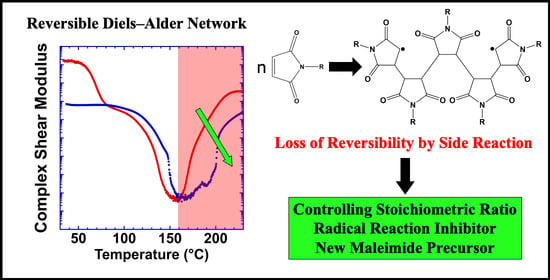
Understanding the Effect of Side Reactions on the Recyclability of Furan–Maleimide Resins Based on Thermoreversible Diels–Alder Network
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
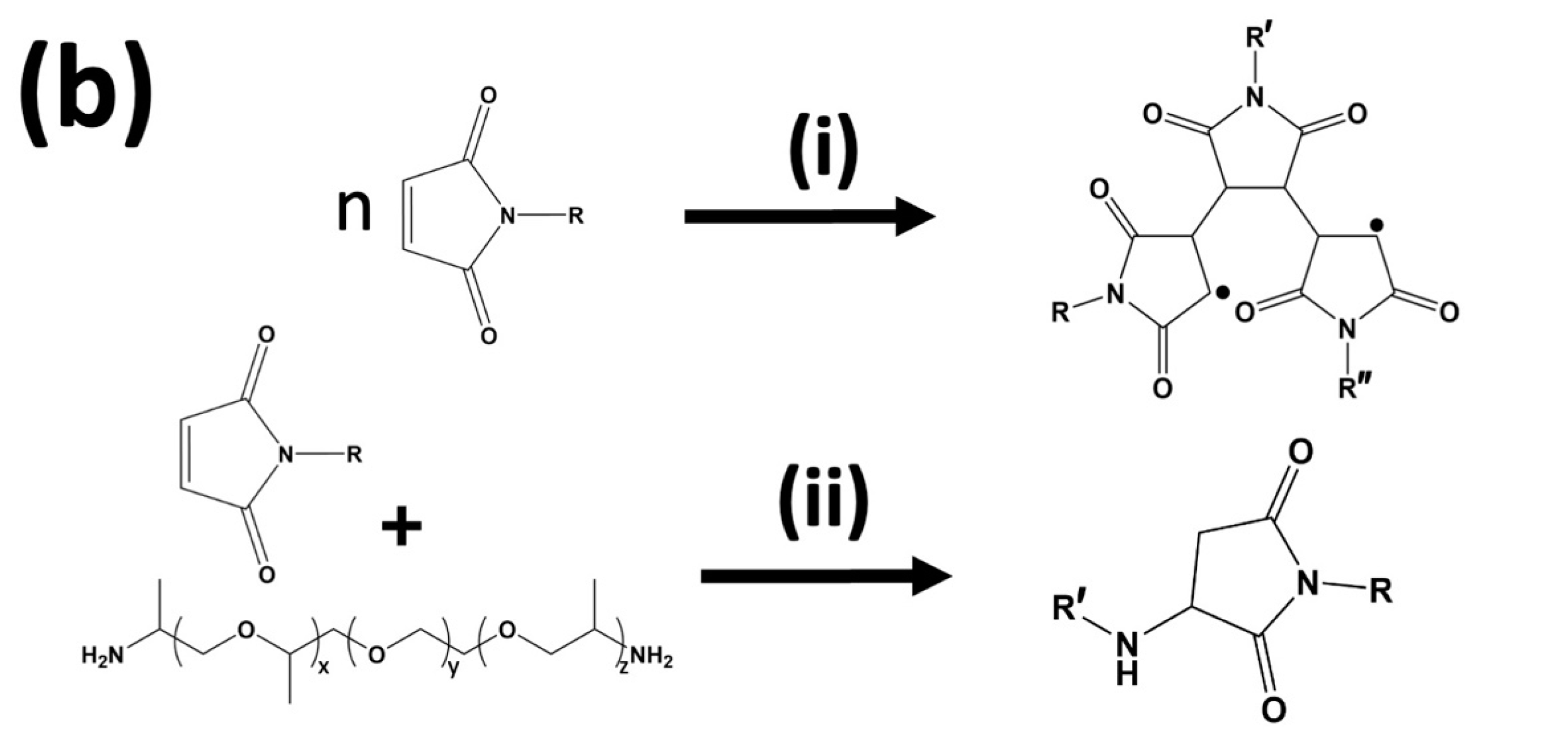
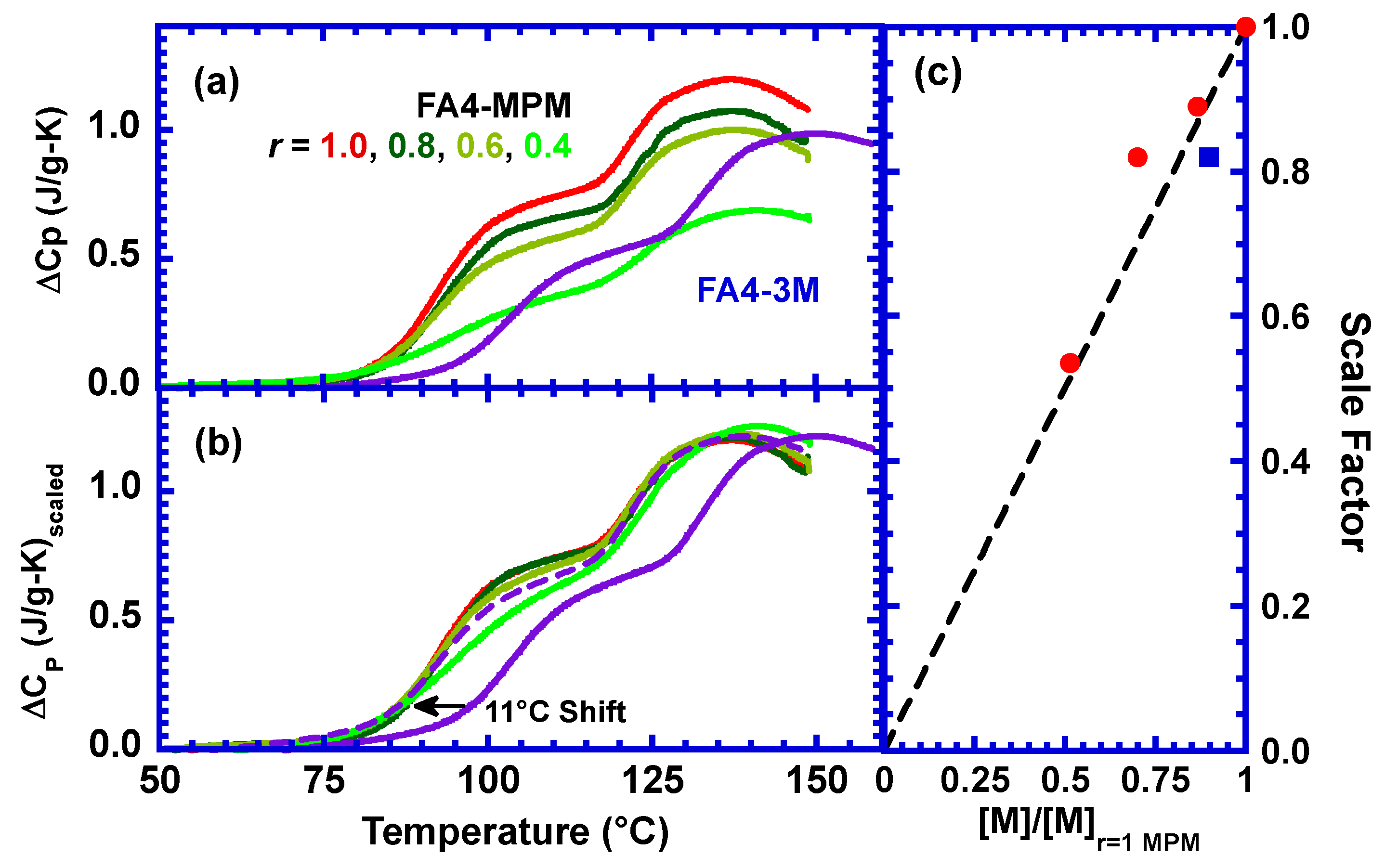
3.1. Effect of Stoichiometric Ratios on the Side Reaction
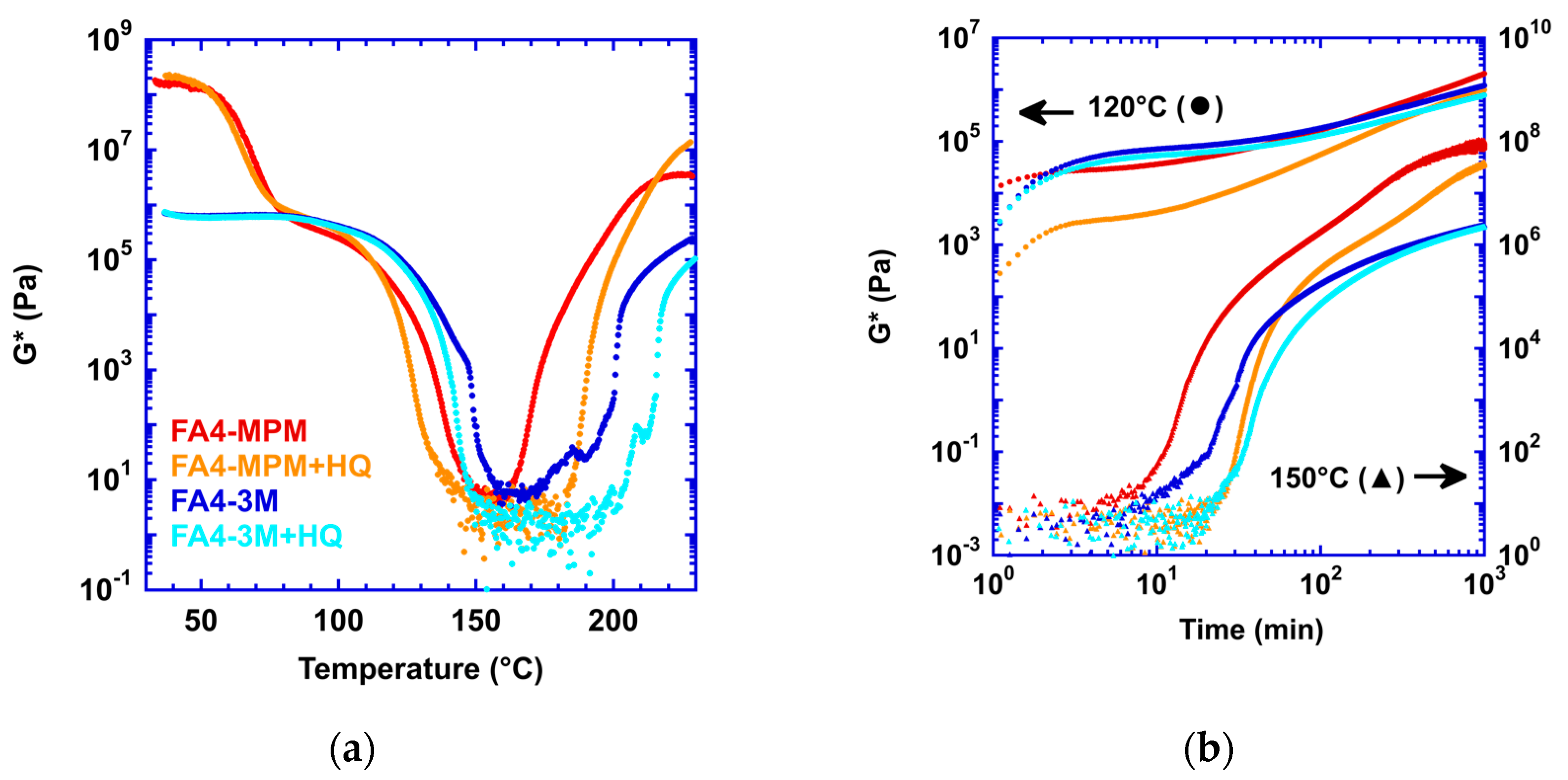
3.2. Effect of a Free Radical Inhibitor on Side Reaction
3.3. Effect of a Different Maleimide Precursor on the Side Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Adzima, B.J.; Aguirre, H.A.; Kloxin, C.J.; Scott, T.F.; Bowman, C.N. Rheological and chemical analysis of reverse gelation in a covalently crosslinked Diels-Alder polymer network. Macromolecules 2008, 41, 9112–9117. [Google Scholar] [CrossRef] [PubMed]
- Froidevaux, V.; Borne, M.; Laborbe, E.; Auvergne, R.; Gandini, A.; Boutevin, B. Study of the Diels–Alder and retro-Diels–Alder reaction between furan derivatives and maleimide for the creation of new materials. RSC Adv. 2015, 5, 37742–37754. [Google Scholar] [CrossRef]
- Lin, C.; Sheng, D.; Liu, X.; Xu, S.; Ji, F.; Dong, L.; Zhou, Y.; Yang, Y. Effect of different sizes of graphene on Diels-Alder self-healing polyurethane. Polymer 2019, 182, 121822. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; He, Z.; Liu, L.; Niu, H. Thermoreversible cross-linking of ethylene/propylene copolymers based on Diels–Alder chemistry: The cross-linking reaction kinetics. Polym. Chem. 2020, 11, 5851–5860. [Google Scholar] [CrossRef]
