Lignocelluloses-Based Furan-Acetone Adducts as Wood Adhesives for Plywood Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Carbohydrate-Based Furan-Acetone Adducts
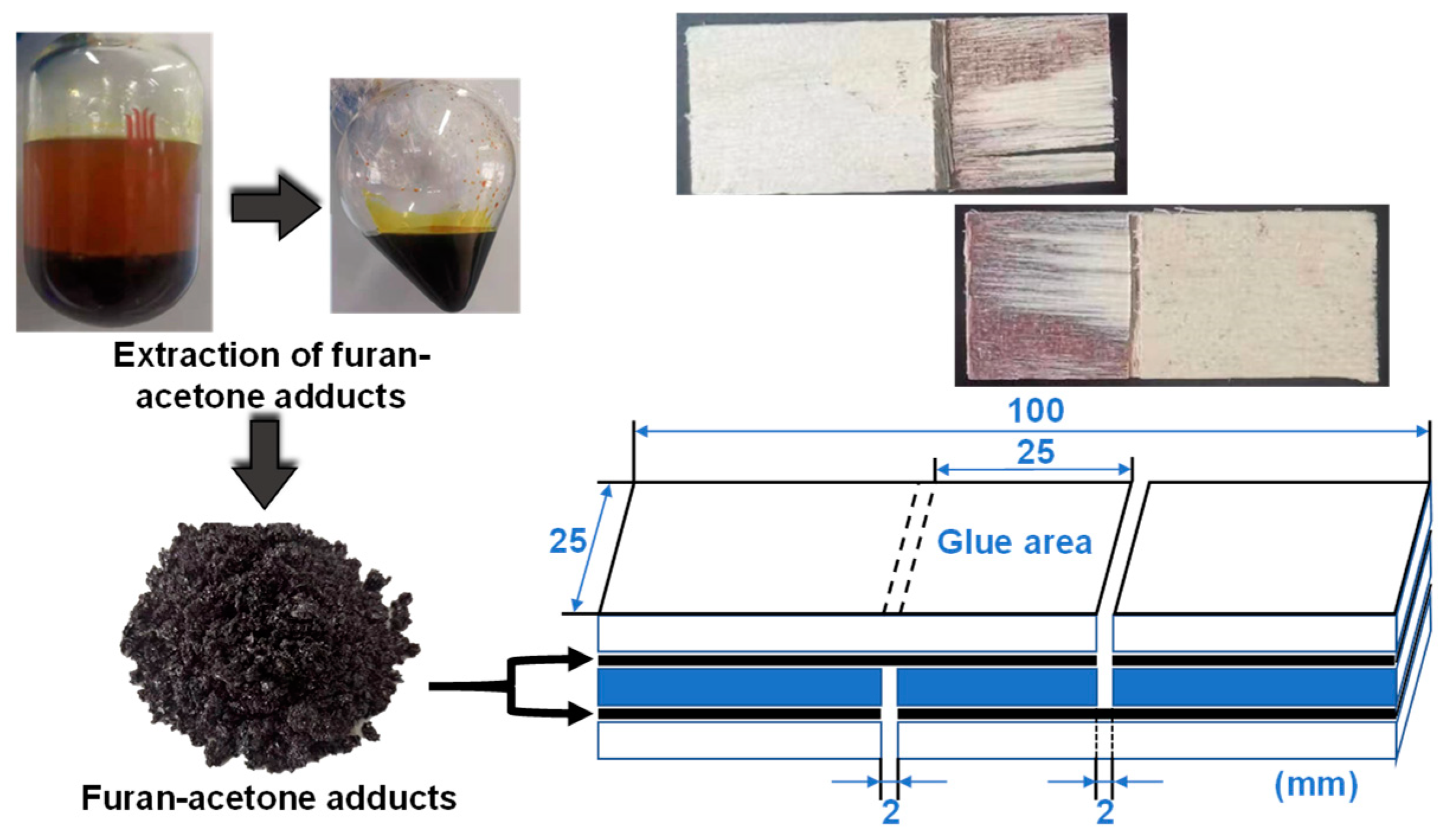
2.3. Preparation of Adhesives and Three-Layer Plywood
2.4. Analysis and Characterization of Adhesives
3. Results and Discussion
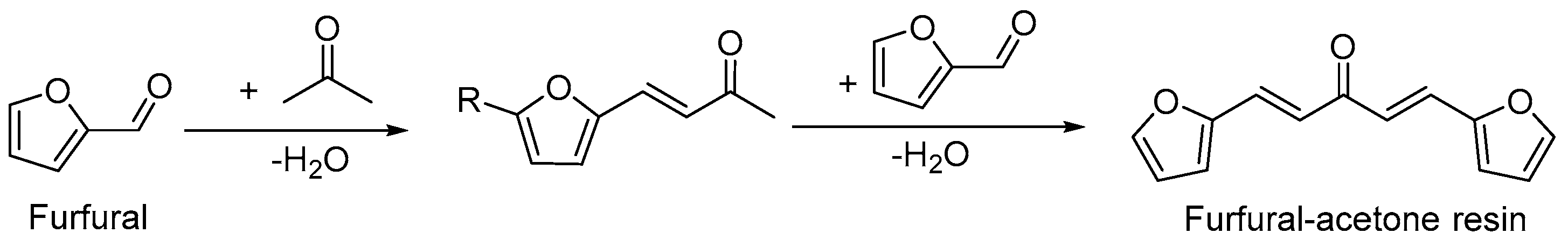
3.1. Synthesis and Identification of Furan-Acetone Adducts from Biomass
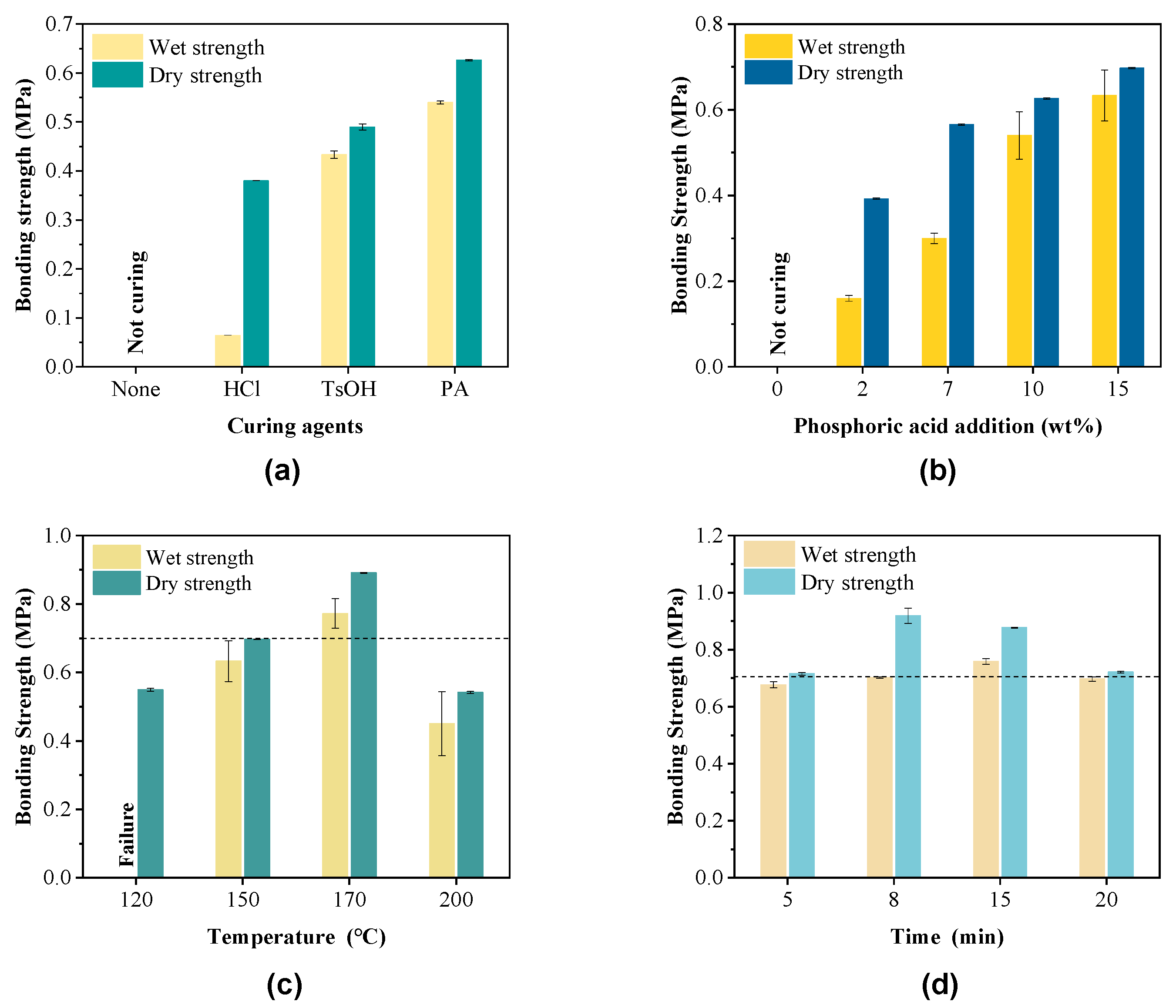
3.2. Application of Furan-Acetone Adducts as Adhesives
3.3. Modification of Furan-Acetone Adhesives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norhazaedawati, B.; SaifulAzry, S.O.A.; Lee, S.H.; Ilyas, R.A. 4—Wood-Based Panel Industries. In Oil Palm Biomass for Composite Panels; Sapuan, S.M., Paridah, M.T., SaifulAzry, S.O.A., Lee, S.H., Eds.; Elsevier: Selangor, Malaysia, 2022; pp. 69–86. ISBN 978-0-12-823852-3. [Google Scholar]
- Kristak, L.; Antov, P.; Bekhta, P.; Lubis, M.A.R.; Iswanto, A.H.; Reh, R.; Sedliacik, J.; Savov, V.; Taghiyari, H.R.; Papadopoulos, A.N.; et al. Recent Progress in Ultra-Low Formaldehyde Emitting Adhesive Systems and Formaldehyde Scavengers in Wood-Based Panels: A Review. Wood Mater. Sci. Eng. 2022, 20, 1–20. [Google Scholar] [CrossRef]
- Hussin, M.H.; Abd Latif, N.H.; Hamidon, T.S.; Idris, N.N.; Hashim, R.; Appaturi, J.N.; Brosse, N.; Ziegler-Devin, I.; Chrusiel, L.; Fatriasari, W.; et al. Latest Advancements in High-Performance Bio-Based Wood Adhesives: A Critical Review. J. Mater. Res. Technol.-JMRT 2022, 21, 3909–3946. [Google Scholar] [CrossRef]
