The Influence of Starch Sweeteners on Functional Properties of Cellulose Fat Mimetics: Rheological and Textural Aspects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fat Mimetics
2.3. Preparation of Mixture of Fat Mimetics and Starch Sweeteners
2.4. Rheological Determination
2.5. Textural Determination
2.6. Statistical Analysis
3. Results and Discussion
3.1. Rheological Properties of MCG Fat Mimetics
3.2. Rheological Properties of Mixtures of MCG Fat Mimetics and Starch Sweeteners
3.2.1. Flow Properties of Mixtures of MCG Fat Mimetics and Starch Sweeteners
3.2.2. Viscoelastic Properties of Mixtures of MCG Fat Mimetics and Starch Sweeteners
3.2.3. Creep and Recovery Curves of Mixtures of MCG Fat Mimetics and Starch Sweeteners
3.3. Textural Properties of the Mixtures of MCG Fat Mimetics and Starch Sweeteners
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, A.R.; Nicholson, R.A.; Marangoni, A.G. Applications of fat mimetics for the replacement of saturated and hydrogenated fat in food products. Curr. Opin. Food Sci. 2020, 33, 61–68. [Google Scholar] [CrossRef]
- Akoh, C.C. Fat replacer. Food Technol. 1998, 52, 47–52. [Google Scholar]
- Cho, S.S.; Prosky, L. Application of complex carbohydrates to food products fat mimetics. In Complex Carbohydrates in Food; Cho, S.S., Prosky, L., Dreher, M.L., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1999; pp. 411–429. [Google Scholar]
- Ognen, C.F.; Darie, N.; Ognean, M. Fat Replacers. J. Agroaliment. Process Technol. 2006, 12, 433–442. [Google Scholar]
- Borderias, A.J.; Sanchez–Alonso, I.; Perez–Mateos, M. New Applications of Fibres in Foods: Addition to Fishery products. Trends Food Sci. Tech. 2005, 16, 458–465. [Google Scholar] [CrossRef]
