Abstract
The formulation in which biochemical enzymes are administered in polymer science plays a key role in retaining their catalytic activity. The one-step synthesis of polymers with highly sequence-controlled enzymes is a strategy employed to provide enzymes with higher catalytic activity and thermostability in material sustainability. Enzyme-catalyzed chain growth polymerization reactions using activated monomers, protein–polymer complexation techniques, covalent and non-covalent interaction, and electrostatic interactions can provide means to develop formulations that maintain the stability of the enzyme during complex material processes. Multifarious applications of catalytic enzymes are usually attributed to their efficiency, pH, and temperature, thus, progressing with a critical structure-controlled synthesis of polymer materials. Due to the obvious economics of manufacturing and environmental sustainability, the green synthesis of enzyme-catalyzed materials has attracted significant interest. Several enzymes from microorganisms and plants via enzyme-mediated material synthesis have provided a viable alternative for the appropriate synthesis of polymers, effectively utilizing the one-step approach. This review analyzes more and deeper strategies and material technologies widely used in multi-enzyme cascade platforms for engineering polymer materials, as well as their potential industrial applications, to provide an update on current trends and gaps in the one-step synthesis of materials using catalytic enzymes.
1. Introduction
Biocatalysis, which is broadly defined as the utilization of enzymes to catalyze chemical reactions, is utilized in numerous fields of application. Since the beginning of the twenty-first century, enzymes have been gaining popularity and a wide range of biocatalysts are employed in an increasing number of processes operating at up to an industrial scale, particularly in the field of synthesizing polymeric materials and synthetic chemistry [1]. Synthetic chemists are beginning to appreciate the significance of enzymes associated with the extreme selectivity of these natural (or engineered) catalysts, along with the benefits of using them under environmentally acceptable reaction conditions. Materials’ service performances in a variety of disciplines, including biomedical devices [2], energy storage accumulators [3], and environmental cleanup systems [4], are greatly influenced by the synthesis of the technologies applied. Significant work has been put into creating various modification techniques to finely adjust the surface structures and properties of materials [5,6], endowing those materials with abundant functions and properties. Conventional modification techniques, on the other hand, frequently suffer from difficult chemical or physical processes, high solvent or energy consumption, or required preparation for the substrate materials [7], hence the need to develop means that produce sustainable synergy between the material and its practical properties. Practically, a desirable modification strategy should include the following characteristics: natural origin, environmentally benign procedure, mild reaction condition, strong binding forces to the substrate surface, simplicity of implementation, and adaptable functionalization accessibility.
Enzyme-catalyzed polymer synthesis has received a lot of attention lately due to its possible usage in fuel cells and organic synthesis. The implementation of far more complex synthesis schemes is made possible using multiple enzymes. By using or manipulating the metabolic networks of biological systems for catalysis, one-step synthesis can be carried out in those systems. Enzyme use in chemistry has a long history, including applications in the textile, food and detergent industries [8]. Likewise, the advent of on-demand enzyme production, manipulation, and modification contributed to the development of current biocatalysis [9,10]. As an alternative, isolated biocatalysts can be used to perform multi-enzymatic catalysis in vitro. Both methods, in vivo and in vitro, offer unique benefits, drawbacks, and difficulties that will be demonstrated using current instances. The current method is very helpful in modifying the polymeric structures of materials that would otherwise be impossible via chemical synthesis due to the mild circumstances in which the enzyme-catalyzed process is carried out. Additionally, multi-enzymatic tandem processes that power life exhibit unparalleled catalytic efficiencies due to biological reactor characteristics such as compartmentalization, nano-confinement, and out-of-equilibrium dynamics [11].
In addition to the tremendous incentive to develop chemical methods that are more ecologically friendly, faster access to proteins and the ability to alter them significantly influenced the shift in industrial thought [12,13]. Enzymes are capable of competing with traditional chemical (catalytic) processes for a variety of chemical changes, even ones that were previously regarded as unnatural. An excellent illustration is the discovery of the catalytic activity of aldoxime dehydratases’ in eliminating the Kemp process of the deprotonation of a benzisoxazole ring in base catalysis, for which, except from chemical bases, only recently developed proteins were recognized to be efficient catalysts [14]. Enantioselective methods using hydrolases made it possible to employ enzymes in the synthesis of asymmetric polymers, particularly when biocatalytic (dynamic) kinetic resolutions were involved. The main enzymatic techniques that are widely applicable in the one-step synthesis of polymeric materials are discussed in this review, with an emphasis on the reactions created in the last decade.
2. One-Step General Approach
The one-step approach refers to the process that produces the desired product by running a single reaction, especially where there are alternative pathways to the same product involving two or more reactions in a sequence. The excellent chemo, regio-, and stereoselectivity of enzymes is the driving force behind the interest in employing them in one-step synthesis. Enzymatic techniques used in polymeric reactions are underpinned by ideas comparable to those used in organic synthesis. The one-step technique has recently emerged as a key technology for the synthesis of stable, affordable, and environmentally friendly supported materials or composites [15,16]. According to Liu et al. [17,18] who employed the one-step synthesis in 3D NaV2O5 mesocrystal as mode materials, the advantages of the method are that it can control the synthesis conditions of the dual metal sulfide composites, simplify the experimental process, and shorten the reaction time. This technique can avoid the problematic instability of a particular polymer substance or other intermediates, as well as skip the tedious purification processes. Comparing the one-step general approach to a multi-step method, the one-step method is facile and secured, controlled material size, fast, easy and efficient synthesis, while the multi-step synthesis may require long chains, and complicated methods leading to low product yield as well as large energy utilization. The development of novel processing and synthetic processes has made it possible to combine several preparation procedures into the one-step approach. To develop consistent metal–silica–polymer core-shell–shell templates for metal-carbon yolk-shell nanostructures, Priestley et al. developed a simple one-step Stöber technique [19,20]. The restricted volume one-step synthesis of silver-decorated polymer colloids with antibacterial and sensing characteristics was reported. The sensitivity of the colloids to hydrogen peroxide reveals a linear response across a wide concentration range and possesses strong antibacterial characteristics [21]. Recently, numerous investigations have been conducted to improve the thermal conductivity of phase change materials (PCMs) [22]. These PCMs are potential alternatives for thermal energy storage because they may selectively absorb and emit enormous amounts of heat during the process of phase shifting [23,24,25,26]. Figure 1 indicates that while PEG or octadecanol acts as the PCM, silica aerogel acts as a supportive substance to prevent PCMs from leaking during the phase transition process. The porous channels of the 3D silica aerogel are specifically occupied by the hydrolytic polycondensation of bridging silsesquioxane precursor and organic PCMs in situ, which makes the production of silica aerogel easier. Finally, without additional solvent exchange or surface modification, the composite PCMs are made utilizing the APD method. The in-situ one-step preparation of a monolithic silica aerogel-based composite phase change materials for thermal protection was constructed. Due to the synergistic combination of the monolithic silica aerogel-based composite PCMs’ high latent heat capacity and poor thermal conductivity, the period of heat preservation might be prolonged. This material has the potential to be directly applied to thermal protection and insulation devices [22].