- Gandini, A. The furan/maleimide Diels–Alder reaction: A versatile click–unclick tool in macromolecular synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Orozco, F.; Li, J.; Ezekiel, U.; Niyazov, Z.; Floyd, L.; Lima, G.M.R.; Winkelman, J.G.M.; Moreno-Villoslada, I.; Picchioni, F.; Bose, R.K. Diels-Alder-based thermo-reversibly crosslinked polymers: Interplay of crosslinking density, network mobility, kinetics and stereoisomerism. Eur. Polym. J. 2020, 135, 109882. [Google Scholar] [CrossRef]
- Scheltjens, G.; Diaz, M.M.; Brancart, J.; Van Assche, G.; Van Mele, B. A self-healing polymer network based on reversible covalent bonding. React. Funct. Polym. 2013, 73, 413–420. [Google Scholar] [CrossRef]
- Tungare, A.V. Curing Chemistry-Network Property-Chemorheology Relations in Bismaleimide Resins; Syracuse University: New York, NY, USA, 1990; p. 286. [Google Scholar]
- Tungare, A.V.; Martin, G.C. Analysis of the curing behavior of bismaleimide resins. J. Appl. Polym. Sci. 1992, 46, 1125–1135. [Google Scholar] [CrossRef]
- Brown, I.M.; Sandreczki, T.C. Crosslinking reactions in maleimide and bis(maleimide) polymers. An ESR study. Macromolecules 1990, 23, 94–100. [Google Scholar] [CrossRef]
- Hopewell, J.L.; Hill, D.J.T.; Pomery, P.J. Electron spin resonance study of the homopolymerization of aromatic bismaleimides. Polymer 1998, 39, 5601–5607. [Google Scholar] [CrossRef]