- Dreher, M. Food Sources and Uses of Dietary Fiber. In Complex Carbohydrates in Foods; Cho, S.S., Prosky, L., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1999; pp. 327–371. [Google Scholar]
- Humphreys, W.M. Fiber–Based Fat Mimetics: Microcrystalline Cellulose. In Handbook of Fat Replacers; Chapter 7A; Roller, S., Ed.; CRC Press: Boca Raton, FL, USA, 1996; pp. 131–144. [Google Scholar]
- Marangoni, A.G.; Van Duynhoven, J.P.; Acevedo, N.C.; Nicholson, R.A.; Patel, A.R. Advances in our understanding of the structure and functionality of edible fats and fat mimetics. Soft Matter 2020, 16, 289–306. [Google Scholar] [CrossRef]
- Glisic, M.; Cabrol, M.B.; Baltić, M.Z.; Maksimovic, Z. Cellulose hydrocolloids in meat products: Current status and challenges in developing functional food. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 854, p. 012030. [Google Scholar] [CrossRef]
- BeMiller, J.N. Carbohydrate Chemistry for Food Scientists; AACC International: Eagan, MN, USA, 2007; pp. 222–243. [Google Scholar]
- O’Brien, N. Overview. In Alternative Sweeteners, 3rd, ed.; O’Brien Nabors, L., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Castro-Muñoz, R.; Correa-Delgado, M.; Córdova-Almeida, R.; Lara-Nava, D.; Chávez-Muñoz, M.; Velásquez-Chávez, V.F.; Ahmad, M.Z. Natural sweeteners: Sources, extraction and current uses in foods and food industries. Food Chem. 2022, 370, 130991. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Sweeteners as food additives in the XXI century: A review of what is known, and what is to come. Food Chem. Toxicol. 2017, 107, 302–317. [Google Scholar] [CrossRef]
- Edwards, C.H.; Rossi, M.; Corpe, C.P.; Butterworth, P.J. The role of sugars and sweeteners in food, diet and health: Alternatives for the future. Trends Food Sci. Technol. 2016, 56, 158–166. [Google Scholar] [CrossRef]