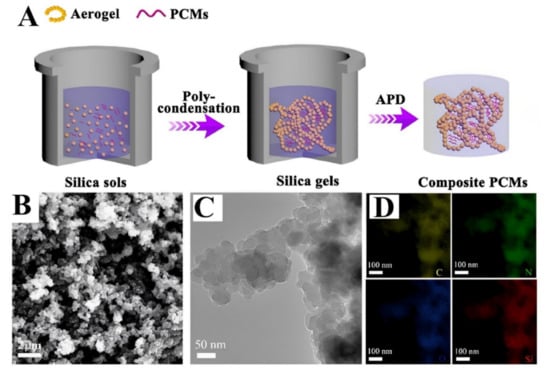
Figure 1.
Phase change materials to improve thermal conductivity prepared in a one-step synthesis. (A) Illustration of the silica aerogel-based composite PCMs’ schematic synthesis, (B) a scanning electron microscope image of the silica aerogel, and (C) a transmission electron microscope image, consisting of primary nanoparticles (approximately 30 nm) and aggregated nanoparticles in a linked, three-dimensional porous structure. (D) TEM mapping images of the silica aerogel consisting of evenly distributed C, N, O, and Si elements [22], with permission from Elsevier, copyright (2020).
Novel materials are synthesized using end-functionalized hydrogenated polymers derived from nitrile-butadiene rubber (NBR), and are well suited for use as adhesives and sealing materials in a variety of applications [27]. According to Liu et al. [28], the one-step synthesis of end-functionalized hydrogenated nitrile-butadiene rubber by combining the functional metathesis with hydrogenation has an advantage of short synthesis path, excellent yield, and complies with the regulations of green chemistry. Due to its mild conditions, environmental tolerance, high efficiency, and adjustable performance, the olefin metathesis reaction has attracted substantial attention recently for the synthesis of rubber [29,30]. High internal phase emulsions (HIPEs), double emulsions, and other complex emulsions make excellent templates for the development of porous polymeric materials. The surfactant-free emulsions with erasable triggered phase inversions conducted via a one-step approach revealed that the polymer can be employed repeatedly for porous materials and be tunable for complicated emulsions because CO2 triggering is erasable [31]. The one-step process makes creating porous 3D scaffolds and particles out of the block copolymer very convenient. Based on a one-step approach for lipase immobilization, Lu et al. synthesized SiO2-coated Fe3O4 nanoparticle/polyacrylonitrile beads to immobilize C. antartica lipase B, with an immobilization yield of up to 98.4% [32].
Specifically, the one-step synthesis of polymeric materials has been conducted in the hydrothermal synthesis of carbon dot–polymer composites [33], SnO2 electrodes by UV curing technology [34], photoluminescent core/shell polystyrene particles [35], the radiation of gel polymer electrolytes [36], magnetic chitosan polymer composite films [37], nitrogen and sulfur–codoped graphene electrodes [38], vegetable oil-based acrylate prepolymers [39], the biodegradation and biocompatibility of polyesters [40], transparent high refractive index sulfur-containing polymers [41], the synthesis of Mn3O4 on carbon cloth [42], and recyclable crosslinked polymer networks [43]. The one-step reaction is the preferred approach for modifying polymer materials due of its advantages of ease of use and mild conditions. Additionally, a one-step synthesis of a foundational material also greatly reduces cost and improves prospective economic feasibility from the perspective of product development.
3. Enzymes Catalyzed One-Step Synthesis
3.1. Free Enzymes
Enzyme-catalyzed reactions can be carried out in gentle conditions with respect to temperature, pressure, and pH as well as in solvents that are more environmentally friendly (such as water) or in mixed solvent systems (Figure 2A). Enzymatic approaches have been substantial in their applications as catalysts for polymeric one-step synthesis. A facile enzymatic one-step preparation of fluorescent conjugated polymers of phenols using horseradish peroxidase (HRP) as the catalyst resulted in the total polyphenols exhibiting fluorescence signals with significant shifts rendering them valuable for use in fluorescence quenching-based sensors [44]. Additionally, surface modified electrospun microfibers as suitable supports for protein immobilization using HRP was reported [45]. The one-step precipitation polymerization of high catalytic and recyclable DNAzyme was used to develop complex catalytic functionalized poly-N-isopropylacrylamide (pNIPAM) microgels [46] (Figure 2B). A recyclable bi-enzyme system for glucose sensing was formed by the Mg2+-dependent DNAzyme and the hemin-G-quadruplex horseradish peroxidase (HRP)-mimicking DNAzyme, with the microgel catalysts maintaining 80–91% after eight times of recycling [46]. The recovery of α (alpha)-amylase from an Aspergillus oryzae culture supernatant in one easy step by creating an insoluble combination with the Eudragit® copolymer provides an efficient method that allows the recycling of the polymer after precipitation [47]. An anionic cellulase was joined with a copolymer poly(ethyleneglycol)-graft-poly (N, N-dimethylaminoethyl methacrylate) (PEG-g-PDMAEMA) with hydrophilic and cationic chains to develop a water-soluble cellulase/PEG-g-PDMAEMA complex. Charged copolymers could be successfully inhibited by turning the cellulase system based on a synergetic polymer pair system on/off and recover positively charged polymers [48]. Additionally, the enzymatic grafting of ferulic acid with cellulose to enhance matrice–fiber compatibility in bio-composites was achieved via one-step synthesis. The resulting bio-composites demonstrated a 12% drop in Young modulus, and a 23% improvement in the elongation under maximum stress, indicating a material with higher mechanical resistance [49]. Multiple oligonucleotide partzymes constitute multicomponent nucleic acid enzymes (MNAzymes), which only associate to develop catalytic complexes when a target nucleic acid is present. According to Hanpanich et al. [50], LNA-modified MNAzymes aided by cationic copolymer for the one-step isothermal RNA detection improved the robustness of the MNAzyme which could pave the way for the design of an alternative rapid, accurate, isothermal, and a protein-free RNA diagnostic tool, which is anticipated to have significant therapeutic implications. Table 1 shows the overview of some free and immobilized enzymes employed in the generation of polymer materials via the one-step approach.
