A Comprehensive Compilation of Graphene/Fullerene Polymer Nanocomposites for Electrochemical Energy Storage
Abstract
1. Introduction
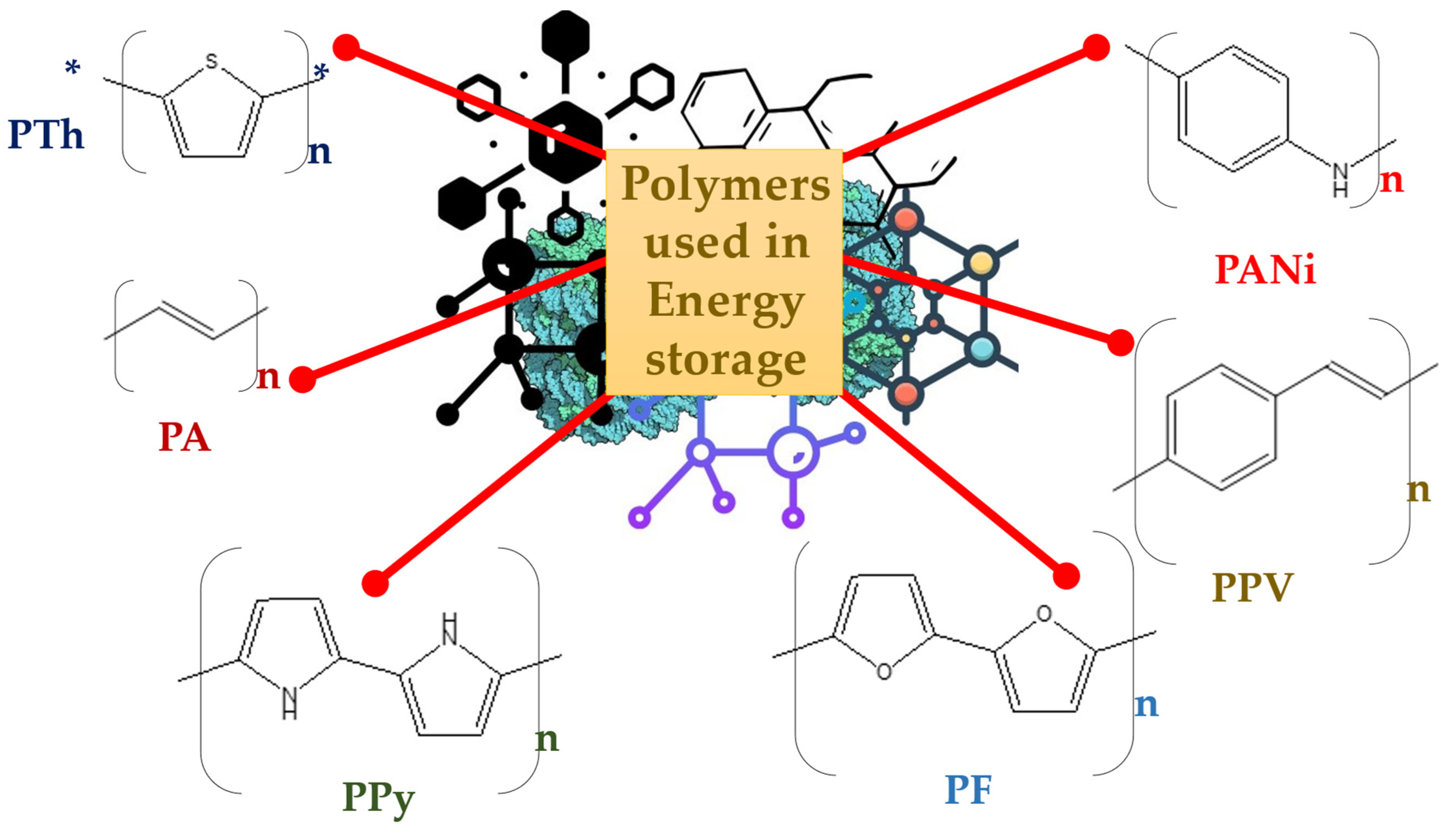
2. Carbon Polymer Nanocomposites Used for Energy Storage
3. Graphene-Based Nanocomposites for Electrochemical Energy Storage
4. Fullerene-Based Polymer Nanocomposites for Energy Storage
5. Future Recommendations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Energy, U.U.S. Energy Information Administration, Renewable Energy; US EIA: Washington, DC, USA, 2018.
- Zhao, P. Research on Power Transmission Direction and Scale of Interconnected Grid Considering Uncertainty. Master’s Thesis, North China Electric Power University, Beijing, China, 2019. [Google Scholar]
- Board, E. The Growth of Electricity Sector in India from 1947–2020; Central Electricity Authority of India, Government of India: New Delhi, India, 2020.
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The Role of Renewable Energy in the Global Energy Transformation. Energy Strateg. Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Nelson, J.P.; Bolin, W.D. Basics and Advances in Battery Systems. IEEE Trans. Ind. Appl. 1995, 31, 419–428. [Google Scholar] [CrossRef]
- Anthony, L.S.; Vasudevan, M.; Perumal, V.; Ovinis, M.; Raja, P.B.; Edison, T.N.J.I. Bioresource-Derived Polymer Composites for Energy Storage Applications: Brief Review. J. Environ. Chem. Eng. 2021, 9, 105832. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-Ion Batteries: Outlook on Present, Future, and Hybridized Technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Abdin, Z.; Khalilpour, K.R. Single and Polystorage Technologies for Renewable-Based Hybrid Energy Systems. In Polygeneration with Polystorage for Chemical and Energy Hubs; Elsevier: Amsterdam, The Netherlands, 2019; pp. 77–131. [Google Scholar]
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Evanko, B.; Boettcher, S.W.; Yoo, S.J.; Stucky, G.D. Redox-Enhanced Electrochemical Capacitors: Status, Opportunity, and Best Practices for Performance Evaluation. ACS Energy Lett. 2017, 2, 2581–2590. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor Devices Based on Graphene Materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Velasco, A.; Ryu, Y.K.; Boscá, A.; Ladrón-de-Guevara, A.; Hunt, E.; Zuo, J.; Pedrós, J.; Calle, F.; Martinez, J. Recent Trends in Graphene Supercapacitors: From Large Area to Microsupercapacitors. Sustain. Energy Fuels 2021, 5, 1235–1254. [Google Scholar] [CrossRef]
- Zhu, J.; Childress, A.S.; Karakaya, M.; Dandeliya, S.; Srivastava, A.; Lin, Y.; Rao, A.M.; Podila, R. Defect-Engineered Graphene for High-Energy- and High-Power-Density Supercapacitor Devices. Adv. Mater. 2016, 28, 7185–7192. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, E. Functionalized and Tip-Open Carbon Nanotubes for High-Performance Symmetric Supercapacitors. Dalt. Trans. 2021, 50, 12982–12989. [Google Scholar] [CrossRef]
- Lu, Z.; Raad, R.; Safaei, F.; Xi, J.; Liu, Z.; Foroughi, J. Carbon Nanotube Based Fiber Supercapacitor as Wearable Energy Storage. Front. Mater. 2019, 6, 138. [Google Scholar] [CrossRef]
- Komatsubara, K.; Suzuki, H.; Inoue, H.; Kishibuchi, M.; Takahashi, S.; Marui, T.; Umezawa, S.; Nakagawa, T.; Nasu, K.; Maetani, M.; et al. Highly Oriented Carbon Nanotube Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 1521–1532. [Google Scholar] [CrossRef]
- Miao, Y.-E.; Huang, Y.; Zhang, L.; Fan, W.; Lai, F.; Liu, T. Electrospun Porous Carbon Nanofiber@MoS 2 Core/Sheath Fiber Membranes as Highly Flexible and Binder-Free Anodes for Lithium-Ion Batteries. Nanoscale 2015, 7, 11093–11101. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Liu, M.; Miao, Y.-E.; Huang, Y.; Liu, T. Electrospun Nickel-Decorated Carbon Nanofiber Membranes as Efficient Electrocatalysts for Hydrogen Evolution Reaction. Electrochim. Acta 2015, 159, 1–7. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Velamakanni, A.; Piner, R.D.; Ruoff, R.S. Microwave Assisted Exfoliation and Reduction of Graphite Oxide for Ultracapacitors. Carbon N. Y. 2010, 48, 2118–2122. [Google Scholar] [CrossRef]
- Zhu, Y.; Stoller, M.D.; Cai, W.; Velamakanni, A.; Piner, R.D.; Chen, D.; Ruoff, R.S. Exfoliation of Graphite Oxide in Propylene Carbonate and Thermal Reduction of the Resulting Graphene Oxide Platelets. ACS Nano 2010, 4, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Beaudin, M.; Zareipour, H.; Schellenberglabe, A.; Rosehart, W. Energy Storage for Mitigating the Variability of Renewable Electricity Sources: An Updated Review. Energy Sustain. Dev. 2010, 14, 302–314. [Google Scholar] [CrossRef]
- Arani, A.A.K.; Karami, H.; Gharehpetian, G.B.; Hejazi, M.S.A. Review of Flywheel Energy Storage Systems Structures and Applications in Power Systems and Microgrids. Renew. Sustain. Energy Rev. 2017, 69, 9–18. [Google Scholar] [CrossRef]
- Elliman, R.; Gould, C.; Al-Tai, M. Review of Current and Future Electrical Energy Storage Devices. In Proceedings of the 2015 50th International Universities Power Engineering Conference (UPEC), Stoke-on-Trent, UK, 1–4 September 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1–5. [Google Scholar]
- Hannan, M.A.; Hoque, M.M.; Mohamed, A.; Ayob, A. Review of Energy Storage Systems for Electric Vehicle Applications: Issues and Challenges. Renew. Sustain. Energy Rev. 2017, 69, 771–789. [Google Scholar] [CrossRef]
