Abstract
A polymeric inclusion membrane (PIM) consisting of matrix CTA (cellulose triacetate), ONPPE (o-nitrophenyl pentyl ether) and phosphonium salts (Cyphos 101, Cyphos 104) was used for separation of Cu(II), Zn(II) and Ni(II) ions. Optimum conditions for metal separation were determined, i.e., the optimal concentration of phosphonium salts in the membrane, as well as the optimal concentration of chloride ions in the feeding phase. On the basis of analytical determinations, the values of parameters characterizing transport were calculated. The tested membranes most effectively transported Cu(II) and Zn(II) ions. The highest recovery coefficients (RF) were found for PIMs with Cyphos IL 101. For Cu(II) and Zn(II), they are 92% and 51%, respectively. Ni(II) ions practically remain in the feed phase because they do not form anionic complexes with chloride ions. The obtained results suggest that there is a possibility of using these membranes for separation of Cu(II) over Zn(II) and Ni(II) from acidic chloride solutions. The PIM with Cyphos IL 101 can be used to recover copper and zinc from jewellery waste. The PIMs were characterized by AFM and SEM microscopy. The calculated values of the diffusion coefficient indicate that the boundary stage of the process is the diffusion of the complex salt of the metal ion with the carrier through the membrane.
1. Introduction
Separation of metal ions and low-molecular-weight organic compounds using polymer inclusion membranes has been proposed as an environmentally friendly separation technology [1,2,3,4,5,6,7]. Membrane separation has evolved into a more rapidly developing and diverse field. Today, there are many types of membranes with different applications. Membrane processes, the latest achievement in process engineering, can successfully replace many ineffective, conventional separation methods and in some cases complement them, improving the parameters of the output fluxes and the economics of the treatment [8,9,10,11,12,13]. PIMs have been applied in separation processes, e.g., precious metals [14,15], lanthanides [16], alkali metals [17,18,19] and heavy metals [18,20,21,22,23,24]. PIMs can also be used for the separation of copper(II) and zinc(II) from water and soil solutions [25,26].
A PIM consists of a carrier, a plasticizer and a matrix [7]. In recent years, ionic liquids, including quaternary phosphonium salts, have been proposed for use as ion carriers through PIMs [5,27,28,29]. Using ionic liquids such as, for example, Cyphos 101 and Cyphos 104, it is possible to remove iron and zinc ions from chloride waste aqueous solutions [5,27,28,29,30], cadmium, copper [31,32] and chromium(VI) ions [33,34], as well as to separate the noble metal Au(III) [35,36] and Pd(II) ions [37].
There is a huge demand for jewellery products in the modern world. Approximately 890 tonnes of gold jewellery products were purchased in the first half of 2022 [38]. There is also an increase in demand for jewellery made from metal alloys that do not contain gold. One such alloy is the so-called new silver (also known as high-nickel brass, argentan, paktong, alpacca and German silver). This alloy was invented in the 17th century in China. Today, it is known and used worldwide. It is particularly appreciated by jewellers because it perfectly imitates real silver. Unlike silver, new silver does not darken when exposed to the elements and does not cause allergic reactions. In addition to jewellery products, the alloy is widely used in a variety of industries, e.g., electronics, electrical engineering (production of resistors), telecommunication and radio technology, in the manufacture of precision apparatus, musical instruments, medical equipment, crockery and even in the manufacture of boat fittings [39,40].
The new silver, contrary to its name, does not contain pure silver in its composition. The chemical composition of this alloy can vary [41]. The composition of new silver according to ASTM B 122M is given in Table 1.

Table 1.
Chemical composition of new silver [41].
The main metals used are zinc, copper and nickel (Table 1). These metals are widely used in various industries. For example, copper is used in electrical engineering for the manufacture of cables and wires; in the energy and chemical industries for the manufacture of radiators, chemical apparatus and heat exchangers; and in the automotive industry [42], among others. Nickel is a very good catalyst for reduction processes, commonly used in the hydrogenation of fats and is also used as an additive in alloy steels, primarily stainless steel [43,44,45]. Zinc, on the other hand, has widespread technological applications in the galvanizing process to make steel corrosion-resistant, in the manufacture of batteries, roofing and is used as a catalyst in the production of India rubber [46]. Zinc is a component of many alloys, e.g., brass and tombac (with copper) as well as zamak (with aluminium) [47,48].
A major challenge facing the modern world is the management of scrap metal alloy waste. In principle, all metals sourced from scrapyards undergo pyrometallurgical recycling in smelters or foundries [49,50]. However, the hydrometallurgical process, also known as extractive metallurgy, is used more and more often in order to reduce air pollution from greenhouse gases [51]. Hydrometallurgical processes using the traditional liquid–liquid extraction method are used to produce gold, platinum, palladium, lanthanides, copper, cobalt and nickel [52,53]. Ionic liquids are becoming increasingly important in the extraction of metal ions [54]. In extraction, quaternary phosphonium salts are used to separate uranium(VI) and thorium(IV) from other rare elements [55], to separate cobalt from nickel, magnesium and calcium [56], as well as in the recovery of PGMs (platinum group metals) from spent catalytic converters [57] and Ag(I) from acidic nitrate environments [58].
The aim of this work is to compare the effectiveness of polymeric inclusion membranes based on CTA with phosphonium ionic liquids (Cyphos 101 and Cyphos 104) in terms of Cu(II) recovery from three-component Cu-Zn-Ni mixtures. These phosphonium ionic liquids have not yet been used as ion carriers in PIM for the separation of copper ions from the mixture of Cu(II)-Zn(II)-Ni(II) ions, as well as for the recovery of Cu(II) from waste jewellery (new silver waste). This work also aimed to determine the optimal conditions for Cu(II) separation using PIMs. Therefore, the effect of chloride ion concentration in the feed phase and carrier concentration in PIMs were studied.