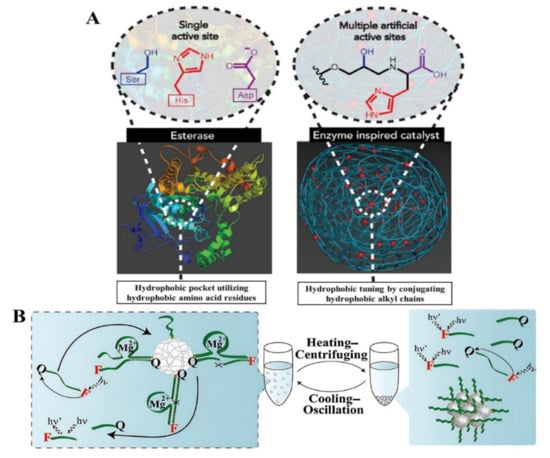
Figure 2.
(A) Typical enzyme-catalyzed reactions, comparing the natural catalytic active site and triad of esterases and the artificial enzyme mimic integrated into the polystyrene Merrifield resin, demonstrating various catalytic sites and hydrophobic tailoring enabled by functionalized resins [51], with permission from Elsevier, copyright (2017). (B) A representation of the heating–centrifuging and cooling–oscillation cycle for the recycling of pNIPAM/DNAzyme microgels [46] with permission from Wiley periodicals Inc. (2022).

Table 1.
Overview of the use of some enzymes in the one-step/pot synthesis of polymer materials.
3.2. Immobilized Enzymes
The immobilization of enzyme polymer synthesis on or within a support is crucial for both scientific and commercial purposes [74,75,76,77,78]. Most conventional immobilization techniques involve the chemical grafting, embedding, and adsorption of enzymes onto organic or inorganic carriers [79]. Magnetic nanoparticles [80], mesoporous silica [81], and organometallic skeletons [82] are a few immobilized carriers that have been reported. They have the potential to be used in numerous sustainable applications due to their reusability, simple operation, strong regio-selectivity, and moderate reaction conditions [83,84,85]. A stimulus responsive to control enzyme activity for one-step synthesis was designed, and the use of immobilized enzymes in stimulus-sensitive hydrogels, hydrogel particles, or hydrogel coatings was regarded as another common strategy [86]. It was reported that the degree of gel swelling can be changed to affect enzyme activity, and the following factors are most likely to contribute to the changes in enzyme activity upon deswelling: altered substrate accessibility to the active site; altered enzyme conformation and moving flexibility as a result of packing the enzyme being packed within the hydrogel meshes; changed enzyme environment (hydrophilicity, presence of charged groups); and modified substrate/product diffusion due to variations in water content [86]. Küchler et al. [87] immobilized an enzyme on silicate glass through simple adsorption of dendronized polymer–enzyme conjugates by employing a one-step synthesis technique and concluded that the methodology could be considered as a promising platform for localizing active enzymes on solid supports. Recently, insoluble substrates for bioconversion efficiency have been frequently low due to the enzyme’s restricted access to the insoluble substrate when it is mounted on insoluble supports. Hence, it is possible to think of stimuli-responsive polymers as carrier materials as a workable solution to the aforementioned issues via one-step synthesis. Polymers that respond to stimuli can react to changes in the environment fast and correctly. According to their reactive situations, stimuli-responsive polymers may be classified as redox-responsive polymers, temperature-sensitive polymers, photo-responsive polymers, and pH-responsive polymers [88,89]. Huang et al. [89] reported that one-step immobilization of β-glucosidase by recyclable UCST-responsive polymers are a potential agent for green biocatalysis. There is strong synthetic usefulness and economic interest in polymers having end functional groups, such as macromonomers and telehelic end functionalized macromolecules. They can be synthesized using a variety of techniques such as chemically modifying the ends of their polymer chains and polymerizations. Candida antartica lipase B immobilized for the one-step lipase-catalyzed production of α,ϖ-thiophene-capped poly(-caprolactone) macromonomers is potentially very significant to produce ɛ-CL based polymers with various end functionalities [90]. Thus, enzyme immobilization from free enzymes and on polymer surfaces as carrier materials could maximize the one-step polymerization and synthesis of polymeric materials for efficient scalable laboratory, industrial, and environmental utilization.
4. Cascade Reaction Systems in One-Step Synthesis
A biocatalytic “cascade” or “domino” reaction is what is referred to when an enzyme-catalyzed first step results in the development of an unstable intermediate which then spontaneously performs additional reactions to generate the stable product. Therefore, we would want to define a biocatalytic cascade as any reaction system that uses at least one biocatalyst and involves two or more simultaneous transformations occurring in the same reaction vessel. This includes enzyme-initiated spontaneous sequences, chemo-enzymatic sequences, and multi-enzymatic sequences [91].
4.1. Multi-Enzyme Cascade Reaction Systems
Multi-enzyme systems of remarkable complexity have been developed and offer considerable advantages including the demand of time, reversible reactions driven to completion, reduced costs and chemicals for product recovery, and minimal exposure to a concentration of unstable compounds. Top-down research on the composition and operation of a multi-enzyme complex has received a lot of attention [92]. Additionally, the bottom-up design of artificial multi-enzyme systems, which entails the selection of enzymes, design of polymer linkers and catalytic link sites, and controllable assembly for desired structure and function, has a considerable measure of work to conduct than natural multi-enzyme complexes, which have evolved over the years [93]. The only disadvantage of a multi-enzyme cascade reaction will be the poor stability and reusability of the enzyme with a tendency to inactivate in toxic media or very high temperatures. Enzymatic cascade reactions, which combine many enzyme reactions in a single vessel without isolating intermediates, hold considerable promise for the development of sustainable chemical processes [94]. Catalyzing two or more enzymes in a cascade system is required for many in vivo biological processes. The results of the previous enzyme catalysis may be used as the substrate for the subsequent enzyme catalytic reaction. Due to the selectivity and high efficiency of enzyme catalysis, complex transformations may be efficiently accomplished by a sequence of enzyme cascade catalytic reactions without any intermediate interruption [95,96,97]. Enzyme catalytic reactions have been applied to the synthesis of polymers, and this has generated considerable interest. Figure 3 shows a biocatalytic one-step retrosynthesis system for multi-enzyme cascade development. To fabricate functional polyamides with a variety of topologies and stimuli-responsive properties, Zhuang et al. [98] developed a cascaded step-growth polymerization. These polymers in the existence of biologically important reductants can degrade molecules through backbone degradation with adjustable kinetics. It has been reported that biocatalysis in polymersomes can be used to compartmentalize multi-enzyme cascades with incompatible reaction steps. The results indicated that reintegrating extremely selective protein channels into the membrane prevents cross-inhibitions in cascade reactions. However, the need to maintain compartmentalization while reducing mass transport restrictions across the polymer membranes is difficult [94,99]. In a biocatalytic one-step cascade synthesis of L-tyrosine derivatives using benzenes, NH3 and pyruvate as substituted starting materials, crude gasoline blends comprising of toluene were utilized to yield more than 97% of 3-methyl-L-tyrosine [100]. Additionally, in a biological cascade reaction, an enzyme cascade system with glucose oxidase (Gox) enclosed in a metal organic framework (ZIF-8) for the oxygen-tolerant RAFT polymerization catalysis of reusable biomimetic mineralization via a one-step facile synthesis highlights the processes in maintaining similar composite activity and comparable to the original composites following five reuses. In this regard, the polymerization process and the first-order kinetic standards are both suitable to influence how the molar mass and conversion relate to each other [101]. Fischereder et al. reported a one-step cascade fashion involving two enzymatic reaction steps performed simultaneously by employing transaminases and strictosidine synthases in the stereoselective cascade to C3-methylated derivatives. Depending on the enzymes used in a Pictet–Spengler reaction catalyzed by strictosidine synthases, the selected amines condensed with secologanin produced pure diastereomeric products (>98% excess) [102].