- Abbasi, H.; Antunes, M.; Velasco, J.I. Recent Advances in Carbon-Based Polymer Nanocomposites for Electromagnetic Interference Shielding. Prog. Mater. Sci. 2019, 103, 319–373. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.; Hui, D.; Bhattacharyya, D. Graphene-Based Materials and Their Composites: A Review on Production, Applications and Product Limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Dang, Z.-M.; Yuan, J.-K.; Yao, S.-H.; Liao, R.-J. Flexible Nanodielectric Materials with High Permittivity for Power Energy Storage. Adv. Mater. 2013, 25, 6334–6365. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Ghosh, S.; Perla, V.K.; Pal, T.; Mallick, K. Laboratory Based Synthesis of the Pure Form of Gananite (BiF 3 ) Nanoparticles: A Potential Material for Electrochemical Supercapacitor Application. New J. Chem. 2019, 43, 18369–18376. [Google Scholar] [CrossRef]
- Al-Saleh, M.H. Electrically Conductive Carbon Nanotube/Polypropylene Nanocomposite with Improved Mechanical Properties. Mater. Des. 2015, 85, 76–81. [Google Scholar] [CrossRef]
- Tang, H.; Chen, G.-X.; Li, Q. Epoxy-Based High-k Composites with Low Dielectric Loss Caused by Reactive Core-Shell-Structured Carbon Nanotube Hybrids. Mater. Lett. 2016, 184, 143–147. [Google Scholar] [CrossRef]
- Baek, J.E.; Kim, J.Y.; Jin, H.M.; Kim, B.H.; Lee, K.E.; Kim, S.O. Single-Step Self-Assembly of Multilayer Graphene Based Dielectric Nanostructures. FlatChem 2017, 4, 61–67. [Google Scholar] [CrossRef]
- Lin, B.; Li, Z.-T.; Yang, Y.; Li, Y.; Lin, J.-C.; Zheng, X.-M.; He, F.-A.; Lam, K.-H. Enhanced Dielectric Permittivity in Surface-Modified Graphene/PVDF Composites Prepared by an Electrospinning-Hot Pressing Method. Compos. Sci. Technol. 2019, 172, 58–65. [Google Scholar] [CrossRef]
- Mohanapriya, M.K.; Deshmukh, K.; Chidambaram, K.; Ahamed, M.B.; Sadasivuni, K.K.; Ponnamma, D.; AlMaadeed, M.A.-A.; Deshmukh, R.R.; Pasha, S.K.K. Polyvinyl Alcohol (PVA)/Polystyrene Sulfonic Acid (PSSA)/Carbon Black Nanocomposite for Flexible Energy Storage Device Applications. J. Mater. Sci. Mater. Electron. 2017, 28, 6099–6111. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, D.; Yuan, Y.; Wang, Y.; Cheng, Z.; Liu, Y.; Xie, Z. A Lightweight and Conductive MXene/Graphene Hybrid Foam for Superior Electromagnetic Interference Shielding. Chem. Eng. J. 2020, 381, 122696. [Google Scholar] [CrossRef]
- Al-Saleh, M.H. Carbon-Based Polymer Nanocomposites as Dielectric Energy Storage Materials. Nanotechnology 2019, 30, 062001. [Google Scholar] [CrossRef]
- Cai, C.; Liu, L.; Fu, Y. Processable Conductive and Mechanically Reinforced Polylactide/Graphene Bionanocomposites through Interfacial Compatibilizer. Polym. Compos. 2019, 40, 389–400. [Google Scholar] [CrossRef]
- Ishaq, S.; Moussa, M.; Kanwal, F.; Ehsan, M.; Saleem, M.; Van, T.N.; Losic, D. Facile Synthesis of Ternary Graphene Nanocomposites with Doped Metal Oxide and Conductive Polymers as Electrode Materials for High Performance Supercapacitors. Sci. Rep. 2019, 9, 5974. [Google Scholar] [CrossRef]
- Idumah, C.I.; Hassan, A. Emerging Trends in Graphene Carbon Based Polymer Nanocomposites and Applications. Rev. Chem. Eng. 2016, 32, 223–264. [Google Scholar] [CrossRef]
- Miller, E.E.; Hua, Y.; Tezel, F.H. Materials for Energy Storage: Review of Electrode Materials and Methods of Increasing Capacitance for Supercapacitors. J. Energy Storage 2018, 20, 30–40. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A Review on Recent Advances in Hybrid Supercapacitors: Design, Fabrication and Applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Farzana, R.; Rajarao, R.; Bhat, B.R.; Sahajwalla, V. Performance of an Activated Carbon Supercapacitor Electrode Synthesised from Waste Compact Discs (CDs). J. Ind. Eng. Chem. 2018, 65, 387–396. [Google Scholar] [CrossRef]
- Tian, J.; Wu, S.; Yin, X.; Wu, W. Novel Preparation of Hydrophilic Graphene/Graphene Oxide Nanosheets for Supercapacitor Electrode. Appl. Surf. Sci. 2019, 496, 143696. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, X.; Ge, C.; Zhou, W.; Zhu, Y.; Xu, B. Branched Carbon Nanotube/Carbon Nanofiber Composite for Supercapacitor Electrodes. Mater. Lett. 2019, 246, 174–177. [Google Scholar] [CrossRef]
- Genc, R.; Alas, M.O.; Harputlu, E.; Repp, S.; Kremer, N.; Castellano, M.; Colak, S.G.; Ocakoglu, K.; Erdem, E. High-Capacitance Hybrid Supercapacitor Based on Multi-Colored Fluorescent Carbon-Dots. Sci. Rep. 2017, 7, 11222. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-W.; Kalimuthu, P.; Veerakumar, P.; Lin, K.-C.; Chen, S.-M.; Ramachandran, R.; Mariyappan, V.; Chitra, S. Recent Developments in Carbon-Based Nanocomposites for Fuel Cell Applications: A Review. Molecules 2022, 27, 761. [Google Scholar] [CrossRef] [PubMed]
- Siwal, S.S.; Zhang, Q.; Devi, N.; Thakur, V.K. Carbon-Based Polymer Nanocomposite for High-Performance Energy Storage Applications. Polymers 2020, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Sarang, K.T.; Miranda, A.; An, H.; Oh, E.-S.; Verduzco, R.; Lutkenhaus, J.L. Poly(Fluorene- Alt -Naphthalene Diimide) as n-Type Polymer Electrodes for Energy Storage. ACS Appl. Polym. Mater. 2019, 1, 1155–1164. [Google Scholar] [CrossRef]
- Li, J.; Han, S.; Zhang, C.; Wei, W.; Gu, M.; Meng, L. High-Performance and Reactivation Characteristics of High-Quality, Graphene-Supported SnS 2 Heterojunctions for a Lithium-Ion Battery Anode. ACS Appl. Mater. Interfaces 2019, 11, 22314–22322. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Lee, S.W.; Moon, K.H.; Cho, S. Fabrication of Solid-State Asymmetric Supercapacitors Based on Aniline Oligomers and Graphene Electrodes with Enhanced Electrochemical Performances. ACS Omega 2019, 4, 1244–1253. [Google Scholar] [CrossRef]
- Han, C.; Shi, R.; Zhou, D.; Li, H.; Xu, L.; Zhang, T.; Li, J.; Kang, F.; Wang, G.; Li, B. High-Energy and High-Power Nonaqueous Lithium-Ion Capacitors Based on Polypyrrole/Carbon Nanotube Composites as Pseudocapacitive Cathodes. ACS Appl. Mater. Interfaces 2019, 11, 15646–15655. [Google Scholar] [CrossRef]