2. Experimental Procedure
2.1. Materials
Two phosphonium ionic liquids, i.e., trihexyl(tetradecyl)phosphoniumchloride (Cyphos 101) (Figure 1) and trihexyl(tetradecyl)phosphoniumbis-(2,4,4-trimethylphentyl)phospinate (Cyphos 104) (Figure 1), cellulose triacetate (CTA), o-nitrophenylpentyl ether (ONPPE) and dichloromethane (DCM) were purchased from Sigma-Aldrich (Poznan, Poland). All inorganic reagents were purchased from POCh (Gliwice, Poland). Solutions of metal ions were prepared by dissolving appropriate amounts of metal in deionized water.
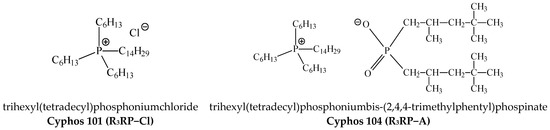
Figure 1.
The carrier structures.
Some of ionic liquid physical properties are presented in Table 2.

Table 2.
Physical properties of phosphonium ionic liquid used as carriers in tested PIMs.
The spent jewellery waste (Figure 2) was obtained from a local market (jewellery workshops). A mass of 1g of the alloy was leached for 6 h with concentrated HCl at a temperature of 60 °C. The obtained solution was filtered and diluted to 100 cm3. The content of copper, zinc and nickel were determined using the AAS method. For transport tests, the solution was diluted.

Figure 2.
The new silver jewellery waste.
2.2. Transport of Metal Ions across PIMs
PIMs were prepared in accordance with the previously used procedure [5,6,24,27,28,31,32]. PIMs are made of three basic substances: a polymer matrix (CTA), a carrier (phosphorus ionic liquid) and a plasticizer (ONPPE). A portion of the 3-component solution in DCM was placed in a 7.0 cm diameter petri dish. The DMC was evaporated overnight, and the resulting film was separated from the glass plate. The membranes were soaked in distilled water to achieve homogeneity. The effective membrane area exposed to the two phases was 4.9 cm2.
Surface characterization of PIMs was performed by atomic force microscopy (AFM) using an atomic force multimode scanning probe microscope IIIa (Digital Instruments Vecco Metrology Group, Santa Barbara, CA, USA).
In addition, PIMs were characterized using scanning electron microscopy (SEM). SEM images of the films were acquired using a Hitachi SU 3500 SEM/EDS (energy dispersive spectroscopy) microscope operated at 10.0 kV. Membranes are visualized in 5.0 × 5.0 μm2 images.
The thickness of the PIM was measured with a digital micrometer (Panametrics® Magna-Mike® 8500 (San Diego, CA, USA)) with an accuracy of 0.1 µm.
The feed phase was an aqueous chloride solution of copper(II), zinc(II) and nickel(II) ions with a concentration of C0 = 0.01 mol/dm3 for each metal ion. The feed phase is buffered at pH = 2 by adding the appropriate amount of HCl. The receiving phase was 2M HCl. The concentrations of the metal ions were measured over a specific time of transport at 324.7, 213.9 and 232.0 nm for copper, zinc and nickel, respectively.
2.3. Calculation Formulae
Transport through PIMs can be described by initial flux (J0), selectivity coefficient (S) and recovery coefficient (RF, %) [59,60,61]. Table 3 summarizes the formulae used to calculate transport efficiency as well as to characterize membranes

Table 3.
The formulae used to calculate transport efficiency as well as to characterize membranes.
3. Results and Discussion
3.1. Membrane Characteristics
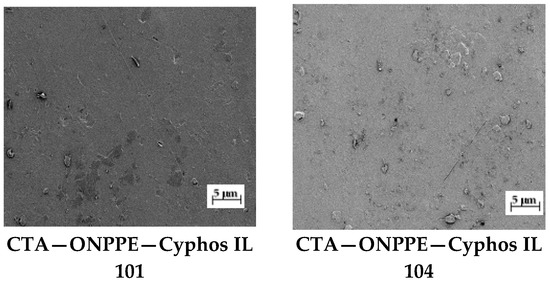
Figure 3.
SEM picture of PIMs. Magnification ×150.

Figure 4.
AFM images of PIMs with Cyphos IL.
The CTA membrane has well-defined pores that disappear completely after the addition of a plasticizer (ONPPE) and a carrier (Cyphos 101 and 104) (Figure 3). Similar images were obtained by Arous et al., who studied the structure of membranes based on CTA with tributyl phosphate (TBP) as a carrier and o-NPOE (o-nitrophenyl octyl ether) as a plasticizer [62]. The plasticizer is an integral part of the membrane. Plasticizers have the ability to interact with polymer chains, resulting in the desired flexibility and smoothness of the system [2,7,62].
The membrane had a dense and homogeneous structure. Furthermore, the roughness of the film surface could be visually confirmed in the image. Carriers were able to crystallize in the membrane. Their molecules migrated to the membrane surface, causing the roughness and porosity of the membrane. Mahanty [63] and Ugur [20] also found no pores in polymer inclusion membranes in SEM images shown at the µm scale.
The membrane roughness was calculated using NanoScope v.5.12 AFM image processing software from AFM images using the definitions shown in Table 3 (Equations (6) and (7)), and they are presented in Table 4.

Table 4.
The roughness parameters for PIM with Cyphos.
As can be seen (Table 4), membranes containing Cyphos 101 as a carrier are characterized by higher roughness compared to membranes with Cyphos 104.
3.2. The Effect of Carrier Concentration on Cu(II) Transport through PIMs
In order to determine the effect of the type and amount of carrier on the rate of Cu(II) transfer through PIMs, transport processes were carried out using CTA membranes with Cyphos IL 101 and Cyphos IL 104 as carriers. Table 5 and Table 6 show the percentage composition of the membranes and the rate constants for the reaction of Cu(II) with both carriers during transport through PIMs.

