Latest Advances in Highly Efficient Dye-Based Photoinitiating Systems for Radical Polymerization
Abstract
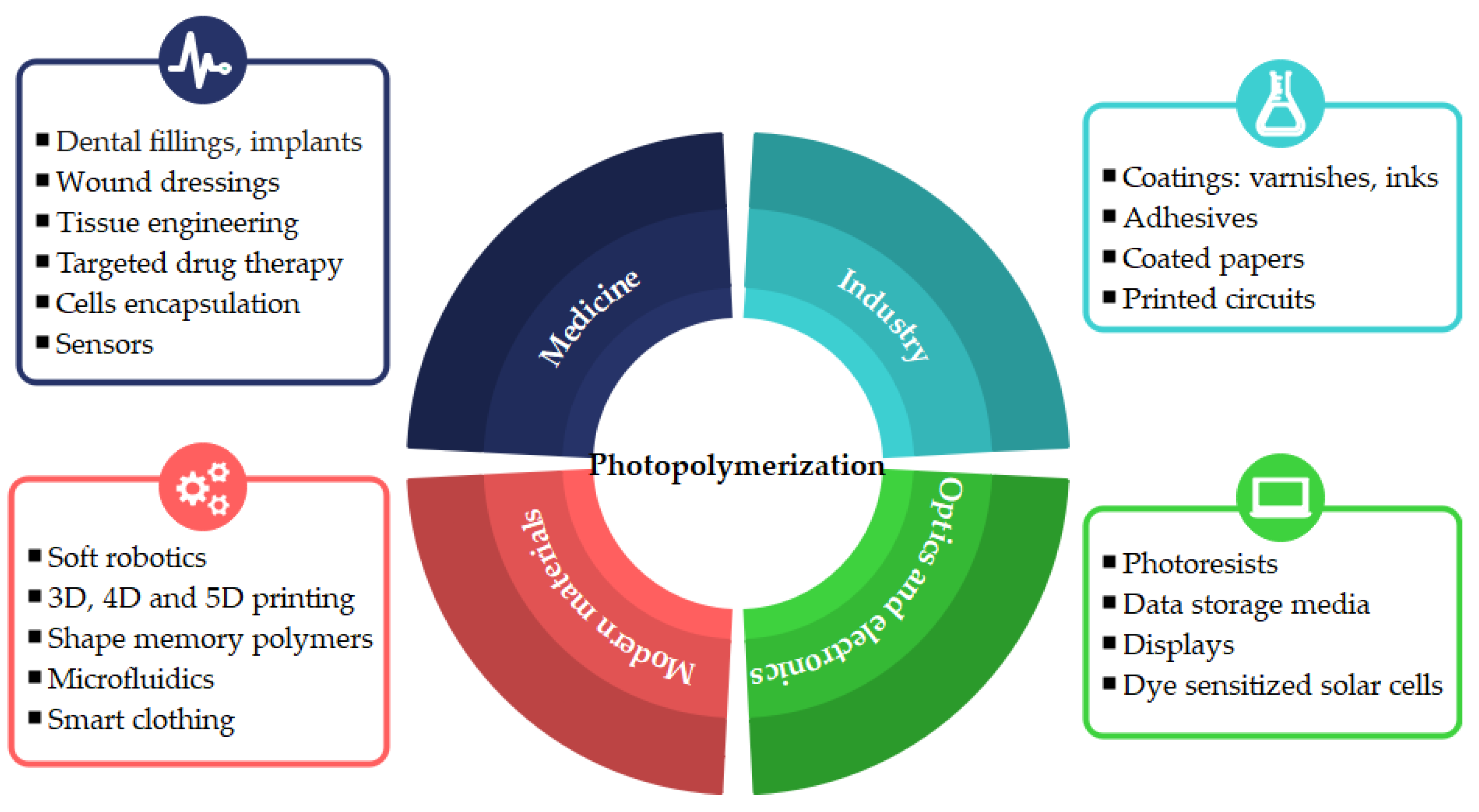
:1. Introduction
2. Photoinitiated Radical Polymerization
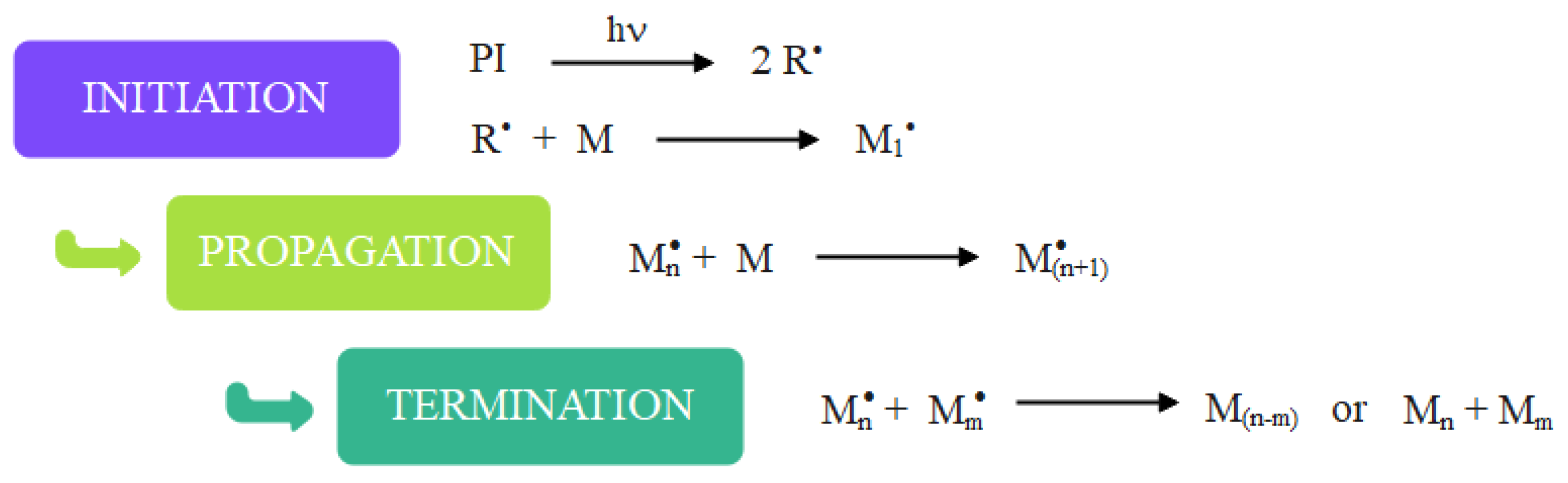
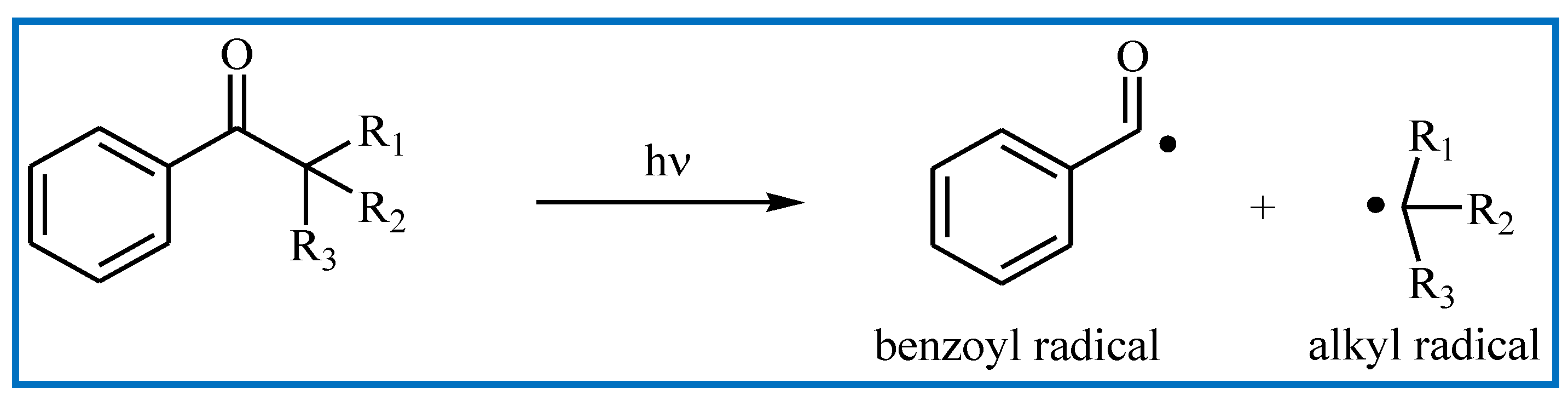
2.1. Mechanism of Photopolymerization
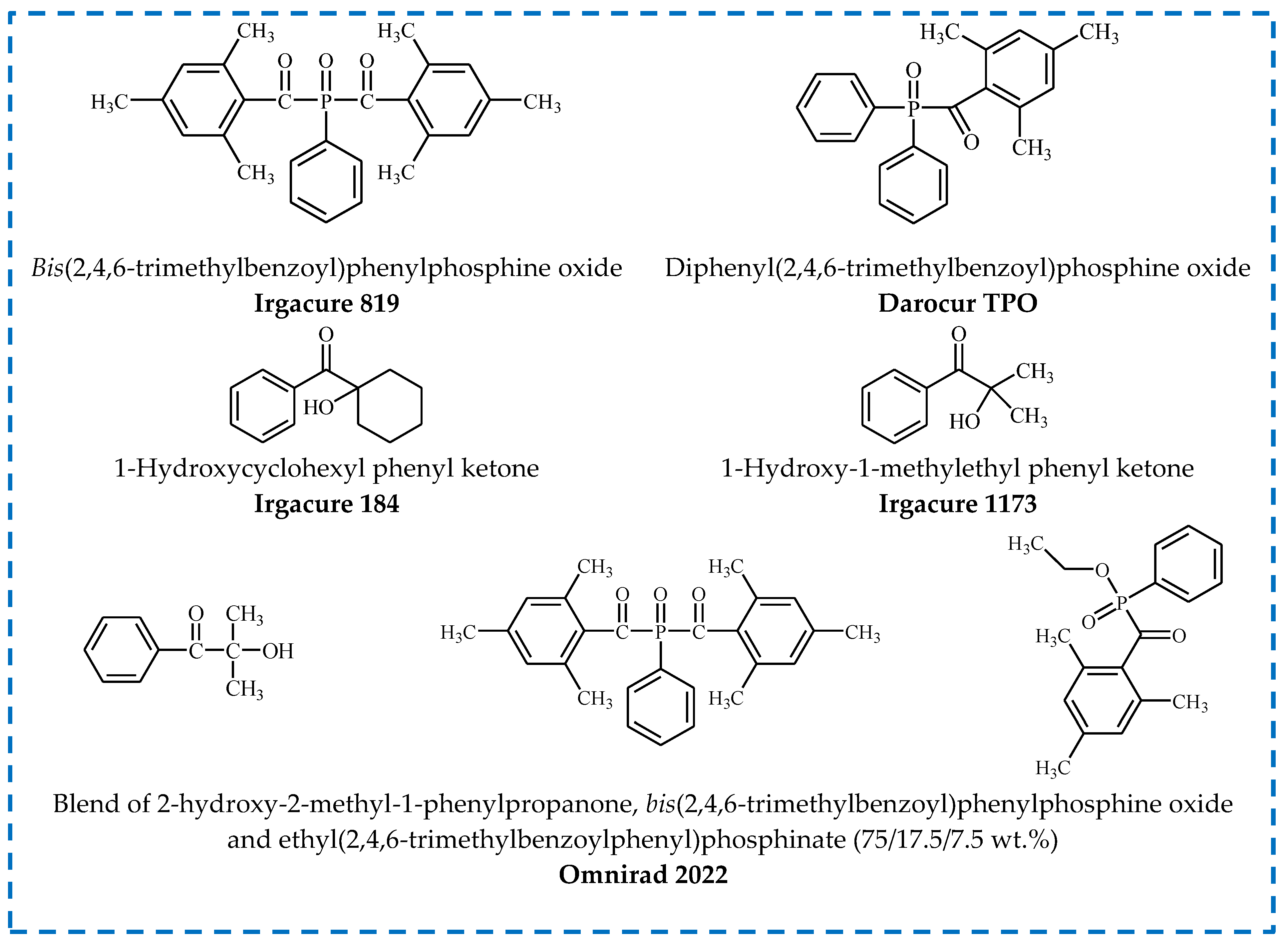
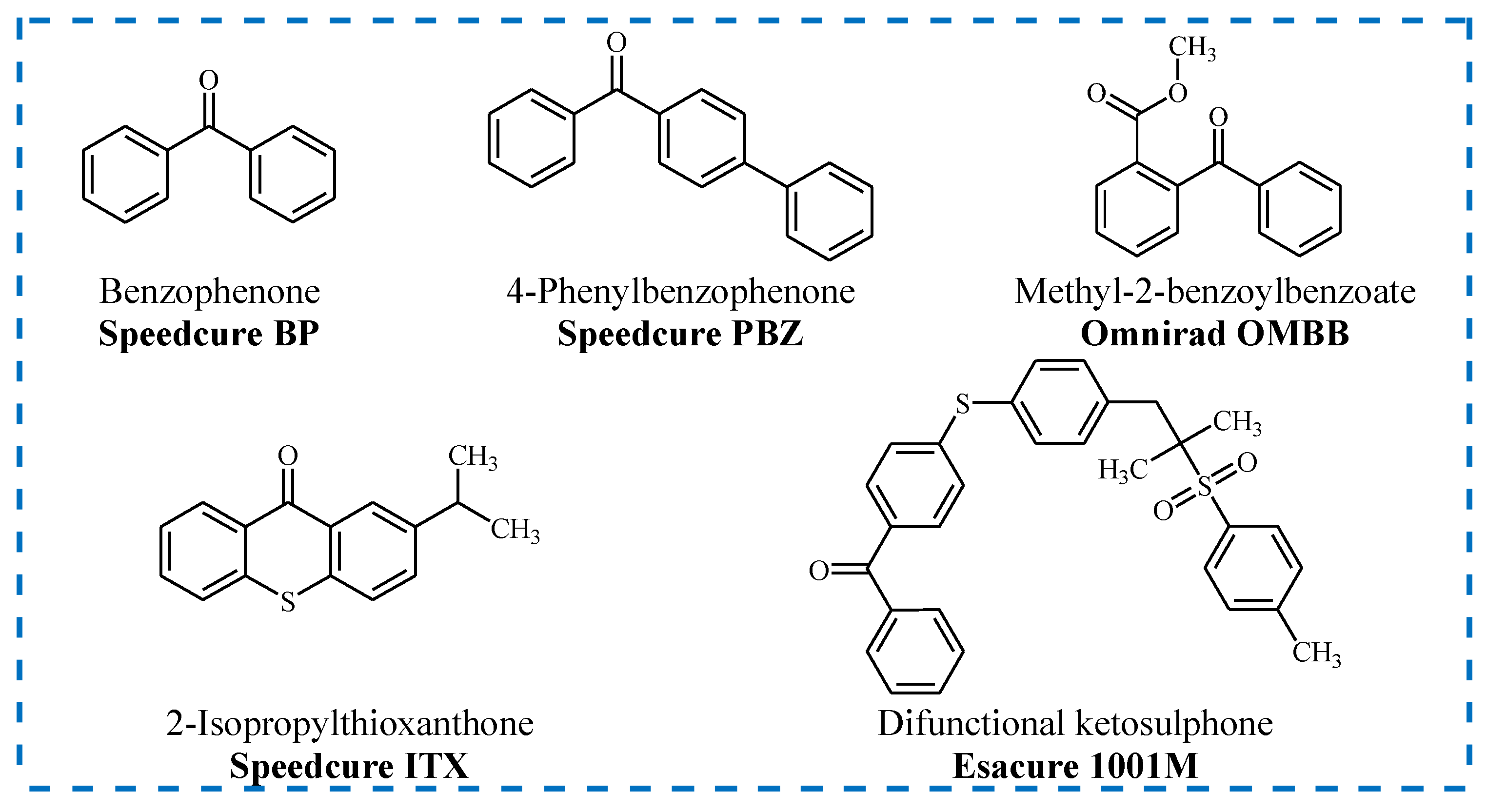
2.2. Types of Photoinitiators