- Li, X.; Zhao, T.; Chen, Q.; Li, P.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Wei, B.; Zhu, H. Flexible All Solid-State Supercapacitors Based on Chemical Vapor Deposition Derived Graphene Fibers. Phys. Chem. Chem. Phys. 2013, 15, 17752. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Zhou, S.; Zhao, N.; Wong, C.-P. Facile and Scalable Fabrication of Three-Dimensional Cu(OH) 2 Nanoporous Nanorods for Solid-State Supercapacitors. J. Mater. Chem. A 2015, 3, 17385–17391. [Google Scholar] [CrossRef]
- Khomenko, V.; Frackowiak, E.; Béguin, F. Determination of the Specific Capacitance of Conducting Polymer/Nanotubes Composite Electrodes Using Different Cell Configurations. Electrochim. Acta 2005, 50, 2499–2506. [Google Scholar] [CrossRef]
- Fan, L.-Z.; Hu, Y.-S.; Maier, J.; Adelhelm, P.; Smarsly, B.; Antonietti, M. High Electroactivity of Polyaniline in Supercapacitors by Using a Hierarchically Porous Carbon Monolith as a Support. Adv. Funct. Mater. 2007, 17, 3083–3087. [Google Scholar] [CrossRef]
- Fan, H.; Liu, W.; Shen, W. Honeycomb-like Composite Structure for Advanced Solid State Asymmetric Supercapacitors. Chem. Eng. J. 2017, 326, 518–527. [Google Scholar] [CrossRef]
- Guo, W.; Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Superior Radical Polymer Cathode Material with a Two-Electron Process Redox Reaction Promoted by Graphene. Energy Environ. Sci. 2012, 5, 5221–5225. [Google Scholar] [CrossRef]
- Sun, M.; Li, H.; Wang, J.; Wang, G. Promising Graphene/Carbon Nanotube Foam@π-Conjugated Polymer Self-Supporting Composite Cathodes for High-Performance Rechargeable Lithium Batteries. Carbon N. Y. 2015, 94, 864–871. [Google Scholar] [CrossRef]
- Song, Z.; Xu, T.; Gordin, M.L.; Jiang, Y.-B.; Bae, I.-T.; Xiao, Q.; Zhan, H.; Liu, J.; Wang, D. Polymer–Graphene Nanocomposites as Ultrafast-Charge and -Discharge Cathodes for Rechargeable Lithium Batteries. Nano Lett. 2012, 12, 2205–2211. [Google Scholar] [CrossRef]
- Ji, L.; Meduri, P.; Agubra, V.; Xiao, X.; Alcoutlabi, M. Graphene-Based Nanocomposites for Energy Storage. Adv. Energy Mater. 2016, 6, 1502159. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-Based Materials for Supercapacitor Electrodes–A Review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, S.; Zhang, Z.; Liu, H.; Xiao, J.; Wan, L.; Luo, J.; Wang, S.; Liu, Y. Scalable Synthesis of Freestanding Sandwich-Structured Graphene/Polyaniline/Graphene Nanocomposite Paper for Flexible All-Solid-State Supercapacitor. Sci. Rep. 2015, 5, 9359. [Google Scholar] [CrossRef]
- Biswas, S.; Drzal, L.T. Multilayered Nanoarchitecture of Graphene Nanosheets and Polypyrrole Nanowires for High Performance Supercapacitor Electrodes. Chem. Mater. 2010, 22, 5667–5671. [Google Scholar] [CrossRef]
- Park, J.H.; Ko, J.M.; Park, O.O.; Kim, D.-W. Capacitance Properties of Graphite/Polypyrrole Composite Electrode Prepared by Chemical Polymerization of Pyrrole on Graphite Fiber. J. Power Sources 2002, 105, 20–25. [Google Scholar] [CrossRef]
- Chee, W.K.; Lim, H.N.; Harrison, I.; Chong, K.F.; Zainal, Z.; Ng, C.H.; Huang, N.M. Performance of Flexible and Binderless Polypyrrole/Graphene Oxide/Zinc Oxide Supercapacitor Electrode in a Symmetrical Two-Electrode Configuration. Electrochim. Acta 2015, 157, 88–94. [Google Scholar] [CrossRef]
- Lim, Y.S.; Tan, Y.P.; Lim, H.N.; Huang, N.M.; Tan, W.T.; Yarmo, M.A.; Yin, C.-Y. Potentiostatically Deposited Polypyrrole/Graphene Decorated Nano-Manganese Oxide Ternary Film for Supercapacitors. Ceram. Int. 2014, 40, 3855–3864. [Google Scholar] [CrossRef]
- Xiong, P.; Huang, H.; Wang, X. Design and Synthesis of Ternary Cobalt Ferrite/Graphene/Polyaniline Hierarchical Nanocomposites for High-Performance Supercapacitors. J. Power Sources 2014, 245, 937–946. [Google Scholar] [CrossRef]
- Guo, X.; Feng, B.; Gai, L.; Zhou, J. Reduced Graphene Oxide/Polymer Dots-Based Flexible Symmetric Supercapacitors Delivering an Output Potential of 1.7 V with Electrochemical Charge Injection. Electrochim. Acta 2019, 293, 399–407. [Google Scholar] [CrossRef]
- Akhina, H.; Mohammed Arif, P.; Gopinathan Nair, M.R.; Nandakumar, K.; Thomas, S. Development of Plasticized Poly (Vinyl Chloride)/Reduced Graphene Oxide Nanocomposites for Energy Storage Applications. Polym. Test. 2019, 73, 250–257. [Google Scholar] [CrossRef]
- Suneetha, R.B.; Selvi, P.; Vedhi, C. Synthesis, Structural and Electrochemical Characterization of Zn Doped Iron Oxide/Grapheneoxide/Chitosan Nanocomposite for Supercapacitor Application. Vacuum 2019, 164, 396–404. [Google Scholar] [CrossRef]
- Chabi, S.; Peng, C.; Yang, Z.; Xia, Y.; Zhu, Y. Three Dimensional (3D) Flexible Graphene Foam/Polypyrrole Composite: Towards Highly Efficient Supercapacitors. RSC Adv. 2015, 5, 3999–4008. [Google Scholar] [CrossRef]
- Azizi, E.; Arjomandi, J.; Lee, J.Y. Reduced Graphene Oxide/Poly(1,5 Dihydroxynaphthalene)/TiO2 Nanocomposite Conducting Polymer Coated on Gold as a Supercapacitor Electrode. Electrochim. Acta 2019, 298, 726–734. [Google Scholar] [CrossRef]
- Pan, Q.; Zhao, J.; Qu, W.; Liu, R.; Li, N.; Xing, B.; Jiang, S.; Pang, M.; Zhao, L.; Zhang, Y.; et al. Facile Synthesis of the 3D Framework Si@N-Doped C/Reduced Graphene Oxide Composite by Polymer Network Method for Highly Stable Lithium Storage. J. Phys. Chem. Solids 2019, 133, 92–99. [Google Scholar] [CrossRef]
- Arukula, R.; Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Cumulative Effect of Bimetallic Alloy, Conductive Polymer and Graphene toward Electrooxidation of Methanol: An Efficient Anode Catalyst for Direct Methanol Fuel Cells. J. Alloys Compd. 2019, 771, 477–488. [Google Scholar] [CrossRef]