Table 5.
Percentage composition of the membranes, membrane thickness (d) and reaction rate constants (k) of Cu(II) ion transport through the CTA—Cyphos 101—ONPPE membrane.

Table 6.
Percentage composition of the membranes, membrane thickness (d) and reaction rate constants (k) of Cu(II) ion transport through the CTA—Cyphos 104—ONPPE membrane.
The membrane thickness was also measured at at least 20 different points of the membrane surface and presented in Table 5 and Table 6. As the percentage of the carrier in PIMs increases, the thickness of the membranes also increases (Table 5 and Table 6). Thickness of the PIM with Cyphos 101 ranges from 25.1 μm (15% IL) to 45.7 μm (60% IL). Depending on the percentage of carrier in the membrane, the thickness of the PIM with Cyphos 104 ranges from 31.4 to 52.1 μm. The greater thickness of the PIM with Cyphos 104 can be explained by the higher molar mass of this carrier.
3.3. Effect of Cl− Ion Concentration in the Feed Phase
Next, the influence of chloride ion concentration in the feed phase on the transport of Cu(II), Zn(II) and Ni(II) was studied. The concentration of chloride ions was increased in the feed phase by adding an appropriate amount of 4M NaCl solution. Figure 5 shows the dependence on the permeability coefficients for metal ion transport (P, μm/s) as the function of Cl− concentration.
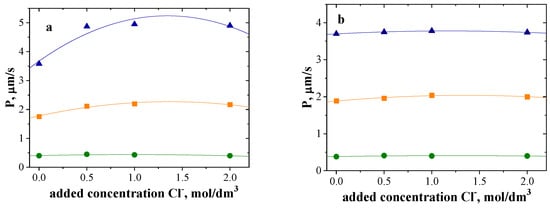
Figure 5.
The dependence on the permeability coefficients as the function of Cl− concentration; the feed phase: ▲Cu(II), ■Zn(II) and ●Ni(II) in HCl; the receiving phase: 2M HCl. The initial concentration of each metal ion was 0.01 M. (a) PIM with Cyphos 101. (b) PIM with Cyphos 104.
In the case of the membrane with Cyphos 101, slightly increasing the concentration of added chloride ions in the feed phase (from 0 to 0.5 mol/dm3) increases the permeability coefficient, especially of the Cu(II) and Zn(II) ions (Figure 5). Further increases in Cl− ion concentration do not result in significant differences in metal ion transport (Figure 5a). In the case of the PIM with Cyphos 104, increasing the added concentration of Cl− ions in the feed phase does not significantly affect the transport of each metal ion (Figure 5b). For both tested membranes, Ni(II) was transported in a negligible amount.
In further tests, the concentration of added chloride ions in the feed phase was 0.5 mol/dm3.
3.4. Separation of Copper(II), Zinc(II) and Nickel(II) Ions Using PIMs with Cyphos IL
Competitive transport of investigated metal ions from aqueous chloride solutions through CTA polymer membrane with Cyphos IL into 2 mol/dm3 HCl was investigated.
The change in the concentration of metal ions, both in the feed and receiving phase over time, is shown in Figure 6.
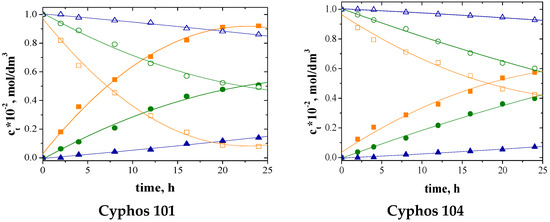
Figure 6.
The concentration of metal ions in the feed and receiving phases during transport across PIM with Cyphos; feed phase: ☐—Cu, ○—Zn, △—Ni; receiving phase: ■—Cu(II), ●—Zn(II), ▲—Ni(II).
Parameters characterizing transport calculated on the basis of analytical determinations from Equations (3) and (4) (Table 3) are presented in Table 7.

Table 7.
Initial fluxes, selectivity order and selectivity coefficient for competitive transport of Cu(II), Zn(II) and Ni(II) ions across PIMs. Membrane: 55% carrier (Cyphos IL 101, Cyphos IL 104), 40% CTA, 5% ONPPE.
The data in Table 7 show that the initial Cu(II) flux values are the highest for all supports, indicating that Cu(II) ions are transported at the highest rate. Ni(II) ion transport is the least. The initial value of the metal ion transport flux also depends on the type of carrier used. The Cyphos 101 PIM showed the highest initial flux values. In the case of PIM with Cyphos 104, the initial fluxes of Zn(II) and Cu(II) ions are slightly lower than for the membrane with Cyphos 101. However, for Ni(II) ions, these values are comparable. It can also be speculated that the higher volume of anions (A) in Cyphos IL 104, and thus their lower mobility, lowers J0 values.
Comparing the values of the initial fluxes (Table 7) with the roughness values (Table 4) of the membranes, it can be concluded that the increase in roughness increases the transport of each metal ion, which is consistent with the literature data [64]. According to the literature, membranes with a more heterogeneous surface show a greater capacity to transport metal ions compared to homogeneous membranes [64,65].
According to Arous [62,65], the amorphous structure of a PIM indicates that the compound is transported through the membrane by diffusion of the metal–carrier complex. At the interface of the feeding phase and the PIM surface, metal ions combine with carrier molecules present in the membrane to form complex salts. The complex salt diffuses to the opposite surface of the membrane, where metal ions are released to the receiving phase. The reaction responsible for ion transport involves anion exchange. This exchange is more efficient when CYPHOS 101 (Table 7) is used as a carrier, perhaps due to the more mobile chloride anion.
Figure 7 presents the proposed mechanism of the transport of metal ions through PIMs with Cyphos IL.