- Cuvellier, A.; Verhelle, R.; Brancart, J.; Vanderborght, B.; Van Assche, G.; Rahier, H. The influence of stereochemistry on the reactivity of the Diels–Alder cycloaddition and the implications for reversible network polymerization. Polym. Chem. 2019, 10, 473–485. [Google Scholar] [CrossRef]
- Rozenberg, B.A.; Dzhavadyan, E.A.; Morgan, R.; Shin, E. High-performance bismaleimide matrices: Cure kinetics and mechanism. Polym. Adv. Technol. 2002, 13, 837–844. [Google Scholar] [CrossRef]
- Iredale, R.J.; Ward, C.; Hamerton, I. Modern advances in bismaleimide resin technology: A 21st century perspective on the chemistry of addition polyimides. Prog. Polym. Sci. 2017, 69, 1–21. [Google Scholar] [CrossRef]
- Diaz, M.M.; Van Assche, G.; Maurer, F.H.J.; Van Mele, B. Thermophysical characterization of a reversible dynamic polymer network based on kinetics and equilibrium of an amorphous furan-maleimide Diels-Alder cycloaddition. Polymer 2017, 120, 176–188. [Google Scholar] [CrossRef]
- Marref, M.; Mignard, N.; Jegat, C.; Taha, M.; Belbachir, M.; Meghabar, R. Epoxy-amine based thermoresponsive networks designed by Diels-Alder reactions. Polym. Int. 2013, 62, 87–98. [Google Scholar] [CrossRef]
- Berg, G.J.; Gong, T.; Fenoli, C.R.; Bowman, C.N. A Dual-Cure, Solid-State Photoresist Combining a Thermoreversible Diels–Alder Network and a Chain Growth Acrylate Network. Macromolecules 2014, 47, 3473–3482. [Google Scholar] [CrossRef]
- Roels, E.; Terryn, S.; Brancart, J.; Verhelle, R.; Van Assche, G.; Vanderborght, B. Additive Manufacturing for Self-Healing Soft Robots. Soft Robot. 2020, 7, 711–723. [Google Scholar] [CrossRef]
