Wastewater from the Arenga Starch Industry as a Potential Medium for Bacterial Cellulose and Cellulose Acetate Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Cellulose Production
2.3. Cellulose Acetate Production
2.3.1. Determination of the Percentage of CA Yield
2.3.2. Determination of the Acetyl Content and the DS of CA
2.4. Product Characterizations
2.4.1. Fourier Transform Infrared (FT−IR)
2.4.2. 1H and 13C Nuclear Magnetic Resonance (1H NMR and 13C NMR)
2.4.3. X-ray Diffractometry (XRD)
2.4.4. Scanning Electron Microscope (SEM)
2.4.5. Statistical Data Analysis
3. Results and Discussion
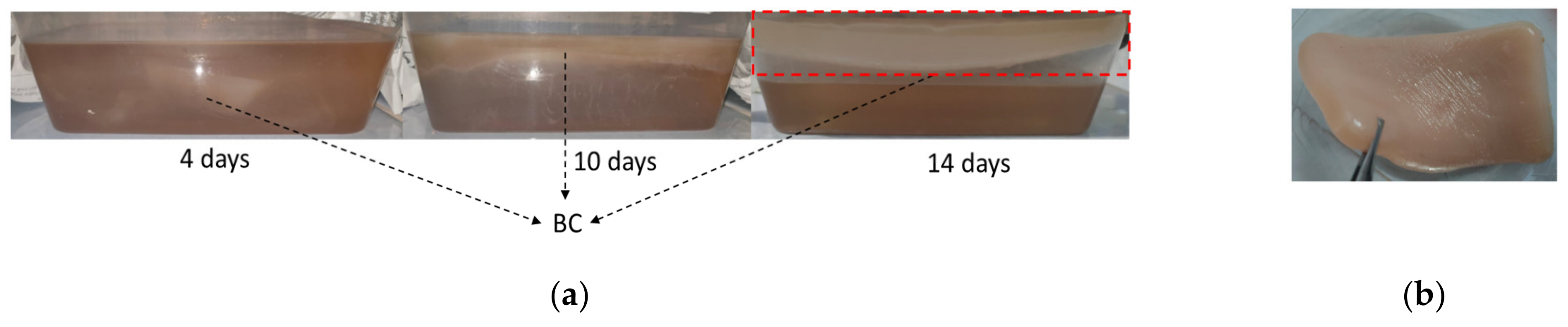
3.1. Production of BC in WWAS Medium
3.2. Cellulose Acetate Production
3.3. Product Characterization
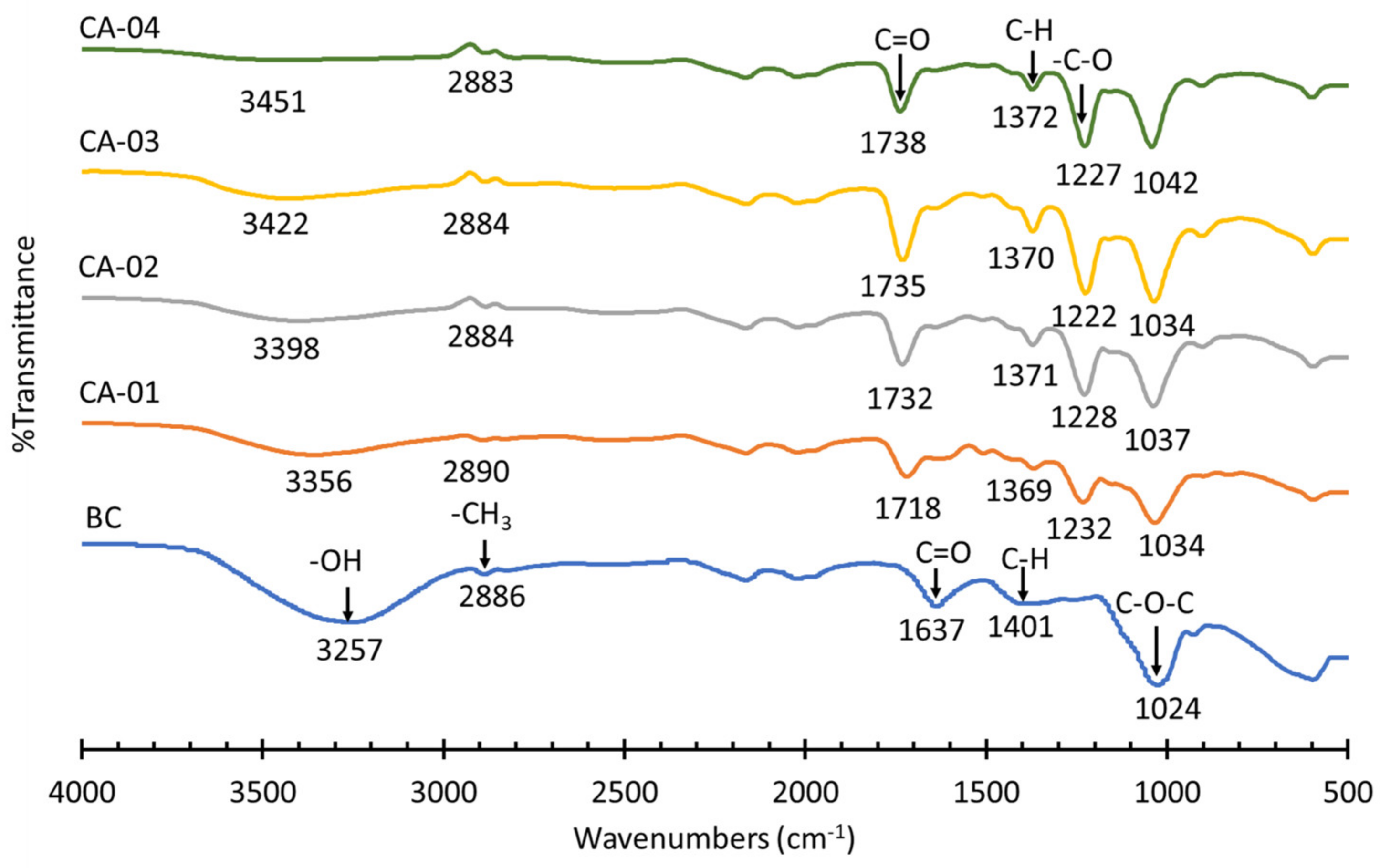
3.3.1. FT–IR Spectroscopic Analysis
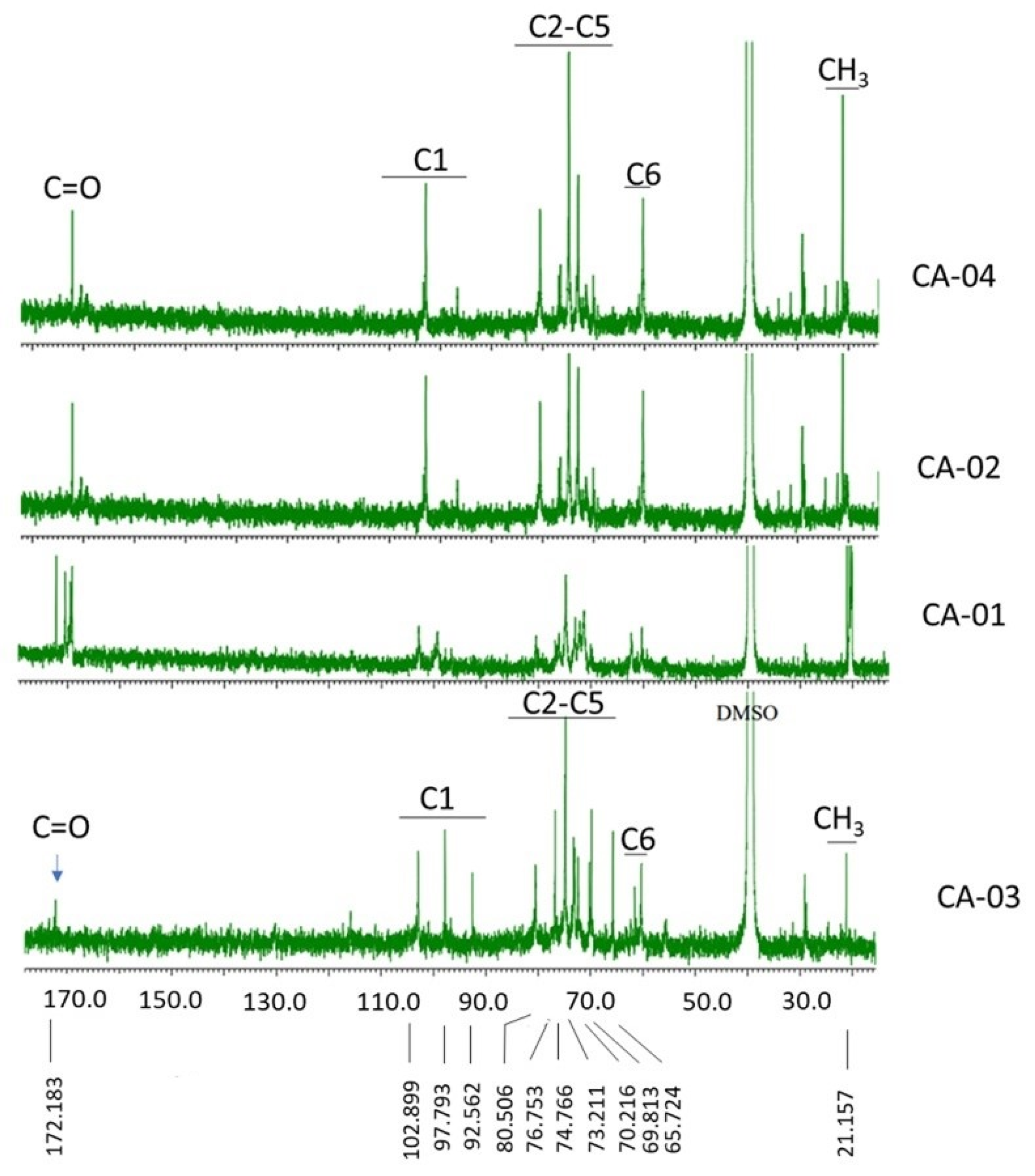
3.3.2. NMR Analysis
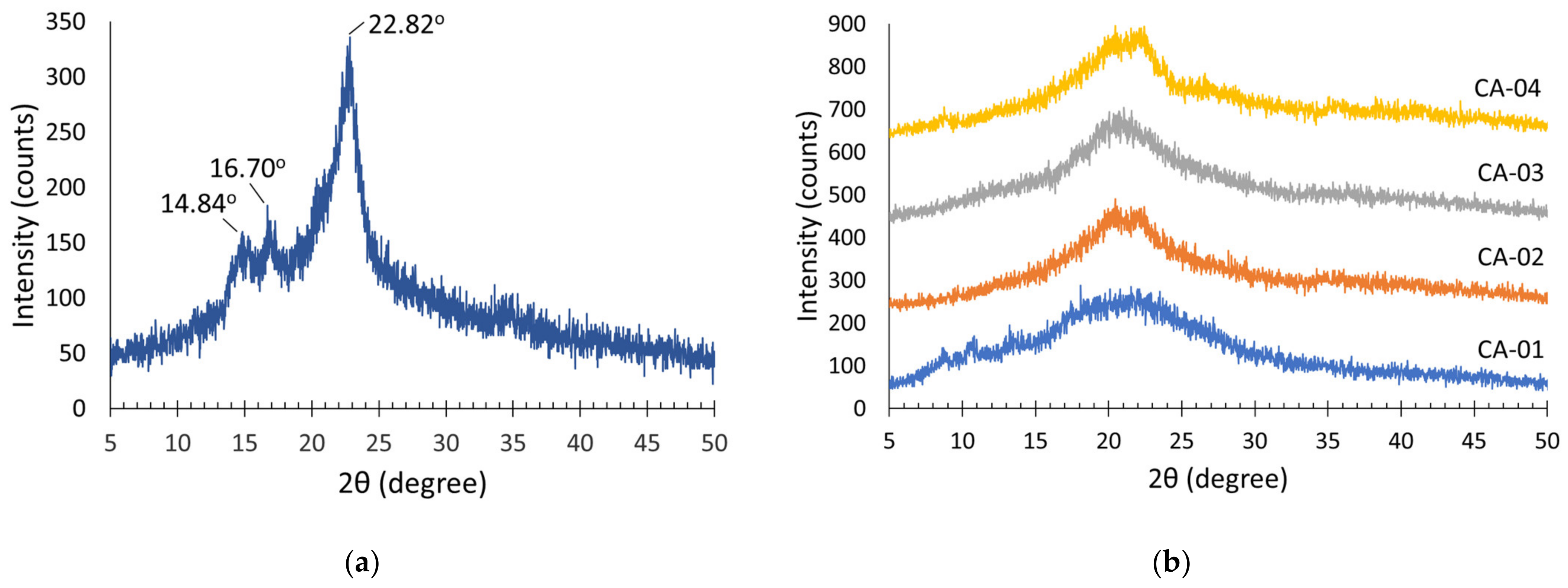
3.3.3. X-ray Diffraction (XRD) Analysis
3.3.4. Scanning Electron Microscope (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Recent Developments in Sugar Palm (Arenga pinnata) Based Biocomposites and Their Potential Industrial Applications: A Review. Renew. Sustain. Energy Rev. 2016, 54, 533–549. [Google Scholar] [CrossRef]
- Ishak, M.R.; Sapuan, S.M.; Leman, Z.; Rahman, M.Z.A.; Anwar, U.M.K.; Siregar, J.P. Sugar Palm (Arenga pinnata): Its Fibres, Polymers and Composites. Carbohydr. Polym. 2013, 91, 699–710. [Google Scholar] [CrossRef]
- Barlina, R.; Liwu, S.; Manaroinsong, E. Potensi Dan Teknologi Pengolahan Komoditas Aren Sebagai Produk Pangan Dan Nonpangan/Potential and Technology Processing of Palm Sugar Commodity as Food and Non-Food Products. J. Penelit. Pengemb. Pertan. 2020, 39, 35. [Google Scholar] [CrossRef]
- Firdayati, M.; Handajani, M. Studi Karakteristik Dasar Limbah Industri Tepung Aren. J. Infrasruktur Lingkung. Binaan 2005, 1, 22–29. [Google Scholar]
- Rahina, E.N.; Rudatin, W. Metode Kombinasi Menurunkan Kadar BOD5 Dan COD Limbah Cair Tepung Aren. Higeia J. Public Health Res. Dev. 2020, 4, 656–666. [Google Scholar] [CrossRef]
- Ramdiana, R. Pengaruh Variasi Komposisi Pada Campuran Limbah Cair Aren Dan Kotoran Sapi Terhadap Produksi Biogas. Eksergi 2017, 14, 12. [Google Scholar] [CrossRef]
