High-Performance Polyamide Reverse Osmosis Membrane Containing Flexible Aliphatic Ring for Water Purification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
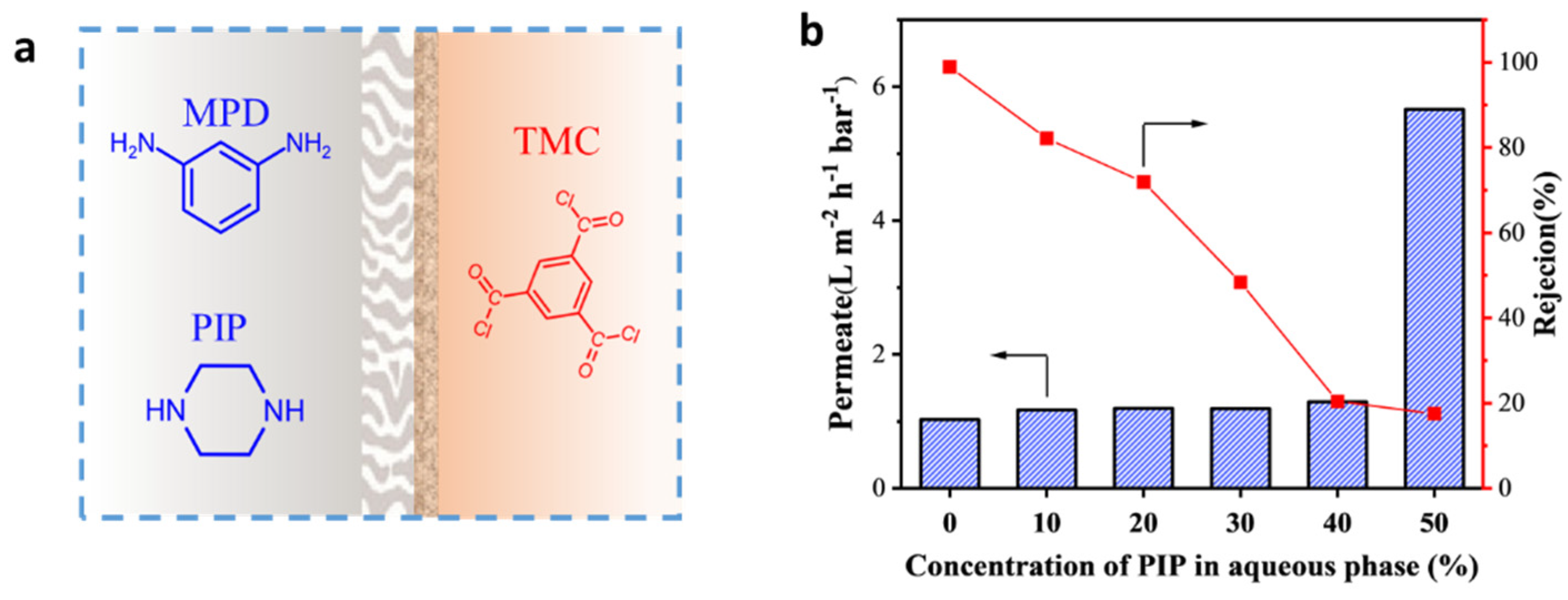
2.2. Preparation of RO Membranes
2.3. Characterization
2.4. Performance Evaluation
2.5. Molecular Simulation
3. Results and Discussion
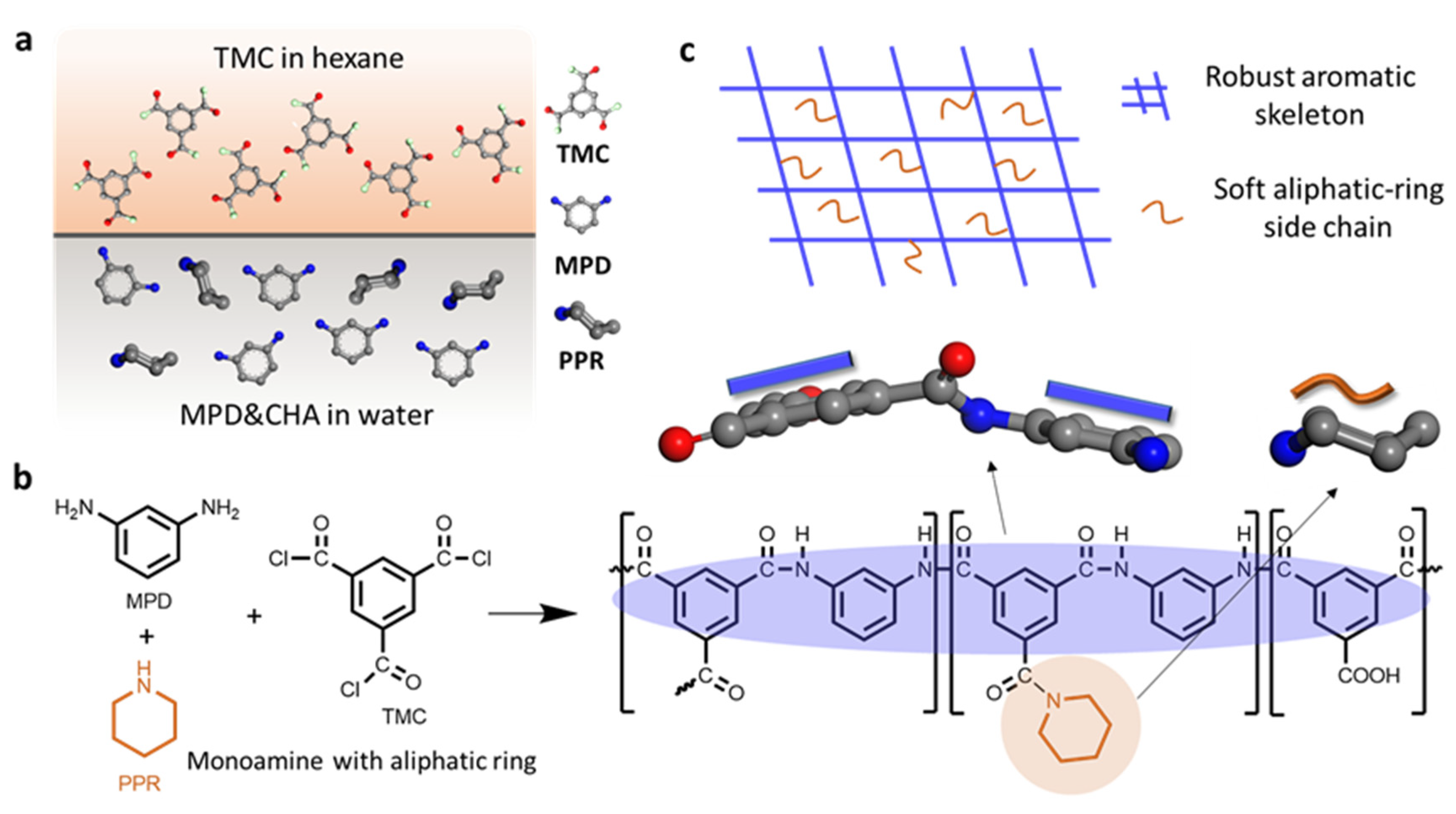
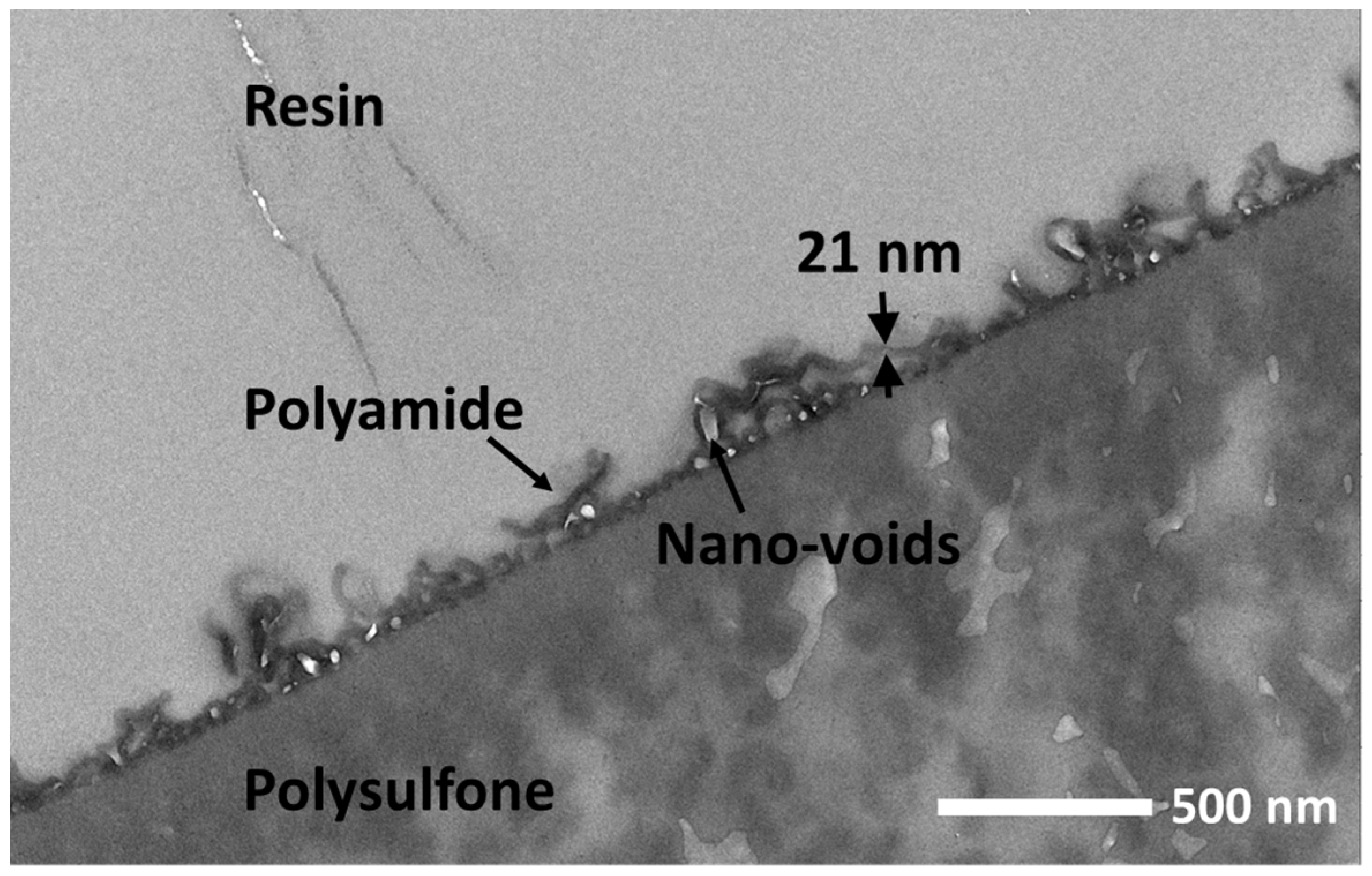
3.1. Chemical Composition and Structural Properties of the RO Membrane
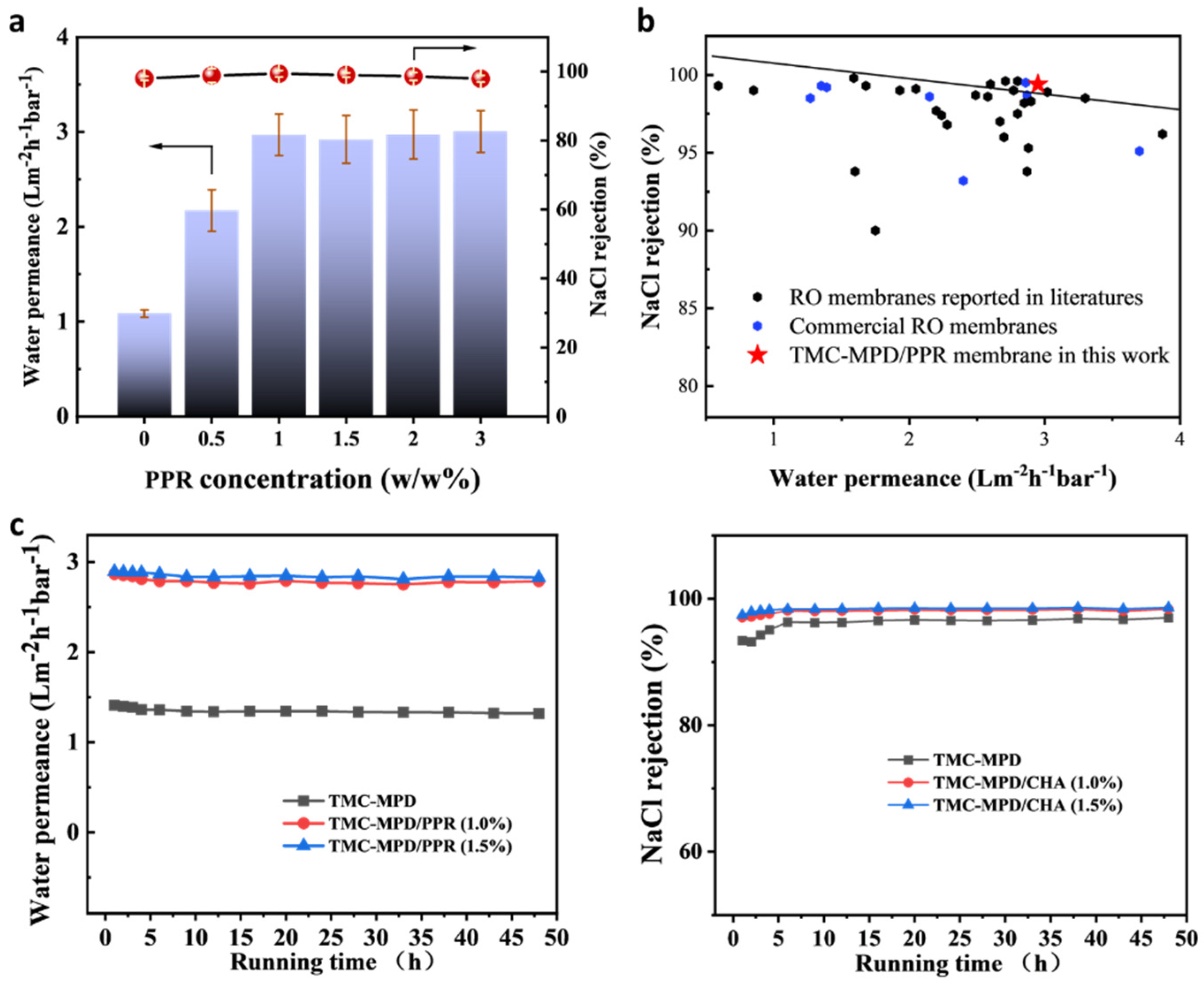
3.2. Separation Performance
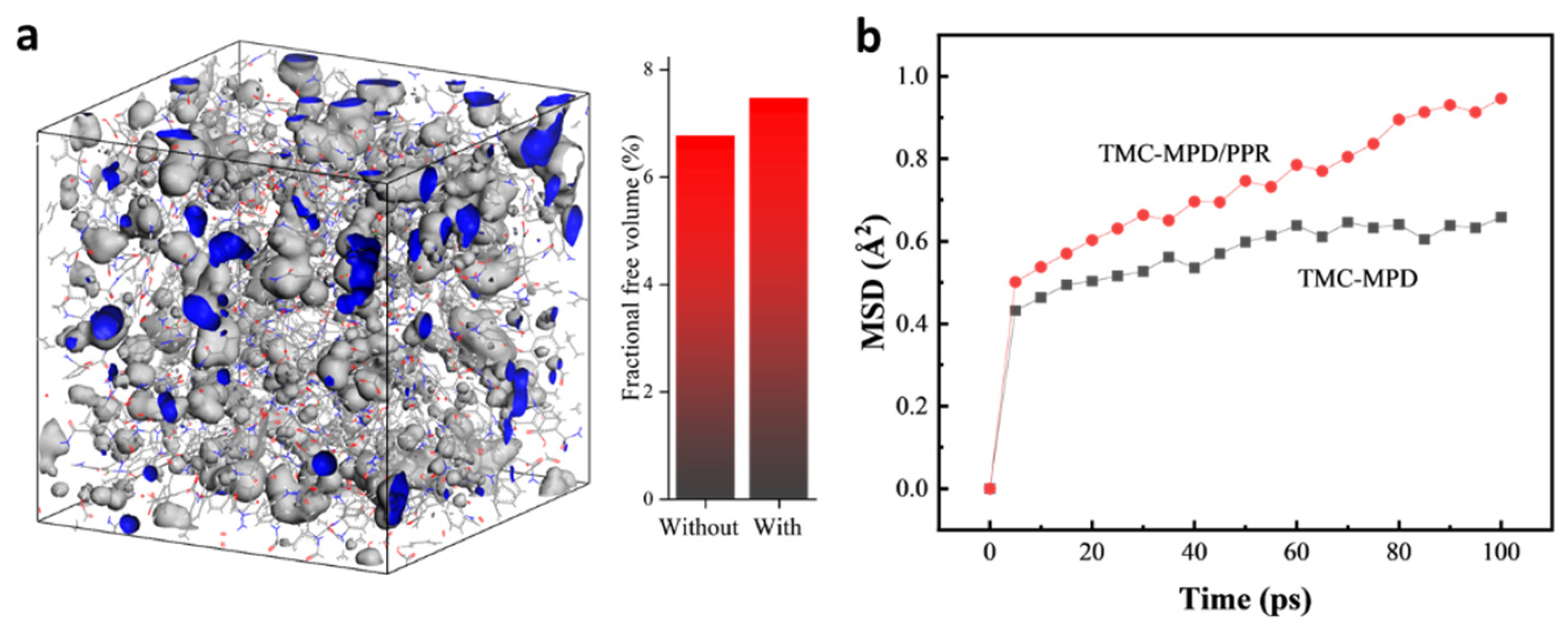
3.3. Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mueller, J.T.; Gasteyer, S. The widespread and unjust drinking water and clean water crisis in the United States. Nat. Commun. 2021, 12, 3544. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, Y.; Feng, Z.Y.; Rui, X.B.; Zhang, T.; Zhang, Z.E. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [PubMed]
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Karabelas, A.J.; Koutsou, C.P.; Kostoglou, M.; Sioutopoulos, D.C. Analysis of specific energy consumption in reverse osmosis desalination processes. Desalination 2018, 431, 15–21. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; McGovern, R.K.; Dave, S.H.; Lienhard, J.H.; Grossman, J.C. Quantifying the potential of ultra-permeable membranes for water desalination. Energ. Environ. Sci. 2014, 7, 1134–1141. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Tang, C.Y.Y. The upper bound of thin-film composite (TFC) polyamide membranes for desalination. J. Membr. Sci. 2019, 590, 117297. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef]
- Jiang, C.; Fei, Z.H.; Zhang, M.M.; Ma, X.P.; Hou, Y.F. Preparation of advanced reverse osmosis membrane by a wettability-transformable interlayer combining with N-acyl imidazole chemistry. J. Membr. Sci. 2022, 644, 120085. [Google Scholar] [CrossRef]
- Lu, X.L.; Elimelech, M. Fabrication of desalination membranes by interfacial polymerization: History, current efforts, and future directions. Chem. Soc. Rev. 2021, 50, 6290–6307. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, S.F.; Peng, X.S.; Zhang, L.; Gao, C.J. Polyamide membranes with nanoscale Turing structures for water purification. Science 2018, 360, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.; Matsuura, T. Progress in transport theory and characterization method of Reverse Osmosis (RO) membrane in past fifty years. Desalination 2018, 434, 2–11. [Google Scholar] [CrossRef]
- Paul, M.; Jons, S.D. Chemistry and fabrication of polymeric nanofiltration membranes: A review. Polymer 2016, 103, 417–456. [Google Scholar] [CrossRef]