- Acylphosphine oxides (APOs);
- Benzil ketals (BKs);
- Benzoin ethers (BEs);
- α-Hydroxyalkyl ketones (HPs);
- Mixtures of the above-mentioned compounds (blends) and others.
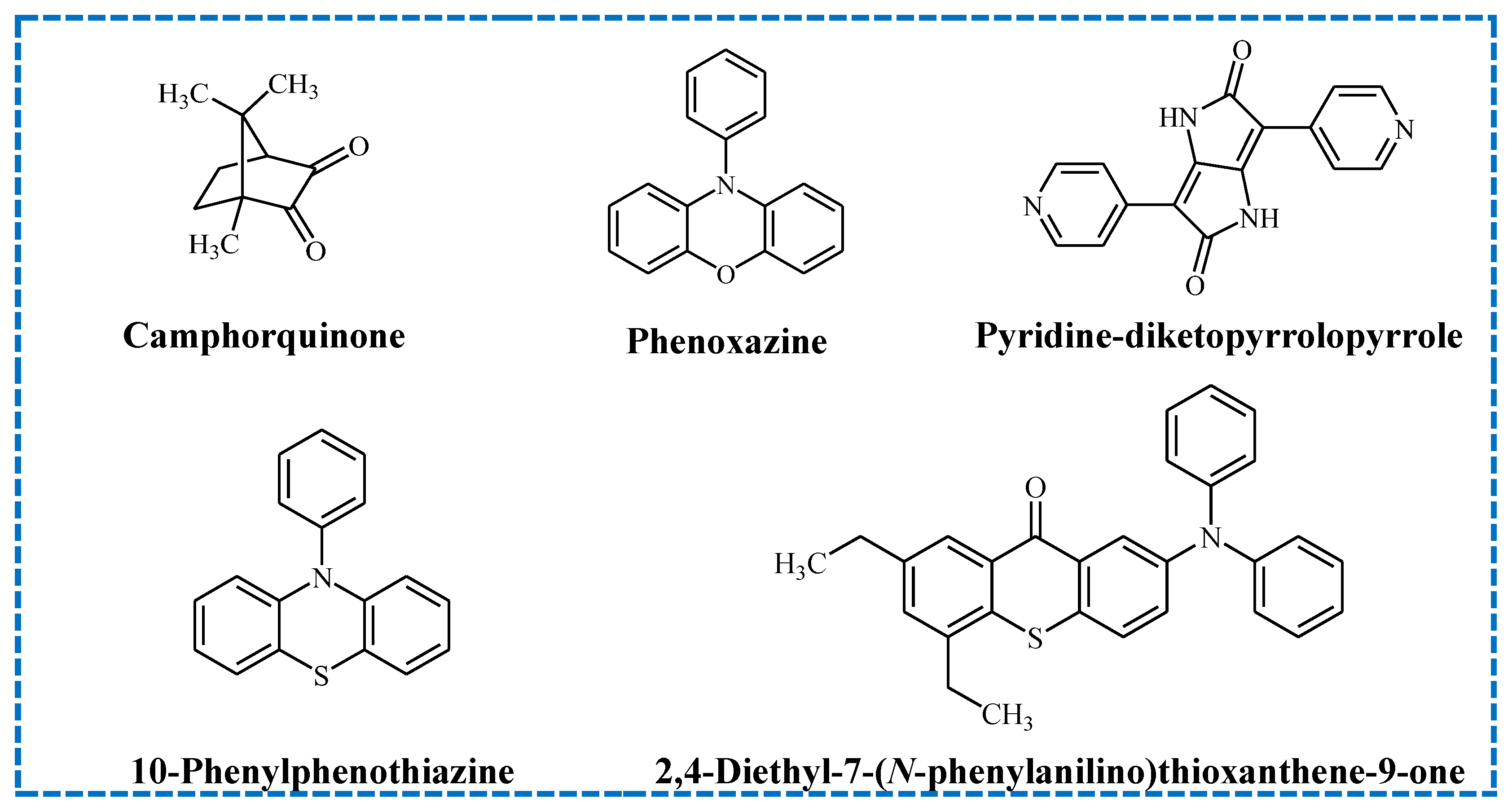
- Anthraquinones (AQs);
- Benzophenones (BPs) and their substituted analogues;
- Camphorquinones (CQs);
- Thioxanthones (TXs);
- Mixtures of the above-mentioned compounds (blends) and others.