- Bengtsson, B.E.; Triet, T. Tapioca-Starch Wastewater Toxicity Characterized by Microtox and Duckweed Tests. Ambio 1994, 23, 473–477. [Google Scholar]
- Shuler, M.L.; Kargi, F. Bioprocess Engineering Basic Concepts Second Edition; Prentice Hall PTR: Hoboken, NJ, USA, 2002; ISBN 0-13-081908-5. [Google Scholar]
- Gayathri, G.; Srinikethan, G. Bacterial Cellulose Production by K. Saccharivorans BC1 Strain Using Crude Distillery Effluent as Cheap and Cost Effective Nutrient Medium. Int. J. Biol. Macromol. 2019, 138, 950–957. [Google Scholar] [CrossRef]
- Jahan, F.; Kumar, V.; Saxena, R.K. Distillery Effluent as a Potential Medium for Bacterial Cellulose Production: A Biopolymer of Great Commercial Importance. Bioresour. Technol. 2018, 250, 922–926. [Google Scholar] [CrossRef]
- Ye, J.; Zheng, S.; Zhang, Z.; Yang, F.; Ma, K.; Feng, Y.; Zheng, J.; Mao, D.; Yang, X. Bacterial Cellulose Production by Acetobacter Xylinum ATCC 23767 Using Tobacco Waste Extract as Culture Medium. Bioresour. Technol. 2019, 274, 518–524. [Google Scholar] [CrossRef]
- Çakar, F.; Kati, A.; Özer, I.; Demirbağ, D.D.; Şahin, F.; Aytekin, A.Ö. Newly Developed Medium and Strategy for Bacterial Cellulose Production. Biochem. Eng. J. 2014, 92, 35–40. [Google Scholar] [CrossRef]
- Machado, R.T.A.; Meneguin, A.B.; Sábio, R.M.; Franco, D.F.; Antonio, S.G.; Gutierrez, J.; Tercjak, A.; Berretta, A.A.; Ribeiro, S.J.L.; Lazarini, S.C.; et al. Komagataeibacter Rhaeticus Grown in Sugarcane Molasses-Supplemented Culture Medium as a Strategy for Enhancing Bacterial Cellulose Production. Ind. Crops Prod. 2018, 122, 637–646. [Google Scholar] [CrossRef]
- Keshk, S.; Sameshima, K. The Utilization of Sugar Cane Molasses With/without the Presence of Lignosulfonate for the Production of Bacterial Cellulose. Appl. Microbiol. Biotechnol. 2006, 72, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Çakar, F.; Özer, I.; Aytekin, A.Ö.; Şahin, F. Improvement Production of Bacterial Cellulose by Semi-Continuous Process in Molasses Medium. Carbohydr. Polym. 2014, 106, 7–13. [Google Scholar] [CrossRef]
- Bae, S.O.; Shoda, M. Production of Bacterial Cellulose by Acetobacter Xylinum BPR2001 Using Molasses Medium in a Jar Fermentor. Appl. Microbiol. Biotechnol. 2005, 67, 45–51. [Google Scholar] [CrossRef]