- Safaei, A.; Terryn, S.; Vanderborght, B.; Van Assche, G.; Brancart, J. The Influence of the Furan and Maleimide Stoichiometry on the Thermoreversible Diels-Alder Network Polymerization. Polymers 2021, 13, 2522. [Google Scholar] [CrossRef]
- Mojtabai, K.D.; Lindholm, S.J.; McReynolds, B.T.; Penners, N.; McCoy, J.D.; Chowdhury, S.; Lee, Y. Diels–Alder Augmented Epoxies with Plasmonic Nanoparticle Fillers for Efficient Photothermal Depolymerization. ACS Appl. Polym. Mater. 2022, 4, 2703–2711. [Google Scholar] [CrossRef]
- Okihara, M.; Okuma, K.; Kawamura, A.; Miyata, T. Photoresponsive Gelation of Four-Armed Poly(ethylene glycol) with Photodimerizable Groups. Gels 2022, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Orozco, F.; Niyazov, Z.; Garnier, T.; Migliore, N.; Zdvizhkov, A.T.; Raffa, P.; Moreno-Villoslada, I.; Picchioni, F.; Bose, R.K. Maleimide Self-Reaction in Furan/Maleimide-Based Reversibly Crosslinked Polyketones: Processing Limitation or Potential Advantage? Molecules 2021, 26, 2230. [Google Scholar] [CrossRef] [PubMed]
- Chandran, M.S.; Krishna, M.; Rai, S.; Krupashankara, M.S.; Salini, K. Cure Kinetics and Activation Energy Studies of Modified Bismaleimide Resins. ISRN Polym. Sci. 2012, 2012, 309861. [Google Scholar] [CrossRef]
- Cantamessa, F.; Damonte, G.; Monticelli, O.; Arrigo, R.; Fina, A. Thermoreversible Cross-Linked Rubber Prepared via Melt Blending and Its Nanocomposites. ACS Appl. Polym. Mater. 2022, 4, 4796–4807. [Google Scholar] [CrossRef] [PubMed]