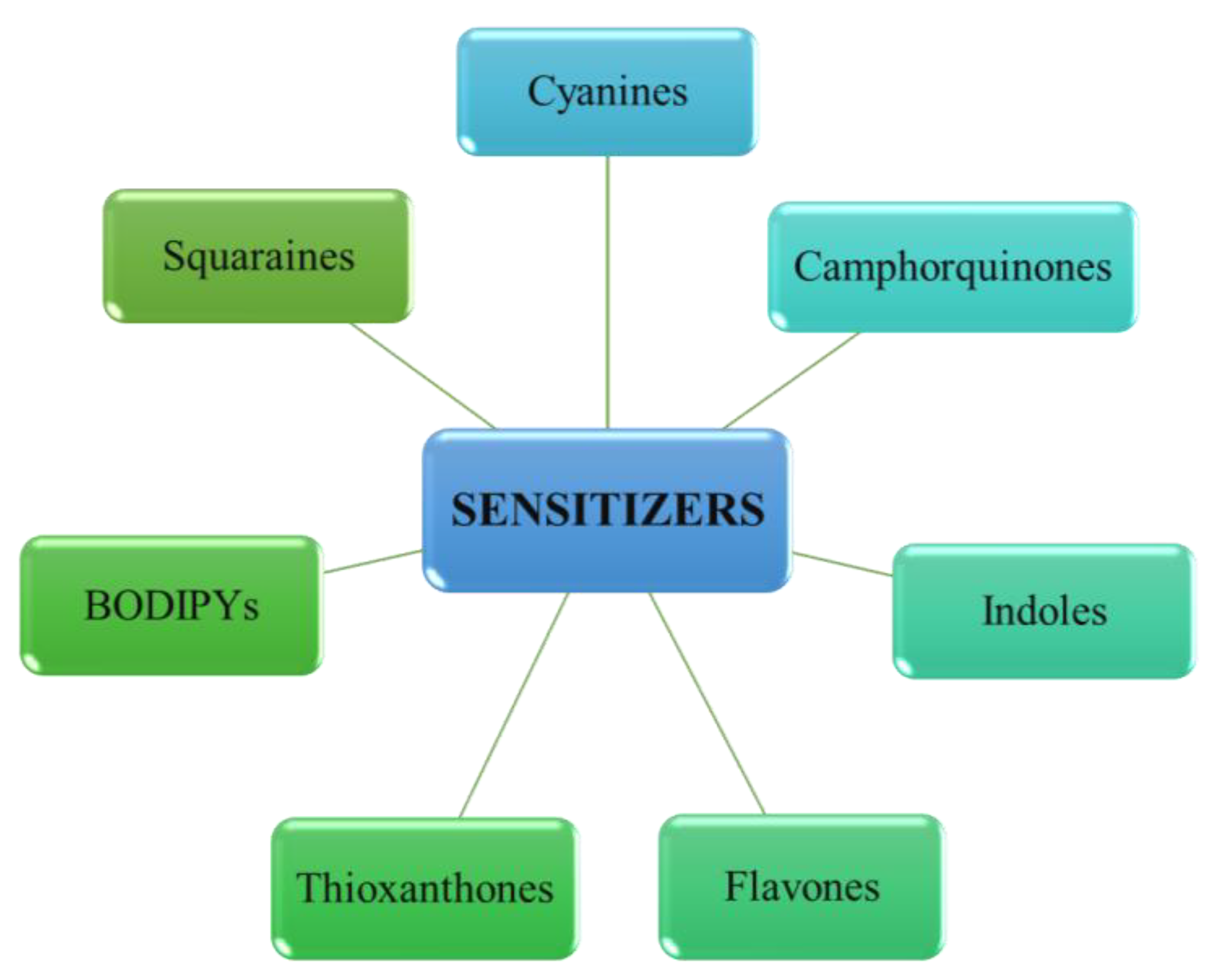
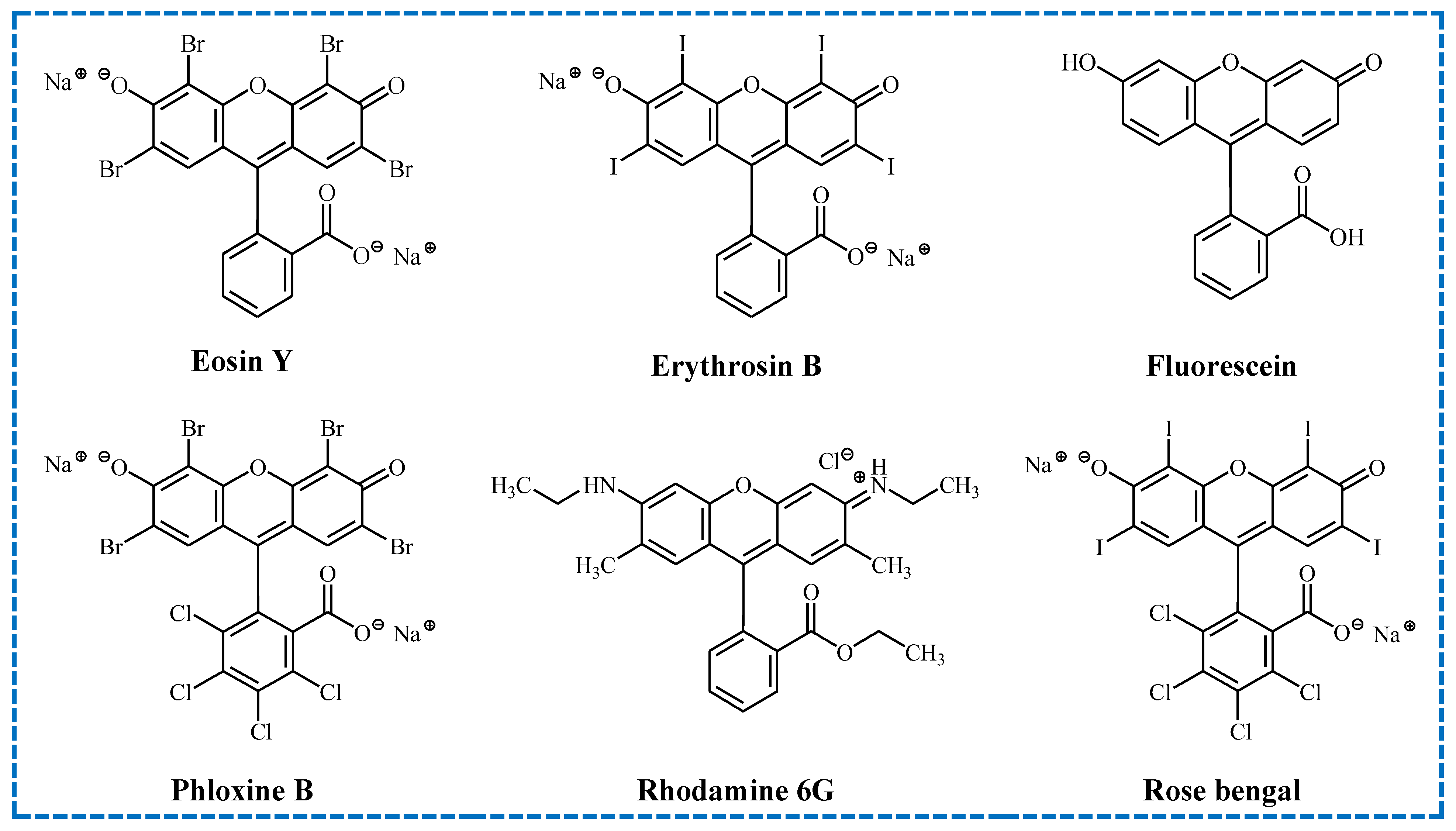
3. Dyes as Effective Sensitizers in Photoinitiating Systems
4. Novel Series of Dye-Photosensitized Systems for Radical Polymerization Reactions
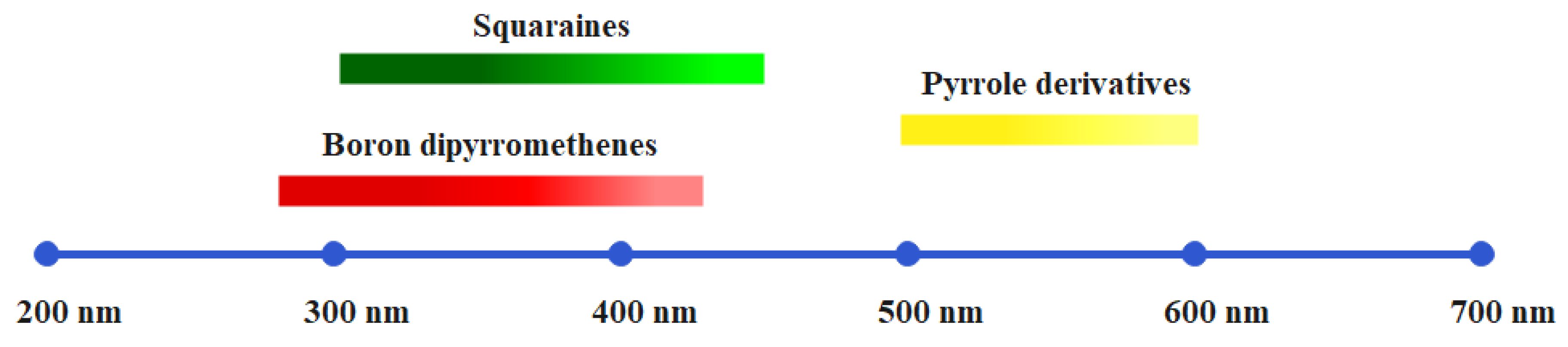
4.1. Squaraine-Based Photoinitiators
4.2. Photoinitiators Containing a Pyrrole Unit
4.3. BODIPY-Based Photoinitiators
4.4. Summary of the Effectiveness of the New Dye-Based Photoinitiating Systems
5. Requirements and Challenges for Radical Photoinitiators
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced electron transfer reactions for macromolecular syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef] [PubMed]
- Buglioni, L.; Raymenants, F.; Slattery, A.; Zondag, S.D.A.; Noël, T. Technological innovations in photochemistry for organic synthesis: Flow chemistry, high-troughput experimentation, scale-up, and photoelectrochemistry. Chem. Rev. 2022, 122, 2752–2906. [Google Scholar] [CrossRef] [PubMed]
- Rahal, M.; Graff, B.; Toufaily, J.; Hamieh, T.; Ibrahim-Ouali, M.; Dumur, F.; Lalevée, J. Naphthyl-naphthalimides as high-performance visible light photoinitiators for 3D printing and photocomposites synthesis. Catalysts 2021, 11, 1269. [Google Scholar] [CrossRef]
- Berneth, H.; Bruder, F.K.; Fäcke, T.; Hansen, S.; Kawamura, K.; Pitzer, L.; Kern, S.; Wewer, B.; Rölle, T. A new three-component photo-initiating system for visible light recording of volume holograms with single-pulsed laser. Polymers 2021, 13, 3517. [Google Scholar] [CrossRef]
- Kabatc, J.; Iwińska, K.; Balcerak, A.; Kwiatkowska, D.; Skotnicka, A.; Czech, Z.; Bartkowiak, M. Onium salts improve the kinetics of photopolymerization of acrylate activated with visible light. RSC Adv. 2020, 10, 24817–24829. [Google Scholar] [CrossRef]
- Sun, G.; Wu, X.; Liu, R. A comprehensive investigation of acrylates photopolymerization shrinkage stress from micro and macro perspectives by real time MIR-photo-rheology. Prog. Org. Coat. 2021, 155, 106229. [Google Scholar] [CrossRef]
- Scanone, A.C.; Casado, U.; Schroeder, W.F.; Hoppe, C.E. Visible-light photopolymerization of epoxy-terminated poly(dimethylsiloxane) blends: Influence of the cycloaliphatic monomer content on the curing behavior and network properties. Eur. Polym. J. 2020, 134, 109841. [Google Scholar] [CrossRef]
- Pan, J.; Tao, Y.; Zhao, L.; Yu, X.; Zhao, X.; Wu, T.; Liu, L. Green preparation of quartenized vinylimidazole-based anion exchange membrane by photopolymerization. Sep. Purif. Technol. 2021, 276, 119220. [Google Scholar] [CrossRef]
- Bonardi, A.-H.; Zahouily, S.; Dietlin, C.; Graff, B.; Dumur, F.; Ibrahim-Ouali, M.; Gigmes, D.; Lalevée, J. Thermal initiators as additives for photopolymerization of methacrylates upon blue light. Coatings 2020, 10, 478. [Google Scholar] [CrossRef]
- Sun, A.; He, X.; Ji, X.; Hu, D.; Pan, M.; Zhang, L.; Qian, Z. Current research progress of photopolymerized hydrogels in tissue engineering. Chin. Chem. Lett. 2021, 32, 2117–2126. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Nie, J.; Zhu, X. Visible light and water-soluble photoinitiating system based on the charge transfer complex for free radical photopolymerization. J. Photochem. Photobiol. A Chem. 2020, 402, 112803. [Google Scholar] [CrossRef]
- Rahal, M.; Graff, B.; Toufaily, J.; Hamieh, T.; Dumur, F.; Lalevée, J. Design of keto-coumarin based photoinitiator for free radical photopolymerization: Towards 3D printing and photocomposites applications. Eur. Polym. J. 2021, 154, 110559. [Google Scholar] [CrossRef]
- Bongiovanni, R.; Vacche, S.D.; Vitale, A. Photoinduced processes as a way to sustainable polymers and innovation in polymeric materials. Polymers 2021, 13, 2293. [Google Scholar] [CrossRef] [PubMed]
- Accioni, F.; Vázquez, J.; Merinero, M.; Begines, B.; Alcudia, A. Latest trends in surface modification for dental implantology: Innovative developments and analytical applications. Pharmaceutics 2022, 14, 455. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Kar, M.; Dinda, P.; Mandal, T.K. Ionic liquid-based unconventional photoinitiators for aqueous polymerization. Eur. Polym. J. 2022, 162, 110870. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, S.; Paul, S.; Thomas, P.; Roy, B.; Mitra, P.; Roy, S.; Banerjee, A. In situ self-assembly and photopolymerization for hetero-phase synthesis and patterning of conducting materials using soft oxometalates in thermo-optical tweezers. J. Mater. Chem. C 2017, 5, 6718–6728. [Google Scholar] [CrossRef] [Green Version]
- Choong, Y.Y.C.; Maleksaeedi, S.; Eng, H.; Yu, S.; Wei, J.; Su, P.-C. High speed 4D printing of shape memory polymers with nanosilica. Appl. Mater. Today 2020, 18, 100515. [Google Scholar] [CrossRef]
- Datta, M.; Maraz, K.M.; Rahman, N.; Khan, R.A. Application of polymer in biomedical implication. GSC Biol. Pharm. Sci. 2021, 14, 98–114. [Google Scholar] [CrossRef]
- Yu, P.; Xu, Y.-X.; Liu, Y.-S. Polymerization shrinkage and shrinkage stress of bulk-fill and non-bulk-fill resin-based composites. J. Dent. Sci. 2021, 17, 1212–1216. [Google Scholar] [CrossRef]
- Dikova, T.; Maximov, J.; Todorov, V.; Georgiev, G.; Panov, V. Optimization of photopolymerization process of dental composites. Processes 2021, 9, 779. [Google Scholar] [CrossRef]
- Hasanain, F.A.; Nassar, H.M.; Ajaj, R.A. Effect of light curing distance on microhardness profiles of bulk-fill resin composites. Polymers 2022, 14, 528. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Yeazel, T.R.; Asheghali, D.; Petersen, S.R.; Dort, S.; Gall, K.; Becker, M.L. Fabrication of biomedical scaffolds using biodegradable polymers. Chem. Rev. 2021, 121, 11238–11304. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-F. Development of hydrogels with self-healing properties for delivery of bioactive agents. Mol. Pharm. 2021, 18, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Khudyakov, I.V. Fast photopolymerization of acrylate coatings: Achievements and problems. Prog. Org. Coat. 2018, 121, 151–159. [Google Scholar] [CrossRef]
- Besse, V.; Derbanne, M.A.; Pham, T.-N.; Cook, W.D.; Le Pluart, L. Photopolymerization study and adhesive properties of self-etch adhesives containing bis(acyl)phosphine oxide initiator. Dent. Mater. 2016, 32, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Li, W.; Zhan, Y.; Tian, L.; Tian, H. Two preparation processes for anti-corrosion and self-healing epoxy coatings containing the poly(calcium alginate) microcapsules loaded with tung oil. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128600. [Google Scholar] [CrossRef]
- Prescient & Strategic Intelligence. Available online: https://www.psmarketresearch.com/market-analysis/paints-and-coatings-market (accessed on 27 February 2022).