- Baruah, B.; Kumar, A. PEDOT:PSS/MnO2/RGO Ternary Nanocomposite Based Anode Catalyst for Enhanced Electrocatalytic Activity of Methanol Oxidation for Direct Methanol Fuel Cell. Synth. Met. 2018, 245, 74–86. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Zhao, J.; Ren, W.; Chen, Z.-G.; Tan, J.; Wu, Z.-S.; Gentle, I.; Lu, G.Q.; Cheng, H.-M. Fabrication of Graphene/Polyaniline Composite Paper via In Situ Anodic Electropolymerization for High-Performance Flexible Electrode. ACS Nano 2009, 3, 1745–1752. [Google Scholar] [CrossRef]
- Gómez, H.; Ram, M.K.; Alvi, F.; Villalba, P.; Stefanakos, E.L.; Kumar, A. Graphene-Conducting Polymer Nanocomposite as Novel Electrode for Supercapacitors. J. Power Sources 2011, 196, 4102–4108. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Y.; Wang, X. Controllable Preparation of Polyaniline-Graphene Nanocomposites Using Functionalized Graphene for Supercapacitor Electrodes. Chemistry 2015, 21, 10408–10415. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wei, T.; Shao, B.; Fan, Z.; Qian, W.; Zhang, M.; Wei, F. Preparation of a Graphene Nanosheet/Polyaniline Composite with High Specific Capacitance. Carbon N. Y. 2010, 48, 487–493. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.; Zu, S.-Z.; Han, B.-H.; Wei, Z. Hierarchical Nanocomposites of Polyaniline Nanowire Arrays on Graphene Oxide Sheets with Synergistic Effect for Energy Storage. ACS Nano 2010, 4, 5019–5026. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wen, N.; Wang, X.; Zheng, Y. A High-Performance Hierarchical Graphene@Polyaniline@Graphene Sandwich Containing Hollow Structures for Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2015, 3, 475–482. [Google Scholar] [CrossRef]
- Chi, K.; Zhang, Z.; Xi, J.; Huang, Y.; Xiao, F.; Wang, S.; Liu, Y. Freestanding Graphene Paper Supported Three-Dimensional Porous Graphene–Polyaniline Nanocomposite Synthesized by Inkjet Printing and in Flexible All-Solid-State Supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 16312–16319. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xu, Q.; Wang, K.; Chen, J.; Chen, Z. Fabrication of Free-Standing Hierarchical Carbon Nanofiber/Graphene Oxide/Polyaniline Films for Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 200–209. [Google Scholar] [CrossRef]
- Liu, A.; Li, C.; Bai, H.; Shi, G. Electrochemical Deposition of Polypyrrole/Sulfonated Graphene Composite Films. J. Phys. Chem. C 2010, 114, 22783–22789. [Google Scholar] [CrossRef]
- Aphale, A.; Maisuria, K.; Mahapatra, M.K.; Santiago, A.; Singh, P.; Patra, P. Hybrid Electrodes by In-Situ Integration of Graphene and Carbon-Nanotubes in Polypyrrole for Supercapacitors. Sci. Rep. 2015, 5, 14445. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, H.P.; Sydlik, S.A.; Swager, T.M. Supercapacitors from Free-Standing Polypyrrole/Graphene Nanocomposites. J. Phys. Chem. C 2013, 117, 10270–10276. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Tang, J.; Tang, W. Three-Dimensional, Chemically Bonded Polypyrrole/Bacterial Cellulose/Graphene Composites for High-Performance Supercapacitors. Chem. Mater. 2015, 27, 7034–7041. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, X.; Xie, C.; Wan, G.; Zhang, H.; Youhong, T. Flexible, Free-Standing TiO 2 –Graphene–Polypyrrole Composite Films as Electrodes for Supercapacitors. J. Phys. Chem. C 2015, 119, 3903–3910. [Google Scholar] [CrossRef]
- Fan, X.; Yang, Z.; He, N. Hierarchical Nanostructured Polypyrrole/Graphene Composites as Supercapacitor Electrode. RSC Adv. 2015, 5, 15096–15102. [Google Scholar] [CrossRef]
- Alvi, F.; Basnayaka, P.A.; Ram, M.K.; Gomez, H.; Stefanako, E.; Goswami, Y.; Kumar, A. Graphene-Polythiophene Nanocomposite as Novel Supercapacitor Electrode Material. J. New Mater. Electrochem. Syst. 2011, 15, 89–95. [Google Scholar] [CrossRef]
- Alvi, F.; Ram, M.K.; Basnayaka, P.; Stefanakos, E.; Goswami, Y.; Hoff, A.; Kumar, A. Electrochemical Supercapacitors Based on Graphene-Conducting Polythiophenes Nanocomposite. ECS Trans. 2011, 35, 167–174. [Google Scholar] [CrossRef]
- Alvi, F.; Ram, M.K.; Basnayaka, P.A.; Stefanakos, E.; Goswami, Y.; Kumar, A. Graphene–Polyethylenedioxythiophene Conducting Polymer Nanocomposite Based Supercapacitor. Electrochim. Acta 2011, 56, 9406–9412. [Google Scholar] [CrossRef]
- Lehtimäki, S.; Suominen, M.; Damlin, P.; Tuukkanen, S.; Kvarnström, C.; Lupo, D. Preparation of Supercapacitors on Flexible Substrates with Electrodeposited PEDOT/Graphene Composites. ACS Appl. Mater. Interfaces 2015, 7, 22137–22147. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhou, T.; Wang, Z.; Xue, W.; Feng, C.; Zhang, F.; Huang, X.; Yang, D.; Théato, P.; Li, Y. Radical Polymer Grafted Graphene for High-Performance Li+/Na+ Organic Cathodes. J. Power Sources 2021, 511, 230363. [Google Scholar] [CrossRef]
- Ma, J.; Kong, Y.; Luo, Y.; Huang, Y.; Han, S. Flexible Polyimide Nanorod/Graphene Framework as an Organic Cathode for Rechargeable Sodium-Ion Batteries. J. Phys. Chem. C 2021, 125, 6564–6569. [Google Scholar] [CrossRef]
- Kobayashi, H.; Oizumi, K.; Tomai, T.; Honma, I. Graphene and Polyethyleneimine Bilayer Wrapping onto Quinone Molecular Crystal Cathode Materials for Aqueous Zinc-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 4707–4711. [Google Scholar] [CrossRef]
- Wang, N.; Hou, D.; Li, Q.; Zhang, P.; Wei, H.; Mai, Y. Two-Dimensional Interface Engineering of Mesoporous Polydopamine on Graphene for Novel Organic Cathodes. ACS Appl. Energy Mater. 2019, 2, 5816–5823. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Yang, G.; Bu, F.; Li, K.; Shakir, I.; Xu, Y. Dispersion–Assembly Approach to Synthesize Three-Dimensional Graphene/Polymer Composite Aerogel as a Powerful Organic Cathode for Rechargeable Li and Na Batteries. ACS Appl. Mater. Interfaces 2017, 9, 15549–15556. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Kim, D.-G.; Kim, H.J.; Lee, J.H.; Baik, J.-H.; Lee, J.-C. Novel Composite Polymer Electrolytes Containing Poly(Ethylene Glycol)-Grafted Graphene Oxide for All-Solid-State Lithium-Ion Battery Applications. J. Mater. Chem. A 2014, 2, 13873–13883. [Google Scholar] [CrossRef]