- Mora, M.R.; Dando, R. The sensory properties and metabolic impact of natural and synthetic sweeteners. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1554–1583. [Google Scholar] [CrossRef]
- Hobbs, L. Sweeteners from starch: Production, properties and uses. In Starch; Academic Press: Cambridge, MA, USA, 2009; pp. 797–832. [Google Scholar]
- Blanchard, P.H.; Katz, F.R. Starch Hydrolysates. In Food Polysaccharides and Their Applications, 2nd ed.; Stephen, A.M., Phillips, G.O., Williams, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- White, J.S. Sucrose, HFCS, and Fructose: History, Manufacture, Composition, Applications, and Production, Chapter 2. In Fructose, High Fructose Corn Syrup, Sucrose and Health; Humana Press: New York, NY, USA, 2014; pp. 13–33. [Google Scholar]
- Khorshidian, N.; Shadnoush, M.; Zabihzadeh Khajavi, M.; Sohrabvandi, S.; Yousefi, M.; Mortazavian, A.M. Fructose and high fructose corn syrup: Are they a two-edged sword? Int. J. Food Sci. Nutr. 2021, 72, 592–614. [Google Scholar] [CrossRef]
- Osberger, T.F.; Linn, H.R. Pure fructose and its applications in reduced-calorie foods. In Low Calorie and Special Dietary Foods; CRC Press: Boca Raton, FL, USA, 2018; pp. 115–124. [Google Scholar]
- Mauer, L.J. Deliquescence of crystalline materials: Mechanism and implications for foods. Curr. Opin. Food Sci. 2022, 46, 100865. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Chen, M.; Goff, H.D.; Zhong, F.; Sharif, H.R.; Li, Y. Functionality and nutritional aspects of microcrystalline cellulose in food. Carbohyd Polym. 2017, 172, 159–174. [Google Scholar] [CrossRef]
- Krawczyk, G.; Venables, A.; Tuason, D. Microcrystalline cellulose. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 740–759. [Google Scholar]
- Rudrarajua, V.S.; Wyandtb, C.M. Rheological characterization of Microcrystalline Cellulose/Sodiumcarboxymethyl cellulose hydrogels using a controlled stress rheometer: Part I. Int. J. Pharm. 2005, 292, 53–61. [Google Scholar] [CrossRef]
