Astaxanthin-Loaded Pickering Emulsions Stabilized by Nanofibrillated Cellulose: Impact on Emulsion Characteristics, Digestion Behavior, and Bioaccessibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AX-Loaded Emulsion Preparation
2.3. Gastrointestinal Tract Model (GIT Model)
2.4. Storage Stability
2.5. Microstructure
2.6. Physicochemical Properties of Emulsions
2.6.1. Determination of AX Content
2.6.2. Encapsulation Efficiency (EE)
2.6.3. Particle Size Measurements
2.6.4. ζ-Potential Measurements
2.6.5. Color Measurement
2.6.6. Viscosity Measurements
2.7. Determination of AX Content after the GIT Model
2.8. Statistical Analysis
3. Results and Discussion
3.1. Emulsion Properties after Storage for 30 Days
3.2. Influence of Emulsifier Concentrations on the Physicochemical Properties of Lipid Droplets in Gastrointestinal Fate
3.2.1. Initial Stage
3.2.2. Oral Stage
3.2.3. Gastric Stage
3.2.4. Small Intestinal Stage
3.3. Influence of Emulsifier Concentrations on Fat Digestibility
3.4. Influence of Emulsifier Concentrations on AX Bioaccessibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, Z.-Z.; Li, M.-Y.; Wang, Y.-T.; Zhu, M.-J. Astaxanthin from Phaffia Rhodozyma: Microencapsulation with Carboxymethyl Cellulose Sodium and Microcrystalline Cellulose and Effects of Microencapsulated Astaxanthin on Yogurt Properties. LWT 2018, 96, 152–160. [Google Scholar] [CrossRef]
- Martínez-Álvarez, Ó.; Calvo, M.M.; Gómez-Estaca, J. Recent Advances in Astaxanthin Micro/Nanoencapsulation to Improve Its Stability and Functionality as a Food Ingredient. Mar. Drugs 2020, 18, 406. [Google Scholar] [CrossRef] [PubMed]
- Niizawa, I.; Espinaco, B.Y.; Zorrilla, S.E.; Sihufe, G.A. Natural Astaxanthin Encapsulation: Use of Response Surface Methodology for the Design of Alginate Beads. Int. J. Biol. Macromol. 2019, 121, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxid. Med. Cell. Longev. 2019, 2019, 3849692. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.X.; Xiong, F. Astaxanthin and Its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Liu, C.; Tan, Y.; Xu, Y.; McCleiments, D.J.; Wang, D. Formation, Characterization, and Application of Chitosan/Pectin-Stabilized Multilayer Emulsions as Astaxanthin Delivery Systems. Int. J. Biol. Macromol. 2019, 140, 985–997. [Google Scholar] [CrossRef]