- Zhang, Y.; Si, L.; Zhou, B.; Zhao, B.; Zhu, Y.; Zhu, L.; Jiang, X. Synthesis of Novel Graphene Oxide/Pristine Graphene/Polyaniline Ternary Composites and Application to Supercapacitor. Chem. Eng. J. 2016, 288, 689–700. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, J.U.; Jo, J.W.; Bae, S.; Kim, K.T.; Jo, W.H. In-Situ Preparation of Graphene/Poly(Styrenesulfonic Acid-Graft-Polyaniline) Nanocomposite via Direct Exfoliation of Graphite for Supercapacitor Application. Carbon N. Y. 2016, 105, 191–198. [Google Scholar] [CrossRef]
- Bulin, C.; Yu, H.; Ge, X.; Xin, G.; Xing, R.; Li, R.; Zhang, B. Preparation and Supercapacitor Performance of Functionalized Graphene Aerogel Loaded with Polyaniline as a Freestanding Electrode. J. Mater. Sci. 2017, 52, 5871–5881. [Google Scholar] [CrossRef]
- Hong, X.; Zhang, B.; Murphy, E.; Zou, J.; Kim, F. Three-Dimensional Reduced Graphene Oxide/Polyaniline Nanocomposite Film Prepared by Diffusion Driven Layer-by-Layer Assembly for High-Performance Supercapacitors. J. Power Sources 2017, 343, 60–66. [Google Scholar] [CrossRef]
- Ke, F.; Liu, Y.; Xu, H.; Ma, Y.; Guang, S.; Zhang, F.; Lin, N.; Ye, M.; Lin, Y.; Liu, X. Flower-like Polyaniline/Graphene Hybrids for High-Performance Supercapacitor. Compos. Sci. Technol. 2017, 142, 286–293. [Google Scholar] [CrossRef]
- Li, R. Graphene-Enabled Improved Supercapacitor Performance of Polyaniline Nanofiber Composites. Int. J. Electrochem. Sci. 2017, 12, 144–154. [Google Scholar] [CrossRef]
- Qin, G.; Zhang, H.; Liao, H.; Li, Z.; Tian, J.; Lin, Y.; Zhang, D.; Wu, Q. Novel Graphene Nanosheet-Wrapped Polyaniline Rectangular-like Nanotubes for Flexible All-Solid-State Supercapacitors. J. Mater. Sci. 2017, 52, 10981–10992. [Google Scholar] [CrossRef]
- Tabrizi, A.G.; Arsalani, N.; Namazi, H.; Ahadzadeh, I. Vanadium Oxide Assisted Synthesis of Polyaniline Nanoarrays on Graphene Oxide Sheets and Its Application in Supercapacitors. J. Electroanal. Chem. 2017, 798, 34–41. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, D.; Hu, N.; Yang, C.; Li, M.; Wei, H.; Yang, Z.; Su, Y.; Zhang, Y. Three-Dimensional Structures of Graphene/Polyaniline Hybrid Films Constructed by Steamed Water for High-Performance Supercapacitors. J. Power Sources 2017, 342, 1–8. [Google Scholar] [CrossRef]
- Ates, M.; El-Kady, M.; Kaner, R.B. Three-Dimensional Design and Fabrication of Reduced Graphene Oxide/Polyaniline Composite Hydrogel Electrodes for High Performance Electrochemical Supercapacitors. Nanotechnology 2018, 29, 175402. [Google Scholar] [CrossRef]
- Ji, J.; Li, R.; Li, H.; Shu, Y.; Li, Y.; Qiu, S.; He, C.; Yang, Y. Phytic Acid Assisted Fabrication of Graphene/Polyaniline Composite Hydrogels for High-Capacitance Supercapacitors. Compos. Part B Eng. 2018, 155, 132–137. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, H.; Xing, R.; Ge, X.; Sun, H.; Li, R.; Zhang, Q. Multi-Growth Site Graphene/Polyaniline Composites with Highly Enhanced Specific Capacitance and Rate Capability for Supercapacitor Application. Electrochim. Acta 2018, 260, 504–513. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Z.; Zhong, W.; Yang, W. Hydrothermal Direct Synthesis of Polyaniline, Graphene/Polyaniline and N-Doped Graphene/Polyaniline Hydrogels for High Performance Flexible Supercapacitors. J. Mater. Chem. A 2018, 6, 9245–9256. [Google Scholar] [CrossRef]
- Wu, X.; Tang, L.; Zheng, S.; Huang, Y.; Yang, J.; Liu, Z.; Yang, W.; Yang, M. Hierarchical Unidirectional Graphene Aerogel/Polyaniline Composite for High Performance Supercapacitors. J. Power Sources 2018, 397, 189–195. [Google Scholar] [CrossRef]
- Liu, J.; Du, P.; Wang, Q.; Liu, D.; Liu, P. Mild Synthesis of Holey N-Doped Reduced Graphene Oxide and Its Double-Edged Effects in Polyaniline Hybrids for Supercapacitor Application. Electrochim. Acta 2019, 305, 175–186. [Google Scholar] [CrossRef]
- Moyseowicz, A.; Gryglewicz, G. Hydrothermal-Assisted Synthesis of a Porous Polyaniline/Reduced Graphene Oxide Composite as a High-Performance Electrode Material for Supercapacitors. Compos. Part B Eng. 2019, 159, 4–12. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, D.; Chen, Y.; Huang, J.; Zhang, Z.; Zhang, X.; Zhang, B. A Novel Core-Shell Polyaniline/Graphene Oxide/Copper Nanocomposite for High Performance and Low-Cost Supercapacitors. Chem. Pap. 2019, 73, 119–129. [Google Scholar] [CrossRef]
- Du, X.; Shi, X.; Li, Y.; Cao, K. Construction of N, S-co-doped Graphene/Polyaniline Composite as Free-standing Electrode Material. Int. J. Energy Res. 2021, 45, 6227–6238. [Google Scholar] [CrossRef]
- Ge, M.; Hao, H.; Lv, Q.; Wu, J.; Li, W. Hierarchical Nanocomposite That Coupled Nitrogen-Doped Graphene with Aligned PANI Cores Arrays for High-Performance Supercapacitor. Electrochim. Acta 2020, 330, 135236. [Google Scholar] [CrossRef]
- Li, T.; Wang, X.; Liu, P.; Yang, B.; Diao, S.; Gao, Y. Synthesis of Graphene/Polyaniline Copolymer for Solid-State Supercapacitor. J. Electroanal. Chem. 2020, 860, 113908. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, F.; Chen, N.; Tan, L.; Liu, C.; Hu, D.; Zhao, D. Enhanced Capacitive Performance of Polyaniline on Hydroquinone-Functionalized Three-Dimensional Porous Graphene Substrate for Supercapacitors. J. Mater. Sci. Mater. Electron. 2021, 32, 5655–5667. [Google Scholar] [CrossRef]