- Uncertainties Ahead: Impact of Market Conditions on Paints and Coatings. Focus Powder Coat. 2016, 2016, 7. [CrossRef]
- Global paints & coatings market report 2014–2022. Focus Powder Coat. 2019, 2019, 7. [CrossRef]
- The UV curable coatings market is expected to reach USD 10,751.64 million by 2026. Focus Powder Coat. 2021, 2021, 7. [CrossRef]
- Goourey, G.G.; Wong-Wah-Chung, P.; Delor-Jestin, F.; Légeret, B.; Balan, L.; Israëli, Y. Photostability of acrylate photopolymers used as components in recording materials. Polym. Degrad. Stab. 2015, 119, 208–216. [Google Scholar] [CrossRef]
- Ashwathy, G.; Rajesh, C.S.; Sreejith, M.S.; Vijayakumar, K.P.; Sudha Kartha, C. Designing photovoltaic concentrators using holographic lens recorded in nickel ion doped photopolymer material. Sol. Energy 2018, 163, 70–77. [Google Scholar] [CrossRef]
- Pradeep, P.V.; Lijo, P. Review on novel biomaterials and innovative 3D printing techniques in biomedical applications. Mater. Today Proc. 2022, 58, 96–103. [Google Scholar] [CrossRef]
- Wang, H.-J.; Mao, Q.-Y.; Feng, G.; Liu, C.; Yang, M.-Z.; Hao, M.-F.; Meng, Z.-F.; Li, S.-M.; Zhang, Y.-P.; Wang, J.-Y. 3D printing of multi-functional artificial conduits against acute thrombosis and clinical infection. Compos. B Eng. 2022, 230, 109497. [Google Scholar] [CrossRef]
- Dey, D.; Srinivas, D.; Panda, B.; Suraneni, P.; Sitharam, T.G. Use of industrial waste materials for 3D printing of sustainable concrete: A review. J. Clean. Prod. 2022, 340, 130749. [Google Scholar] [CrossRef]
- Ge, Q.; Jian, B.; Li, H. Shaping soft materials via digital light processing-based 3D printing: A review. Forces Mech. 2022, 6, 100074. [Google Scholar] [CrossRef]
- Shehata, N.; Abdelkareem, M.A.; Sayed, E.T.; Egirani, D.E.; Opukumo, A.W. Smart materials: The next generation. Encycl. Smart Mater. 2022, 4, 288–299. [Google Scholar] [CrossRef]
- Mukherjee, A.; Deepmala; Srivastava, P.; Sandhu, J.K. Application of smart materials in civil engineering: A review. Mater. Today Proc. 2021; in press. [Google Scholar] [CrossRef]
- Luo, H.; Li, C.; Shi, C.; Nie, S.; Song, J. Switchable dry adhesive based on shape memory polymer with hemispherical indenters for transfer printing. Theor. Appl. Mech. Lett. 2021, 11, 100308. [Google Scholar] [CrossRef]
- Jeewantha, L.H.J.; Emmanuel, K.D.C.; Herath, H.M.C.M.; Islam, M.M.; Fang, L.; Epaarachchi, J.A. Multi-attribute parametric optimization of shape memory polymer properties for adaptive orthopaedic plasters. Materialia 2022, 21, 101325. [Google Scholar] [CrossRef]
- Li, D.; Qing, L.; Li, M.; Cheng, H.; Yang, G.; Fu, Q.; Sun, Y. Ultra-fast self-repairing of anti-corrosive coating based on synergistic effect between cobalt octoate and linseed oil. Prog. Org. Coat. 2022, 166, 106776. [Google Scholar] [CrossRef]
- Hou, S.; Li, K.; Wu, Z.; Li, F.; Shi, C. Quantative evaluation on self-healing capacity of cracked concrete by water permeability test—A review. Cem. Concr. Compos. 2022, 127, 104404. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Yeo, L.P.; Ong, A.J.; Zhiwei, W.; Mandler, D.; Magdassi, S.; Tok, A.I.Y. Electrochromic smart glass coating on functional nano-frameworks for effective building energy conservation. Mater. Today Energy 2020, 18, 100496. [Google Scholar] [CrossRef]
- Jiang, Z.; Ouyang, T.; Ding, L.; Li, W.; Li, W.; Balogun, M.S.J.T. 3D self-bonded porous graphite fiber monolith for phase change material composite with high thermal conductivity. Chem. Eng. J. 2022, 438, 135496. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-healing mechanisms in smart protective coatings: A review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Xue, T.; Lu, H.; Yuan, H.; He, Y.; Nie, J.; Zhu, X. A bis-acrylate functionalized enone as photoinitiator and crosslinker in photopolymerization. Prog. Org. Coat. 2022, 162, 106587. [Google Scholar] [CrossRef]
- Bae, C.-J.; Ramachandran, A.; Chung, K.; Park, S. Ceramic stereolitography: Additive manufacturing for 3D complex ceramic structures. J. Korean Ceram. Soc. 2017, 54, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Luu, T.; Jia, Z.; Kanaev, A.; Museur, L. Effect of light intensity on the free-radical photopolymerization kinetics of 2-hydroxyethyl methacrylate: Experiments and Simulations. J. Phys. Chem. B 2020, 124, 6857–6866. [Google Scholar] [CrossRef]
- Petko, F.; Świeży, A.; Ortyl, J. Photoinitiating systems and kinetics of frontal photopolymerization processes—The prospects for efficient preparation of composites and thick 3D structures. Polym. Chem. 2021, 12, 4593–4612. [Google Scholar] [CrossRef]
- Lang, M.; Hirner, S.; Wiesbrock, F.; Fuchs, P. A review on modeling cure kinetics and mechanisms of photopolymerization. Polymers 2022, 14, 2074. [Google Scholar] [CrossRef]
- Ito, S.; Tanaka, Y.; Yoshikawa, H.; Ishibashi, Y.; Miyasaka, H.; Masuhara, H. Confinement of photopolymerization and solidification with radiation pressure. J. Am. Che. Soc. 2011, 133, 14472–14475. [Google Scholar] [CrossRef]
- Ribas-Massonis, A.; Cicujano, M.; Duran, J.; Besalú, E.; Poater, A. Free-radical photopolymerization for curing products for refinish coatings market. Polymers 2022, 14, 2856. [Google Scholar] [CrossRef]
- Wu, Y.; Ke, J.; Dai, C.; Wang, J.; Huang, C.; Situ, Y.; Huang, H. Large-molecular-weight acyldiphenylphosphine oxides as low mobility type I photoinitiator for radical polymerization. Eur. Polym. J. 2022, 175, 111380. [Google Scholar] [CrossRef]
- Wu, Y.P.; Li, R.; Huang, C.; Wu, J.; Sun, X.; Situ, Y. New acyl phosphine oxides as high-performance and low migration type I photoinitiators of radical polymerization. Prog. Org. Coat. 2022, 168, 106876. [Google Scholar] [CrossRef]
- Tomal, W.; Pilch, M.; Chachaj-Brekiesz, A.; Ortyl, J. Development of new high-performance biphenyl and terphenyl derivatives as versatile photoredox photoinitiating systems and their applications in 3D printing photopolymerization processes. Catalysts 2019, 9, 827. [Google Scholar] [CrossRef] [Green Version]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated polymerization: Advances, challenges, and opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Breloy, L.; Negrell, C.; Mora, A.-S.; Li, W.S.J.; Brezová, V.; Caillol, S.; Versace, D.-L. Vanillin derivative as performing type I photoinitiator. Eur. Polym. J. 2020, 132, 109727. [Google Scholar] [CrossRef]
- Ley, C.; Carré, C.; Ibrahim, A.; Allonas, X. Application of high performance photoinitiating systems for holographic grating recording. In Holographic Materials and Optical Systems; Naydenova, I., Nazarova, D., Babeva, T., Eds.; IntechOpen: London, UK, 2017; Chapter 17; pp. 275–404. Available online: https://www.intechopen.com/chapters/52925 (accessed on 10 March 2022).