- Aaen, R.; Brodin, F.W.; Simon, S.; Heggset, E.B.; Syverud, K. Oil-in-Water Emulsions Stabilized by Cellulose Nanofibrils-The Effects of Ionic Strength and PH. Nanomater 2019, 9, 259. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, J.; Yang, S.; Xue, Y.; Zhang, T.; Wang, J.; Xue, C. The Effect of Various Antioxidants on the Degradation of O/W Microemulsions Containing Esterified Astaxanthins from Haematococcus Pluvialis. J. Oleo Sci. 2015, 64, 515–525. [Google Scholar] [CrossRef]
- Kale, S.N.; Deore, S.L. Emulsion Micro Emulsion and Nano Emulsion: A Review. Syst. Rev. Pharm. 2017, 8, 39–47. [Google Scholar] [CrossRef]
- Tan, C.; McClements, D.J. Application of Advanced Emulsion Technology in the Food Industry: A Review and Critical Evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef]
- Ribeiro, A.; Gonçalves, R.F.S.; Pinheiro, A.C.; Manrique, Y.A.; Barreiro, M.F.; Lopes, J.C.B.; Dias, M.M. In Vitro Digestion and Bioaccessibility Studies of Vitamin E-Loaded Nanohydroxyapatite Pickering Emulsions and Derived Fortified Foods. LWT 2022, 154, 112706. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, Z.; Wu, T. Encapsulation of β-Carotene in Oleogel-in-Water Pickering Emulsion with Improved Stability and Bioaccessibility. Int. J. Biol. Macromol. 2020, 164, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; Aberg, J.; Dai, T.; McClements, D.J. Comparison of Emulsion and Nanoemulsion Delivery Systems: The Chemical Stability of Curcumin Decreases as Oil Droplet Size Decreases. J. Agric. Food Chem. 2020, 68, 9205–9212. [Google Scholar] [CrossRef]
- Xie, L.; Ciftci, O.; Zhang, Y. Encapsulation of Astaxanthin-Enriched Camelina Oil Extract in Ovalbumin/Gum Arabic Stabilized Emulsion with/without Crosslinking by Tannic Acid. ES Food Agrofor. 2020, 1, 77–84. [Google Scholar] [CrossRef]