- Brancart, J.; Verhelle, R.; Mangialetto, J.; Assche, G.V. Coupling the Microscopic Healing Behaviour of Coatings to the Thermoreversible Diels-Alder Network Formation. Coatings 2018, 9, 13. [Google Scholar] [CrossRef]
- Canadell, J.; Fischer, H.; De With, G.; van Benthem, R.A.T.M. Stereoisomeric effects in thermo-remendable polymer networks based on Diels-Alder crosslink reactions. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3456–3467. [Google Scholar] [CrossRef]
- Clarkson, C.M.; McCoy, J.D.; Kropka, J.M. Enthalpy recovery and its relation to shear response in an amine cured DGEBA epoxy. Polymer 2016, 94, 19–30. [Google Scholar] [CrossRef]
- Wimmer, M.G.; Compton, B.G. Semi-solid epoxy feedstocks with high glass transition temperature for material extrusion additive manufacturing. Addit. Manuf. 2022, 54, 102725. [Google Scholar] [CrossRef]
- Tammaro, D. Rheological characterization of complex fluids through a table-top 3D printer. Rheol. Acta 2022, 61, 761–772. [Google Scholar] [CrossRef]
- Yang, K.; Grant, J.C.; Lamey, P.; Joshi-Imre, A.; Lund, B.R.; Smaldone, R.A.; Voit, W. Diels–Alder Reversible Thermoset 3D Printing: Isotropic Thermoset Polymers via Fused Filament Fabrication. Adv. Funct. Mater. 2017, 27, 1700318. [Google Scholar] [CrossRef]
- Imai, Y.; Itoh, H.; Naka, K.; Chujo, Y. Thermally Reversible IPN Organic−Inorganic Polymer Hybrids Utilizing the Diels−Alder Reaction. Macromolecules 2000, 33, 4343–4346. [Google Scholar] [CrossRef]