- Deng, L.; Tang, L.; Qu, J. Synthesis and photopolymerization of novel UV-curable macrophotoinitiators. Prog. Org. Coat. 2020, 141, 105546. [Google Scholar] [CrossRef]
- Liu, S.; Giacoletto, N.; Graff, B.; Morlet-Savary, F.; Nechab, M.; Xiao, P.; Dumur, F.; Lalevée, J. N-naphthalimide ester derivatives as Type I photoinitiators for LED photopolymerization. Mater. Today Chem. 2022, 26, 101137. [Google Scholar] [CrossRef]
- Green, W.A. Industrial Photoinitiators: A Technical Guide; CRC Press: Boca Raton, FL, USA, 2010; pp. 17–43. [Google Scholar]
- Müller, S.M.; Schlögl, S.; Wiesner, T.; Haas, M.; Griesser, T. Recent advances in type I photoinitiators for visible light induced photopolymerization. ChemPhotoChem 2022, 6, e202200091. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Weisbrodt, M.; Schmidt, B.; Kraśkiewicz, A. The effect of type-I photoinitiators on the kinetics of the UV-induced cotelomerization process of acrylate monomers and properties of obtained pressure-sensitive adhesives. Materials 2021, 14, 4563. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Water-soluble photoinitiators in biomedical applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-L.; Chen, Y.-C. Synthesis and free radical photopolymerization of one-component type II photoinitiator based on benzophenone segment. J. Photochem. Photobiol. A Chem. 2022, 429, 113900. [Google Scholar] [CrossRef]
- Kreutzer, J.; Kaya, K.; Yagci, Y. Poly(propylene oxide)-thioxanthone as one-component Type II polymeric photoinitiator for free radical polymerization with low migration behavior. Eur. Polym. J. 2017, 95, 71–81. [Google Scholar] [CrossRef]
- Xue, T.; Li, Y.; Zhao, X.; Nie, J.; Zhu, X. A facile synthesized benzophenone Schiff-base ligand as efficient type II visible light photoinitiator. Prog. Org. Coat. 2021, 157, 106329. [Google Scholar] [CrossRef]
- Mustroph, H. Bring back order in the polymethine dye medley: Classification, structure and spectra. Dyes Pigm. 2022, 208, 110783. [Google Scholar] [CrossRef]
- Balcerak, A.; Kabatc, J. Recent progress in the development of highly active dyeing photoinitiators based on 1,3-bis(p-substituted phenylamino)squaraines for radical polymerization of acrylates. Polym. Chem. 2022, 13, 1787–1812. [Google Scholar] [CrossRef]
- Kabatc, J. The influence of a radical structure on the kinetics of photopolymerization. J. Polym. Sci. A Polym. Chem. 2017, 55, 1575–1589. [Google Scholar] [CrossRef] [Green Version]
- Balcerak, A.; Kabatc, J. The photooxidative sensitization of bis(p-substituted diphenyl)iodonium salts in the radical polymerization of acrylates. RSC Adv. 2019, 9, 28490. [Google Scholar] [CrossRef] [Green Version]
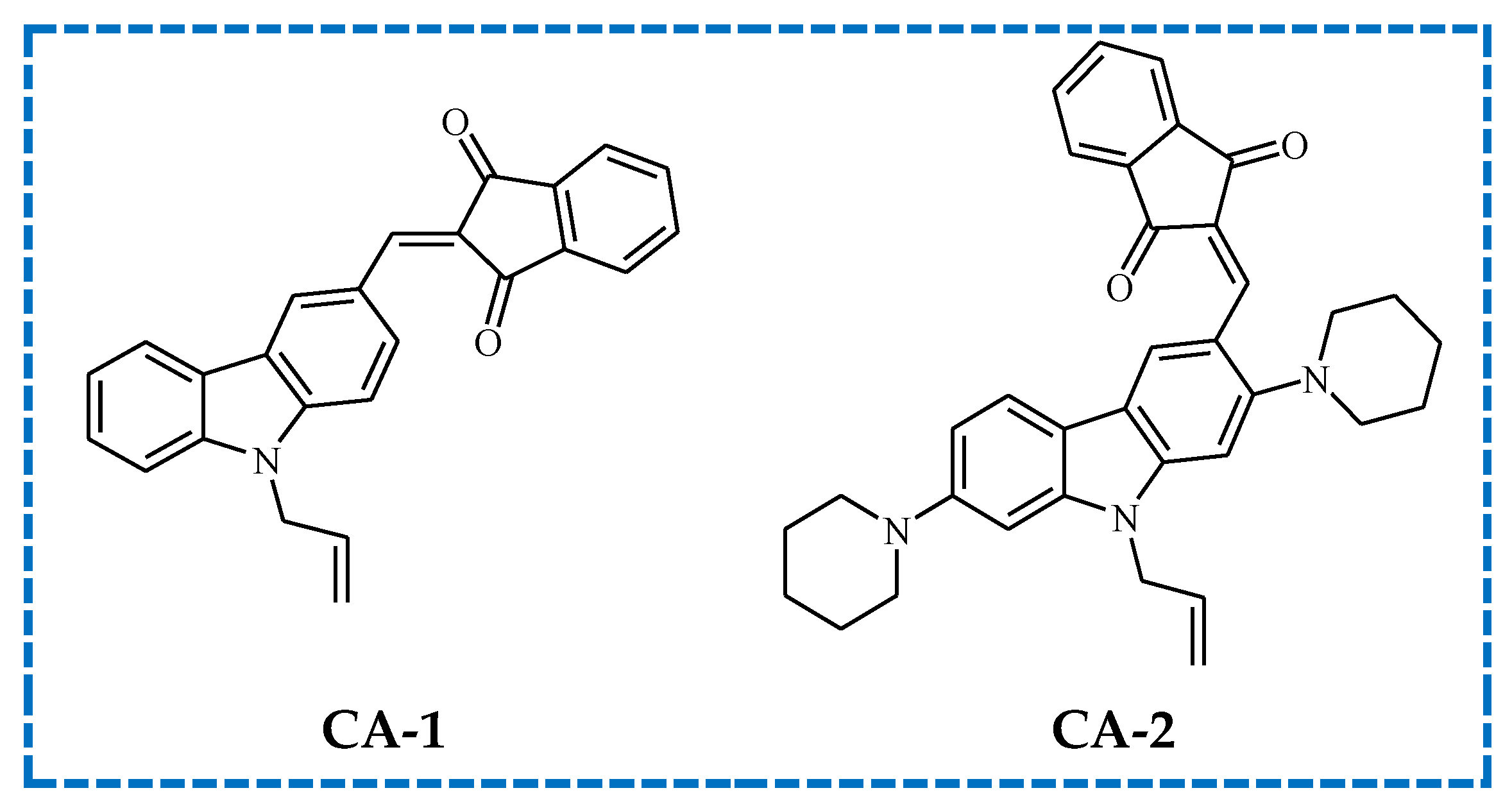
- Liao, W.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. Design, synthesis and properties of carbazole-indenedione based photobleachable photoinitiators for photopolymerization. J. Photochem. Photobiol. A Chem. 2023, 435, 114297. [Google Scholar] [CrossRef]
- Deng, L.; Qu, J. Synthesis and properties of novel bis-chalcone-based photoinitiators for LED polymerization with photobleaching and low migration. Prog. Org. Coat. 2023, 174, 107240. [Google Scholar] [CrossRef]
- Pigot, C.; Noirbent, G.; Brunel, D.; Dumur, F. Recent advances on push-pull organic dyes as visible light photoinitiators of polymerization. Eur. Polym. J. 2020, 133, 109797. [Google Scholar] [CrossRef]
- Sun, K.; Chen, H.; Zhang, Y.; Morlet-Savary, F.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. High-performance sunlight induced polymerization using novel push-pull dyes with high light absorption properties. Eur. Polym. J. 2021, 151, 110410. [Google Scholar] [CrossRef]
- Guo, Z. Research advances in UV-curable self-healing coatings. RSC Adv. 2022, 12, 32429–32439. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Felipe, C.; Oliveira, J.; Etxebarria, I.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. State-of-the-art and future challenges of UV curable polymer-based smart materials for printing technologies. Adv. Mater. Technol. 2019, 4, 1800618. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Jia, Y.; Tian, K.; Xu, Z.; Jiao, J.; Li, R.; Wu, Y.; Cao, L.; Wang, H. UV-triggered self-healing of a single robust SiO2 microcapsule based on cationic polymerization for potential application in aerospace coatings. ACS Appl. Mater. Interfaces 2016, 8, 21046–21054. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Lee, T.H.; Kim, J.C.; Lee, K.C.; Lee, S.-H.; Noh, S.M.; Park, Y.I. Dual monitoring of cracking and healing in self-healing coatings using microcapsules loaded with two fluorescent dyes. Molecules 2019, 24, 1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, D.H.; Bae, H.E.; Bae, M.J.; Lee, S.-H.; Cheong, I.W.; Park, Y.I.; Jeong, J.-E.; Kim, J.C. Fast, localized and low-energy consumption self-healing of automotive clearcoats using a photothermal effect triggered by NIR radiation. ACS Appl. Polym. Mater. 2022, 4, 3802–3810. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, X.; Jiang, Y.; Lin, J.; Graff, B.; Hu, S.; Lalevée, J.; Liao, S. Organocatalytic PET-RAFT polymerization with a low ppm of organic photocatalyst under visible light. Polym. Chem. 2022, 13, 209–219. [Google Scholar] [CrossRef]