- Ma, H.Y.; Burger, C.; Hsiao, B.S.; Chu, B. Highly Permeable Polymer Membranes Containing Directed Channels for Water Purification. Acs Macro Lett. 2012, 1, 723–726. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, X.H.; Tang, C.Y.Y. Recent development of novel membranes for desalination. Desalination 2018, 434, 37–59. [Google Scholar] [CrossRef]
- Liu, T.Y.; Liu, Z.H.; Zhang, R.X.; Wang, Y.; Van der Bruggen, B.; Wang, X.L. Fabrication of a thin film nanocomposite hollow fiber nanofiltration membrane for wastewater treatment. J. Membr. Sci. 2015, 488, 92–102. [Google Scholar] [CrossRef]
- Hu, R.R.; He, Y.J.; Zhang, C.M.; Zhang, R.J.; Li, J.; Zhu, H.W. Graphene oxide-embedded polyamide nanofiltration membranes for selective ion separation. J. Mater. Chem. A 2017, 5, 25632–25640. [Google Scholar] [CrossRef]
- Zheng, J.F.; Li, M.; Yu, K.; Hu, J.H.; Zhang, X.; Wang, L.J. Sulfonated multiwall carbon nanotubes assisted thin-film nanocomposite membrane with enhanced water flux and anti-fouling property. J. Membr. Sci. 2017, 524, 344–353. [Google Scholar] [CrossRef]
- Yang, L.B.; Wang, Z.; Zhang, J.L. Highly permeable zeolite imidazolate framework composite membranes fabricated via a chelation-assisted interfacial reaction. J. Mater. Chem. A 2017, 5, 15342–15355. [Google Scholar] [CrossRef]