- Nikolić, I.; Dokić, L.; Rakić, D.; Tomović, V.; Maravić, N.; Vidosavljević, S.; Šereš, Z.; Šoronja-Simović, D. The role of two types of continuous phases based on cellulose during textural, color, and sensory characterization of novel food spread with pumpkin seed flour. J. Food Process Preserv. 2018, 42, e13684. [Google Scholar] [CrossRef]
- Mezger, T. The Rheology Handbook: For Users of Rotational and Oscillation Rheometers; Vincentz Verlag: Hannover, Germany, 2002. [Google Scholar]
- Steffe, F.J. Rheological Methods in Food Process Engineering, 2nd ed; Freeman Press: East Lansing, MI, USA, 1996. [Google Scholar]
- Sozer, N. Rheological properties of rice pasta dough supplemented with proteins and gums. Food Hydrocolloid 2009, 23, 849–855. [Google Scholar] [CrossRef]
- Vithanage, C.R.; Grimson, M.J.; Smith, B.G. The effect of temperature on the rheology of butter, a spreadable blend and spreads. J. Texture Stud. 2009, 40, 346–369. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C. Gluten–free doughs: Rheological properties, testing procedures–methods and potential problems. In Gluten–Free Food Science and Technology; Gallagher, E., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2009; pp. 52–83. [Google Scholar]
- Selaković, A.; Nikolić, I.; Dokić, L.; Šoronja-Simović, D.; Šimurina, O.; Zahorec, J.; Šereš, Z. Enhancing rheological performance of laminated dough with whole wheat flour by vital gluten addition. LWT Food Sci. Technol. 2021, 138, 110604. [Google Scholar] [CrossRef]