- Treat, N.J.; Sprafke, H.; Kramer, J.W.; Clark, P.G.; Barton, B.E.; de Alaniz, J.R.; Fors, B.P.; Hawker, C.J. Metal-free atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 16096–16101. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Liu, Y.; Miller, K.A.; Zhu, H.; Egap, E. Lead halide perovskite nanocrystals as photocatalysts for PET-RAFT polymerization under visible and near-infrared irradiation. ACS Macro Lett. 2020, 9, 725–730. [Google Scholar] [CrossRef]
- Ballard, N.; Asua, J.M. Can we push rapid reversible deactivation radical polymerizations toward immortality? ACS Marco Lett. 2020, 9, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, X.-Y.; Su, Z.-Q. Rational design of biomolecules/polymer hybrids by reversible deactivation radical polymerization (RDRP) for biomedical applications. Chin. J. Polym. Sci. 2021, 39, 1093–1109. [Google Scholar] [CrossRef]
- Tao, H.; Xia, L.; Chen, G.; Zeng, T.; Nie, X.; Zhang, Z.; You, Y. PET-RAFT polymerization catalyzed by small organic molecule under green light irradiation. Polymers 2019, 11, 892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellotti, V.; Simonutti, R. New light in polymer science; photoinduced reversible addition-fragmentation chain transfer polymerization (PET-RAFT) as innovative strategy for the synthesis of advanced materials. Polymers 2021, 13, 1119. [Google Scholar] [CrossRef] [PubMed]
- Nomeir, B.; Fabre, O.; Ferji, K. Effect of tertiary amines on the photoinduced electron transfer-reversible addition-fragmentation chain transfer (PET-RAFT) polymerization. Macromolecules 2019, 52, 6898–6903. [Google Scholar] [CrossRef]
- Allegrezza, M.L.; Konkolewicz, D. PET-RAFT polymerization: Mechanistic perspectives for future materials. ACS Macro Lett. 2021, 10, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.-H.; Caldona, E.B.; Advincula, R.C. PET-RAFT polymerization under flow chemistry and surface-initiated reactions. Polym. Int. 2023, 72, 145–157. [Google Scholar] [CrossRef]
- Phommalysack-Lovan, J.; Chu, Y.; Boyer, C.; Xu, J. PET-RAFT polymerization: Towards green and precision polymer manufacturing. Chem. Commun. 2018, 54, 6591–6606. [Google Scholar] [CrossRef]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef]
- Figg, C.A.; Hickman, J.D.; Scheutz, G.M.; Shanmugam, S.; Carmean, R.N.; Tucker, B.S.; Boyer, C.; Sumerlin, B.S. Color-coding visible light polymerizations to elucidate the activation of trithiocarbonates using eosin Y. Macromolecules 2018, 51, 1370–1376. [Google Scholar] [CrossRef]
- Lin, C.; Katla, S.K.; Perez-Mercader, J. Enhanced fluorescence emission from rhodamine 6G dye through polymerization-induced self-assembly. J. Photochem. Photobiol. A Chem. 2021, 406, 112992. [Google Scholar] [CrossRef]
- Wu, C.; Corrigan, N.; Lim, C.-H.; Jung, K.; Zhu, J.; Miyake, G.; Xu, J.; Boyer, C. Guiding the design of organic photocatalyst for PET-RAFT polymerization: Halogenated xanthene dyes. Macromolecules 2019, 52, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhu, N.; Guo, K. Advances in organocatalyzed atom transfer radical polymerization. Adv. Polym. Technol. 2019, 2019, 7971683. [Google Scholar] [CrossRef] [Green Version]
- Discekici, E.H.; Anastasaki, A.; de Alaniz, J.R.; Hawker, C.J. Evolution and future directions of metal-free atom transfer radical polymerization. Macromolecules 2018, 51, 7421–7434. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Fang, C.; Fantin, M.; Malhotra, N.; So, W.Y.; Peteanu, L.A.; Isse, A.A.; Gennaro, A.; Liu, P.; Matyjaszewski, K. Mechanism of photoinduced metal-free atom transfer radical polymerization: Experimental and computational studies. J. Am. Chem. Soc. 2016, 138, 2411–2425. [Google Scholar] [CrossRef] [PubMed]
- Aklujkar, P.S.; Rao, A.R. Developments in the components of metal-free photoinitiated organocatalyzed-atom transfer radical polymerization (O-ATRP). ChemistrySelect 2020, 5, 14884–14899. [Google Scholar] [CrossRef]
- Taskin, O.S.; Yilmaz, G.; Tasdelen, M.A.; Yagci, Y. Photoinduced reverse atom transfer radical polymerization of methyl methacrylate using camphorquinone/benzhydrol system. Polym. Int. 2014, 63, 902–907. [Google Scholar] [CrossRef]
- Yang, L.; Huang, Y.; Peng, Y.; Liu, F.; Zhang, Q.; He, H.; Wang, J.; Jiang, L.; Zhou, Y. Pyridine-diketopyrrolopyrole-based novel metal-free visible-light organophotoredox catalyst for atom-transfer radical polymerization. J. Phys. Chem. A 2020, 124, 1068–1075. [Google Scholar] [CrossRef]