- Revin, V.; Liyaskina, E.; Nazarkina, M.; Bogatyreva, A.; Shchankin, M. Cost-Effective Production of Bacterial Cellulose Using Acidic Food Industry by-Products. Braz. J. Microbiol. 2018, 49, 151–159. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Hua, J.; Jia, S.; Zhang, J.; Liu, H. Production of Nano Bacterial Cellulose from Waste Water of Candied Jujube-Processing Industry Using Acetobacter Xylinum. Carbohydr. Polym. 2015, 120, 115–119. [Google Scholar] [CrossRef]
- Wu, J.M.; Liu, R.H. Thin Stillage Supplementation Greatly Enhances Bacterial Cellulose Production by Gluconacetobacter Xylinus. Carbohydr. Polym. 2012, 90, 116–121. [Google Scholar] [CrossRef]
- Sar, T.; Yesilcimen Akbas, M. Potential Use of Olive Oil Mill Wastewater for Bacterial Cellulose Production. Bioengineered 2022, 13, 7659–7669. [Google Scholar] [CrossRef]
- Zhong, C.; Zhang, G.C.; Liu, M.; Zheng, X.T.; Han, P.P.; Jia, S.R. Metabolic Flux Analysis of Gluconacetobacter Xylinus for Bacterial Cellulose Production. Appl. Microbiol. Biotechnol. 2013, 97, 6189–6199. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Park, J.K. Production of Bacterial Cellulose from Alternative Cheap and Waste Resources: A Step for Cost Reduction with Positive Environmental Aspects. Korean J. Chem. Eng. 2020, 37, 925–937. [Google Scholar] [CrossRef]
- Ullah, H.; Badshah, M.; Mäkilä, E.; Salonen, J.; Shahbazi, M.A.; Santos, H.A.; Khan, T. Fabrication, Characterization and Evaluation of Bacterial Cellulose-Based Capsule Shells for Oral Drug Delivery. Cellulose 2017, 24, 1445–1454. [Google Scholar] [CrossRef]
- Jonas, R.; Farah, L.F. Production and Application of Microbial Cellulose. Polym. Degrad. Stab. 1998, 59, 101–106. [Google Scholar] [CrossRef]
- Lin, S.-P.; Loira Calvar, I.; Catchmark, J.M.; Liu, J.-R.; Demirci, A.; Cheng, K.-C. Biosynthesis, Production and Applications of Bacterial Cellulose. Cellulose 2013, 20, 2191–2219. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial Cellulose Production, Properties and Applications with Different Culture Methods—A Review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef]
- Ross, P.; Mayer, R.; Benziman, M. Cellulose Biosynthesis and Function in Bacteria. Microbiol. Rev. 1991, 55, 35–58. [Google Scholar] [CrossRef]
- Lee, K.Y.; Buldum, G.; Mantalaris, A.; Bismarck, A. More than Meets the Eye in Bacterial Cellulose: Biosynthesis, Bioprocessing, and Applications in Advanced Fiber Composites. Macromol. Biosci. 2014, 14, 10–32. [Google Scholar] [CrossRef]