- Mirmazloum, I.; Ladányi, M.; Omran, M.; Papp, V.; Ronkainen, V.-P.; Pónya, Z.; Papp, I.; Némedi, E.; Kiss, A. Co-Encapsulation of Probiotic Lactobacillus Acidophilus and Reishi Medicinal Mushroom (Ganoderma Lingzhi) Extract in Moist Calcium Alginate Beads. Int. J. Biol. Macromol. 2021, 192, 461–470. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving Emulsion Formation, Stability and Performance Using Mixed Emulsifiers: A Review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Kutzli, I.; Griener, D.; Gibis, M.; Grossmann, L.; Baier, S.K.; Weiss, J. Improvement of Emulsifying Behavior of Pea Proteins as Plant-Based Emulsifiers via Maillard-Induced Glycation in Electrospun Pea Protein–Maltodextrin Fibers. Food Funct. 2020, 11, 4049–4056. [Google Scholar] [CrossRef]
- Nishizawa, N.; Kawamura, A.; Kohri, M.; Nakamura, Y.; Fujii, S. Polydopamine Particle as a Particulate Emulsifier. Polymers 2016, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering Emulsions: Preparation Processes, Key Parameters Governing Their Properties and Potential for Pharmaceutical Applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions Principles, Practices, and Techniques; CRC Press: Boca Raton, FL, USA, 2005; Volume 53, ISBN 9788578110796. [Google Scholar]
- Perumal, A.B.; Nambiar, R.B.; Moses, J.A.; Anandharamakrishnan, C. Nanocellulose: Recent Trends and Applications in the Food Industry. Food Hydrocoll. 2022, 127, 107484. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Ravishankar, K.; Dhamodharan, R. Preparation of Nanofibrillated Cellulose and Nanocrystalline Cellulose from Surgical Cotton and Cellulose Pulp in Hot-Glycerol Medium. Cellulose 2019, 26, 3127–3141. [Google Scholar] [CrossRef]
- Parés, D.; Pèlach, M.À.; Toldrà, M.; Saguer, E.; Tarrés, Q.; Carretero, C. Nanofibrillated Cellulose as Functional Ingredient in Emulsion-Type Meat Products. Food Bioprocess Technol. 2018, 11, 1393–1401. [Google Scholar] [CrossRef]