- Jia, T.; Huang, S.; Bohra, H.; Wang, M. Examining derivatives of quinacridone, diketopyrrolopyrrole and indigo as the visible-light organic photocatalystsfor metal-free atom transfer radical polymerization. Dyes Pigm. 2019, 165, 223–230. [Google Scholar] [CrossRef]
- Pearson, R.M.; Lim, C.-H.; McCarthy, B.G.; Musgrave, C.B.; Miyake, G.M. Organocatalyzed atom transfer radical polymerization using N-aryl phenoxazines as photoredox catalysts. J. Am. Chem. Soc. 2016, 138, 11399–11407. [Google Scholar] [CrossRef] [Green Version]
- Bhattacherjee, A.; Sneha, M.; Lewis-Borrell, L.; Amoruso, G.; Oliver, T.A.A.; Tyler, J.; Clark, I.P.; Orr-Ewing, A.J. Singlet and triplet contributions to the excited-state activities of dihydrophenazine, phenoxazine and phenothiazine organocatalysts used in atom transfer radical polymerization. J. Am. Chem. Soc. 2021, 143, 3613–3627. [Google Scholar] [CrossRef] [PubMed]
- Hola, E.; Fiedor, P.; Dzienia, A.; Ortyl, J. Visible-light amine thioxanthone derivatives as photoredox catalysts for photopolymerization processes. ACS Appl. Polym. Mater. 2021, 3, 5547–5558. [Google Scholar] [CrossRef]
- Allushi, A.; Kutahya, C.; Aydogan, C.; Kreutzer, J.; Yilmaz, G.; Yagci, Y. Conventional type II photoinitiators as activators for photoinduced metal-free atom transfer radical polymerization. Polym. Chem. 2017, 8, 1972–1977. [Google Scholar] [CrossRef]
- Soly, S.; Mistry, B.; Murthy, C.N. Photo-mediated metal-free atom transfer radical polymerization: Recent advances in organocatalysts and perfection towards polymer synthesis. Polym. Int. 2022, 71, 159–168. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on pyrene-based photoinitiators of polymerization. Eur. Polym. J. 2020, 126, 109564. [Google Scholar] [CrossRef]
- Giacoletto, N.; Ibrahim-Ouali, M.; Dumur, F. Recent advances on squaraine-based photoinitiators of polymerization. Eur. Polym. J. 2021, 150, 110427. [Google Scholar] [CrossRef]
- Lynch, D.E.; Hamilton, D.G. Microreview: Pyrrol-3-yl-squaraines (including indol-3-yl squaraines). J. Heterocycl. Chem. 2018, 55, 1249–1262. [Google Scholar] [CrossRef]
- Topa-Skwarczyńska, M.; Galek, M.; Jankowska, M.; Morlet-Savary, F.; Graff, B.; Lalevée, J.; Popielarz, R.; Ortyl, J. Development of the first panchromatic BODIPY-based one-component iodonium salts for initiating the photopolymerization process. Polym. Chem. 2021, 12, 6873–6893. [Google Scholar] [CrossRef]
- Balcerak, A.; Kabatc, J.; Czech, Z.; Nowak, M.; Mozelewska, K. High-performance UV-Vis light induces radical photopolymerization using novel 2-aminobenzothiazole-based photosensitizers. Materials 2021, 14, 7814. [Google Scholar] [CrossRef]
- Balcerak, A.; Kwiatkowska, D.; Kabatc, J. Novel photoinitiators based on difluoroborate complexes of squaraine dyes for radical polymerization of acrylates upon visible light. Polym. Chem. 2022, 13, 220–234. [Google Scholar] [CrossRef]
- Skotnicka, A.; Kabatc, J. New BODIPY dyes based on benzoxazole as photosensitizers in radical polymerization of acrylate monomers. Materials 2022, 15, 662. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhu, Y.; Wu, J.; Hu, B.; Pang, Z.; Lu, Z.; Zhao, S.; Huang, Y. Squaraine dyes containing diphenylamine group: Effects of different type structures on material properties and organic photovoltaics performances. Dyes Pigm. 2019, 171, 107763. [Google Scholar] [CrossRef]
- Balcerak, A.; Iwińska, K.; Kabatc, J. Novel 1,3-bis(p-substituted phenylamino)squaraine dyes. The synthesis and spectroscopic studies. Dyes Pigm. 2019, 170, 107596. [Google Scholar] [CrossRef]
- Gomes, V.S.D.; Boto, R.E.F.; Almeida, P.; Coutinho, P.J.G.; Pereira, M.R.; Gonçalves, M.S.T.; Reis, L.V. Squaraine dyes as serum albumins probes: Synthesis, photophysical experiments and molecular docking studies. Bioorg. Chem. 2021, 115, 105221. [Google Scholar] [CrossRef] [PubMed]
- Jachak, M.; Khopkar, S.; Mehta, V.; Bhise, R.; Shankarling, G. Synthesis of A2-D2-A1-D1 type red-emitting unsymmetrical squaraine dye: Influence of additional pyridine moiety of photophysical, electrochemical, photo and thermal stability. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 273, 121019. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, X.; He, J.; Xie, M.; Zhou, Y.; Li, Z. Photostable squaraine dimers for organic solar cells with a high open circuit voltage exceeding 1.0 V. Dyes Pigm. 2021, 194, 109633. [Google Scholar] [CrossRef]
- Vega, M.; Gomila, R.M.; Pons, J.; Frontera, A.; Rotger, C.; Costa, A. Synthesis and fluorescence of N-squaraine dianions derived from electron-deficient primary anilines. Dyes Pigm. 2022, 207, 110746. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Gao, X.; Cui, M. Squaraine dye based prostate-specific membrane antigen probes for near-infrared fluorescence imaging of prostate cancer. Dyes Pigm. 2022, 208, 110822. [Google Scholar] [CrossRef]