- Jiang, C.; Tian, L.; Hou, Y.; Niu, Q.J. Nanofiltration membranes with enhanced microporosity and inner-pore interconnectivity for water treatment: Excellent balance between permeability and selectivity. J. Membr. Sci. 2019, 586, 192–201. [Google Scholar] [CrossRef]
- Culp, T.E.; Khara, B.; Brickey, K.P.; Geitner, M.; Zimudzi, T.J.; Wilbur, J.D.; Jons, S.D.; Roy, A.; Paul, M.; Ganapathysubramanian, B.; et al. Nanoscale control of internal inhomogeneity enhances water transport in desalination membranes. Science 2021, 371, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhang, L.P.; Li, P.; Sun, H.X.; Hou, Y.F.; Niu, Q.J. Ultrathin Film Composite Membranes Fabricated by Novel In Situ Free Interfacial Polymerization for Desalination. Acs Appl. Mater. Inter. 2020, 12, 25304–25315. [Google Scholar] [CrossRef] [PubMed]
- Karan, S.; Jiang, Z.W.; Livingston, A.G. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.H.; Yao, Z.K.; Yang, Z.; Guo, H.; Xu, Z.L.; Tang, C.Y.Y.; Elimelech, M. Nanofoaming of Polyamide Desalination Membranes to Tune Permeability and Selectivity. Environ. Sci. Technol. Lett. 2018, 5, 123–130. [Google Scholar] [CrossRef]