- Arias, A.; González-Rodríguez, S.; Vetroni Barros, M.; Salvador, R.; de Francisco, A.C.; Moro Piekarski, C.; Moreira, M.T. Recent Developments in Bio-Based Adhesives from Renewable Natural Resources. J. Clean. Prod. 2021, 314, 127892. [Google Scholar] [CrossRef]
- Pizzi, A. Wood Products and Green Chemistry. Ann. For. Sci. 2016, 73, 185–203. [Google Scholar] [CrossRef]
- Hemmilä, V.; Adamopoulos, S.; Karlsson, O.; Kumar, A. Development of Sustainable Bio-Adhesives for Engineered Wood Panels—A Review. RSC Adv. 2017, 7, 38604–38630. [Google Scholar] [CrossRef]
- Hussin, M.H.; Samad, N.A.; Abd Latif, N.H.; Rozuli, N.A.; Yusoff, S.B.; Gambier, F.; Brosse, N. Production of Oil Palm (Elaeis Guineensis) Fronds Lignin-Derived Non-Toxic Aldehyde for Eco-Friendly Wood Adhesive. Int. J. Biol. Macromol. 2018, 113, 1266–1272. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Wang, C.; Chu, F.; Xu, F.; Zhang, D. Synthesis of Lignin-Based Polyacid Catalyst and Its Utilization to Improve Water Resistance of Urea-Formaldehyde Resins. Polymers 2020, 12, 175. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Tarrés, Q.; Delgado-Aguilar, M.; Rodríguez, A.; Espinach, F.X.; Mutjé, P. Approaching a New Generation of Fiberboards Taking Advantage of Self Lignin as Green Adhesive. Int. J. Biol. Macromol. 2018, 108, 927–935. [Google Scholar] [CrossRef]
- Wu, H.; Liao, D.; Chen, X.; Du, G.; Li, T.; Essawy, H.; Pizzi, A.; Zhou, X. Functionalized Natural Tannins For Preparation of a Novel Non-Isocyanate Polyurea-Based Adhesive. Polym. Test. 2023, 117, 107853. [Google Scholar] [CrossRef]