- Zhu, M.; Huan, S.; Liu, S.; Li, Z.; He, M.; Yang, G.; Liu, S.; McClements, D.J.; Rojas, O.J.; Bai, L. Recent Development in Food Emulsion Stabilized by Plant-Based Cellulose Nanoparticles. Curr. Opin. Colloid Interface Sci. 2021, 56, 101512. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Ilyin, S.O. Morphology and Rheology of Heavy Crude Oil/Water Emulsions Stabilized by Microfibrillated Cellulose. Energy Fuels 2021, 35, 6527–6540. [Google Scholar] [CrossRef]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Suspension Containing Microfibrillated Cellulose. U.S. Patent No. 4,487,634, 11 December 1984. [Google Scholar]
- Fujisawa, S.; Togawa, E.; Kuroda, K. Nanocellulose-Stabilized Pickering Emulsions and Their Applications. Sci. Technol. Adv. Mater. 2017, 18, 959–971. [Google Scholar] [CrossRef]
- Mitbumrung, W.; Suphantharika, M.; McClements, D.J.; Winuprasith, T. Encapsulation of Vitamin D3 in Pickering Emulsion Stabilized by Nanofibrillated Mangosteen Cellulose: Effect of Environmental Stresses. J. Food Sci. 2019, 84, 3213–3221. [Google Scholar] [CrossRef]
- Winuprasith, T.; Khomein, P.; Mitbumrung, W.; Suphantharika, M.; Nitithamyong, A.; McClements, D.J. Encapsulation of Vitamin D3 in Pickering Emulsions Stabilized by Nanofibrillated Mangosteen Cellulose: Impact on In Vitro Digestion and Bioaccessibility. Food Hydrocoll. 2018, 83, 153–164. [Google Scholar] [CrossRef]
- Rungraung, N.; Jain, S.; Mitbumrung, W.; Khomein, P.; Suphantharika, M.; McClements, D.J.; Winuprasith, T. Controlling the in Vitro Gastrointestinal Digestion of Emulsified Lipids by Encapsulation within Nanocellulose-Fortified Alginate Beads. Food Struct. 2022, 32, 100266. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Winuprasith, T.; Suphantharika, M. Properties and Stability of Oil-in-Water Emulsions Stabilized by Microfibrillated Cellulose from Mangosteen Rind. Food Hydrocoll. 2015, 43, 690–699. [Google Scholar] [CrossRef]
- Lv, S.; Zhou, H.; Bai, L.; Rojas, O.J.; McClements, D.J. Development of Food-Grade Pickering Emulsions Stabilized by a Mixture of Cellulose Nanofibrils and Nanochitin. Food Hydrocoll. 2021, 113, 106451. [Google Scholar] [CrossRef]