- Angioloni, A.; Collar, C. Small and large deformation viscoelastic behaviour of selected fibre blends with gelling properties. Food Hydrocolloid 2009, 23, 742–748. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture, Viscosity and Food. In Food Texture and Viscosity: Concept and Measurement; Chapter 1; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Hedayati, S.; Shahidi, F.; Koocheki, A.; Farahnaky, A.; Majzoobi, M. Functional properties of granular cold-water swelling maize starch: Effect of sucrose and glucose. Int. J. Food Sci. Technol. 2016, 51, 2416–2423. [Google Scholar] [CrossRef]
- Anderson, M.J.; Whitcomb, P.J. DOE Simplified: Practical Tools for Effective Experimentation, 2nd ed.; Productivity Press: New York, NY, USA, 2007. [Google Scholar]
- Gómez, M.; Moraleta, A.; Oliete, B. Effect of fibre size on the quality of fibre–enriched layer cakes. LWT Food Sci. Technol. 2010, 43, 33–38. [Google Scholar] [CrossRef]
- Segura, J.A.; Herrera, M.L.; Añón, M.C. Margarines: A rheological study. J. Am. Oil Chem. Soc. 1995, 72, 375–378. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Yuan, R. Applications of natural polymer-based hydrogels in the food industry. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 357–410. [Google Scholar]
- Tadros, T. Application of rheology for assessment and prediction of the long–term physical stability of emulsions. Adv. Colloid. Interfac. 2004, 108–109, 227–258. [Google Scholar] [CrossRef]
- Luckham, P.F.; Ukeje, M.A. Effect of Particle Size Distribution on the Rheology of Dispersed Systems. J. Colloid. Interf. Sci. 1999, 220, 347–356. [Google Scholar] [CrossRef]
- Hoefler, C.A. Hydrocolloids; Eagan Press Handbook Series; Eagan Press: Eagan, MN, USA, 2004. [Google Scholar]
- Buck, A.W. High Fructose Corn Syrup. In Alternative Sweeteners, 3rd ed.; O’Brien Nabors, L., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2001. [Google Scholar]