- Pang, H.; Ma, C.; Shen, Y.; Sun, Y.; Li, J.; Zhang, S.; Cai, L.; Huang, Z. Novel Bionic Soy Protein-Based Adhesive with Excellent Prepressing Adhesion, Flame Retardancy, and Mildew Resistance. Acs Appl. Mater. Interfaces 2021, 13, 38732–38744. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, T.; Jiang, H.; Zhang, X.; Li, J.; Shi, S.Q.; Gao, Q. Biomimetic Lignin-Protein Adhesive with Dynamic Covalent/Hydrogen Hybrid Networks Enables High Bonding Performance and Wood-Based Panel Recycling. Int. J. Biol. Macromol. 2022, 214, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gu, J.; Tan, H.; Zhang, Y.; Huo, P. Physicochemical Properties of Starch Adhesives Enhanced by Esterification Modification with Dodecenyl Succinic Anhydride. Int. J. Biol. Macromol. 2018, 112, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, N.S.; Hashim, R.; Sulaiman, O.; Nasir, M.; Amini, M.H.M.; Hiziroglu, S. Partial Replacement of Urea-Formaldehyde with Modified Oil Palm Starch Based Adhesive to Fabricate Particleboard. Int. J. Adhes. Adhes. 2018, 84, 1–8. [Google Scholar] [CrossRef]
- Chrobak, J.; Iłowska, J.; Chrobok, A. Formaldehyde-Free Resins for the Wood-Based Panel Industry: Alternatives to Formaldehyde and Novel Hardeners. Molecules 2022, 27, 4862. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Pinkl, S.; Gindl-Altmutter, W. Application of Surface Chemical Functionalized Cellulose Nanocrystals to Improve the Performance of UF Adhesives Used in Wood Based Composites—MDF Type. Carbohydr. Polym. 2019, 206, 11–20. [Google Scholar] [CrossRef]
