparticle Interactions on Physicochemical Properties. Polymer 2012, 53, 5723–5736. [Google Scholar] [CrossRef]
- Qi, W.; Ghoroghchian, P.; Li, G.; Hammer, D.; Therien, M. Aqueous Self-Assembly of Poly(ethylene oxide)-block-Poly(ε-caprolactone) (PEO-b-PCL) Copolymers: Disparate Diblock Copolymer Compositions Give Rise to Nano- and Meso-Scale Bilayered Vesicles. Nanoscale 2013, 5, 10908–10915. [Google Scholar] [CrossRef]
- Xin, H.; Chen, L.; Gu, J.; Ren, X.; Wei, Z.; Luo, J.; Chen, Y.; Jiang, X.; Sha, X.; Fang, X. Enhanced Anti-Glioblastoma Efficacy by PTX-Loaded PEGylated Poly(ε-Caprolactone) Nanoparticles: In Vitro and in Vivo Evaluation. Int. J. Pharm. 2010, 402, 238–247. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Wang, H.; Wu, S.; Zhao, K.; Sun, H.; Kong, D.; Wang, C.; Leng, X.; Zhu, D. Preparation and Evaluation of PCL-PEG-PCL Polymeric Nanoparticles for Doxorubicin Delivery against Breast Cancer. RSC Adv. 2016, 6, 54727–54737. [Google Scholar] [CrossRef]
- Hakemi, P.; Ghadi, A.; Mahjoub, S.; Zabihi, E.; Tashakkorian, H. Ratio Design of Docetaxel/Quercetin Co-Loading-to-Nanocarrier: Synthesis of PCL–PEG–PCL Copolymer, Study of Drug Release Kinetic and Growth Inhibition of Human Breast Cancer (MCF-7) Cell Line. Russ. J. Appl. Chem. 2021, 94, 388–401. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Z.; Wu, S.; Han, Y.; Wang, H.; Sun, H.; Kong, D.; Leng, X.; Wang, C.; Zhang, L.; et al. Micelle or Polymersome Formation by PCL-PEG-PCL Copolymers as Drug Delivery Systems. Chin. Chem. Lett. 2017, 28, 1905–1909. [Google Scholar] [CrossRef]
- Mirzaghavami, P.S.; Khoei, S.; Khoee, S.; Shirvalilou, S. Folic Acid-Conjugated Magnetic Triblock Copolymer Nanoparticles for Dual Targeted Delivery of 5-Fluorouracil to Colon Cancer Cells. Cancer Nanotechnol. 2022, 13, 1–18. [Google Scholar] [CrossRef]
- Ni, R.; Duan, D.; Li, B.; Li, Z.; Li, L.; Ming, Y.; Wang, X.; Chen, J. Dual-Modified PCL-PEG Nanoparticles for Improved Targeting and Therapeutic Efficacy of Docetaxel against Colorectal Cancer. Pharm. Dev. Technol. 2021, 26, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, X.; Xie, L.; Ding, D.; Hu, Y.; Qian, X.; Yu, L.; Ding, Y.; Jiang, X.; Liu, B. Preparation and Evaluation of PEG-PCL Nanoparticles for Local Tetradrine Delivery. Int. J. Pharm. 2009, 379, 158–166. [Google Scholar] [CrossRef]
- Jalilzadeh, N.; Samadi, N.; Salehi, R.; Dehghan, G.; Iranshahi, M.; Dadpour, M.R.; Hamishehkar, H. Novel Nano-Vehicle for Delivery and Efficiency of Anticancer Auraptene against Colon Cancer Cells. Sci. Rep. 2020, 10, 1606. [Google Scholar] [CrossRef]
- Chen, L.; Li, K.; Liu, Q.; Quiles, J.L.; Filosa, R.; Kamal, M.A.; Wang, F.; Kai, G.; Zou, X.; Teng, H.; et al. Protective Effects of Raspberry on the Oxidative Damage in HepG2 Cells through Keap1/Nrf2-Dependent Signaling Pathway. Food Chem. Toxicol. 2019, 133, 110781. [Google Scholar] [CrossRef]
- Kim, D.H.; Khan, H.; Ullah, H.; Hassan, S.T.S.; Šmejkal, K.; Efferth, T.; Mahomoodally, M.F.; Xu, S.; Habtemariam, S.; Filosa, R.; et al. MicroRNA Targeting by Quercetin in Cancer Treatment and Chemoprotection. Pharmacol. Res. 2019, 147, 104346. [Google Scholar] [CrossRef]
- Tan, B.J.; Liu, Y.; Chang, K.L.; Lim, B.K.W.; Chiu, G.N.C. Perorally Active Nanomicellar Formulation of Quercetin in the Treatment of Lung Cancer. Int. J. Nanomed. 2012, 7, 651–661. [Google Scholar] [CrossRef]
- Xu, G.Y.; Shi, H.S.; Ren, L.B.; Gou, H.F.; Gong, D.Y.; Gao, X.; Huang, N. Enhancing the Anti-Colon Cancer Activity of Quercetin by Self-Assembled Micelles. Int. J. Nanomed. 2015, 10, 2051–2063. [Google Scholar] [CrossRef]