- Joseph, E.; Singhvi, G. Chapter 4—Multifunctional Nanocrystals for Cancer Therapy: A Potential Nanocarrier. In Nanomaterials for Drug Delivery and Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 91–116. ISBN 978-0-12-816505-8. [Google Scholar]
- Winuprasith, T.; Suphantharika, M. Microfibrillated Cellulose from Mangosteen (Garcinia mangostana L.) Rind: Preparation, Characterization, and Evaluation as an Emulsion Stabilizer. Food Hydrocoll. 2013, 32, 383–394. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Z.-C.; Deng, Y.-Y.; Dong, H.; Zhang, Y.; Tang, X.-J.; Li, P.; Liu, G.; Zhang, M.-W. Comparison of the Effects of Different Food-Grade Emulsifiers on the Properties and Stability of a Casein-Maltodextrin-Soybean Oil Compound Emulsion. Molecules 2020, 25, 458. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar, A.C.; de Paula, J.T.; Mundo, J.L.M.; Martínez, J.; McClements, D.J. Influence of Type of Natural Emulsifier and Microfluidization Conditions on Capsicum Oleoresin Nanoemulsions Properties and Stability. J. Food Process Eng. 2021, 44, e13660. [Google Scholar] [CrossRef]
- Schvartz, T.; Shoseyov, O. Defibrillated Microcrystalline Cellulose as an Efficient Emulsion Stabilizer—Study of Food-Grade Pickering Emulsions Resistant to Extreme Conditions. LWT 2022, 155, 113006. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Gorbacheva, S.N.; Yadykova, A.Y. Rheology and Tribology of Nanocellulose-Based Biodegradable Greases: Wear and Friction Protection Mechanisms of Cellulose Microfibrils. Tribol. Int. 2023, 178, 108080. [Google Scholar] [CrossRef]
- Xhanari, K.; Syverud, K.; Chinga-Carrasco, G.; Paso, K.; Stenius, P. Structure of Nanofibrillated Cellulose Layers at the o/w Interface. J. Colloid Interface Sci. 2011, 356, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Winuprasith, T. Properties and Stability of Tween 20−stabilized Emulsions Containing Nanocellulose. Food Appl. Biosci. J. 2021, 9, 28–41. [Google Scholar]