- Matsumae, T.; Horito, M.; Kurushima, N.; Yazaki, Y. Development of Bark-Based Adhesives for Plywood: Utilization of Flavonoid Compounds from Bark and Wood. II. J. Wood Sci. 2019, 65, 9. [Google Scholar] [CrossRef]
- Karagiannidis, E.; Markessini, C.; Athanassiadou, E. Micro-Fibrillated Cellulose in Adhesive Systems for the Production of Wood-Based Panels. Molecules 2020, 25, 4846. [Google Scholar] [CrossRef]
- Coumans, F.J.A.G.; Overchenko, Z.; Wiesfeld, J.J.; Kosinov, N.; Nakajima, K.; Hensen, E.J.M. Protection Strategies for the Conversion of Biobased Furanics to Chemical Building Blocks. ACS Sustain. Chem. Eng. 2022, 10, 3116–3130. [Google Scholar] [CrossRef]
- Moses, V.; Narula, A.; Chetan, N.; Mishra, R.K. Hydroxymethyl Furfural (HMF) a High Strength Cellulose Resin for Wood Composite Laminates. Heliyon 2022, 8, e12081. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-Phase Catalytic Processing of Biomass-Derived Oxygenated Hydrocarbons to Fuels and Chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef]
- Lai, F.; Yan, F.; Wang, P.; Li, C.; Shen, X.; Zhang, Z. Efficient Conversion of Carbohydrates and Biomass into Furan Compounds by Chitin/Ag Co-Modified H3PW12O40 Catalysts. J. Clean. Prod. 2021, 316, 128243. [Google Scholar] [CrossRef]
- Shuai, L.; Pan, X. Method for Producing Liquid Hydrocarbon Fuels Directly from Lignocellulosic Biomass. U.S. Patent No. 9,487,712, 8 November 2016. [Google Scholar]
- National Standard of the People’s Republic of China GB/T 9846; Plywood for General Use. Standardization Administration of the Peoples Republic of China: Beijing, China, 2015.