- White, J.S.; Osberger, T.F. Crystalline Fructose. In Alternative Sweeteners, 3rd ed.; O’Brien Nabors, L., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Onyango, C.; Unbehend, G.; Lindhauer, M.G. Effect of cellulose–derivatives and emulsifiers on creep–recovery and crumb properties of gluten–free bread prepared from sorghum and gelatinized cassava starch. Food Res. Int. 2009, 42, 949–955. [Google Scholar] [CrossRef]
- TA.HD. Texture Analyser Product Specification; Stable Micro Systems, Ltd.: Godalming, UK, 2004. [Google Scholar]
- Roopa, B.S.; Bhattacharya, S. Alginate gels: I. Characterization of textural attributes. J. Food Eng. 2008, 85, 123–131. [Google Scholar] [CrossRef]
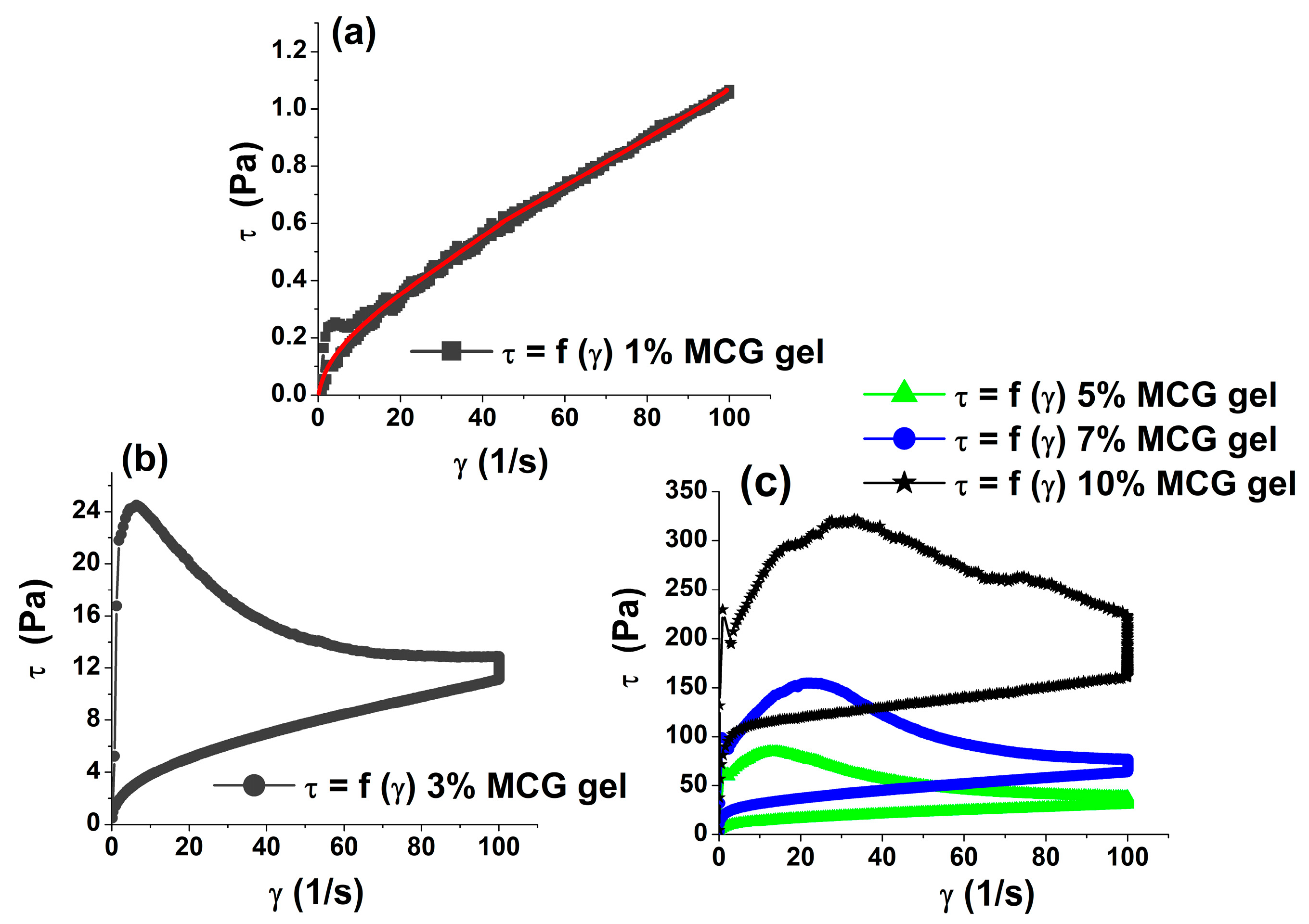

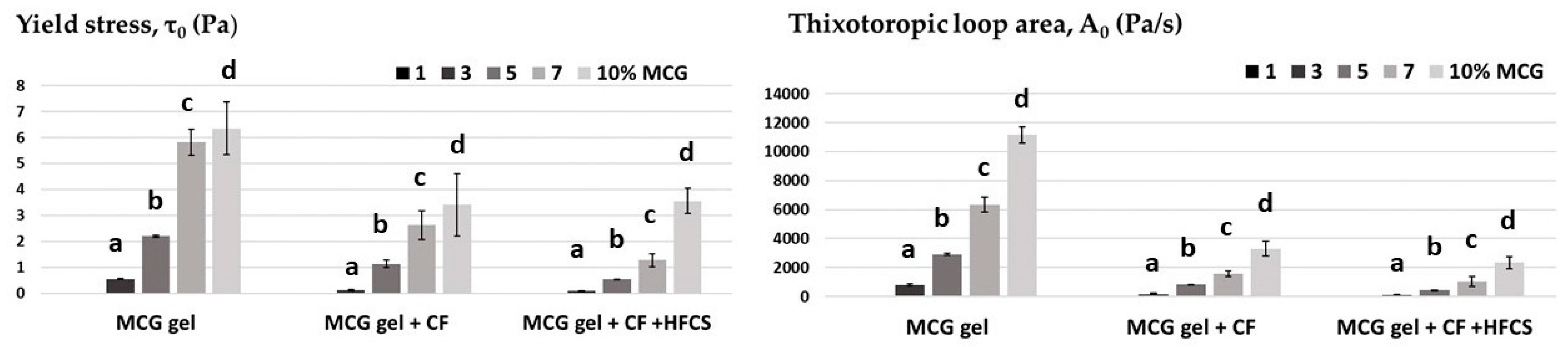
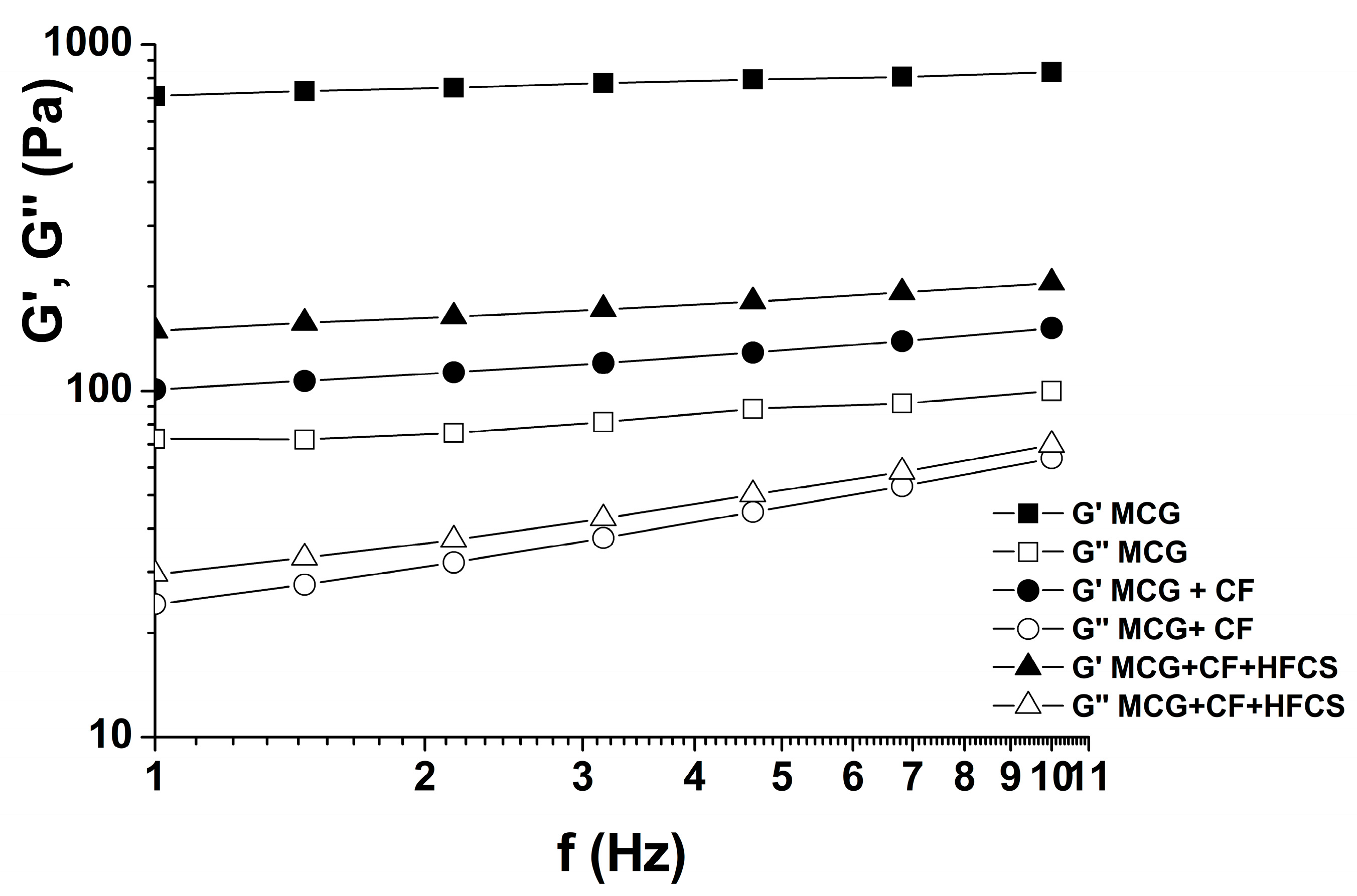
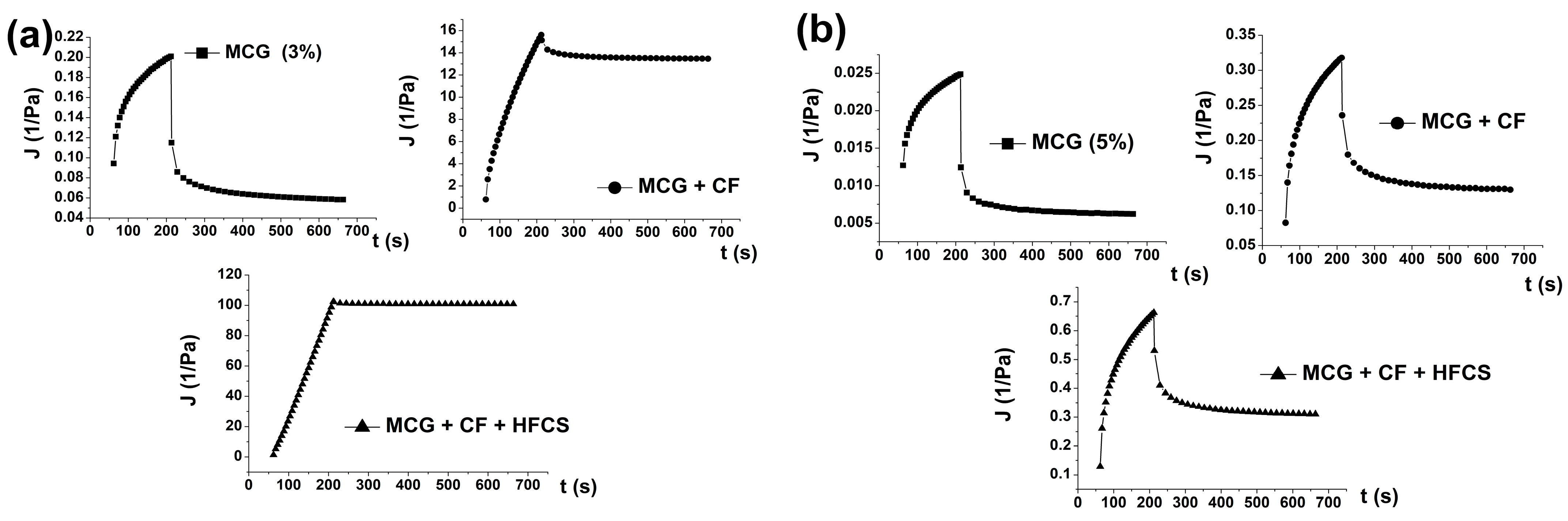

| MCG Fat Mimetics Concentration % | Yield Stress, τ0 (Pa) * | Thixotropic Loop Area, A0 (Pa/s) * | Tan δ *, G″/G′ |
|---|---|---|---|
| 1 | 0.033 ± 0.01 a | 23.16 ± 4.42 a | 0.656 ± 0.065 e |
| 3 | 0.546 ± 0.05 b | 784.00 ± 98.85 b | 0.263 ± 0.010 d |
| 5 | 2.195 ± 0.01 c | 2909.00 ± 62.45 c | 0.140 ± 0.008 c |
| 7 | 5.521 ± 1.42 d | 6037.33 ± 509.56 d | 0.124 ± 0.003 b |
| 10 | 7.203 ± 0.50 e | 6717.33 ± 567.71 e | 0.107 ±0.006 a |
| Samples | MCG Fat Mimetic Concentration (%) | ||||
|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 10 | |
| MCG gel | 0.556 ± 0.065 a | 0.263 ± 0.021 a | 0.144 ± 0.008 a | 0.124 ± 0.003 a | 0.107 ± 0.006 a |
| MCG + CF | 0.934 ± 0.011 b | 0.672 ± 0.016 b | 0.453 ± 0.006 b | 0.320 ± 0.002 b | 0.139 ± 0.006 b |
| MCG + CF + HFCS | 0.961 ± 0.013 b | 0.804 ± 0.018 c | 0.585 ± 0.001 c | 0.458 ± 0.009 c | 0.299 ± 0.006 c |
| Sample of Fat Mimetics | Creep Phase | |||||
|---|---|---|---|---|---|---|
| J0 10−2 (1/Pa) | J1 10−2 (1/Pa) | η0 102 (Pas) | λ1 (s) | Jmax 10−2 (1/Pa) | R2 | |
| 3% MCG | 9.25 | 6.45 | 10.88 | 92.34 | 19.55 | 0.7970 |