- Paukkonen, H.; Ukkonen, A.; Szilvay, G.; Yliperttula, M.; Laaksonen, T. Hydrophobin-Nanofibrillated Cellulose Stabilized Emulsions for Encapsulation and Release of BCS Class II Drugs. Eur. J. Pharm. Sci. 2017, 100, 238–248. [Google Scholar] [CrossRef]
- Jain, S.; Winuprasith, T.; Suphantharika, M. Encapsulation of Lycopene in Emulsions and Hydrogel Beads Using Dual Modified Rice Starch: Characterization, Stability Analysis and Release Behaviour during in-Vitro Digestion. Food Hydrocoll. 2020, 104, 105730. [Google Scholar] [CrossRef]
- Costa, C.; Medronho, B.; Filipe, A.; Mira, I.; Lindman, B.; Edlund, H.; Norgren, M. Emulsion Formation and Stabilization by Biomolecules: The Leading Role of Cellulose. Polymers 2019, 11, 1570. [Google Scholar] [CrossRef]
- Qi, J.; Song, L.; Zeng, W.; Liao, J. Citrus Fiber for the Stabilization of O/W Emulsion through Combination of Pickering Effect and Fiber-Based Network. Food Chem. 2021, 343, 128523. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, J.; Ge, L.; Xie, W.; Wu, D. Pickering Emulsion Stabilized with Fibrous Nanocelluloses: Insight into Fiber Flexibility-Emulsifying Capacity Relations. Carbohydr. Polym. 2021, 255, 117483. [Google Scholar] [CrossRef] [PubMed]
- Mitbumrung, W.; Rungraung, N.; Muangpracha, N.; Akanitkul, P.; Winuprasith, T. Approaches for Extracting Nanofibrillated Cellulose from Oat Bran and Its Emulsion Capacity and Stability. Polymers 2022, 14., 327. [Google Scholar] [CrossRef]
- Chantrapornchai, W.; Clydesdale, F.; McClements, D.J. Theoretical and Experimental Study of Spectral Reflectance and Color of Concentrated Oil-in-Water Emulsions. J. Colloid Interface Sci. 1999, 218, 324–330. [Google Scholar] [CrossRef]
- Fitri, I.A.; Mitbumrung, W.; Akanitkul, P.; Rungraung, N.; Kemsawasd, V.; Jain, S.; Winuprasith, T. Encapsulation of β-Carotene in Oil-in-Water Emulsions Containing Nanocellulose: Impact on Emulsion Properties, In Vitro Digestion, and Bioaccessibility. Polymers 2022, 14, 1414. [Google Scholar]
- Zheng, B.; Zhang, Z.; Chen, F.; Luo, X.; McClements, D.J. Impact of Delivery System Type on Curcumin Stability: Comparison of Curcumin Degradation in Aqueous Solutions, Emulsions, and Hydrogel Beads. Food Hydrocoll. 2017, 71, 187–197. [Google Scholar] [CrossRef]