- Hareesh, K.; Rondiya, S.R.; Dzade, N.Y.; Dhole, S.D.; Williams, J.; Sergey, S. Polymer-Wrapped Reduced Graphene Oxide/Nickel Cobalt Ferrite Nanocomposites as Tertiary Hybrid Supercapacitors: Insights from Experiment and Simulation. J. Sci. Adv. Mater. Devices 2021, 6, 291–301. [Google Scholar] [CrossRef]
- Kung, C.-Y.; Wang, T.-L.; Lin, H.-Y.; Yang, C.-H. A High-Performance Covalently Bonded Self-Doped Polyaniline–Graphene Assembly Film with Superior Stability for Supercapacitors. J. Power Sources 2021, 490, 229538. [Google Scholar] [CrossRef]
- Macherla, N.; Singh, K.; Santosh, M.S.; Kumari, K.; Lekkala, R.G.R. Heat Assisted Facile Synthesis of Nanostructured Polyaniline/Reduced Crumbled Graphene Oxide as a High-Performance Flexible Electrode Material for Supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125982. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, H.; Huang, H.; Li, L.; Yu, X. Graphene Oxide/Polypyrrole/Polyaniline Composite Hydrogel Synthesized by Vapor-Liquid Interfacial Method for Supercapacitors. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 626, 127125. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Xu, X.; Wang, C. Facile Route for the Preparation of Functionalized Reduced Graphene Oxide/Polyaniline Composite and Its Enhanced Electrochemical Performance. ECS J. Solid State Sci. Technol. 2021, 10, 031003. [Google Scholar] [CrossRef]
- Naoi, K.; Naoi, W.; Aoyagi, S.; Miyamoto, J.; Kamino, T. New Generation “Nanohybrid Supercapacitor Technology”. Acc. Chem. Res. 2013, 46, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent Advancements in Supercapacitor Technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Libich, J.; Máca, J.; Vondrák, J.; Čech, O.; Sedlaříková, M. Supercapacitors: Properties and Applications. J. Energy Storage 2018, 17, 224–227. [Google Scholar] [CrossRef]
- Gong, S.; Li, X.; Sheng, M.; Liu, S.; Zheng, Y.; Wu, H.; Lu, X.; Qu, J. High Thermal Conductivity and Mechanical Strength Phase Change Composite with Double Supporting Skeletons for Industrial Waste Heat Recovery. ACS Appl. Mater. Interfaces 2021, 13, 47174–47184. [Google Scholar] [CrossRef]
- Yin, G.-Z.; Yang, X.-M.; Hobson, J.; López, A.M.; Wang, D.-Y. Bio-Based Poly (Glycerol-Itaconic Acid)/PEG/APP as Form Stable and Flame-Retardant Phase Change Materials. Compos. Commun. 2022, 30, 101057. [Google Scholar] [CrossRef]
- Conway, B.E. Similarities and Differences between Supercapacitors and Batteries for Storing Electrical Energy. In Electrochemical Supercapacitors; Springer: Boston, MA, USA, 1999; pp. 11–31. [Google Scholar]
- Hu, C.-C.; Chang, K.-H.; Lin, M.-C.; Wu, Y.-T. Design and Tailoring of the Nanotubular Arrayed Architecture of Hydrous RuO 2 for Next Generation Supercapacitors. Nano Lett. 2006, 6, 2690–2695. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.-L.; Simon, P. Ultrahigh-Power Micrometre-Sized Supercapacitors Based on Onion-like Carbon. Nat. Nanotechnol. 2010, 5, 651–654. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.-M. Towards Greener and More Sustainable Batteries for Electrical Energy Storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Mousty, C.; Leroux, F. LDHs as Electrode Materials for Electrochemical Detection and Energy Storage: Supercapacitor, Battery and (Bio)-Sensor. Recent Pat. Nanotechnol. 2012, 6, 174–192. [Google Scholar] [CrossRef]
- Chen, G.Z. Supercapacitor and Supercapattery as Emerging Electrochemical Energy Stores. Int. Mater. Rev. 2017, 62, 173–202. [Google Scholar] [CrossRef]
- Bao, L.; Zang, J.; Li, X. Flexible Zn2SnO4/MnO2 Core/Shell Nanocable−Carbon Microfiber Hybrid Composites for High-Performance Supercapacitor Electrodes. Nano Lett. 2011, 11, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Augustyn, V.; Wen, J.; Zhang, Y.; Shen, M.; Dunn, B.; Lu, Y. High-Performance Supercapacitors Based on Intertwined CNT/V2O5 Nanowire Nanocomposites. Adv. Mater. 2011, 23, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.S.; Lee, Y.-G.; Hong, Y.-S.; Park, Y.J.; Wu, X.; Kim, K.M.; Kang, M.G.; Park, N.-G.; Chang, S.H. Poly(Ethylenedioxythiophene) (PEDOT) as Polymer Electrode in Redox Supercapacitor. Electrochim. Acta 2004, 50, 843–847. [Google Scholar] [CrossRef]
- Kim, Y.-T.; Tadai, K.; Mitani, T. Highly Dispersed Ruthenium Oxide Nanoparticles on Carboxylated Carbon Nanotubes for Supercapacitor Electrode Materials. J. Mater. Chem. 2005, 15, 4914. [Google Scholar] [CrossRef]
- Sen, P.; De, A.; Chowdhury, A.D.; Bandyopadhyay, S.K.; Agnihotri, N.; Mukherjee, M. Conducting Polymer Based Manganese Dioxide Nanocomposite as Supercapacitor. Electrochim. Acta 2013, 108, 265–273. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Chen, X.; Du, X. Electrochemical Supercapacitor Electrode Material Based on Poly(3,4-Ethylenedioxythiophene)/Polypyrrole Composite. J. Power Sources 2007, 163, 1120–1125. [Google Scholar] [CrossRef]
- Chen, H.-W.; Hsu, C.-Y.; Chen, J.-G.; Lee, K.-M.; Wang, C.-C.; Huang, K.-C.; Ho, K.-C. Plastic Dye-Sensitized Photo-Supercapacitor Using Electrophoretic Deposition and Compression Methods. J. Power Sources 2010, 195, 6225–6231. [Google Scholar] [CrossRef]
- White, A.M.; Slade, R.C.T. Electrochemically and Vapour Grown Electrode Coatings of Poly(3,4-Ethylenedioxythiophene) Doped with Heteropolyacids. Electrochim. Acta 2004, 49, 861–865. [Google Scholar] [CrossRef]
- Snook, G.A.; Peng, C.; Fray, D.J.; Chen, G.Z. Achieving High Electrode Specific Capacitance with Materials of Low Mass Specific Capacitance: Potentiostatically Grown Thick Micro-Nanoporous PEDOT Films. Electrochem. Commun. 2007, 9, 83–88. [Google Scholar] [CrossRef]
- Liu, K.; Hu, Z.; Xue, R.; Zhang, J.; Zhu, J. Electropolymerization of High Stable Poly(3,4-Ethylenedioxythiophene) in Ionic Liquids and Its Potential Applications in Electrochemical Capacitor. J. Power Sources 2008, 179, 858–862. [Google Scholar] [CrossRef]