| 3% MCG + CF | 82.02 | 32.25 | 0.12 | 92.44 | 1734 | 0.6955 |
| 3% MCG + CF + HFCS | 130.1 | 58.22 | 0.22 | 92.31 | 9761 | 0.6936 |
| 5% MCG | 1.26 | 0.81 | 87.28 | 92.33 | 2.43 | 0.9960 |
| 5% MCG + CF | 8.06 | 10.96 | 6.41 | 92.39 | 33.21 | 0.9970 |
| 5% MCG + CF + HFCS | 13.53 | 22.26 | 3.15 | 92.46 | 67.45 | 0.9949 |
| 7% MCG | 0.44 | 0.30 | 235.35 | 92.33 | 0.90 | 0.9969 |
| 7% MCG + CF | 3.44 | 3.59 | 20.16 | 92.36 | 10.90 | 0.9971 |
| 7% MCG + CF + HFCS | 4.27 | 5.01 | 15.72 | 92.28 | 15.39 | 0.9970 |
| 10% MCG | 0.15 | 0.11 | 613.71 | 92.42 | 0.35 | 0.9965 |
| 10% MCG + CF | 1.12 | 0.96 | 73.45 | 92.31 | 2.89 | 0.9939 |
| 10% MCG + CF + HFCS | 1.16 | 0.95 | 71.25 | 92.40 | 2.92 | 0.9918 |
| Recovery phase | ||||||
| J0 10−2 (1/Pa) | J1 10−2 (1/Pa) | η0 102 (Pas) | λ1 (s) | R2 | ||
| 3% MCG | 11.13 | 1.84 | 11.90 | 288.55 | 0.7210 | |
| 3% MCG + CF | 17.34 | 5.17 | 0.43 | 288.55 | 0.6089 | |
| 3% MCG + CF + HFCS | 974.6 | 31.69 | 0.69 | 288.45 | 0.6970 | |
| 5% MCG | 1.15 | 0.94 | 17.62 | 288.45 | 0.8950 | |
| 5% MCG + CF | 24.77 | 4.49 | 4.88 | 288.55 | 0.8081 | |
| 5% MCG + CF + HFCS | 54.10 | 10.43 | 2.10 | 288.50 | 0.8930 | |
| 7% MCG | 0.47 | 0.13 | 240.30 | 288.60 | 0.9700 | |
| 7% MCG + CF | 7.52 | 1.27 | 18.42 | 288.55 | 0.9761 | |
| 7% MCG + CF + HFCS | 11.41 | 2.12 | 12.92 | 288.55 | 0.9388 | |
| 10% MCG | 0.21 | 0.34 | 414.60 | 288.45 | 0.9886 | |
| 10% MCG + CF | 1.81 | 0.51 | 65.10 | 288.60 | 0.9166 | |
| 10% MCG + CF + HFCS | 1.90 | 0.56 | 69.49 | 288.55 | 0.9006 |
| Yield Stress | Thixotropic Area | Firmness | Consistency | Cohesiveness | Index of Viscosity | Tan δ | Newtonian Viscosity | |
|---|---|---|---|---|---|---|---|---|
| Yield stress | 1.0000 | 0.9157 | 0.9268 | 0.9224 | 0.8894 | 0.9183 | −0.7798 | 0.8190 |