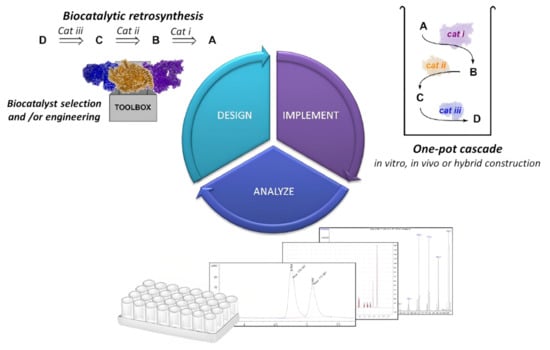
Figure 3.
A multi-enzyme cascade development cycle that has been analyzed and implemented. Whereas a wider variety of biocatalysts are now readily available, both in vitro and in vivo de novo enzyme pathway development is becoming easier using a biocatalytic retrosynthetic strategy [103], with permission from the American Chemical Society, copyright (2017).
4.2. Synthetic Cascade Reaction Systems
As a fast-growing field, non-natural synthetic cascades enable the synthesis of complex useful compounds from simple precursors by combining consecutive biocatalytic events. Cascade reaction-based synthetic strategies are very helpful since they typically bypass several reaction work-up phases and the purification of all intermediary products [104]. Köhler et al. [105] reported on synthetic cascades that are enabled by combining biocatalyst with artificial metalloenzymes, as a homogeneous catalytic means to address synthetic challenges. The alternatives for biocatalytic retrosynthesis of a target molecule increase along with the toolbox of accessible biocatalysts, opening up new pathways including enzymatic conversions. Care must be taken while implementing such cascade reactions, especially when deciding whether to build the pathway in vitro or in vivo [103] (Figure 4). Biocatalysis has unquestionably advanced beyond one-step transformations to de novo cascade reactions which can be carried out in a “one-pot”, either with isolated catalysts or whole-cell systems and can even incorporate chemo-catalytic stages. Biocatalytic retrosynthetic design, which is expanding in breadth and applications as additional biocatalysts are researched and developed, allows for the anticipation of complexity cascades [106,107,108].
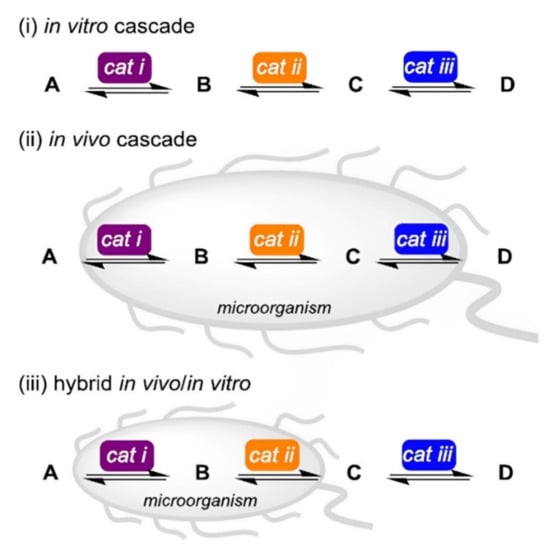
Figure 4.
Comparison of (i) in vitro reaction and (ii) in vivo reaction with (iii) hybrid cascade reactions, utilizing potent and selective biocatalysts cat i–iii to develop product D from starting material A. Several parameters such as the availability of genetic sequences and recombinant enzymes, the need for cofactors, the absorption and release of substrates and products, and the metabolic stability influence which of these three possibilities is best for a given application [103], with permission from the American Chemical Society, copyright (2017).
These synthetic cascades, which involve converting basic starting materials, such as frequent metabolic intermediates, into complicated target structures, are predicted to become more advanced as a result of access to a wider variety of various biocatalysts. Biocatalysts can now mediate a wide range of organic and chemical reactions, due to advances in multi-enzyme discovery and screening techniques as well as faster and less expensive gene synthesis [109,110,111,112,113]. The result is a continually expanding catalytic toolbox made up of naturally occurring or engineered enzymes [114,115,116], and synthetic enzymes [117,118] that can catalyze a wider variety of chemical transformations. Citrate synthase was tested using a label-free fluorescent enzyme assay based on CoA-Au(I) co-ordination polymers and applied in a multi-enzyme logic gate cascade. It was determined that using the TCA cycle’s cascade enzymatic interactions to process chemical synthesis revealed a simple and affordable logic system with faster detection [119]. Figure 5A shows a cascaded reaction mechanism and operation, Figure 5B shows a catalytic cascade reaction of glucose oxidation and heating-induced recycling of microgels, and Figure 5C,D indicate a hybrid multi-enzyme mimetic via a cascade reaction. According to Chauboon et al. [120], enabling effective flavin reduction and hydride transfer in a one-pot bioconversion of L-Arabinose to L-Ribulose in an enzymatic cascade is favorable toward a complete yield of the products. Sperl and Sieber [121] reviewed the status and recent advances of multi-enzyme cascade reactions and highlighted that employing artificial enzyme cascades for the integrated and other materials that can be controlled to synthesize a variety of desired chemicals regardless of the starting material available is the objective for the near future to sustainably improve life on earth. By eliminating the time-consuming isolation and reaction purification of intermediates, cascade reactions are unquestionably better than traditional step-by-step synthesis in terms of costs and waste. Additionally, higher yields can be obtained, and the atom economy is improved. Management and altering of adverse reaction equilibria as well as the potential manipulation of unstable intermediates are further advantages of cascade reactions [122,123]. The one-pot synthesis of (R)-α-Hydroxy ketones from meso- or racemic epoxides via enantioselective cascade biocatalysis revealed a green and high-yielding technique that could be useful for the preparation of certain chemical synthesis of which polymer materials could be efficient [124]. Thus, enzyme cascade systems are efficient and feasible approaches in the one-step synthesis of polymer, hybrid systems and other starter materials.