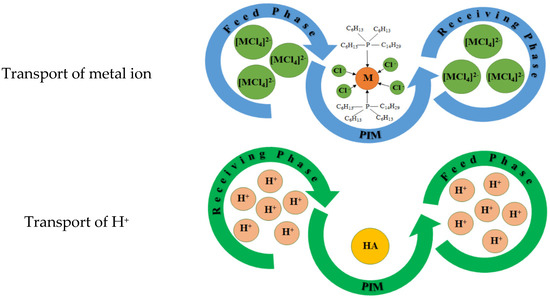
Figure 7.
Schematic transport across PIMs with Cyphos IL.
The proposed transport mechanism can be described as follows:
At the interface feed phase/membrane, the Cu(II) and Zn(II) ions with carrier molecules form complex salts (Equation (9)):
For Cyphos IL 101: [MCl4]2− + 2 R3RP-Cl ↔ (R3RP)2MCl4 + 2 Cl−
For Cyphos IL 104: [MCl4]2− + 2 R3RP-A ↔ (R3RP)2MCl4 + 2 A−
For Cyphos IL 104: [MCl4]2− + 2 R3RP-A ↔ (R3RP)2MCl4 + 2 A−
At the interface membrane/receiving phase:
For Cyphos IL 101: (R3RP)2MCl4 ↔ [MCl4]2− + 2 R3RP-Cl
For Cyphos IL 104: (R3RP)2MCl4 ↔ [MCl4]2− + 2 R3RP-A
For Cyphos IL 104: (R3RP)2MCl4 ↔ [MCl4]2− + 2 R3RP-A
At the same time, hydrogen ions combine with the anions of the studied ionic liquids (A) and are transported from the receiving phase to the feed phase.
Cu(II) and Zn(II) ions are effectively transported to the receiving phase (Equations (9) and (10)), as they can form [MCl4]2– chloro-complexes in the feed phase. Ni(II) ions are present in chloride solutions as Ni2+ and NiCl+ [66] and are incapable of forming anionic complexes. Regardless of the type of carrier used, transport of Ni(II) through the PIM with both carriers is inefficient, as evidenced by the low J0 values (Table 7).
3.5. Metal Ion Diffusion Coefficients
Figure 8 shows plots of (C0 − Ct) as a function of time for the transport of each metal ion through PIM with Cyphos IL. These graphs were used to calculate the ion diffusion coefficients from Equation (8) (Table 3).
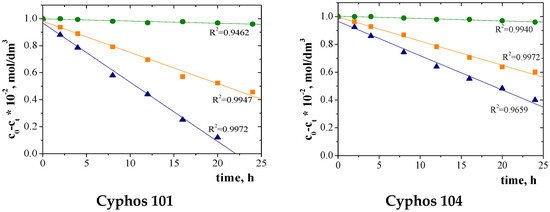
Figure 8.
Plots of (C0 − Ct) against time for ▲Cu(II), ■Zn(II) and ●Ni(II) ion transport across PIMs with Cyphos IL.
The values of diffusion coefficients are presented in Table 8.

Table 8.
Diffusion coefficients for transport of Cu(II), Zn(II) and Ni(II) ions through PIMs with Cyphos IL.
The values of diffusion coefficients range from 10−7 to 10−8 cm2/s (Table 8) and are comparable to the diffusion coefficients of metal ions through the membrane CTA presented in the literature [59,67,68].
3.6. Recovery of Metal
The recovery coefficient (RF) was calculated from Equation (5) (Table 3). Figure 9 shows the RF coefficients of metal ions.
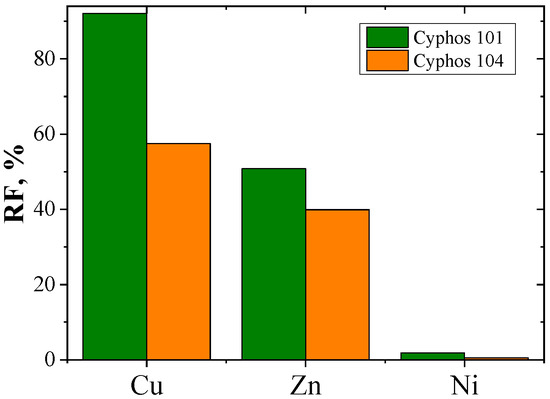
Figure 9.
Recovery coefficient (RF) of copper, zinc and nickel after 24-hour transport through PIMs with Cyphos IL.
The values of the recovery coefficient (RF) depend on the carrier used in the membrane (Figure 9). The highest recovery coefficients (RF) were found for PIMs with Cyphos IL 101. For Cu(II) and Zn(II), they are 92% and 51%, respectively. For PIMs with Cyphos IL 104, the RF values of Cu(II) and Zn(II) ions are 58% and 40%, respectively. The RF value for Ni(II) ions did not exceed 2% (PIMs with Cyphos IL 101). These ions practically remain in the feed phase.
3.7. Recovery of Metal from Jewellery Waste Solutions
In the next step, attempts have been made to utilize the PIMs with Cyphos IL 101 for the separation of the Cu(II) and Zn(II) ions from jewellery waste. The content of Cu(II), Zn(II) and Ni(II) in the tested sample of new silver waste was 56%, 20% and 7.5%, respectively.
The copper(II), zinc(II) and nickel(II) concentrations in the starting solution for transport tests were 0.008 mol/dm3, 0.003 mol/dm3 and 0.001 mol/dm3, respectively. The values of initial fluxes (J0) for the transport of metal ions from the solution after leaching waste new silver, the selectivity coefficient (S) and the recovery coefficient (RF) are shown in Table 9.

Table 9.