- Islam, M.U.; Ullah, M.W.; Khan, S.; Shah, N.; Park, J.K. Strategies for Cost-Effective and Enhanced Production of Bacterial Cellulose. Int. J. Biol. Macromol. 2017, 102, 1166–1173. [Google Scholar] [CrossRef]
- Cielecka, I.; Ryngajłło, M.; Maniukiewicz, W.; Bielecki, S. Highly Stretchable Bacterial Cellulose Produced by Komagataeibacter Hansenii si1. Polymers 2021, 13, 4455. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Saha, N.; Saha, P. Characterization of Bacterial Cellulose Produced Using Media Containing Waste Apple Juice. Appl. Biochem. Microbiol. 2018, 54, 649–657. [Google Scholar] [CrossRef]
- Budhiono, A.; Rosidi, B.; Taher, H.; Iguchi, M. Kinetic Aspects of Bacterial Cellulose Formation in Nata-de-Coco Culture System. Carbohydr. Polym. 1999, 40, 137–143. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Putaux, J.L.; Caro, G.; Mondragon, I.; Gañán, P. Structural Characterization of Bacterial Cellulose Produced by Gluconacetobacter Swingsii Sp. from Colombian Agroindustrial Wastes. Carbohydr. Polym. 2011, 84, 96–102. [Google Scholar] [CrossRef]
- Wan Daud, W.R.; Djuned, F.M. Cellulose Acetate from Oil Palm Empty Fruit Bunch via a One Step Heterogeneous Acetylation. Carbohydr. Polym. 2015, 132, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.G.; Maranni, M.; Araujo, L.O.; Maciel, B.M.; Canassa, T.; Caires, A.R.L.; Cena, C. Preparation and Phytotoxicity Evaluation of Cellulose Acetate Nanoparticles. Polymers 2022, 14, 5022. [Google Scholar] [CrossRef] [PubMed]
- Steinmeier, H. 3. Acetate Manufacturing, Process and Technology 3.1 Chemistry of Cellulose Acetylation. Macromol. Symp. 2004, 208, 49–60. [Google Scholar] [CrossRef]
- Sassi, J.F.; Chanzy, H. Ultrastructural Aspects of the Acetylation of Cellulose. Cellulose 1995, 2, 111–127. [Google Scholar] [CrossRef]
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in Cellulose Ester Performance and Application. Prog. Polym. Sci. 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Bahmid, N.A.; Syamsu, K.; Maddu, A. Production of Cellulose Acetate from Oil Palm Empty Fruit Bunches Cellulose. Chem. Process Eng. Res. 2013, 17, 12–20. [Google Scholar]