- Tian, Y.; Ying, X.; Zhou, C.; Zhang, S.; Zhao, S.; Li, K.; Xu, M.; Yu, H.-Y.; Shao, G.; Fang, J.-K. Novel organic dyes for dye-sensitized solar cells based on 1-(2-ethylhexyl)pyrrole as a π-bridge. Dyes Pigm. 2020, 182, 108655. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on visible light pyrrole-derived photoinitiators of polymerization. Eur. Polym. J. 2022, 173, 111254. [Google Scholar] [CrossRef]
- Shi, T.; Yin, G.; Wang, X.; Xiong, Y.; Peng, Y.; Li, S.; Zeng, Y.; Wang, Z. Recent advances in the syntheses of pyrroles. Green Synth. Catal. 2022, in press. [CrossRef]
- Rawat, P.; Bharati, P.; Gautam, A.; Kumar, M.; Singh, R.; Prakash; Ram, A.; Gautam, S.; Darwari, A.; Mishra, A.; et al. Design and synthesis of pyrazole, pyrazolone, and 1,3,4-oxiadiazole derivatives having pyrrole motif as a source of new antimicrobial and anticancer agents. J. Mol. Struct. 2023, 1272, 134087. [Google Scholar] [CrossRef]
- Bumagina, N.A.; Antina, E.V.; Ksenofontov, A.A.; Antina, L.A.; Kalyagin, A.A.; Berezin, M.B. Basic structural modifications for improving the practical properties of BODIPY. Coord. Chem. Rev. 2022, 469, 214684. [Google Scholar] [CrossRef]
- Wang, J.; Yu, C.; Hao, E.; Jiao, L. Conformationally restricted and ring-fused aza-BODIPYs as promising near infrared absorbing and emitting dyes. Coord. Chem. Rev. 2022, 470, 214709. [Google Scholar] [CrossRef]
- Yuan, L.; Su, Y.; Cong, H.; Yu, B.; Shen, Y. Application of multifunctional small molecule fluorescent probe BODIPY in life science. Dyes Pigm. 2022, 208, 110851. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Kang, L.J.; Pandey, U.K.; Luscombe, C.K.; Thilagar, P. Triarylborane-BODIPY conjugate: An efficient non-fullerene electron acceptor for bulk heterojunction organic solar cell. Sol. Energy 2021, 230, 242–249. [Google Scholar] [CrossRef]
- You, J.; Cao, D.; Hu, T.; Ye, Y.; Jia, X.; Li, H.; Hu, X.; Dong, Y.; Ma, Y.; Wang, T. Novel Norrish type I flavonoid photoinitiator for safe LED light with high activity and low toxicity by inhibiting the ESIPT process. Dyes Pigm. 2021, 184, 108865. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Sun, K.; Brunel, D.; Gigmes, D.; Morlet-Savary, F.; Zhang, Y.; Liu, S.; Xiao, P.; Dumur, F.; et al. Photoinitiators derived from natural product scaffolds: Monochalcones in three-component photoinitiating systems and their applications in 3D printing. Polym. Chem. 2020, 11, 4647–4659. [Google Scholar] [CrossRef]
- Noirbent, G.; Dumur, F. Photoinitiators of polymerization with reduced environmental impact: Nature as an unlimited and renewable source of dyes. Eur. Polym. J. 2021, 142, 110109. [Google Scholar] [CrossRef]
- Waiskopf, N.; Magdassi, S.; Banin, U. Quantum photoinitiators: Toward emerging photocuring applications. J. Am. Chem. Soc. 2021, 143, 577–587. [Google Scholar] [CrossRef]






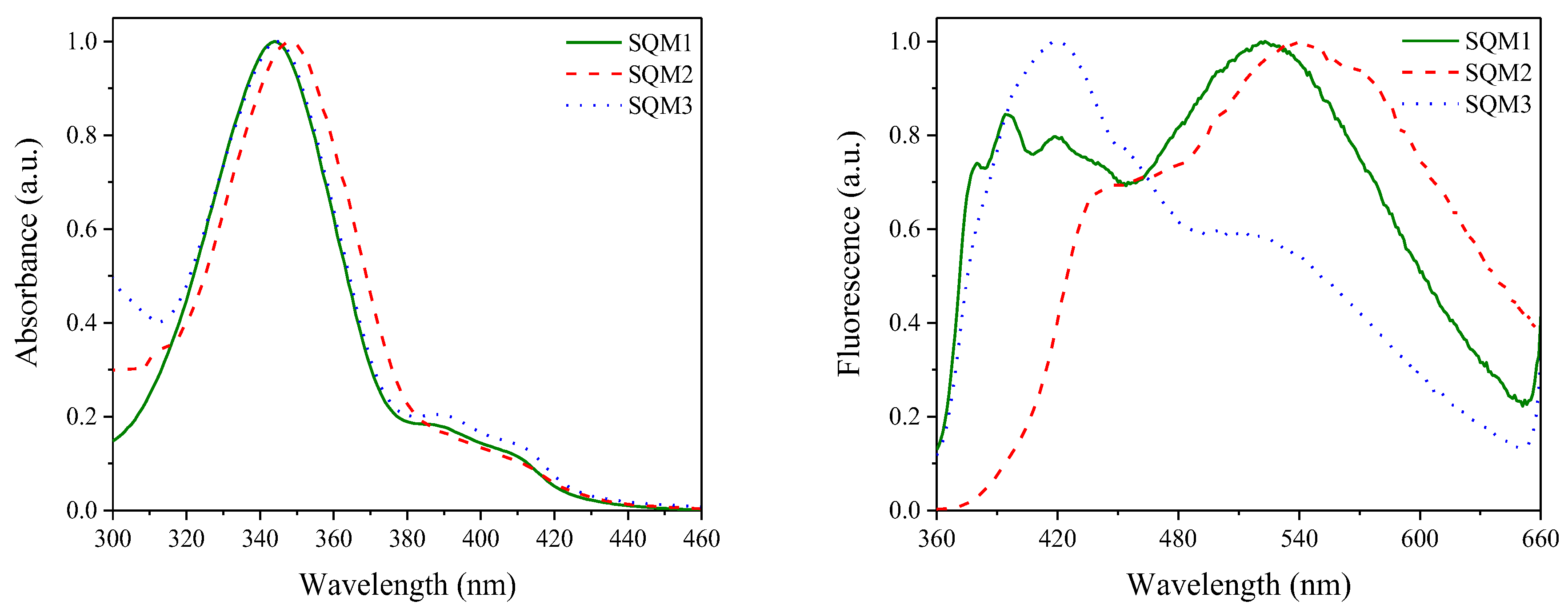
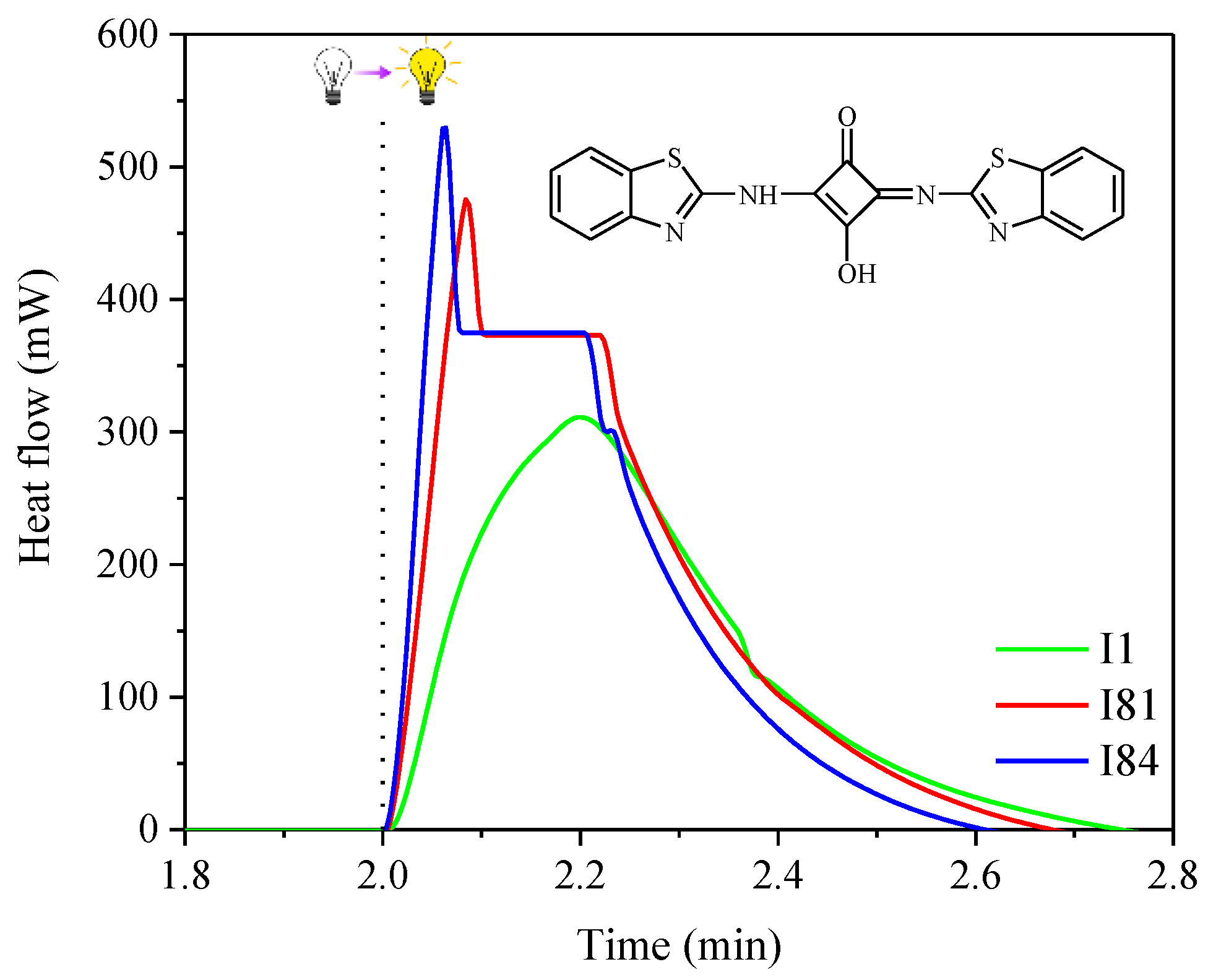


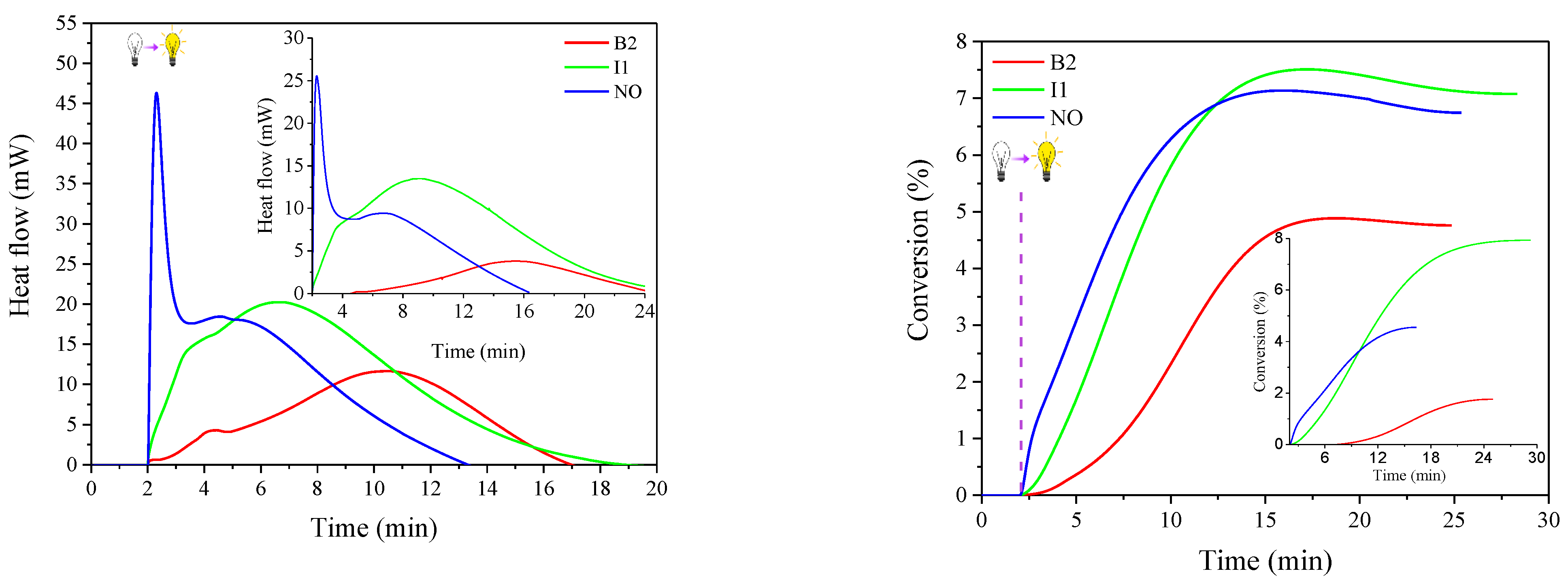
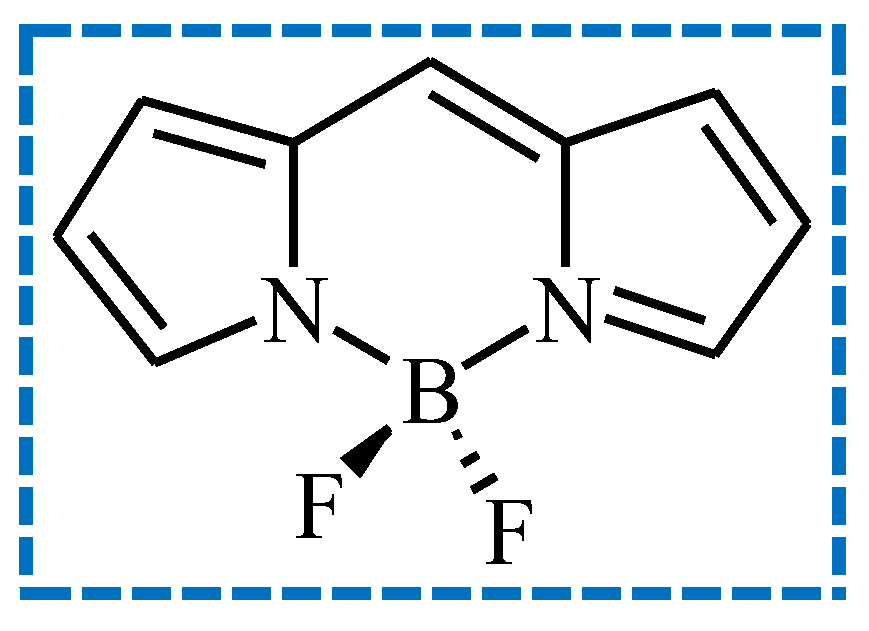
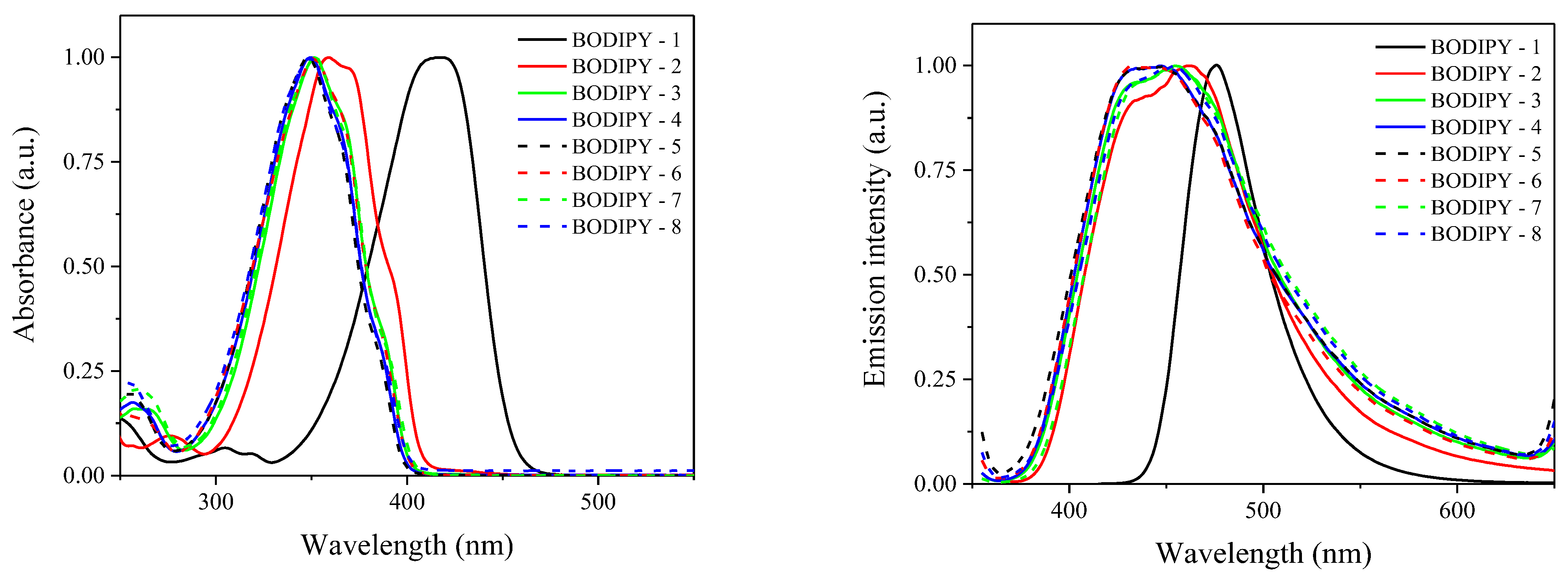
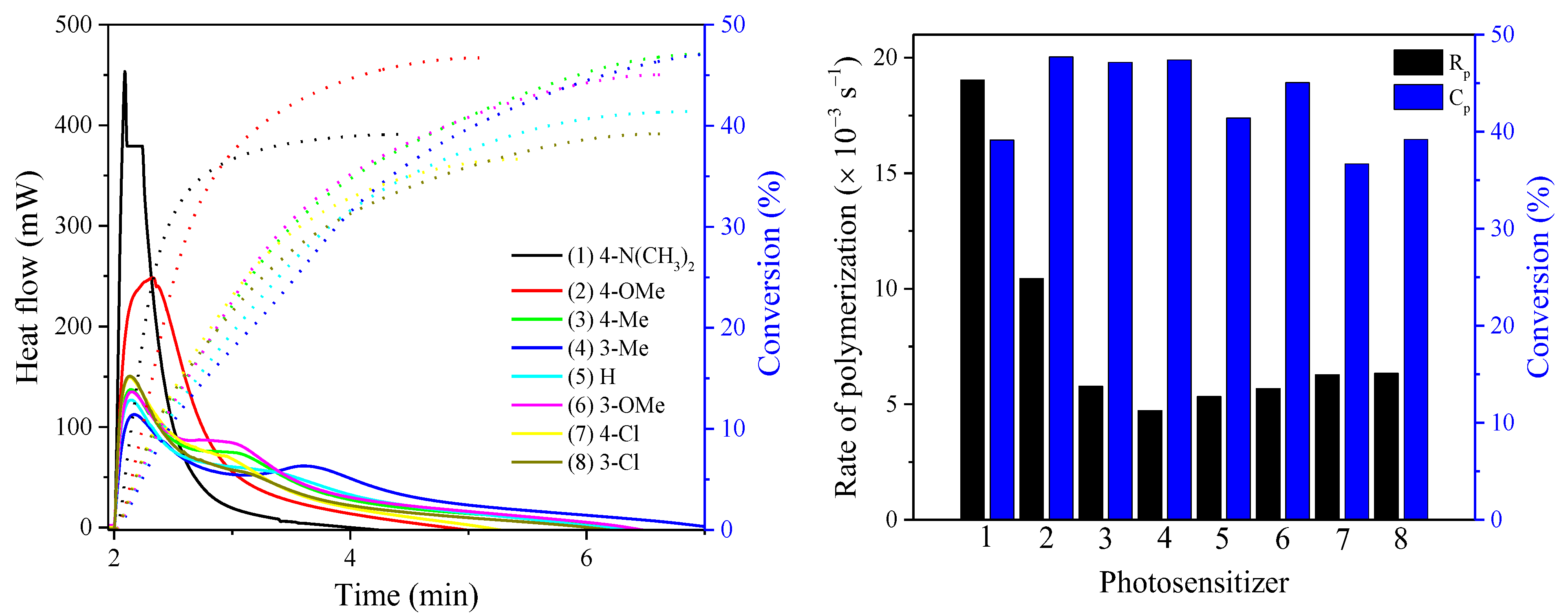

| Sensitizer | λmax (nm) | ε (× 104 M−1 cm−1) | Light Intensity (mW cm−2) | Rp (× 10−3 s−1) | C% (%) |
|---|---|---|---|---|---|
| SQM1 | 344 | 2.45 | 30 | 20.00 | 29.4 |
| SQM2 | 348 | 0.97 | 25.80 | 29.7 | |
| SQM3 | 345 | 0.83 | 23.40 | 35.7 | |
| PSQ1 | 553 | 5.90 | 50 | 0.26 | 12.4 |
| PSQ2 | 562 | 8.40 | 0.36 | 12.0 | |
| BPSQ1 | 553 | 7.70 | 1.25 | 6.3 | |
| BPSQ2 | 562 | 11.00 | 0.56 | 10.7 | |
| BODIPY-1 | 415 | 4.25 | 50 | 19.04 | 39.2 |
| BODIPY-2 | 359 | 3.29 | 10.45 | 47.7 | |
| BODIPY-3 | 351 | 3.12 | 5.78 | 47.1 | |
| BODIPY-4 | 350 | 2.30 | 4.73 | 47.4 | |
| BODIPY-5 | 349 | 2.70 | 5.34 | 41.4 | |
| BODIPY-6 | 351 | 2.67 | 5.68 | 45.1 | |
| BODIPY-7 | 352 | 2.96 | 6.28 | 36.7 | |
| BODIPY-8 | 350 | 2.96 | 6.34 | 39.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balcerak, A.; Kabatc-Borcz, J.; Czech, Z.; Bartkowiak, M. Latest Advances in Highly Efficient Dye-Based Photoinitiating Systems for Radical Polymerization. Polymers 2023, 15, 1148. https://doi.org/10.3390/polym15051148
Balcerak A, Kabatc-Borcz J, Czech Z, Bartkowiak M. Latest Advances in Highly Efficient Dye-Based Photoinitiating Systems for Radical Polymerization. Polymers. 2023; 15(5):1148. https://doi.org/10.3390/polym15051148
Chicago/Turabian StyleBalcerak, Alicja, Janina Kabatc-Borcz, Zbigniew Czech, and Marcin Bartkowiak. 2023. "Latest Advances in Highly Efficient Dye-Based Photoinitiating Systems for Radical Polymerization" Polymers 15, no. 5: 1148. https://doi.org/10.3390/polym15051148
APA StyleBalcerak, A., Kabatc-Borcz, J., Czech, Z., & Bartkowiak, M. (2023). Latest Advances in Highly Efficient Dye-Based Photoinitiating Systems for Radical Polymerization. Polymers, 15(5), 1148. https://doi.org/10.3390/polym15051148