- Patake, V.D.; Lokhande, C.D.; Joo, O.S. Electrodeposited Ruthenium Oxide Thin Films for Supercapacitor: Effect of Surface Treatments. Appl. Surf. Sci. 2009, 255, 4192–4196. [Google Scholar] [CrossRef]
- Poizot, P.; Dolhem, F. Clean Energy New Deal for a Sustainable World: From Non-CO2 Generating Energy Sources to Greener Electrochemical Storage Devices. Energy Environ. Sci. 2011, 4, 2003. [Google Scholar] [CrossRef]
- Stenger-Smith, J.D.; Webber, C.K.; Anderson, N.; Chafin, A.P.; Zong, K.; Reynolds, J.R. Poly(3,4-Alkylenedioxythiophene)-Based Supercapacitors Using Ionic Liquids as Supporting Electrolytes. J. Electrochem. Soc. 2002, 149, A973. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Rao, M.M.; Venugopal, N.; Kim, K.-B. Hydrothermal Synthesis of SnO2–V2O5 Mixed Oxide and Electrochemical Screening of Carbon Nano-Tubes (CNT), V2O5, V2O5–CNT, and SnO2–V2O5–CNT Electrodes for Supercapacitor Applications. J. Power Sources 2007, 166, 578–583. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/Polyaniline Nanofiber Composites as Supercapacitor Electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Wang, H.; Yan, X.; Piao, G. A High-Performance Supercapacitor Based on Fullerene C 60 Whisker and Polyaniline Emeraldine Base Composite. Electrochim. Acta 2017, 231, 264–271. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Joseph, S.; Saianand, G.; Gopalan, A.-I.; Sarkar, S.; Srinivasan, S.; Park, D.-H.; Kim, S.; Talapaneni, S.N.; Ramadass, K.; et al. Highly Ordered Iron Oxide-Mesoporous Fullerene Nanocomposites for Oxygen Reduction Reaction and Supercapacitor Applications. Microporous Mesoporous Mater. 2019, 285, 21–31. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, C.; Yan, Z.; Zhu, Y.; Peng, Z.; Hauge, R.H.; Natelson, D.; Tour, J.M. 3-Dimensional Graphene Carbon Nanotube Carpet-Based Microsupercapacitors with High Electrochemical Performance. Nano Lett. 2013, 13, 72–78. [Google Scholar] [CrossRef]
- Xiong, S.; Yang, F.; Jiang, H.; Ma, J.; Lu, X. Covalently Bonded Polyaniline/Fullerene Hybrids with Coral-like Morphology for High-Performance Supercapacitor. Electrochim. Acta 2012, 85, 235–242. [Google Scholar] [CrossRef]
- Xiong, S.; Phua, S.L.; Dunn, B.S.; Ma, J.; Lu, X. Covalently Bonded Polyaniline−TiO2 Hybrids: A Facile Approach to Highly Stable Anodic Electrochromic Materials with Low Oxidation Potentials. Chem. Mater. 2010, 22, 255–260. [Google Scholar] [CrossRef]
- Dou, F.; Silva, C.; Zhang, X. The Role of Acceptor-Rich Domain in Optoelectronic Properties of Photovoltaic Diodes Based on Polymer Blends. Chem. Phys. Lett. 2013, 583, 92–96. [Google Scholar] [CrossRef]
- Ramadan, A.; Anas, M.; Ebrahim, S.; Soliman, M.; Abou-Aly, A.I. Polyaniline/Fullerene Derivative Nanocomposite for Highly Efficient Supercapacitor Electrode. Int. J. Hydrogen Energy 2020, 45, 16254–16265. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Wang, Q.; Xie, H.Y.; Ho, L.P.; Tan, D.M.F.; Diao, Y.Y.; Chen, W.; Xie, X.N. Fabrication of Highly Ordered P3HT:PCBM Nanostructures and Its Application as a Supercapacitive Electrode. Nanoscale 2012, 4, 3725. [Google Scholar] [CrossRef]
- Momodu, D.; Bello, A.; Dangbegnon, J.; Barzeger, F.; Fabiane, M.; Manyala, N. P3HT:PCBM/Nickel-Aluminum Layered Double Hydroxide-Graphene Foam Composites for Supercapacitor Electrodes. J. Solid State Electrochem. 2015, 19, 445–452. [Google Scholar] [CrossRef]
- Kausar, A. Fullerene Reinforced Polymeric Nanocomposites for Energy Storage—Status and Prognoses. Front. Mater. 2022, 9, 150. [Google Scholar] [CrossRef]
- Wu, N.-L. Nanocrystalline Oxide Supercapacitors. Mater. Chem. Phys. 2002, 75, 6–11. [Google Scholar] [CrossRef]
- Muthu, M.; Gopal, J.; Chun, S.; Devadoss, A.J.P.; Hasan, N.; Sivanesan, I. Crustacean Waste-Derived Chitosan: Antioxidant Properties and Future Perspective. Antioxidants 2021, 10, 228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sivanesan, I.; Gopal, J.; Muthu, M.; Shin, J.; Mari, S.; Oh, J. Green Synthesized Chitosan/Chitosan Nanoforms/Nanocomposites for Drug Delivery Applications. Polymers 2021, 13, 2256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, J.W.; Shin, J.; Chun, S.; Muthu, M.; Gopal, J. Evaluating the Anticarcinogenic Activity of Surface Modified/Functionalized Nanochitosan: The Emerging Trends and Endeavors. Polymers 2021, 13, 3138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sivanesan, I.; Muthu, M.; Gopal, J.; Hasan, N.; Kashif Ali, S.; Shin, J.; Oh, J.W. Nanochitosan: Commemorating the Metamorphosis of an ExoSkeletal Waste to a Versatile Nutraceutical. Nanomaterials 2021, 11, 821. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gopal, J.; Muthu, M.; Dhakshanamurthy, T.; Kim, K.J.; Hasan, N.; Kwon, S.J.; Chun, S. Sustainable ecofriendly phytoextract mediated one pot green recovery of chitosan. Sci. Rep. 2019, 9, 13832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gopal, J.; Abdelhamid, H.N.; Hua, P.Y.; Wu, H.F. Chitosan nanomagnets for effective extraction and sensitive mass spectrometric detection of pathogenic bacterial endotoxin from human urine. J. Mater. Chem. B 2013, 1, 2463–2475. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, I.; Gopal, J.; Muthu, M.; Shin, J.; Oh, J.W. Reviewing Chitin/Chitosan Nanofibers and Associated Nanocomposites and Their Attained Medical Milestones. Polymers 2021, 13, 2330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramkumar, R.; Minakshi, M. Fabrication of ultrathin CoMoO4 nanosheets modified with chitosan and their improved performance in energy storage device. Dalton Trans. 2015, 44, 6158–6168. [Google Scholar] [CrossRef]
- Minakshi, M.; Meyrick, D.; Appadoo, D. Maricite (NaMn1/3Ni1/3Co1/3PO4)/activated carbon: Hybrid capacitor. Energy Fuels 2013, 27, 3516–3522. [Google Scholar] [CrossRef]