- Sarkar, A.; Zhang, S.; Holmes, M.; Ettelaie, R. Colloidal Aspects of Digestion of Pickering Emulsions: Experiments and Theoretical Models of Lipid Digestion Kinetics. Adv. Colloid Interface Sci. 2019, 263, 195–211. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zhang, H.; Decker, E.A.; McClements, D.J. Influence of Lipid Type on Gastrointestinal Fate of Oil-in-Water Emulsions: In Vitro Digestion Study. Food Res. Int. 2015, 75, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R.; McClements, D.J. Encapsulation of β-Carotene in Alginate-Based Hydrogel Beads: Impact on Physicochemical Stability and Bioaccessibility. Food Hydrocoll. 2016, 61, 1–10. [Google Scholar] [CrossRef]
- Dima, C.; Dima, S. Bioaccessibility Study of Calcium and Vitamin D3 Co-Microencapsulated in Water-in-Oil-in-Water Double Emulsions. Food Chem. 2020, 303, 125416. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kerr, W.L.; Kong, F. Characterization of Lipid Emulsions during in Vitro Digestion in the Presence of Three Types of Nanocellulose. J. Colloid Interface Sci. 2019, 545, 317–329. [Google Scholar] [CrossRef] [PubMed]
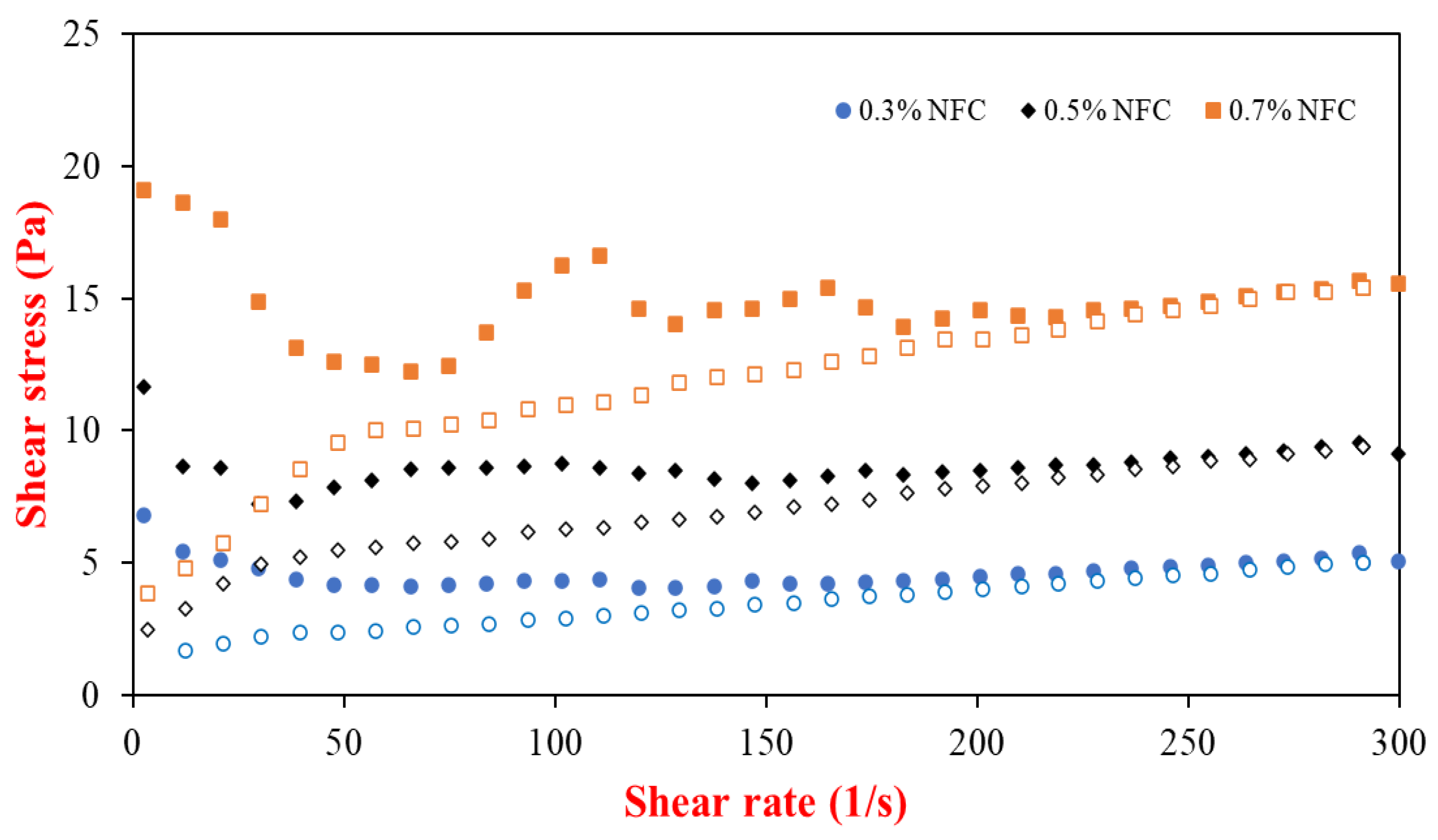

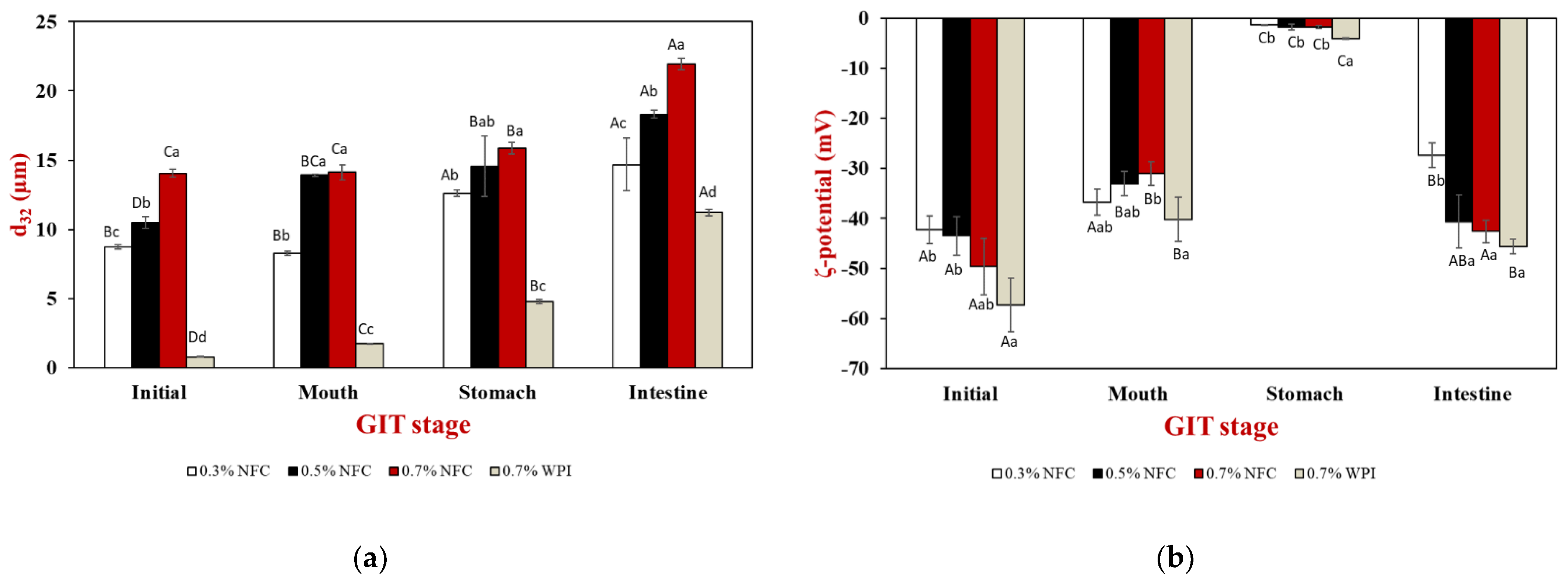
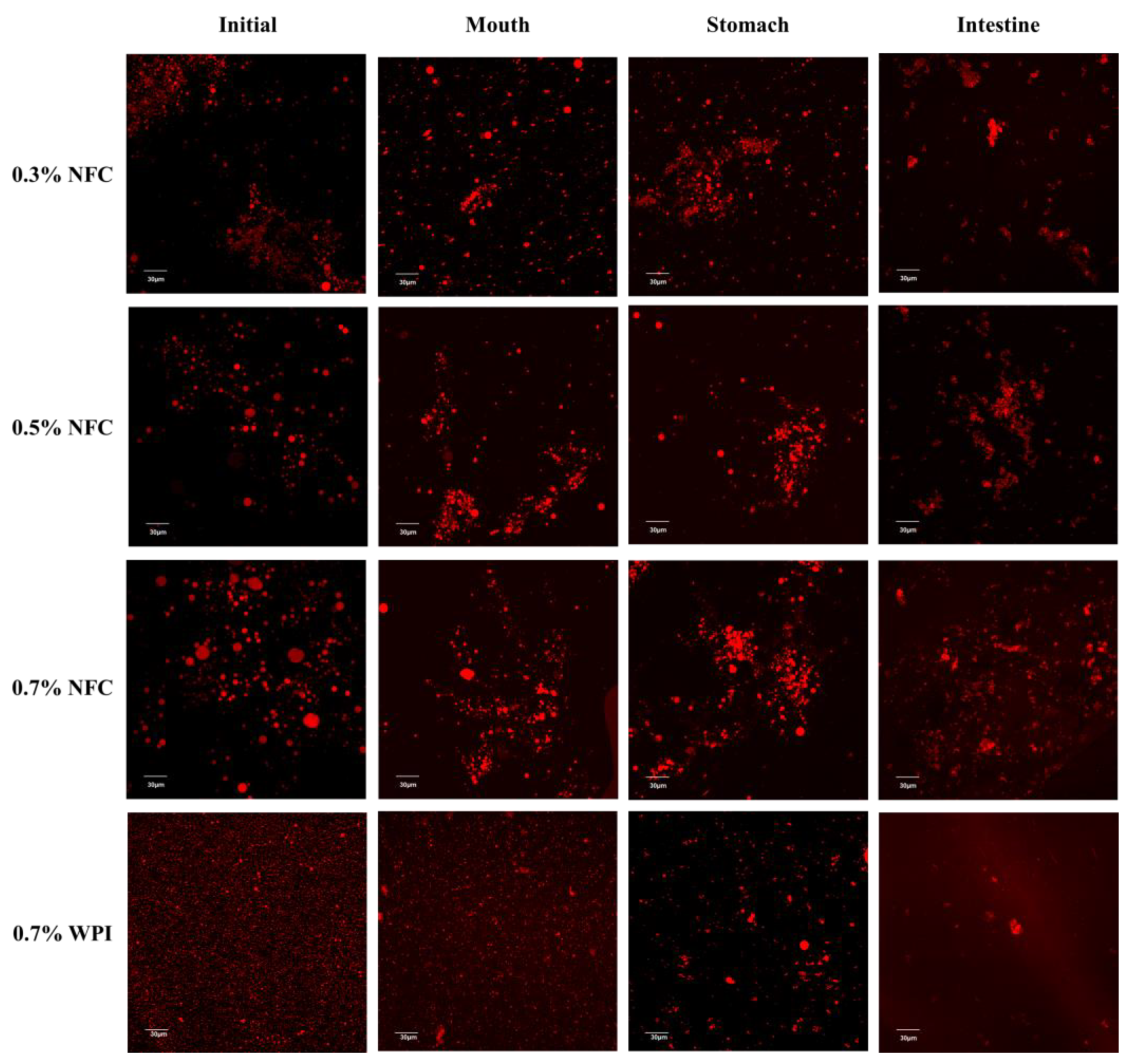


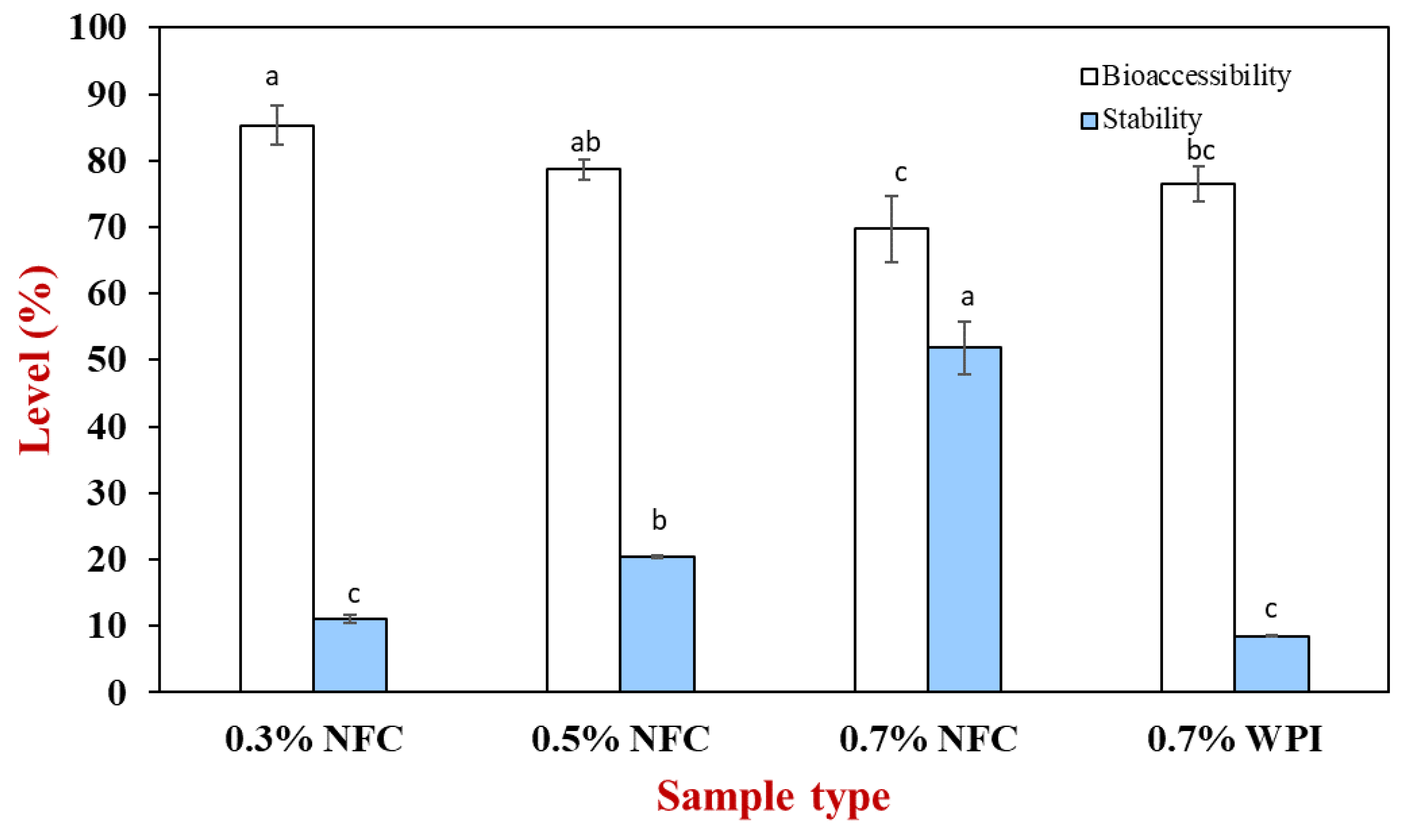
| NFC Concentration (%) | d32 (µm) | ζ–Potential (mV) | ηa,100 (mPa.s) | Encapsulation Efficacy (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 30 | Day 1 | Day 30 | Day 1 | Day 30 | Day 1 | Day 30 | |
| 0.3 | 12.65 ± 0.69 a,A | 13.59 ± 1.06 a,A | −21.88 ± 3.35 b,A | –25.92 ± 2.85 a,B | 41 ± 0.003 a,C | 42 ± 0.001 a,C | 56.33 ± 0.08 a,B | 24.61 ± 0.01 b,C |
| 0.5 | 13.44 ± 0.01 a,A | 14.31 ± 0.70 a,A | −20.58 ± 4.04 b,A | –28.48 ± 3.24 a,B | 84 ± 0.004 b,B | 96 ± 0.008 a,B | 53.75 ± 0.08 a,C | 42.60 ± 0.05 b,B |
| 0.7 | 14.40 ± 1.37 a,A | 14.41 ± 1.48 a,A | –25.58 ± 4.35 b,A | –40.33 ± 3.97 a,A | 158 ± 0.006 a,A | 132 ± 0.017 a,A | 70.94 ± 0.01 a,A | 47.71 ± 0.05 b,A |