- Sridhar, L.M.; Oster, M.O.; Herr, D.E.; Gregg, J.B.D.; Wilson, J.A.; Slark, A.T. Re-usable thermally reversible crosslinked adhesives from robust polyester and poly(ester urethane) Diels–Alder networks. Green Chem. 2020, 22, 8669–8679. [Google Scholar] [CrossRef]
- Phelan, J.C.; Sung, C.S.P. Cure Characterization in Bis(maleimide)/Diallylbisphenol A Resin by Fluorescence, FT-IR, and UV-Reflection Spectroscopy. Macromolecules 1997, 30, 6845–6851. [Google Scholar] [CrossRef]
- Cerdan, K.; Brancart, J.; De Coninck, H.; Van Hooreweder, B.; Van Assche, G.; Van Puyvelde, P. Laser sintering of self-healable and recyclable thermoset networks. Eur. Polym. J. 2022, 175, 111383. [Google Scholar] [CrossRef]
- Joshi, R. Free Radical Scavenging Reactions of Tetrahydroxyquinone: A Pulse Radiolysis Study. ChemistrySelect 2016, 1, 1084–1091. [Google Scholar] [CrossRef]
- Liu, X.; Du, P.; Liu, L.; Zheng, Z.; Wang, X.; Joncheray, T.; Zhang, Y. Kinetic study of Diels–Alder reaction involving in maleimide–furan compounds and linear polyurethane. Polym. Bull. 2013, 70, 2319–2335. [Google Scholar] [CrossRef]
- Wagner, A.; Mühlberger, M.; Paulik, C. Photoinitiator-free photopolymerization of acrylate-bismaleimide mixtures and their application for inkjet printing. J. Appl. Polym. Sci. 2019, 136, 47789. [Google Scholar] [CrossRef]