- Garofalo, C.; Capuano, G.; Sottile, R.; Tallerico, R.; Adami, R.; Reverchon, E.; Carbone, E.; Izzo, L.; Pappalardo, D. Different Insight into Amphiphilic PEG-PLA Copolymers: Influence of Macromolecular Architecture on the Micelle Formation and Cellular Uptake. Biomacromolecules 2014, 15, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, T.; Pappalardo, D.; Finne-Wistrand, A. Redox-Responsive Disulfide Cross-Linked PLA-PEG Nanoparticles. Macromolecules 2017, 50, 7052–7061. [Google Scholar] [CrossRef]
- Caruso, E.; Orlandi, V.T.; Malacarne, M.C.; Martegani, E.; Scanferla, C.; Pappalardo, D.; Vigliotta, G.; Izzo, L. Bodipy-Loaded Micelles Based on Polylactide as Surface Coating for Photodynamic Control of Staphylococcus Aureus. Coatings 2021, 11, 223. [Google Scholar] [CrossRef]
- Adami, R.; Liparoti, S.; Izzo, L.; Pappalardo, D.; Reverchon, E. PLA-PEG Copolymers Micronization by Supercritical Assisted Atomization. J. Supercrit. Fluids 2012, 72, 15–21. [Google Scholar] [CrossRef]
- Ostacolo, L.; Marra, M.; Ungaro, F.; Zappavigna, S.; Maglio, G.; Quaglia, F.; Abbruzzese, A.; Caraglia, M. In Vitro Anticancer Activity of Docetaxel-Loaded Micelles Based on Poly(Ethylene Oxide)-Poly(Epsilon-Caprolactone) Block Copolymers: Do Nanocarrier Properties Have a Role? J. Control. Release 2010, 148, 255–263. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, B.; Xu, P.; Yang, B. Integrated Whole Transcriptome Profiling and Bioinformatics Analysis for Revealing Regulatory Pathways Associated With Quercetin-Induced Apoptosis in HCT-116 Cells. Front. Pharmacol. 2019, 10, 798. [Google Scholar] [CrossRef]
- Cao, H.; Högger, P.; Prieto, M.; Simal-Gandara, J.; Xiao, J. Stability of Quercetin in DMEM and Cell Culture with A549 Cells. eFood 2022, 3, e13. [Google Scholar] [CrossRef]





| Sample | Average Diameter (nm) 1 | |
|---|---|---|
| NP+Q | 25 °C | 37 °C |
| 152.5 ± 77.3 | 128.5 ± 59.0 |
| HCT 116 | IC50 |
|---|---|
| 24 h | >5 µM (low toxicity) |
| 48 h | 3.7 µM |
| 72 h | 2.1 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrentino, N.; Romano, M.P.; Zappavigna, S.; Abate, M.; Del Vecchio, V.; Romano, D.; Germinario, C.; Grifa, C.; Filosa, R.; Pappalardo, D. Poly(ε-caprolactone)-poly(ethylene glycol) Tri-Block Copolymer as Quercetin Delivery System for Human Colorectal Carcinoma Cells: Synthesis, Characterization and In Vitro Study. Polymers 2023, 15, 1179. https://doi.org/10.3390/polym15051179
Ferrentino N, Romano MP, Zappavigna S, Abate M, Del Vecchio V, Romano D, Germinario C, Grifa C, Filosa R, Pappalardo D. Poly(ε-caprolactone)-poly(ethylene glycol) Tri-Block Copolymer as Quercetin Delivery System for Human Colorectal Carcinoma Cells: Synthesis, Characterization and In Vitro Study. Polymers. 2023; 15(5):1179. https://doi.org/10.3390/polym15051179
Chicago/Turabian StyleFerrentino, Nancy, Maria Preziosa Romano, Silvia Zappavigna, Marianna Abate, Vitale Del Vecchio, Dario Romano, Chiara Germinario, Celestino Grifa, Rosanna Filosa, and Daniela Pappalardo. 2023. "Poly(ε-caprolactone)-poly(ethylene glycol) Tri-Block Copolymer as Quercetin Delivery System for Human Colorectal Carcinoma Cells: Synthesis, Characterization and In Vitro Study" Polymers 15, no. 5: 1179. https://doi.org/10.3390/polym15051179
APA StyleFerrentino, N., Romano, M. P., Zappavigna, S., Abate, M., Del Vecchio, V., Romano, D., Germinario, C., Grifa, C., Filosa, R., & Pappalardo, D. (2023). Poly(ε-caprolactone)-poly(ethylene glycol) Tri-Block Copolymer as Quercetin Delivery System for Human Colorectal Carcinoma Cells: Synthesis, Characterization and In Vitro Study. Polymers, 15(5), 1179. https://doi.org/10.3390/polym15051179