| NFC Concentration (%) | Color | |||||||
|---|---|---|---|---|---|---|---|---|
| L* (Lightness) | a* (Redness) | b* (Yellowness) | ΔE (Total Color Change) | |||||
| Day 1 | Day 30 | Day 1 | Day 30 | Day 1 | Day 30 | Day 1 | Day 30 | |
| 0.3 | 54.20 ± 0.17 a,B | 52.63 ± 0.12 b,C | 9.03 ± 0.12 a,B | 7.47 ± 0.06 b,AB | 6.17 ± 0.15 a,A | 5.43 ± 0.06 b,A | – | 2.35 ± 0.13 b,A |
| 0.5 | 56.77 ± 0.06 a,A | 56.20 ± 0.01 b,A | 9.10 ± 0.01 a,AB | 7.50 ± 0.01 b,A | 6.00 ± 0.01 a,A | 5.40 ± 0.01 b,A | – | 1.80 ± 0.02 b,B |
| 0.7 | 54.40 ± 0.10 a,B | 53.80 ± 0.01 b,B | 9.23 ± 0.06 a,A | 7.37 ± 0.06 b,B | 5.57 ± 0.06 a,B | 4.43 ± 0.06 b,B | – | 2.27 ± 0.12 b,A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saechio, S.; Akanitkul, P.; Thiyajai, P.; Jain, S.; Tangsuphoom, N.; Suphantharika, M.; Winuprasith, T. Astaxanthin-Loaded Pickering Emulsions Stabilized by Nanofibrillated Cellulose: Impact on Emulsion Characteristics, Digestion Behavior, and Bioaccessibility. Polymers 2023, 15, 901. https://doi.org/10.3390/polym15040901
Saechio S, Akanitkul P, Thiyajai P, Jain S, Tangsuphoom N, Suphantharika M, Winuprasith T. Astaxanthin-Loaded Pickering Emulsions Stabilized by Nanofibrillated Cellulose: Impact on Emulsion Characteristics, Digestion Behavior, and Bioaccessibility. Polymers. 2023; 15(4):901. https://doi.org/10.3390/polym15040901
Chicago/Turabian StyleSaechio, Supaporn, Ploypailin Akanitkul, Parunya Thiyajai, Surangna Jain, Nattapol Tangsuphoom, Manop Suphantharika, and Thunnalin Winuprasith. 2023. "Astaxanthin-Loaded Pickering Emulsions Stabilized by Nanofibrillated Cellulose: Impact on Emulsion Characteristics, Digestion Behavior, and Bioaccessibility" Polymers 15, no. 4: 901. https://doi.org/10.3390/polym15040901
APA StyleSaechio, S., Akanitkul, P., Thiyajai, P., Jain, S., Tangsuphoom, N., Suphantharika, M., & Winuprasith, T. (2023). Astaxanthin-Loaded Pickering Emulsions Stabilized by Nanofibrillated Cellulose: Impact on Emulsion Characteristics, Digestion Behavior, and Bioaccessibility. Polymers, 15(4), 901. https://doi.org/10.3390/polym15040901