- Galbe, M.; Wallberg, O. Pretreatment for Biorefineries: A Review of Common Methods for Efficient Utilisation of Lignocellulosic Materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.; Pandey, K.K. Effect of Heat Treatment on Color Changes, Dimensional Stability, and Mechanical Properties of Wood. J. Wood Chem. Technol. 2012, 32, 304–316. [Google Scholar] [CrossRef]
- Rosso, L.; Negro, F.; Castro, G.; Cremonini, C.; Zanuttini, R. Moisture Dynamics of Thermally Treated Poplar Plywood. Eur. J. Wood Wood Prod. 2017, 75, 277–279. [Google Scholar] [CrossRef]
- Lin, W.-S.; Lee, W.-J. Influence of Curing Temperature on the Bonding Strength of Heat-Treated Plywood Made with Melamine-Urea-Formaldehyde and Phenol-Formaldehyde Resins. Eur. J. Wood Wood Prod. 2018, 76, 297–303. [Google Scholar] [CrossRef]
- Sonnenschein, M.F.; Wendt, B.L. Efficacy of Polymeric MDI/Polyol Mixtures for Binding Wood Boards. Wood Sci. Technol. 2005, 39, 27–36. [Google Scholar] [CrossRef]
- Perminova, D.A.; Malkov, V.S.; Guschin, V.; Eisenreich, N. Influence of Glyoxal on Curing of Urea-Formaldehyde Resins. Int. J. Adhes. Adhes. 2019, 92, 1–6. [Google Scholar] [CrossRef]
- Hernandez, E.D.; Bassett, A.W.; Sadler, J.M.; La Scala, J.J.; Stanzione, J.F.I. Synthesis and Characterization of Bio-Based Epoxy Resins Derived from Vanillyl Alcohol. ACS Sustain. Chem. Eng. 2016, 4, 4328–4339. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, W.; Zhao, Z.; Guo, M. Novel Whey Protein-Based Aqueous Polymer-Isocyanate Adhesive for Glulam. J. Appl. Polym. Sci. 2011, 120, 220–225. [Google Scholar] [CrossRef]
- Collett, B.M. A Review of Surface and Interfacial Adhesion in Wood Science and Related Fields. Wood Sci. Technol. 1972, 6, 1–42. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Sun, W.; Shuai, L.; Luo, X.; Liu, J. Lignocelluloses-Based Furan-Acetone Adducts as Wood Adhesives for Plywood Production. Polymers 2023, 15, 996. https://doi.org/10.3390/polym15040996
Huang L, Sun W, Shuai L, Luo X, Liu J. Lignocelluloses-Based Furan-Acetone Adducts as Wood Adhesives for Plywood Production. Polymers. 2023; 15(4):996. https://doi.org/10.3390/polym15040996
Chicago/Turabian StyleHuang, Lizhen, Wenchang Sun, Li Shuai, Xiaolin Luo, and Jing Liu. 2023. "Lignocelluloses-Based Furan-Acetone Adducts as Wood Adhesives for Plywood Production" Polymers 15, no. 4: 996. https://doi.org/10.3390/polym15040996
APA StyleHuang, L., Sun, W., Shuai, L., Luo, X., & Liu, J. (2023). Lignocelluloses-Based Furan-Acetone Adducts as Wood Adhesives for Plywood Production. Polymers, 15(4), 996. https://doi.org/10.3390/polym15040996