- Syamsu, K.; Kuryani, T. Pembuatan Biofilm Selulosa Asetat Dari Selulosa Mikrobial Nata De Cassava. Agroindustri Indones. 2014, 214, 126–133. [Google Scholar]
- Homem, N.C.; Amorim, M.T.P. Synthesis of Cellulose Acetate Using as Raw Material Textile Wastes. Mater. Today Proc. 2020, 31, S315–S317. [Google Scholar] [CrossRef]
- Samios, E.; Dart, R.K.; Dawkins, J.V. Preparation, Characterization and Biodegradation Studies on Cellulose Acetates with Varying Degrees of Substitution. Polymer 1997, 38, 3045–3054. [Google Scholar] [CrossRef]
- Kurniawan, T.; Muraza, O.; Hakeem, A.S.; Al-Amer, A.M. Mechanochemical Route and Recrystallization Strategy to Fabricate Mordenite Nanoparticles from Natural Zeolites. Cryst. Growth Des. 2017, 17, 3313–3320. [Google Scholar] [CrossRef]
- Rahmayetty; Whulanza, Y.; Sukirno; Rahman, S.F.; Suyono, E.A.; Yohda, M.; Gozan, M. Use of Candida Rugosa Lipase as a Biocatalyst for L-Lactide Ring-Opening Polymerization and Polylactic Acid Production. Biocatal. Agric. Biotechnol. 2018, 16, 683–691. [Google Scholar] [CrossRef]
- Amor, Y.; Haigler, C.H.; Johnson, S.; Wainscott, M.; Delmer, D.P. A Membrane-Associated Form of Sucrose Synthase and Its Potential Role in Synthesis of Cellulose and Callose in Plants. Proc. Natl. Acad. Sci. USA 1995, 92, 9353–9357. [Google Scholar] [CrossRef]
- Embuscado, M.E.; Marks, J.S.; BeMiller, J.N. Bacterial Cellulose. I. Factors Affecting the Production of Cellulose by Acetobacter Xylinum. Top. Catal. 1994, 8, 407–418. [Google Scholar] [CrossRef]
- Mohainin Mohammad, S.; Abd Rahman, N.; Sahaid Khalil, M.; Rozaimah Sheikh Abdullah, S. An Overview of Biocellulose Production Using Acetobacter Xylinum Culture. Adv. Biol. Res. 2014, 8, 307–313. [Google Scholar] [CrossRef]
- Jagannath, A.; Kalaiselvan, A.; Manjunatha, S.S.; Raju, P.S.; Bawa, A.S. The Effect of pH, Sucrose and Ammonium Sulphate Concentrations on the Production of Bacterial Cellulose (Nata-de-Coco) by Acetobacter Xylinum. World J. Microbiol. Biotechnol. 2008, 24, 2593–2599. [Google Scholar] [CrossRef]
- Hutchens, S.A.; León, R.V.; O’Neill, H.M.; Evans, B.R. Statistical Analysis of Optimal Culture Conditions for Gluconacetobacter Hansenii Cellulose Production. Lett. Appl. Microbiol. 2007, 44, 175–180. [Google Scholar] [CrossRef]