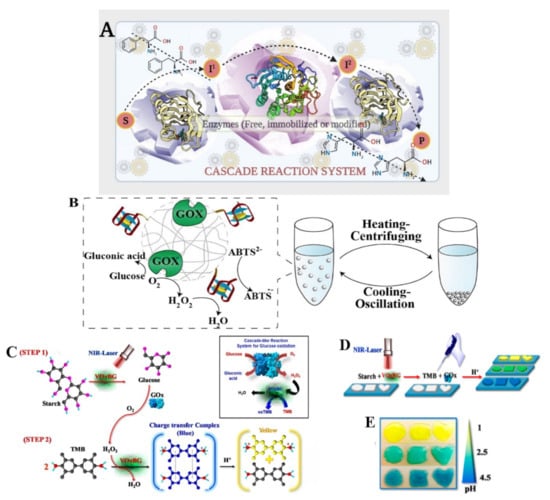
Figure 5.
(A) Illustrating a general cascaded reaction mechanism and its operation. The figure made in Biorender.com. (B) A representation of the recycling use of the microgels through heating/centrifuging and cooling/oscillation cycles, as well as DNA/GOx cascaded catalyzed oxidation of glucose/ABTS2-. After the reaction, the microgels were removed from the reaction mixture using a heating–centrifuging technique and recycled by catalyzing a new DNA substrate following a cooling–oscillation dispersion procedure [46], with permission from Wiley periodicals Inc. (2018). (C) in the presence of an NIR laser, VOxBG catalyzes the conversion of starch into glucose (step I). In the presence of H2O2, GOx catalyzes the oxidation of glucose to produce H2O2, while VOxBG acting as HRP catalyzes the oxidation of TMB to produce colored compounds which changes in response to the different pH values (step II), (D) the photolithography process is illustrated, in a polyacrylamide hydrogel, starch and VOxBG are incorporated, (E) various geometric designs are created by photolithographing the hydrogel [125], with permission from the American Chemical Society, copyright (2020).
4.3. Advantages and Disadvantages of Cascade Reaction Systems
There is a widespread desire to combine several chemical processes into one reaction process. The difficulties of intermediate stability, isolation/purification of desirable chemicals, and solvent exchange are lessened if this is successful [126]. There are significant advantages in terms of space and resource utilization, as well as frequently in terms of process completion time [127]. Even though there are many excellent examples of chemical “one-step” synthesis that combine two or more processes, most of the time, incompatibility of conditions (particularly solvents, pH, and co-catalysts) necessitates performing reactions in sequence. Many of the difficulties in synthetic or semi-synthetic chemical reactions may be overcome by cascading reactions aided by several enzymes. Cascade enzyme reactions offer specific advantages. An additional reaction in a cascade reaction can stabilize partially unstable intermediates produced by enzyme reactions [128]. This has the specific benefit of making systems with reactive substrates easier to implement. By connecting with another process to steer the reaction in the desired direction, enzyme cascades provide the ability to accelerate reactions toward completion [103].
However, when developing new polymeric materials, it is necessary to take the drawbacks of enzyme cascades into account. Cascades may require multi-factorial optimization e.g., using a design of experiments approach [129], particularly where enzymes in the cascade have different cofactor requirements, to achieve the maximum activity. Enzymes are typically evolutionarily optimized for their biosynthetic pathways in vivo, rather than large-scale industrial application, which presents another hurdle for the inclusion of enzyme cascades into larger synthetic pipelines. Poor solvent compatibility with desired products [130], constrained substrate ranges [131], substrate flux sinks [132], and low turnover rates are some difficulties imposed by this challenge. However, to combat these limitations, it is now possible to synthesize a variety of enzymes in the quantities required by industrial applications due to advances in molecular biology techniques and the identification of new enzymes utilizing bioinformatics and added coupling chemical processes with biocatalysis for in vitro processes.
5. One-Step Synthesis for Enzyme Assemblies
5.1. Nano-Enzyme Assemblies
Multi-enzyme catalysis requires enzyme assembly to improve catalytic efficiency and stability while progress in nanotechnology provides new materials and methods for the design of multi-enzyme cascade one-pot synthesis of the polymer [133]. Immobilization, self-assembly, and composite nano enzymes are the most used assembly methods. Figure 6 shows various nano enzyme assemblies employed in the one-step synthesis of polymer materials. By carefully combining copper ions and cysteine (Cys), Tang et al. [134] synthesized biomimetic copper–cysteine nanoparticles (Cu/CysNPs) with a stronger laccase-like activity than natural laccase (Figure 6D,E). The approach of immobilizing an enzyme on zinc sulfide–chitosan hybrid nanoparticles was discovered by Bahri et al. [135] and it considerably improved the materials’ thermal and storage stabilities, and resistance to pH extremes.
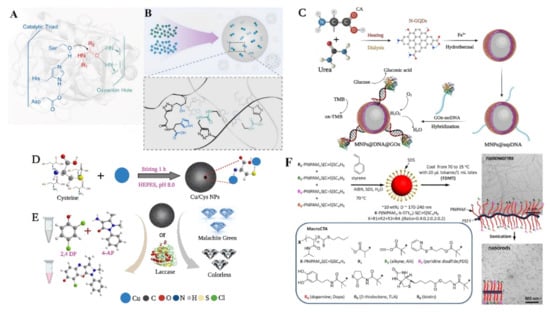
Figure 6.