- Sun, Y.N.; Xue, L.X.; Zhang, Y.J.; Zhao, X.L.; Huang, Y.; Du, X.D. High flux polyamide thin film composite forward osmosis membranes prepared from porous substrates made of polysulfone and polyethersulfone blends. Desalination 2014, 336, 72–79. [Google Scholar] [CrossRef]
- Jiang, C.; Tian, L.; Zhai, Z.; Shen, Y.F.; Dong, W.J.; He, M.; Hou, Y.F.; Niu, Q.J. Thin-film composite membranes with aqueous template-induced surface nanostructures for enhanced nanofiltration. J. Membr. Sci. 2019, 589, 117244. [Google Scholar] [CrossRef]
- Jiang, C.; Ma, X.P.; Zhang, L.P.; Tian, L.; Li, P.; Hou, Y.F.; Niu, Q.J. Thin-film composite membranes with programmable in-plane heterostructure for high degree-of-freedom performance control. J. Membr. Sci. 2022, 653, 120522. [Google Scholar] [CrossRef]
- Song, X.X.; Gan, B.W.; Qi, S.R.; Guo, H.; Tang, C.Y.Y.; Zhou, Y.; Gao, C.J. Intrinsic Nanoscale Structure of Thin Film Composite Polyamide Membranes: Connectivity, Defects, and Structure-Property Correlation. Environ. Sci. Technol. 2020, 54, 3559–3569. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, Z.Y.; Jiang, L.; Fei, Z.H.; Hou, Y.F. Rapid transport of water and monovalent ions through ultrathin polyamide nanofilms for highly efficient mono/bivalent ions separation. Appl. Surf. Sci. 2023, 608, 155025. [Google Scholar] [CrossRef]