- Krystynowicz, A.; Czaja, W.; Wiktorowska-Jezierska, A.; Gonçalves-Miśkiewicz, M.; Turkiewicz, M.; Bielecki, S. Factors Affecting the Yield and Properties of Bacterial Cellulose. J. Ind. Microbiol. Biotechnol. 2002, 29, 189–195. [Google Scholar] [CrossRef]
- Borzani, W.; de Souza, S.J. Mechanism of the Film Thickness Increasing during the Bacterial Production of Cellulose on Non-Agitaded Liquid Media. Biotechnol. Lett. 1995, 17, 1271–1272. [Google Scholar] [CrossRef]
- Sheykhnazari, S.; Tabarsa, T.; Ashori, A.; Shakeri, A.; Golalipour, M. Bacterial Synthesized Cellulose Nanofibers; Effects of Growth Times and Culture Mediums on the Structural Characteristics. Carbohydr. Polym. 2011, 86, 1187–1191. [Google Scholar] [CrossRef]
- Fan, G.; Wang, M.; Liao, C.; Fang, T.; Li, J.; Zhou, R. Isolation of Cellulose from Rice Straw and Its Conversion into Cellulose Acetate Catalyzed by Phosphotungstic Acid. Carbohydr. Polym. 2013, 94, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Araújo, D.; Castro, M.C.R.; Figueiredo, A.; Vilarinho, M.; Machado, A. Green Synthesis of Cellulose Acetate from Corncob: Physicochemical Properties and Assessment of Environmental Impacts. J. Clean. Prod. 2020, 260, 120865. [Google Scholar] [CrossRef]
- Curtis, L.G.; Crowley, J.D. Cellulose Acetate and Related Esters. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1985; pp. 1053–1072. ISBN 0841208913. [Google Scholar]
- Barud, H.S.; de Araújo Júnior, A.M.; Santos, D.B.; de Assunção, R.M.N.; Meireles, C.S.; Cerqueira, D.A.; Rodrigues Filho, G.; Ribeiro, C.A.; Messaddeq, Y.; Ribeiro, S.J.L. Thermal Behavior of Cellulose Acetate Produced from Homogeneous Acetylation of Bacterial Cellulose. Thermochim. Acta 2008, 471, 61–69. [Google Scholar] [CrossRef]
- Mayer, J.M.; Elion, G.R.; Buchanan, C.M.; Sullivan, B.K.; Pratt, S.D.; Kaplan, D.L. Biodegradable Blends of Cellulose Acetate and Starch: Production and Properties. J. Macromol. Sci. Part A 1995, 32, 775–785. [Google Scholar] [CrossRef]
- Battisti, R.; Hafemann, E.; Claumann, C.A.; Machado, R.A.F.; Marangoni, C. Synthesis and Characterization of Cellulose Acetate from Royal Palm Tree Agroindustrial Waste. Polym. Eng. Sci. 2019, 59, 891–898. [Google Scholar] [CrossRef]
- Du, R.; Zhao, F.; Peng, Q.; Zhou, Z.; Han, Y. Production and Characterization of Bacterial Cellulose Produced by Gluconacetobacter Xylinus Isolated from Chinese Persimmon Vinegar. Carbohydr. Polym. 2018, 194, 200–207. [Google Scholar] [CrossRef]