Initial fluxes (J0), selectivity coefficients (S) and recovery coefficients (RF) of Cu(II), Zn(II) and Ni(II) ions after 24 h transport across PIMs with Cyphos IL 101; feed phase: the leaching solution (pH = 2, [Cl−] = 1 mol/dm3); receiving phase: 2M HCl.
Compared to the model solution, in which the concentrations of all metal ions were equal (Table 6), in the real solution, which is dominated by copper, J0 for Cu(II) has a higher value, while J0 for Zn(II) has a slightly lower value (Table 9). The initial flux of Ni(II) ions is negligible. This increases the Cu(II)/Zn(II) separation coefficient to almost 2.5 and enables selective separation of Ni(II) ions from Cu(II) and Zn(II) ions. Ni(II) ions remain in the feed phase.
The recovery coefficient values of Cu(II) and Zn(II) ions are 90% and 35%, respectively.
4. Conclusions
The polymer inclusion membranes with Cyphos IL 101 and Cyphos IL 104 can be used due to their properties for the separation of copper(II), zinc(II) and nickel(II) ions. For all carriers, the initial fluxes of Cu(II) and Zn(II) have the highest value.
The transport efficiency of Cu(II) and Zn(II) ions facilitates the formation of [MCl4]2− chloro-complexes in the chloride feeding phase.
Diffusion coefficient values show that the limiting step of the process is the transfer of the metal complex through the membrane barrier.
The highest recovery coefficients (RF) were found for PIMs with Cyphos IL 101. For Cu(II) and Zn(II), they are 92% and 51%, respectively. The RF value for Ni(II) ions did not exceed 2% (PIMs with Cyphos IL 101). These ions practically remain in the feed phase because they do not form anionic complexes with chloride ions.
The obtained results suggest that there is a possibility of application of these membranes for separation of Cu(II) over Zn(II) and Ni(II) from acidic chloride solutions.
The PIM with Cyphos 101 can be used to recover copper and zinc from jewellery waste. Copper(II) can be selectively transferred from the feed containing high Cu(II) concentration and the low concentration of Zn(II) ions. In this case, the recovery coefficient values of Cu(II) and Zn(II) ions are 90% and 35%, respectively.
From a practical point of view, the Cyphos IL 101 PIM can be used for the selective separation of Cu(II), Zn(II) and Ni(II) ions from waste containing these metals.
Author Contributions
Conceptualization, E.R.-L.; methodology, I.P. and E.R.-L.; software, E.R.-L. and I.P.; validation, E.R.-L. and I.P.; formal analysis, W.U., E.R.-L. and I.P.; investigation, E.R.-L., W.U. and I.P.; data curation, I.P. and E.R.-L.; writing—original draft preparation, I.P. and E.R.-L.; writing—review and editing, E.R.-L. and I.P.; visualization, I.P. and E.R.-L.; project administration, E.R.-L. All authors have read and agreed to the published version of the manuscript.
Funding
Research co-financed by the Operational Program PL03 “Improving Environmental Monitoring and Inspection” within the EEA Grants 2009–2014, No. 309/2015/Wn15/MN-xn-03/D.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
In memory of Elżbieta Radzymińska-Lenarcik (8.11.1955-16.02.2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sgarlata, C.; Arena, G.; Longo, E.; Zhang, D.; Yang, Y.; Bartsch, R.A. Heavy Metal Separation with Polymer Inclusion Membranes. J. Membr. Sci. 2008, 323, 444–451. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and Transport of Metal Ions and Small Organic Compounds Using Polymer Inclusion Membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Zulkefeli, N.S.W.; Weng, S.K.; Abdul Halim, N.S. Removal of Heavy Metals by Polymer Inclusion Membranes. Curr. Pollut. Rep. 2018, 4, 84–92. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M.; Masmoudi, A.; Barillon, R.; Trébouet, D. Supported Ionic Liquid and Polymer Inclusion Membranes for Metal Separation. Sep. Purif. Rev. 2022, 51, 100–116. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Nowak, Ł.; Wiśniewski, M. Removal of Zinc(II) and Iron Ions from Chloride Solutions with Phosphonium Ionic Liquids. Sep. Purif. Technol. 2012, 97, 158–163. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M.; Pyszka, I. Application of Polymer Inclusion Membranes Doped with Alkylimidazole to Separation of Silver and Zinc Ions from Model Solutions and after Battery Leaching. Materials 2020, 13, 3103. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent Trends in Extraction and Transport of Metal Ions Using Polymer Inclusion Membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Eloffy, M.G.; El-Sherif, D.M.; Abouzid, M.; Elkodous, M.A.; El-nakhas, H.S.; Sadek, R.F.; Ghorab, M.A.; Al-Anazi, A.; El-Sayyad, G.S. Proposed Approaches for Coronaviruses Elimination from Wastewater: Membrane Techniques and Nanotechnology Solutions. Nanotechnol. Rev. 2022, 11, 1–25. [Google Scholar] [CrossRef]