- Minakshi, M.; Duraisamy, S.; Tirupathi, P.; Kandhasamy, S.; Munichandraiah, N. Multi-component olivine for lithium-ion hybrid capacitor. Int. J. Electrochem. Sci. 2014, 9, 5974–5992. [Google Scholar]
- Ramkumarar, R.; Sundaram, M.M. A biopolymer gel-decorated cobalt molybdate nanowafer: Effective graft polymer cross-linked with an organic acid for better energy storage. New J. Chem. 2016, 40, 2863–2877. [Google Scholar] [CrossRef]
- Revanappa, S.K.; Soni, I.; Siddalinganahalli, M.; Jayaprakash, G.K.; Flores-Moreno, R.; Bananakere Nanjegowda, C. A Fukui Analysis of an Arginine-Modified Carbon Surface for the Electrochemical Sensing of Dopamine. Materials 2022, 15, 6337. [Google Scholar] [CrossRef]



| Energy Storage Device | Carbon Materials | Polymer Composite | Electrochemical Performance | References |
|---|---|---|---|---|
| Lithium ion Batteries | Graphene sheets | poly(anthraquinonyl sulfide) and polyimide, | Ultrafast-Charge and -Discharge Cathodes | [60] |
| Li-ion/Na-ion batteries | Reduced Graphene oxide | radical polymer poly(2,2,6,6-tetramethylpiperidin-1-oxyl-4-yl methacrylate) (PTMA) | cathodes for Li-ion/Na-ion batteries with high energy storage | [95] |
| Na-ion batteries | Graphene sheets | polyimide nanorod composites(PInd) | superior capacity of 101.3 mAh g–1 at 500 mA g−1 after 1000 cycles. | [96] |
| Zinc-Ion Batteries | Reduced Graphene oxide | Polyethyleneimine + Quinone crystal | superior cyclability of charge/discharge cycle | [97] |
| Li-ion batteries | Reduced Graphene oxide | mesoporous polydopamine (mPDA), | superior cyclability of charge/discharge cycles | [98] |
| Li-ion/Na-ion batteries | Three-dimensional (3D) graphene framework | poly(anthraquinonyl sufide) (PAQS) | highest cathode charging and discharging capacity. | [99] |
| lithium-ion battery | Graphene oxide | polymer electrolytes (1) branched graft copolymer (BCP), poly(ethylene glycol) methyl ether methacrylate (PEGMA) (2) 3-(3,5,7,9,11,13,15-heptaisobutylpentacyclo-[9.5.1.13,9.15,15.17,13]octasiloxane-1-yl)propyl methacrylate (MA-POSS) (3) poly(ethylene glycol)-grafted graphene oxide (PGO) | Batteries with improved thermal and mechanical stabilities with PGO graphene nano hybrid. | [100] |
| Nanocomposites | Specific Capacitance (F g−1) | Current Density (A g−1) | Cycling Performances | Reference | |
|---|---|---|---|---|---|
| Capacity Retention (%) | Cycle Number | ||||
| Graphene oxide (GO)/pristine graphene (PG)/polyaniline (PANI) | 793.7 | 1.0 | 83.8 | 1000 | [101] |
| Graphene (Gr)/poly (styrenesulfonic acid-graft polyaniline) (S-g-A) | 767 | 0.5 | 92 | 5000 | [102] |
| Graphene aerogel (GA) functionalized with p-phenylenediamine/PANI | 810 | 1.0 | 83.2 | 10,000 | [103] |
| rGO/PANI film | 763 | 0.34 | 76.5 | 2000 | [104] |
| Flower-like PANI/graphene | 1510 | 1.0 | 89 | 1500 | [105] |
| Reduced graphene oxide (rGO)/PANI | 952 | 1.0 | 88 | 1000 | [106] |
| rGO/PANI | 850 | 1.0 | 93.2 | 10,000 | [107] |
| PANI/GO-vanadium (V)-ammonium persulfate (APS) | 712 | 0.5 | 83 | 6000 | [108] |
| rGO/PANI fiber films | 1180 | 1.0 | 99 | 10,000 | [109] |
| Graphene/PANI | 311.3 | 0.4 | 88.6 | 1000 | [110] |
| Graphene/PANI hydrogels | 865.6 | 1.0 | 82 | 1000 | [111] |
| 3D multi-growth site graphene (MSG)/PANI | 912 | 1.0 | 89.5 | 10,000 | [112] |
| N-doped graphene/PANI hydrogels | 514.3 | 1.0 | 84.7 | 1000 | [113] |
| Unidirectional graphene aerogel (UGA)/PANI | 538 | 1.0 | 74 | 1000 | [114] |
| rGO/poly (3,4-ethylenedioxythiophene) (PEDOT) | 202.7 | 1.0 | 90 | 9000 | [114] |
| Holey nitrogen-doped graphene oxide (H-NGO)/PANI-10 | 746 | 1.0 | 97 | 2000 | [115] |
| PANI/rGO-HT | 420 | 0.2 | 80 | 6000 | [116] |
| PANI/GO/copper (Cu) | 557.9 | 1.0 | 88 | 1000 | [117] |
| N, S-co-doped graphene hydrogel (N/SGH)/PANI | 236.5 | 0.5 | 95.1 | 1000 | [118] |
| Nitrogen-doped graphene/PANI-35% | 620 | 0.5 | 87.4 | 5000 | [119] |
| Graphene/PANI copolymer | 1701.1 | 0.34 | 94.9 | 5000 | [120] |
| 3D porous graphene/PANI | 542 | 1.14 | 82 | 3000 | [121] |
| Polymer-wrapped rGO/nickel cobalt ferrite | 1286 | 0.5 | 95 | 6000 | [122] |
| Self-doped PANI/bonded graphene | 642.6 | 1.0 | 100 | 5000 | [123] |
| PANI/ /reduced crumbled GO | 299 | 0.5 | 88.5 | 2000 | [124] |
| GO/polypyrrole (PPy)/PANI | 131 | 8.0 | 91 | 2000 | [125] |
| Functionalized rGO/PANI | 421 | 0.6 | 84.6 | 800 | [126] |
| Physical Properties | Graphene | Fullerenes |
|---|---|---|
| Charge carrier mobility | ~200,000 cm2/V·s | ~1.2 × 10−5 cm2/V·s |
| Thermal conductivity | ~5000 W/m·K | 0.4 W/m·K |
| Transparency | ~97.4% | ~85% |
| Specific surface area | ~2630 m2/g | ~300 m2/g |
| Young’s modulus | ~1 TPa | 14 × 109 Pa |
| Tensile strength | ~1100 GPa | <100 GPa |
| Band gap | Zero | ~1.4–3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gopal, J.; Muthu, M.; Sivanesan, I. A Comprehensive Compilation of Graphene/Fullerene Polymer Nanocomposites for Electrochemical Energy Storage. Polymers 2023, 15, 701. https://doi.org/10.3390/polym15030701
Gopal J, Muthu M, Sivanesan I. A Comprehensive Compilation of Graphene/Fullerene Polymer Nanocomposites for Electrochemical Energy Storage. Polymers. 2023; 15(3):701. https://doi.org/10.3390/polym15030701
Chicago/Turabian StyleGopal, Judy, Manikandan Muthu, and Iyyakkannu Sivanesan. 2023. "A Comprehensive Compilation of Graphene/Fullerene Polymer Nanocomposites for Electrochemical Energy Storage" Polymers 15, no. 3: 701. https://doi.org/10.3390/polym15030701
APA StyleGopal, J., Muthu, M., & Sivanesan, I. (2023). A Comprehensive Compilation of Graphene/Fullerene Polymer Nanocomposites for Electrochemical Energy Storage. Polymers, 15(3), 701. https://doi.org/10.3390/polym15030701