- Andrade Alves, J.A.; Lisboa dos Santos, M.D.; Morais, C.C.; Ramirez Ascheri, J.L.; Signini, R.; dos Santos, D.M.; Cavalcante Bastos, S.M.; Ramirez Ascheri, D.P. Sorghum Straw: Pulping and Bleaching Process Optimization and Synthesis of Cellulose Acetate. Int. J. Biol. Macromol. 2019, 135, 877–886. [Google Scholar] [CrossRef]
- Cao, L.; Luo, G.; Tsang, D.C.W.; Chen, H.; Zhang, S.; Chen, J. A Novel Process for Obtaining High Quality Cellulose Acetate from Green Landscaping Waste. J. Clean. Prod. 2018, 176, 338–347. [Google Scholar] [CrossRef]
- Khan, H.; Kadam, A.; Dutt, D. Studies on Bacterial Cellulose Produced by a Novel Strain of Lactobacillus Genus. Carbohydr. Polym. 2020, 229, 1155131. [Google Scholar] [CrossRef]
- Huang, C.; Guo, H.J.; Xiong, L.; Wang, B.; Shi, S.L.; Chen, X.F.; Lin, X.Q.; Wang, C.; Luo, J.; Chen, X. De Using Wastewater after Lipid Fermentation as Substrate for Bacterial Cellulose Production by Gluconacetobacter Xylinus. Carbohydr. Polym. 2016, 136, 198–202. [Google Scholar] [CrossRef]
- Das, A.M.; Ali, A.A.; Hazarika, M.P. Synthesis and Characterization of Cellulose Acetate from Rice Husk: Eco-Friendly Condition. Carbohydr. Polym. 2014, 112, 342–349. [Google Scholar] [CrossRef]
- Nishino, T.; Kotera, M.; Suetsugu, M.; Murakami, H.; Urushihara, Y. Acetylation of Plant Cellulose Fiber in Supercritical Carbon Dioxide. Polymer 2011, 52, 830–836. [Google Scholar] [CrossRef]



| Parameters | Methods | Values * |
|---|---|---|
| pH (25 °C) | A digital pH meter | 6.9 |
| Dissolved oxygen (DO) | SNI 06-6989.14-2004 | 0.73 |
| Total dissolved solid (TDS) | A digital TDS meter | 258 |
| Total suspended solid (TSS) | Gravimetric analysis | 2750 |
| Biology oxygen demand (BOD) | SNI 6989.72-2009 | 3050 |
| Chemical oxygen demand (COD) | SNI 6989.2-2009 | 6394 |
| Ammonia | IK 7.2.1.2 KA (spectrophotometric) | 7.2 |
| Sucrose Addition (g/L) | Initial Medium pH | BC | ||
|---|---|---|---|---|
| Thickness (mm) | Wet Weight (g/L) | Dry Weight (g/L) | ||
| 3.5 | 2 | 22 | 0.16 | |
| 100 | 4.5 | 5 | 44 | 2.2 |
| 5.5 | 1.5 | 16 | 0.016 | |
| 6.5 | - | - | - | |
| 3.5 | 16 | 320.6 | 28.4 | |
| 200 | 4.5 | 21 | 505.6 | 43.6 |
| 5.5 | 8 | 150.2 | 5.4 | |
| 6.5 | 1 | 7.6 | 0.002 | |