- Donat, R.; Eyice, M.İ.; Erden, K.E. Transportation and Extraction of Cu2+ Metal Ions from Acidic Solution by MDLM Technique. Resour. Conserv. Recycl. 2022, 179, 106124. [Google Scholar] [CrossRef]
- Staszak, M. Membrane Technologies for Sports Supplementation. Phys. Sci. Rev. 2022. [Google Scholar] [CrossRef]
- Alsobh, A.; Zin, M.M.; Vatai, G.; Bánvölgyi, S. The Application of Membrane Technology in the Concentration and Purification of Plant Extracts: A Review. Period. Polytech. Chem. Eng. 2022, 66, 394–408. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Varjani, S.; Ding, A.; Bui, X.-T.; Nguyen, D.P. Bio-Membrane Based Integrated Systems for Nitrogen Recovery in Wastewater Treatment: Current Applications and Future Perspectives. Chemosphere 2021, 265, 129076. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.M.; Garg, S.; Bajpai, S. Economic Feasibility of Arsenic Removal Using Nanofiltration Membrane: A Mini Review. Chem. Pap. 2021, 75, 4431–4444. [Google Scholar] [CrossRef]
- Kubota, F.; Kono, R.; Yoshida, W.; Sharaf, M.; Kolev, S.D.; Goto, M. Recovery of Gold Ions from Discarded Mobile Phone Leachate by Solvent Extraction and Polymer Inclusion Membrane (PIM) Based Separation Using an Amic Acid Extractant. Sep. Purif. Technol. 2019, 214, 156–161. [Google Scholar] [CrossRef]
- Fontàs, C.; Tayeb, R.; Tingry, S.; Hidalgo, M.; Seta, P. Transport of Platinum(IV) through Supported Liquid Membrane (SLM) and Polymeric Plasticized Membrane (PPM). J. Membr. Sci. 2005, 263, 96–102. [Google Scholar] [CrossRef]
- Croft, C.F.; Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Separation of Lanthanum(III), Gadolinium(III) and Ytterbium(III) from Sulfuric Acid Solutions by Using a Polymer Inclusion Membrane. J. Membr. Sci. 2018, 545, 259–265. [Google Scholar] [CrossRef]
- Cai, C.; Yang, F.; Zhao, Z.; Liao, Q.; Bai, R.; Guo, W.; Chen, P.; Zhang, Y.; Zhang, H. Promising Transport and High-Selective Separation of Li(I) from Na(I) and K(I) by a Functional Polymer Inclusion Membrane (PIM) System. J. Membr. Sci. 2019, 579, 1–10. [Google Scholar] [CrossRef]
- Smail, F.; Arous, O.; Amara, M.; Kerdjoudj, H. A Competitive Transport across Polymeric Membranes. Study of Complexation and Separation of Ions. Comptes Rendus Chim. 2013, 16, 605–612. [Google Scholar] [CrossRef]
- Paredes, C.; Rodríguez de San Miguel, E. Selective Lithium Extraction and Concentration from Diluted Alkaline Aqueous Media by a Polymer Inclusion Membrane and Application to Seawater. Desalination 2020, 487, 114500. [Google Scholar] [CrossRef]
- Ugur, A.; Sener, I.; Hol, A.; Alpoguz, H.K.; Elci, L. Facilitated Transport of Zn(II) and Cd(II) Ions Through Polymer Inclusion Membranes Immobilized With a Calix [4] Resorcinarene Derivative. J. Macromol. Sci. Part A 2014, 51, 611–618. [Google Scholar] [CrossRef]
- Ayyavoo, S.; Kandasamy, P.; Ramasamy, S. Removal of Mercury in Aqueous Solutions Using Tri N-Butyl Phosphate-Based Polymer Inclusion Membrane. Environ. Eng. Sci. 2022, 39, 650–661. [Google Scholar] [CrossRef]
- Sellami, F.; Kebiche-Senhadji, O.; Marais, S.; Colasse, L.; Fatyeyeva, K. Enhanced Removal of Cr(VI) by Polymer Inclusion Membrane Based on Poly(Vinylidene Fluoride) and Aliquat 336. Sep. Purif. Technol. 2020, 248, 117038. [Google Scholar] [CrossRef]
- Kaya, A.; Onac, C.; Alpoguz, H.K.; Yilmaz, A.; Atar, N. Removal of Cr(VI) through Calixarene Based Polymer Inclusion Membrane from Chrome Plating Bath Water. Chem. Eng. J. 2016, 283, 141–149. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of polymer inclusion membranes doped with 1-hexyl-4-methylimidazole for pertraction of zinc(II) and other transition metal ions. Physicochem. Probl. Miner. Process. 2015, 51, 447–460. [Google Scholar] [CrossRef]
- Ribas, T.C.F.; Croft, C.F.; Almeida, M.I.G.S.; Mesquita, R.B.R.; Kolev, S.D.; Rangel, A.O.S.S. Use of a Polymer Inclusion Membrane and a Chelating Resin for the Flow-Based Sequential Determination of Copper(II) and Zinc(II) in Natural Waters and Soil Leachates. Molecules 2020, 25, 5062. [Google Scholar] [CrossRef]
- Suah, F.B.M.; Roslan, N.A.; Dahlan, N.F.; Mohamed, N. A Use of Polymer Inclusion Membrane as Anion Exchange Membrane for Recovery of Cu(II) Ions Based on an Electrogenerative System. J. Electrochem. Soc. 2018, 165, H310. [Google Scholar] [CrossRef]
- Baczyńska, M.; Regel-Rosocka, M.; Nowicki, M.; Wiśniewski, M. Effect of the Structure of Polymer Inclusion Membranes on Zn(II) Transport from Chloride Aqueous Solutions. J. Appl. Polym. Sci. 2015, 132, 1–11. [Google Scholar] [CrossRef]
- Nowak, Ł.; Regel-Rosocka, M.; Marszałkowska, B.; Wiśniewski, M. Removal of Zn(II) from Chloride Acidic Solutions with Hydrophobic Quaternary Salts. Pol. J. Chem. Technol. 2010, 12, 24–28. [Google Scholar] [CrossRef]