Schematic illustrations of the one-step nano-enzyme synthesis. (A) A hydrolytic enzyme with an oxyanion hole and a catalytic triad. In a Ser–His–Asp motif, the carboxyl group of aspartate forms a hydrogen bond with the imidazole domains of histidine. The hydroxy group of serine that is close by is subsequently deprotonated by the imidazole group to produce a nucleophile, (B) using nanoprecipitation in water to generate polystyrene-supported catalysts with urea and ACT groups. Here, a facile one-step method for synthesizing polystyrene-supported catalytic systems with integrated and tailored ACTs, urea groups, and hydrophobic environments is devised [136], with permission from Elsevier, copyright (2022), (C) fabrication of nanozyme-enzyme multi-catalyst system, reproduced from reference [137], (D,E) illustration of the synthesis of Cu/Cys nanozyme with laccase-like activity, reproduced from reference [134], (F) RAFT-Mediated emulsion polymerization in water followed by TDMT and ultrasonic cutting to generate multifunctional nanoworms and nanorods [138], with permission from the American Chemical Society copyright (2014).
To enhance the stability of free enzymes in reactions, a variety of materials have been developed as support. Metal organic frameworks (MOFs) is one of the first support to be used and the efficient metal organic frameworks of α-amylase and glucoamylase were constructed, which could carry out multi-enzyme one-pot reaction in vitro, with higher catalytic efficiency and stability than the free enzymes [139]. Magnetic support that facilitates recycling has also been developed based on metal materials. For instance, Nobuaki et al. produced a complex made of layered titanate (TiOx), magnetic beads (MB), and horseradish peroxidase (HRP) and realized multiple recycling [140]. Figure 7 shows the fabrication of an enzyme–MOF combination.
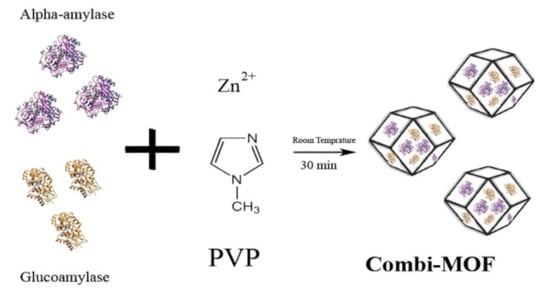
Figure 7.
Fabrication of highly efficient combi-metal organic frameworks [139], adapted with permission from Elsevier, copyright (2018).
In addition, catalytically active materials can also be used for the support itself. Shen et al. developed a flexible strategy for fabricating an effective multi-enzyme cascade system by immobilizing glucose oxidase (GOx) on ferriferous oxide nanocomposites functionalized with nitrogen-doped graphene quantum dots (Fe3O4@N-GQDs) using a DNA-directed immobilization [137]. The new enzyme immobilization method on graphene quantum dots provides a new idea for the design of enzyme catalysts. A recently created catalyst, 3.4Pd1.7 Cu/CALB CLEAs, has 2.6 times the activity of Pd/CALB CLEAs, which were previously employed extensively in the synthesis of (R)-N-[1-(4-(phenylethynyl)phenyl)ethyl] acetamide (Figure 8), verifying that an enzyme–bimetal complex catalyst is a powerful tool for the efficient and green asymmetric synthesis of high value organic compounds [141].
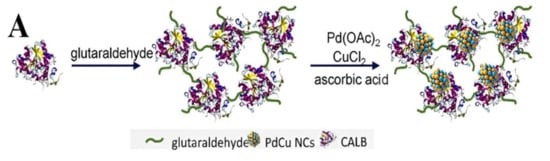
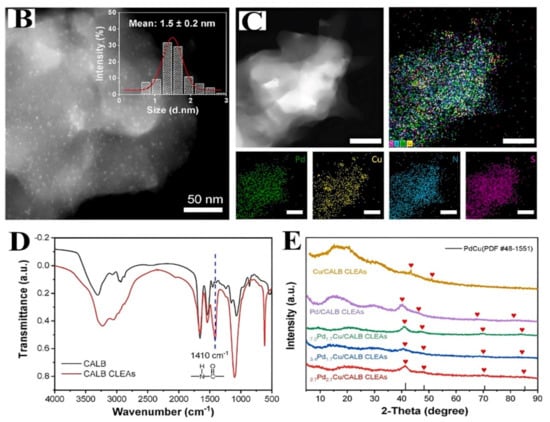
Figure 8.
(A) Illustration of the of the in situ PdCu/CALB CLEA fabrication process. (B) HAADF-STEM picture of the hybrid catalyst composed of PdCu and CALB CLEAs. (C) An elemental map of Pd, Cu, N, and S that corresponds to (D) CALB and CALB CLEAs FT-IR spectra and (E) powder XRD patterns [141], with permission from Elsevier, copyright (2023).
Non-metallic materials are also commonly used as support. Zhou et al. [142] reported a dual-enzyme cascade catalysis which is stabilized by immobilization in ZIF-8/GO composites obtained by GO-assisted co-growth. The low protein adsorption feature, biocompatibility, water solubility, good chemical resistance, and hydrophilicity of polymer material carriers are of concern. The surface functionalized nanofiber membranes were examined as a support material for enzyme immobilization, which increased the storage stability and reusability, and a novel amylase immobilized poly(vinyl alcohol) (PVA) nanofibers were developed [143]. Nanoparticle immobilization has contributed greatly to the biocatalysis of enzymes in different functional forms [144]. In addition to the immobilization of enzymes on substrates by various methods, the self-assembly of enzymes has also been developed, including assembly between different enzymes or with other catalytically active catalysts. This is particularly valuable if one or more enzymes are dimers/multimers, as it can promote enzyme ratios beyond 1:1, which can have benefits, such as bringing the catalytic activity closer to the desired ratio of enzyme activities [145]. For example, the simple one-step immobilization of zinc ions and adenine in self-assembling aqueous solution under mild conditions, while improving their activity and stability, enables multiple recycling, which will be helpful for the development of polymer synthesis catalysts [146]. Additionally, developing hybrid nanocages (Figure 9) made of self-assembling proteins and DNA will enable the development of nanomaterials that combine the benefits of both DNA and protein nanotechnology [147]. Wang et al. [148] achieved the array arrangement of the multi-reactions by alternately equidistant anchoring two model enzymes, glucose oxidase (GOx) and HRP, to the vertices of the rigid DNA tetrahedral unit so that the distance between adjacent cascades was adjusted to an optimal level to obtain high enzyme cascade catalytic efficiency.
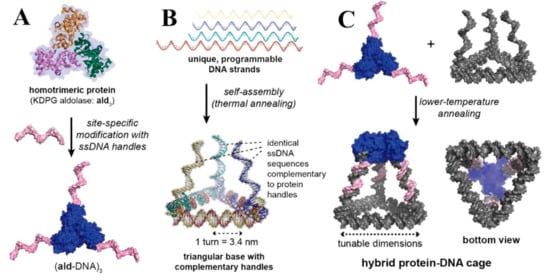
Figure 9.