- Terryn, S.; Brancart, J.; Roels, E.; Verhelle, R.; Safaei, A.; Cuvellier, A.; Vanderborght, B.; Van Assche, G. Structure–Property Relationships of Self-Healing Polymer Networks Based on Reversible Diels–Alder Chemistry. Macromolecules 2022, 55, 5497–5513. [Google Scholar] [CrossRef]
- van den Tempel, P.; Picchioni, F.; Bose, R.K. Designing End-of-Life Recyclable Polymers via Diels-Alder Chemistry: A Review on the Kinetics of Reversible Reactions. Macromol. Rapid Commun. 2022, 43, e2200023. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93. [Google Scholar] [CrossRef]
- Gandini, A.; Silvestre, A.; Coelho, D. Reversible click chemistry at the service of macromolecular materials. Part 4: Diels–Alder non-linear polycondensations involving polyfunctional furan and maleimide monomers. Polym. Chem. 2013, 4, 1364–1371. [Google Scholar] [CrossRef]
- Turkenburg, D.H.; Fischer, H.R. Diels-Alder based, thermo-reversible cross-linked epoxies for use in self-healing composites. Polymer 2015, 79, 187–194. [Google Scholar] [CrossRef]
- van Duin, M.; Picchioni, F.; de With, T.; Niemeijer, B.; Blom, R.; Keizer, J.; Polgar, L.M. Thermoreversible Cross-Linking of Rubber Compounds: From Proof-of-Concept toward an Industrial Process. Rubber Chem. Technol. 2018, 91, 492–508. [Google Scholar]

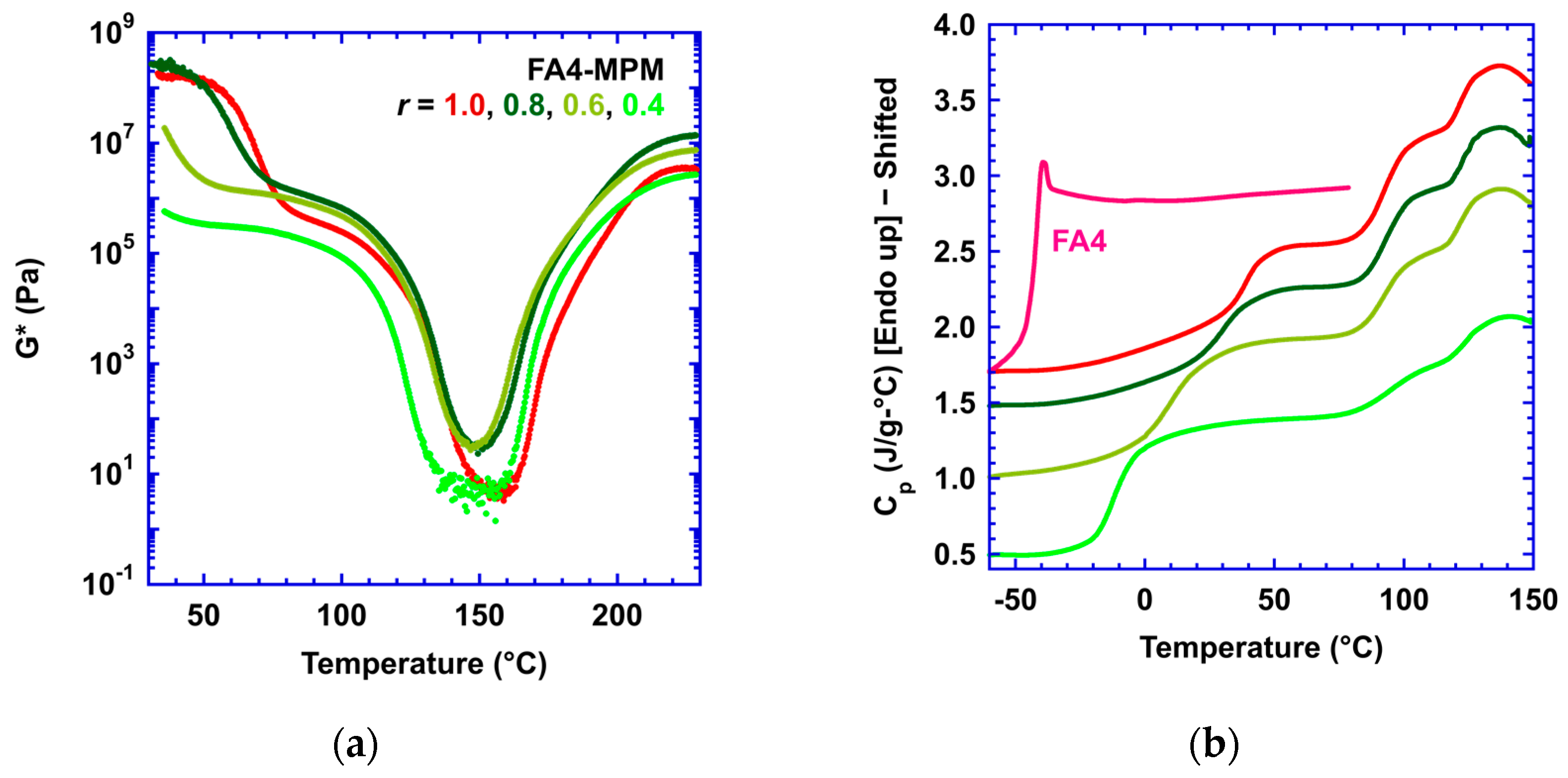
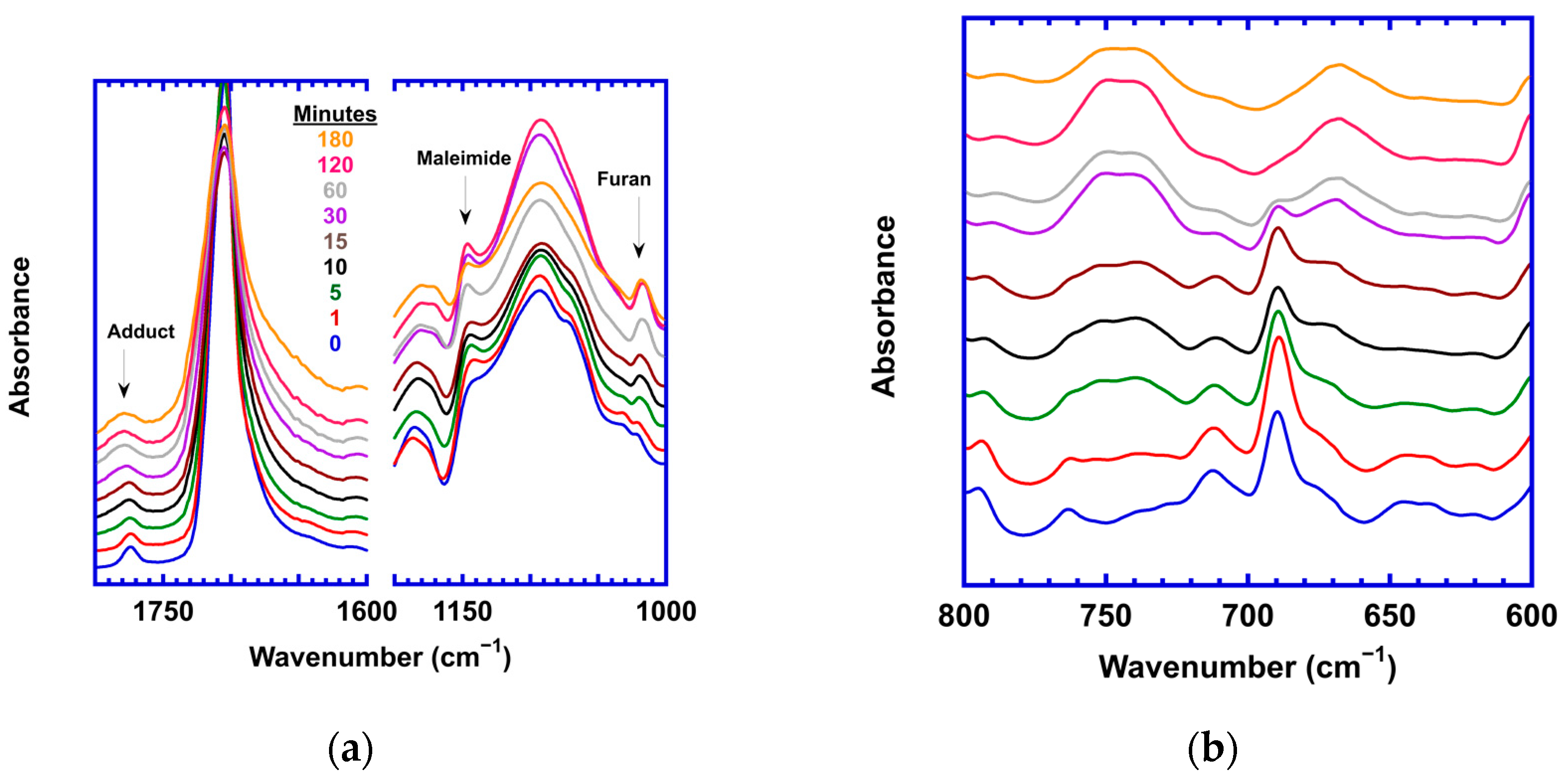
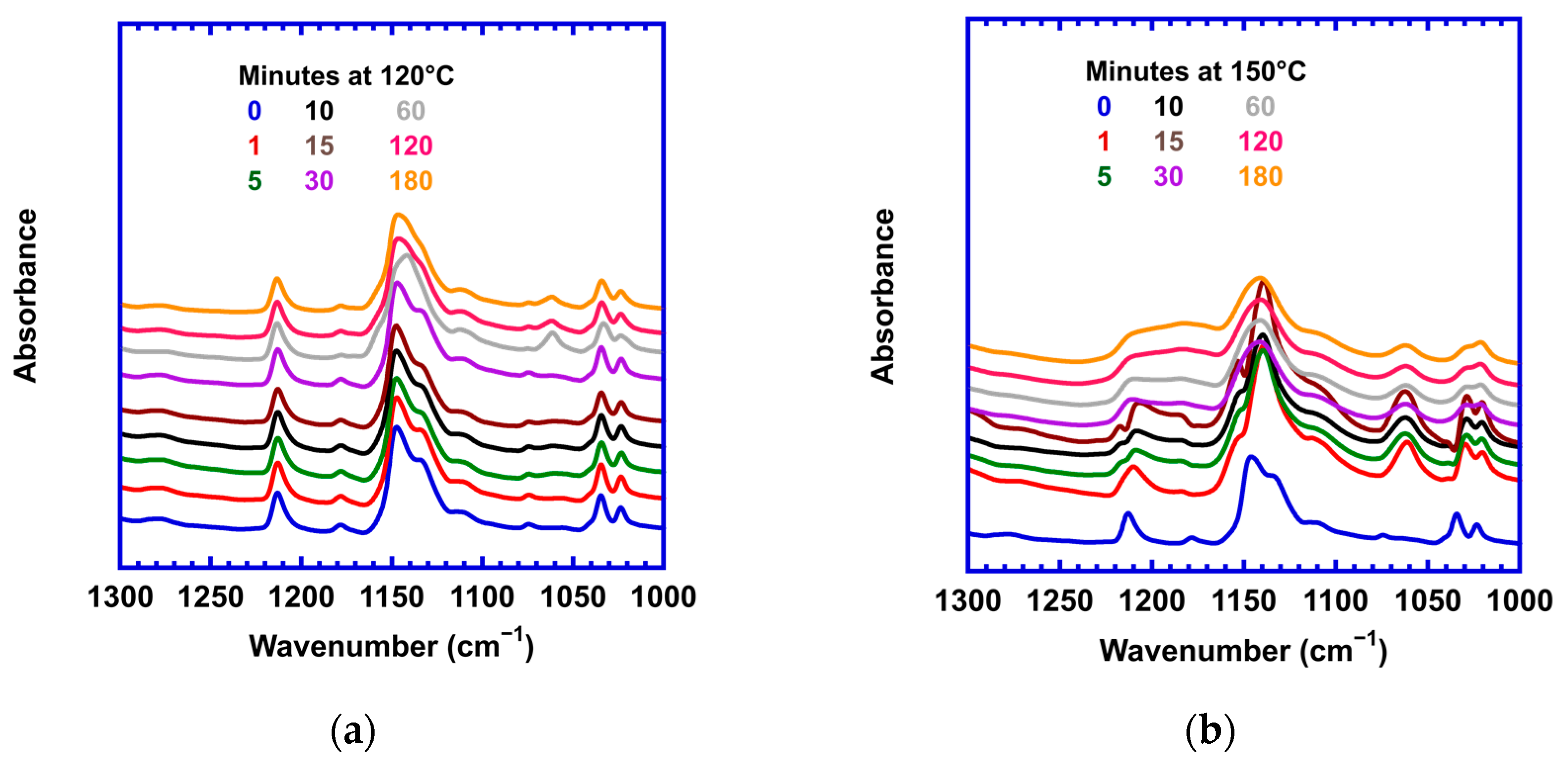
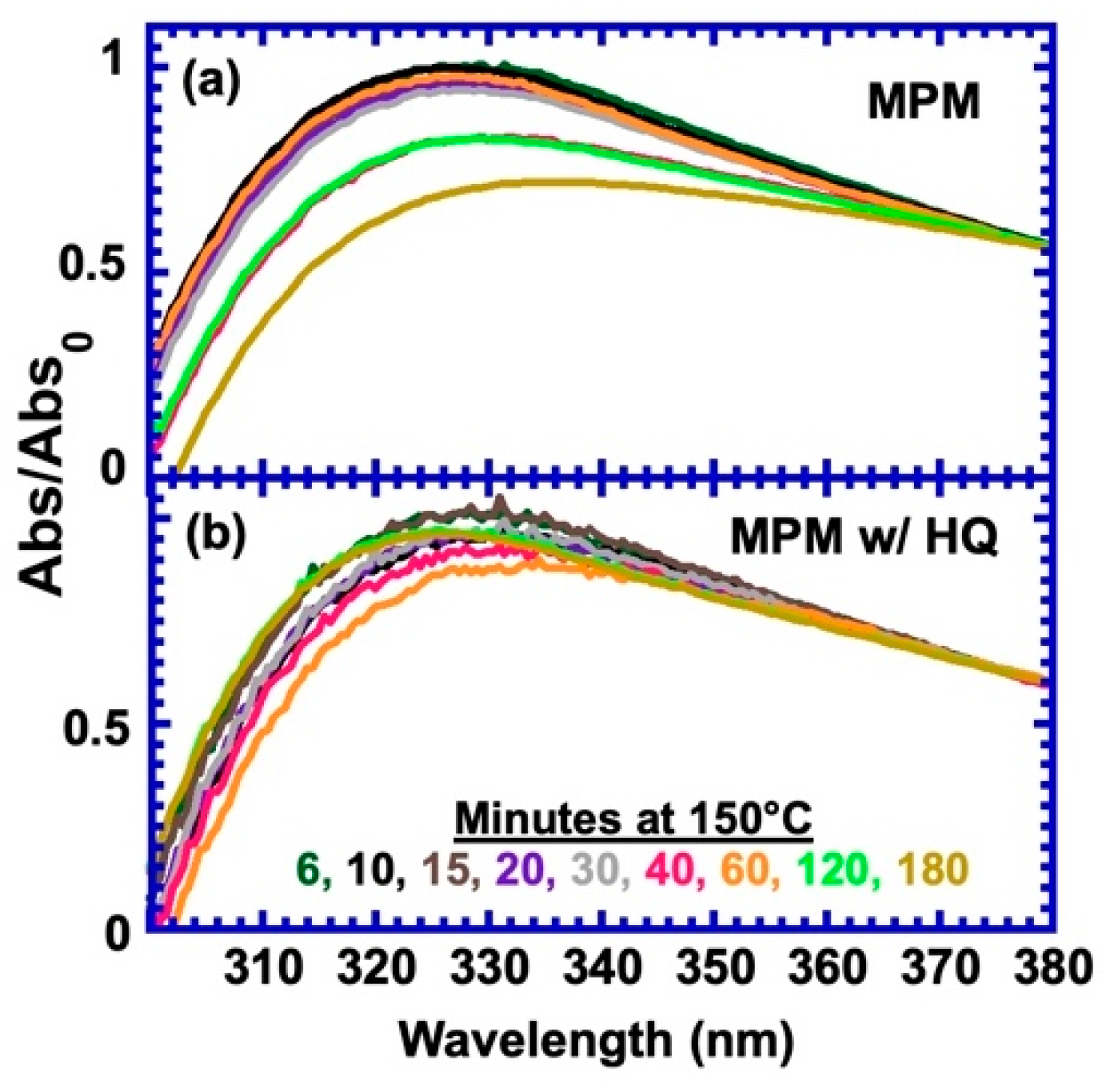


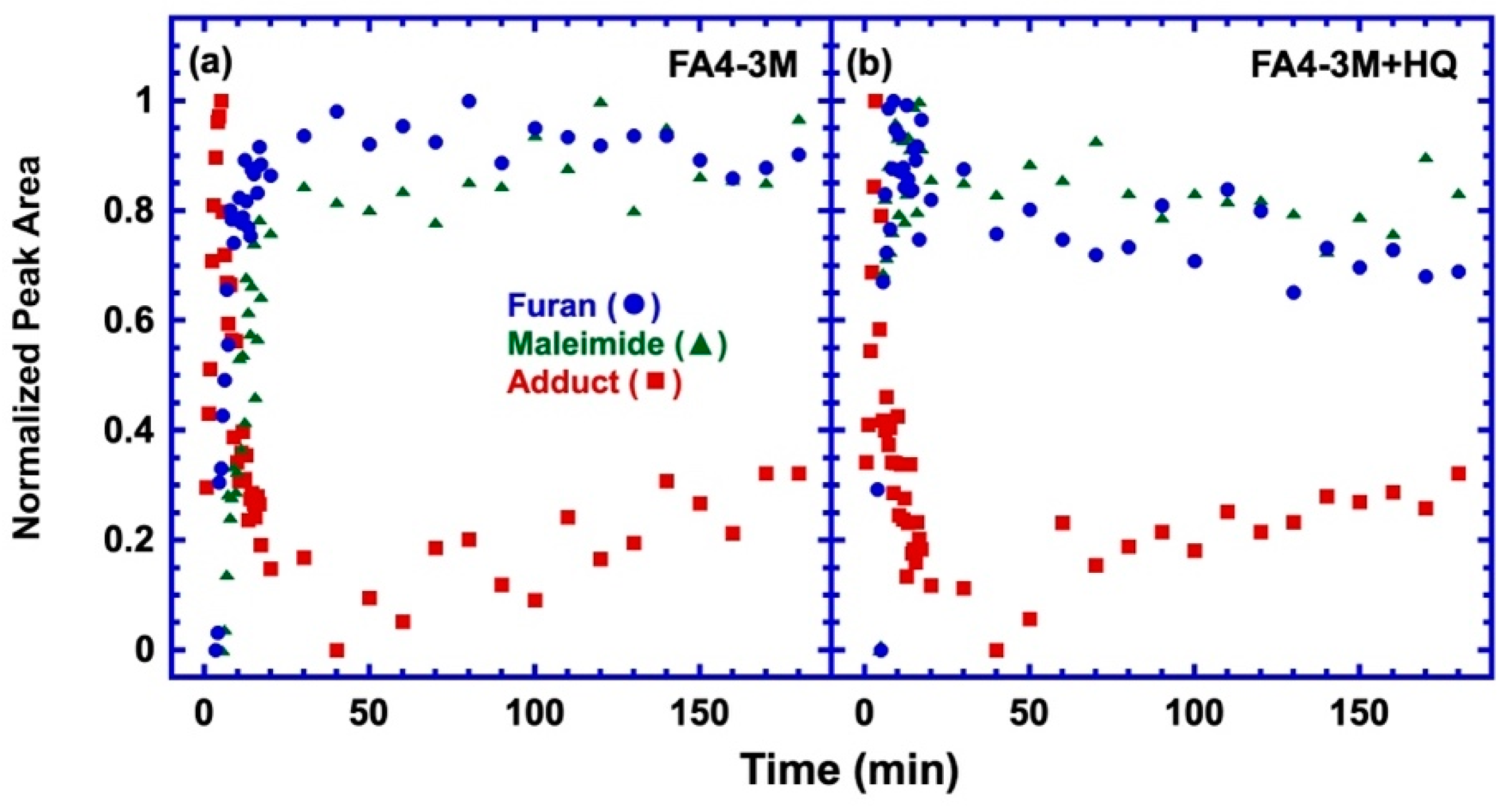

| Stoichiometric Ratio r = [M]/[F] | Calorimetric Tg | Rheological Tg | Flow Temp (Visco. = 104 Pa-s) | Temp. of Min G* | [M] (mol/L) |