- Kogelnig, D.; Regelsberger, A.; Stojanovic, A.; Jirsa, F.; Krachler, R.; Keppler, B.K. A Polymer Inclusion Membrane Based on the Ionic Liquid Trihexyl(Tetradecyl)Phosphonium Chloride and PVC for Solid–Liquid Extraction of Zn(II) from Hydrochloric Acid Solution. Mon. Für Chem.-Chem. Mon. 2011, 142, 769–772. [Google Scholar] [CrossRef]
- Baczynska, M.; Rzelewska, M.; Regel-Rosocka, M.; Wisniewski, M. Transport of Iron Ions from Chloride Solutions Using Cellulose Triacetate Matrix Inclusion Membranes with an Ionic Liquid Carrier. Chem. Pap. 2016, 70, 172–179. [Google Scholar] [CrossRef]
- Pospiech, B. Studies on Extraction and Permeation of Cadmium(II) Using Cyphos IL 104 as Selective Extractant and Ion Carrier. Hydrometallurgy 2015, 154, 88–94. [Google Scholar] [CrossRef]
- Pospiech, B. Application of Phosphonium Ionic Liquids as Ion Carriers in Polymer Inclusion Membranes (PIMs) for Separation of Cadmium(II) and Copper(II) from Aqueous Solutions. J. Solut. Chem. 2015, 44, 2431–2447. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Y.; Zhang, C.; Chen, J. Preparation of PVDF-Based Polymer Inclusion Membrane Using Ionic Liquid Plasticizer and Cyphos IL 104 Carrier for Cr(VI) Transport. J. Membr. Sci. 2011, 372, 314–321. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Alonso, M.; Lopez, F.A.; Lopez-Delgado, A. Pseudo-Emulsion Membrane Strip Dispersion (PEMSD) Pertraction of Chromium(VI) Using CYPHOS IL101 Ionic Liquid as Carrier. Environ. Sci. Technol. 2010, 44, 7504–7508. [Google Scholar] [CrossRef] [PubMed]
- Bonggotgetsakul, Y.Y.; Cattrall, R.W.; Kolev, S.D. Extraction of Gold(III) from Hydrochloric Acid Solutions with a PVC-Based Polymer Inclusion Membrane (PIM) Containing Cyphos® IL 104. Membranes 2015, 5, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Bonggotgetsakul, Y.Y.N.; Cattrall, R.W.; Kolev, S.D. Recovery of Gold from Aqua Regia Digested Electronic Scrap Using a Poly(Vinylidene Fluoride-Co-Hexafluoropropene) (PVDF-HFP) Based Polymer Inclusion Membrane (PIM) Containing Cyphos® IL 104. J. Membr. Sci. 2016, 514, 274–281. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Rzelewska, M.; Baczynska, M.; Janus, M. Removal of palladium(II) from aqueous chloride solutions with cyphos phosphonium ionic liquids as metal ion carriers for liquidliquid extraction and transport across polymer inclusion membranes. Physicochem. Probl. Miner. Process. 2015, 51, 621–631. [Google Scholar] [CrossRef]
- The World Gold Council Report (WGC). Available online: https://www.gold.org/ (accessed on 1 January 2023).
- Hoyer, K.-P.; Schaper, M. Alloy Design for Biomedical Applications in Additive Manufacturing. In TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings; Springer International Publishing: Cham, Switzerland, 2019; pp. 475–484. [Google Scholar]
- Jacobson, D.M.; Sangha, S.P.S.; Gales, A.; Schmid, E.E. The Development of New Silver-Free Brazing Alloys for Steel Tubular Assembly. Weld. J. 2002, 81, 149S–155S. [Google Scholar]
- ASTM B 122M; Standard Specification for Copper-Nickel-Tin Alloy, Copper-Nickel-Zinc Alloy (Nickel Silver), and Copper-Nickel Alloy Plate, Sheet, Strip, and Rolled Bar. ASTM Standards: West Conshohocken, PA, USA, 2020.
- International Copper Study Group. Available online: https://icsg.org/ (accessed on 1 January 2023).
- Hongloi, N.; Prapainainar, P.; Prapainainar, C. Review of Green Diesel Production from Fatty Acid Deoxygenation over Ni-Based Catalysts. Mol. Catal. 2022, 523, 111696. [Google Scholar] [CrossRef]
- Wan Khalit, W.N.A.; Asikin-Mijan, N.; Marliza, T.S.; Safa-Gamal, M.; Shamsuddin, M.R.; Azreena, I.N.; Saiman, M.I.; Taufiq-Yap, Y.H. One-Pot Decarboxylation and Decarbonylation Reaction of Waste Cooking Oil over Activated Carbon Supported Nickel-Zinc Catalyst into Diesel-like Fuels. J. Anal. Appl. Pyrolysis 2022, 164, 105505. [Google Scholar] [CrossRef]
- Kaika, N.; Panopoulou, C.; Anagnostopoulou, E.; Fakas, C.; Lilas, P.; Stavroulaki, D.; Papadogianakis, G. Novel Full Hydrogenation Reaction of Methyl Esters of Palm Kernel and Sunflower Oils Into Methyl Stearate Catalyzed by Rhodium, Ruthenium and Nickel Complexes of Bidentate Hexasulfonated o-Phenylendiphosphite Ligands. Catal. Lett. 2019, 149, 580–590. [Google Scholar] [CrossRef]
- Biryukov, A.I.; Kozaderov, O.A.; Zakharievich, D.A.; Galin, R.G.; Burmistrov, L.O.; Batmanova, T.V.; Zhivulin, V.E. Corrosion of Diffusion Iron–Zinc Coatings (δ-Phase) in an Alkaline Medium. Int. J. Corros. Scale Inhib. 2021, 10, 1677–1688. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Croy, J.R.; Lee, E.; Gutierrez, A.; He, M.; Park, J.S.; Yonemoto, B.T.; Long, B.R.; Blauwkamp, J.D.; Johnson, C.S.; et al. The Quest for Manganese-Rich Electrodes for Lithium Batteries: Strategic Design and Electrochemical Behavior. Sustain. Energy Fuels 2018, 2, 1375–1397. [Google Scholar] [CrossRef]