Hybrid DNA/protein cage architecture. (A) Addition of ssDNA handles to a site-specific reactive residue on an aldolase homotrimer (ald3) protein building block (B) self-assembly of three complementary handles and a triangular base for the protein from four distinct DNA strands and (C) by annealing the protein–DNA conjugate with a triangular base, a tetrahedral cage composed of both protein and DNA molecules is constructed. The cage’s proportions can be adjusted by altering the lengths of the DNA strands in the triangle-shaped base [147], with permission from the American Chemical Society, copyright (2019).
Due to the excellent properties of enzymes as catalysts, enzymatic polymer synthesis has always been an important direction in the synthesis of polymer materials. Based on the above enzyme assembly methods, researchers have developed several multi-enzyme cascade polymerization methods. In order to directly produce highly branched polyesters, Nguyen et al. [149] polymerized bio-based feed fractions such as tall oil fatty acid (TOFA), glycerol, azelaic acid, and pentaerythritol utilizing an immobilized Candida antarctica lipase B and the potential for enzymatic synthesis of alkyds. High value repeating disaccharide units make up the linear polysaccharide known as hyaluronan (HA). Li et al. [150] devised the sequential one-pot multi-enzyme (OPME) method for producing HA and its derivatives in vitro. A developed method for the production of lipophilic esters of hydroxytyrosol, which were synthesized from tyrosol and long-side chain carboxylic acids in a one-pot process by an enzymatic cascade of lipase M and tyrosinase supported by lignin nanoparticles [151]. Additionally, the synthesis of enantiomer-enriched O-heterocycles from alleles catalyzed by a cascade of bifunctional lipase metal bioheterozygotes was also achieved [152] (Figure 10). Thus, it can be inferred that enzymes assembled by various methods have great application prospects in polymer synthesis.
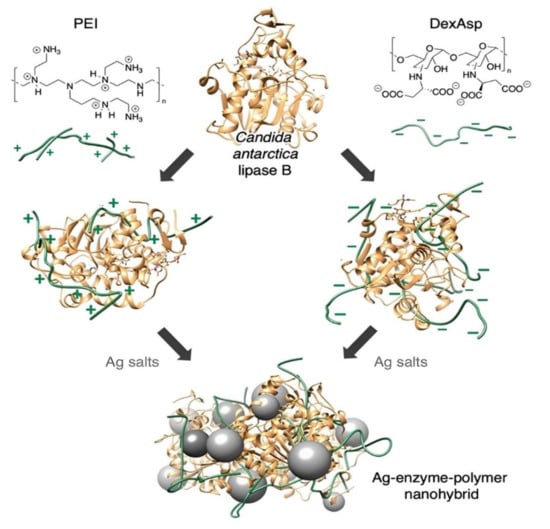
Figure 10.
Synthesis of metal nano-biohybrids using enzyme–polymer conjugates. Illustrating the attempted prevention of mutual deactivation of the coupled enzyme and metal entities through poisoning effects in the nano-biohybrid synthesis by using polymer additives in the structure of the enzyme–metal nano-biohybrids [152], with permission from Wiley periodicals Inc., copyright (2022).
5.2. Self-Assembling Peptides
Self-assembling peptides (SAPs) are ionic-complementary peptides that alternate hydrophilic and hydrophobic amino acid residues, with the advantages of their biocompatibility and ability to efficiently target molecular recognition sites which will be beneficial in one-step synthesis for effective catalysis of polymer materials. At last, SAPs play an important role, which could be used in encapsulation and catalysis. Liang et al. [153] created three oligopeptide bionanozymes with intrinsic hydrolase-like activity by achieving the zinc-induced self-assembly of histidine-rich heptapeptide, which shows great potential for environmental remediation. As for medical application, Dega et al. [154] used self-assembled bovine serum albumin-hydrated manganese phosphate nano-flowers (MnPNF) which works as a biomimic oxidase for GSH detection in human serum. Figure 11 shows the schematic information for designing SAPs. Self-assembling peptides also provide a new approach for the fabrication of integrated multi-enzymatic cascade systems that remains a great challenge. An arginine-rich peptide–Pt nanoparticle cluster (ARP-PtNC) nanozymes that mimic two typical enzymatic cascade systems of uricase/catalase and superoxide dismutase/catalase in natural peroxisome has been reported, which multiplied catalytic activity by introducing peptides [155]. As this section has been extensively reviewed elsewhere [156], it will not be discussed in detail here. Beyond this, it also confirmed that the rational design of peptide–nanozyme complex may provide a versatile and designed strategy to fabricate multi-enzymatic cascade systems, opening new avenues to researchers in this field.
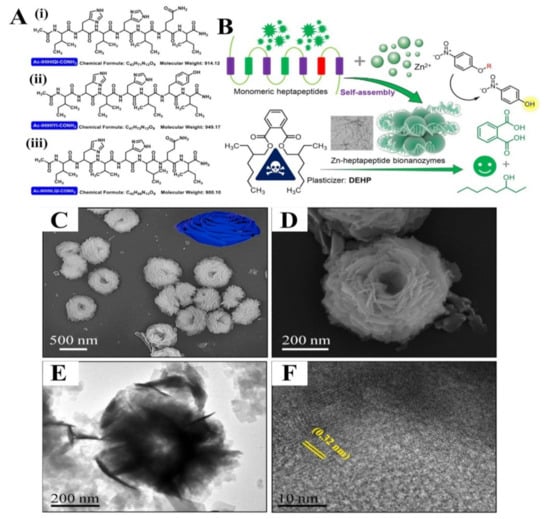
Figure 11.
(A) Structural information of three heptapeptides ((i) Ac-IHIHIQI-CONH2, (ii) Ac-IHIHIYI-CONH2, (iii) Ac-IHVHLQI-CONH2)). (B) Schematic illustration showing the design of Zn-heptapeptide bionanozymes for hydrolysis of p-nitrophenyl esters and degradation of DEHP [153], with permission from Elsevier, copyright (2022). (C,D) SEM with difference magnifications, (E) TEM, and (F) HR-TEM images of BSA-Mn3(PO4)2⋅3H2O hybrid nanoflower (MnPNF) [154], with permission from Elsevier, copyright (2021).