| 3.5 | 12 | 181.2 | 11.6 | |
| 300 | 4.5 | 10 | 166.4 | 8.8 |
| 5.5 | - | - | - | |
| 6.5 | - | - | - | |
| 3.5 | 3.0 | 28 | 0.32 | |
| 400 | 4.5 | 2.5 | 21.2 | 0.20 |
| 5.5 | - | - | - | |
| 6.5 | - | - | - | |
| Sample | The Ratio of BC:AcAn (w/v) | Yield of CA (%) | Acetyl Content of CA (%) | Degree of Substitution (DS) of CA | Solubility | ||
|---|---|---|---|---|---|---|---|
| Water | Acetone | Chloroform | |||||
| CA-01 | 1:1 | 20.46 | 27.87 | 1.444 | - | + | + |
| CA-02 | 1:2 | 52.82 | 35.44 | 2.043 | - | + | + |
| CA-03 | 1:3 | 81.49 | 39.82 | 2.456 | - | + | + |
| CA-04 | 1:4 | 74.13 | 41.65 | 2.645 | - | + | + |
| Sample | C=O | C1 | C2–C5 | C6 | C–Me | |
|---|---|---|---|---|---|---|
| CA-01 | 169.092 | 99.357 | 71.281 | 72.126 | 60.291 | 20.130 |
| 169.418 | 102.880 | 72.990 | 74.833 | 62.297 | 20.245 | |
| 170.407 | 20.339 | |||||
| 172.125 | 20.639 | |||||
| 21.119 | ||||||
| CA-02 | 172.173 | 96.689 | 73.009 | 74.833 | 60.300 | 20.283 |
| 102.899 | 76.513 | 76.849 | 20.667 | |||
| 80.486 | 21.147 | |||||
| 22.184 | ||||||
| 24.555 | ||||||
| CA-03 | 172.183 | 92.562 | 69.813 | 70.216 | 60.320 | 21.157 |
| 97.793 | 72.366 | 73.009 | 61.558 | |||
| 102.899 | 73.211 | 74.766 | 65.724 | |||
| 76.753 | 80.506 | |||||
| CA-04 | 172.163 | 96.689 | 74.766 | 74.862 | 60.310 | 21.138 |
| 97.793 | 61.087 | 22.174 | ||||
| 24.545 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmayetty; Sulaiman, F. Wastewater from the Arenga Starch Industry as a Potential Medium for Bacterial Cellulose and Cellulose Acetate Production. Polymers 2023, 15, 870. https://doi.org/10.3390/polym15040870
Rahmayetty, Sulaiman F. Wastewater from the Arenga Starch Industry as a Potential Medium for Bacterial Cellulose and Cellulose Acetate Production. Polymers. 2023; 15(4):870. https://doi.org/10.3390/polym15040870
Chicago/Turabian StyleRahmayetty, and Fatah Sulaiman. 2023. "Wastewater from the Arenga Starch Industry as a Potential Medium for Bacterial Cellulose and Cellulose Acetate Production" Polymers 15, no. 4: 870. https://doi.org/10.3390/polym15040870
APA StyleRahmayetty, & Sulaiman, F. (2023). Wastewater from the Arenga Starch Industry as a Potential Medium for Bacterial Cellulose and Cellulose Acetate Production. Polymers, 15(4), 870. https://doi.org/10.3390/polym15040870