- Larcin, J.; Maskell, W.C.; Tye, F.L. Leclanché Cell Investigations. Part II: Zinc Potential as a Tool for Studying Intermittent Discharge. Electrochim. Acta 1998, 44, 191–199. [Google Scholar] [CrossRef]
- Cho, M.; Kwon, E.; Park, K. Design and Control of Pneumatic System for Recycling Classification of Non-Ferrous Metals. Int. J. Precis. Eng. Manuf. -Green Technol. 2022, 9, 567–575. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Z.; Wang, C.; Pang, Q.; Hua, L. Research on the Process of Small Sample Non-Ferrous Metal Recognition and Separation Based on Deep Learning. Waste Manag. 2021, 126, 266–273. [Google Scholar] [CrossRef]
- Pospiech, B.; Kujawski, W. Ionic Liquids as Selective Extractants and Ion Carriers of Heavy Metal Ions from Aqueous Solutions Utilized in Extraction and Membrane Separation. Rev. Chem. Eng. 2015, 31, 179–191. [Google Scholar] [CrossRef]
- Kumar, A.; Haddad, R.; Sastre, A.M. Integrated Membrane Process for Gold Recovery from Hydrometallurgical Solutions. AIChE J. 2001, 47, 328–340. [Google Scholar] [CrossRef]
- Imdad, S.; Dohare, R.K. A Critical Review On Heavy Metals Removal Using Ionic Liquid Membranes From The Industrial Wastewater. Chem. Eng. Process.—Process Intensif. 2022, 173, 108812. [Google Scholar] [CrossRef]
- Kadokawa, J. Ionic Liquids—New Aspects for the Future; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Lu, J.; He, K.; Wang, Y.; Chen, G.; Weng, H.; Lin, M. An Effective Process for the Separation of U(VI), Th(IV) from Rare Earth Elements by Using Ionic Liquid Cyphos IL 104. Chin. Chem. Lett. 2022, 33, 3422–3428. [Google Scholar] [CrossRef]
- Wellens, S.; Thijs, B.; Binnemans, K. An Environmentally Friendlier Approach to Hydrometallurgy: Highly Selective Separation of Cobalt from Nickel by Solvent Extraction with Undiluted Phosphonium Ionic Liquids. Green Chem. 2012, 14, 1657–1665. [Google Scholar] [CrossRef]
- Wiecka, Z.; Rzelewska-Piekut, M.; Regel-Rosocka, M. Recovery of Platinum Group Metals from Spent Automotive Converters by Leaching with Organic and Inorganic Acids and Extraction with Quaternary Phosphonium Salts. Sep. Purif. Technol. 2022, 280, 119933. [Google Scholar] [CrossRef]
- Rios-Vera, R.M.; Chagnes, A.; Hernández-Perales, L.; Martínez-Rodríguez, D.E.; Navarro-Segura, D.L.; Gaillon, L.; Sirieix-Plénet, J.; Rizzi, C.; Rollet, A.L.; Avila-Rodriguez, M.; et al. Trihexyl(Tetradecyl)Phosphonium Bis-2,4,4-(Trimethylpentyl)Phosphinate Micellar Behavior in the Extraction of Ag(I) from Acidic Nitrate Media. J. Mol. Liq. 2022, 358, 119132. [Google Scholar] [CrossRef]
- St John, A.M.; Cattrall, R.W.; Kolev, S.D. Determination of the Initial Flux of Polymer Inclusion Membranes. Sep. Purif. Technol. 2013, 116, 41–45. [Google Scholar] [CrossRef]
- Danesi, P.R. Separation of Metal Species by Supported Liquid Membranes. Sep. Sci. Technol. 1984, 19, 857–894. [Google Scholar] [CrossRef]
- Kislik, V.S. (Ed.) Liquid Membranes; Elsevier: Amsterdam, The Netherlands, 2010; p. iv. ISBN 978-0-444-53218-3. [Google Scholar]
- Arous, O.; Amara, M.; Kerdjoudj, H. Synthesis and Characterization of Cellulose Triacetate and Poly(Ethylene Imine) Membranes Containing a Polyether Macrobicyclic: Their Application to the Separation of Copper(II) and Silver(I) Ions. J. Appl. Polym. Sci. 2004, 93, 1401–1410. [Google Scholar] [CrossRef]
- Mahanty, B.N.; Mohapatra, P.K.; Raut, D.R.; Das, D.K.; Behere, P.G.; Afzal, M.; Verboom, W. Polymer Inclusion Membrane Containing a Tripodal Diglycolamide (T-DGA): Characterization and Sorption Isotherm Studies. J. Environ. Chem. Eng. 2016, 4, 1826–1838. [Google Scholar] [CrossRef]
- Kumar, R.; Pandey, A.K.; Sharma, M.K.; Panicker, L.V.; Sodaye, S.; Suresh, G.; Ramagiri, S.V.; Bellare, J.R.; Goswami, A. Diffusional Transport of Ions in Plasticized Anion-Exchange Membranes. J. Phys. Chem. B 2011, 115, 5856–5867. [Google Scholar] [CrossRef]
- Arous, O.; Saoud, F.; Amara, M.; Kerdjoudj, H. Efficient Facilitated Transport of Lead and Cadmium Across a Plasticized Triacetate Membrane Mediated by D2EHPA and TOPO. Mater. Sci. Appl. 2011, 2, 615–623. [Google Scholar] [CrossRef]
- Ji, J.; Cooper, W.C. Nickel Speciation in Aqueous Chloride Solutions. Electrochim. Acta 1996, 41, 1549–1560. [Google Scholar] [CrossRef]
- Tor, A.; Arslan, G.; Muslu, H.; Celiktas, A.; Cengeloglu, Y.; Ersoz, M. Facilitated Transport of Cr(III) through Polymer Inclusion Membrane with Di(2-Ethylhexyl)Phosphoric Acid (DEHPA). J. Membr. Sci. 2009, 329, 169–174. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of Hydrophobic Alkylimidazoles in the Separation of Non-Ferrous Metal Ions across Plasticised Membranes—A Review. Membranes 2020, 10, 331. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).