- Jiang, Z.W.; Karan, S.; Livingston, A.G. Water Transport through Ultrathin Polyamide Nanofilms Used for Reverse Osmosis. Adv. Mater. 2018, 30, e1705973. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Amani, M.; Amjad-Iranagh, S.; Golzar, K.; Sadeghi, G.M.M.; Modarress, H. Study of nanostructure characterizations and gas separation properties of poly(urethane-urea)s membranes by molecular dynamics simulation. J. Membr. Sci. 2014, 462, 28–41. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Fei, Z.; Hou, Y. High-Performance Polyamide Reverse Osmosis Membrane Containing Flexible Aliphatic Ring for Water Purification. Polymers 2023, 15, 944. https://doi.org/10.3390/polym15040944
Jiang C, Fei Z, Hou Y. High-Performance Polyamide Reverse Osmosis Membrane Containing Flexible Aliphatic Ring for Water Purification. Polymers. 2023; 15(4):944. https://doi.org/10.3390/polym15040944
Chicago/Turabian StyleJiang, Chi, Zhaohui Fei, and Yingfei Hou. 2023. "High-Performance Polyamide Reverse Osmosis Membrane Containing Flexible Aliphatic Ring for Water Purification" Polymers 15, no. 4: 944. https://doi.org/10.3390/polym15040944
APA StyleJiang, C., Fei, Z., & Hou, Y. (2023). High-Performance Polyamide Reverse Osmosis Membrane Containing Flexible Aliphatic Ring for Water Purification. Polymers, 15(4), 944. https://doi.org/10.3390/polym15040944