|---|---|---|---|---|---|
| 0.4 | −13 | NA | 97 | 148 | 1.26 |
| 0.6 | 8 | 38 | 116 | 147 | 1.74 |
| 0.8 | 31 | 61 | 119 | 150 | 2.15 |
| 1.0 | 38 | 70 | 111 | 157 | 2.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McReynolds, B.T.; Mojtabai, K.D.; Penners, N.; Kim, G.; Lindholm, S.; Lee, Y.; McCoy, J.D.; Chowdhury, S. Understanding the Effect of Side Reactions on the Recyclability of Furan–Maleimide Resins Based on Thermoreversible Diels–Alder Network. Polymers 2023, 15, 1106. https://doi.org/10.3390/polym15051106
McReynolds BT, Mojtabai KD, Penners N, Kim G, Lindholm S, Lee Y, McCoy JD, Chowdhury S. Understanding the Effect of Side Reactions on the Recyclability of Furan–Maleimide Resins Based on Thermoreversible Diels–Alder Network. Polymers. 2023; 15(5):1106. https://doi.org/10.3390/polym15051106
Chicago/Turabian StyleMcReynolds, Brandon T., Kavon D. Mojtabai, Nicole Penners, Gaeun Kim, Samantha Lindholm, Youngmin Lee, John D. McCoy, and Sanchari Chowdhury. 2023. "Understanding the Effect of Side Reactions on the Recyclability of Furan–Maleimide Resins Based on Thermoreversible Diels–Alder Network" Polymers 15, no. 5: 1106. https://doi.org/10.3390/polym15051106
APA StyleMcReynolds, B. T., Mojtabai, K. D., Penners, N., Kim, G., Lindholm, S., Lee, Y., McCoy, J. D., & Chowdhury, S. (2023). Understanding the Effect of Side Reactions on the Recyclability of Furan–Maleimide Resins Based on Thermoreversible Diels–Alder Network. Polymers, 15(5), 1106. https://doi.org/10.3390/polym15051106