| Thixotropic area | 1.0000 | 0.9812 | 0.9610 | 0.9753 | 0.9577 | −0.6576 | 0.9746 | |
| Firmness | 1.0000 | 0.9920 | 0.9942 | 0.9836 | −0.6835 | 0.9408 | ||
| Consistency | 1.0000 | 0.9887 | 0.9835 | −0.6810 | 0.9071 | |||
| Cohesiveness | 1.0000 | 0.9804 | −0.6290 | 0.9470 | ||||
| Index of viscosity | 1.0000 | −0.6875 | 0.9063 | |||||
| Tan δ | 1.0000 | −0.5233 | ||||||
| Newtonian viscosity | 1.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolić, I.; Petrović, J.; Pajin, B.; Lončarević, I.; Šubarić, D.; Ačkar, Đ.; Miličević, B.; Šereš, Z.; Dokić, L.; Šoronja-Simović, D.; et al. The Influence of Starch Sweeteners on Functional Properties of Cellulose Fat Mimetics: Rheological and Textural Aspects. Polymers 2023, 15, 2982. https://doi.org/10.3390/polym15142982
Nikolić I, Petrović J, Pajin B, Lončarević I, Šubarić D, Ačkar Đ, Miličević B, Šereš Z, Dokić L, Šoronja-Simović D, et al. The Influence of Starch Sweeteners on Functional Properties of Cellulose Fat Mimetics: Rheological and Textural Aspects. Polymers. 2023; 15(14):2982. https://doi.org/10.3390/polym15142982
Chicago/Turabian StyleNikolić, Ivana, Jovana Petrović, Biljana Pajin, Ivana Lončarević, Drago Šubarić, Đurđica Ačkar, Borislav Miličević, Zita Šereš, Ljubica Dokić, Dragana Šoronja-Simović, and et al. 2023. "The Influence of Starch Sweeteners on Functional Properties of Cellulose Fat Mimetics: Rheological and Textural Aspects" Polymers 15, no. 14: 2982. https://doi.org/10.3390/polym15142982
APA StyleNikolić, I., Petrović, J., Pajin, B., Lončarević, I., Šubarić, D., Ačkar, Đ., Miličević, B., Šereš, Z., Dokić, L., Šoronja-Simović, D., & Jozinović, A. (2023). The Influence of Starch Sweeteners on Functional Properties of Cellulose Fat Mimetics: Rheological and Textural Aspects. Polymers, 15(14), 2982. https://doi.org/10.3390/polym15142982