6. One-Step Synthesis Application
6.1. Application in Polymeric Scaffolds
Polymer materials have advanced over the past century, paving the way to potential industrial uses with the advancements in rising attention in the domains of polymer chemistry, nanotechnology, and biotechnology. Enzymes are powerful catalysts, but they are also very delicate molecules that can easily be denaturated beyond their normal environment. The application of one-step synthesis in producing polymer scaffolds has been reported in imidazole scaffolds [157], porous membrane-based scaffolds [158], multiple heterocyclic scaffolds [159], and the construction of β-Lactam scaffolds [160]. The successful fusion of enzymes with adaptable synthetic or natural polymers is made possible by a variety of opportunities provided by polymer chemistry, which can help resolve this problem. The design of functional, stable, and strong biocatalytic hybrid materials (hydrogels, nanoparticles, films, or capsules) has demonstrated effectiveness for a number of applications, including organic synthesis, bioremediation, biosensing, and biomedicine. Figure 12 shows the schematic preparation and printing of a polymeric scaffold for biomedical use. Immobilized enzymes have been employed in polymeric scaffolds [161,162,163,164,165]. Through the chemical modification of the protein’s sequence, by immobilizing the enzyme on stable supports, or by employing a combination of methods, the extensive application of enzymes in polymer scaffolds could be achieved [166]. Recently, the research and development of new scaffolds for tissue engineering have attracted a lot of attention. Particularly, the electrospun nanofibrillar scaffold materials are promising materials for use in biomedical applications [165,167,168] because they are produced from biocompatible and biodegradable polymers. Karahaliloğlu et al. employed an enzyme from the Alcaligenes eutrophus DSM 545 strain to produce cell-friendly bacterial electrospun PHB polymeric scaffolds. Through plasma polymerization modification, the nanofibrillar structures appeared to be effective substrates for cell attachment [169]. In a one-step surgical concept for cartilage and bone tissue engineering, the rapid attachment of resorbable polymeric scaffolds identifies both poly(L-lactide-co-caprolactone) and collagen type I/III as promising scaffold materials [170]. In the one-step synthesis of producing polymer scaffolds, the chain conformation of the polymer must also be considered because bulky and large polymers may obstruct the enzyme’s catalytic pocket and reduce its catalytic effectiveness. Overall, careful consideration of several characteristics is required for the design of an experiment that yields an enzyme–polymer hybrid that is catalytically active, reliable, and stable.
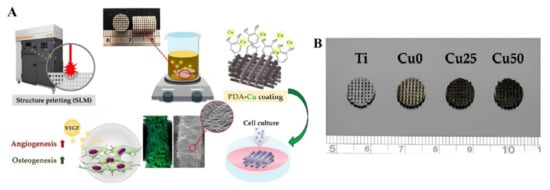
Figure 12.
(A) Schematic representation of the Cu ion grafted by DA coating on the SLM-fabricated Ti6Al4V scaffold, which gave the scaffold the ability to stimulate angiogenesis and osteogenesis by releasing Cu ions. (B) Differential appearance of printed structures before and after PDA/Cu coating showing the changes in scaffold’s appearance [165], with permission from MDPI, Basel, copyright (2022).
In tissue engineering, a scaffold serves as an important component as it functions as a three-dimensional structure for cell interactions, extracellular matrix production, and a transporter for delivering the right cytokines and growth factors to the repair site [171]. One advantage of polymeric scaffold for tissue repair is that it gives the newly formed tissue the appropriate structural and functional support that it needs. However, for scaffold tissue regeneration to be effective, it must be properly shaped on both the outside and inside to support cellular processes such as adhesion, proliferation, and differentiation, it must possess similar mechanical properties to those of the tissue repair site, it must be biocompatible, and must have the same degradation rate in the case of bone regeneration [172,173]. Tissue regeneration employs the use of synthetic polymers as they are biocompatible as well as easy to control in their three-dimensional structure and other surface features. Regardless, the downside to the use of polymeric scaffolds for tissue regeneration is that many scaffold properties need to be designed based on cell types for specific applications as scaffolds with increased surface roughness work best with osteoblast attachment while scaffolds with smooth surfaces work best with endothelial cells and fibroblasts [174].
The incorporation of polymer scaffolds in drug delivery systems has also been researched. A drug delivery system (DDS) involves any method that loads and delivers active compounds to target tissues to enhance the safety and effectiveness of drugs and the design of polymer implants or injectables by material researchers’ function in the repair of tissues as well as the cure of illness [175,176]. The use of these polymer injectables ensures minimal incision sizes and effective anatomical closing, and prevents the post-treatment step of removal surgery which is almost impossible with other thick or solid forms [177]. Additionally, polymer implants permit shape adaptation of the device which makes them advantageous compared to other scaffold-producing materials [178]. Polymeric scaffolds for DDS promote drug protection which in turn prevents side effects as implants are site specific and drug release and drug levels are controlled [179,180]. Biocompatible scaffolds are also used in the delivery of proteins, cells, and genes and these applications improve their capacity for the designs of novel treatments for diseases such as tumors and pathogen-derived microbials, and as a tool for tissue repair in regenerative medicine to stimulate biological responses [175].
6.2. Challenges of the Application of One-Step Synthesis in Scaffolds
Despite their obvious benefits and widespread use, polymer scaffolds generated through one-step synthesis have certain practical drawbacks, including a lack of mechanical strength for load-bearing applications and a failure to scale up. However, different research teams have proposed several strategies to surpass the constraints listed above. In tissue engineering, it has some practical drawbacks, including poor cellular penetration and ingrowth, the toxicity of chemical residues in electrospun fibers or postprocessing, insufficient mechanical strength for load-bearing applications, and a sluggish manufacturing rate [181]. Some scaffold manufacturing strategies could also account for these challenges. For example, the freeze-drying technique has the disadvantage of high energy consumption, the development of uneven hole diameters, and the use of cytotoxic solvents to dissolve the polymers [182]. In addition, the solvent casting method and particulate leaching are time consuming, require extensive time to evaporate the toxic compounds, have limited membrane thickness, and have mechanical properties [183]. Additional challenges such as tissue architecture, simultaneous seeding, and vascularization have also been reported and reviewed extensively [184]. Thus, more hybrid structures and the incorporation of enzyme composites are advocated for the construction of scaffolds to lessen these associated limitations.
7. Conclusions and Prospects
One-step synthesis is an efficient and simple approach for the construction of polymeric or organic/inorganic hybrid functional materials, and there is a need to carefully adjust them to suit industrial standards for high productivity to potentially replace the expensive and environmentally harmful advanced organic chemistry techniques. There have been only modest industrial successes despite scientific advancements and exploratory efforts to fully understand one-step synthesis and its functional dimensions for polymeric materials. The current review focused on the significant factors among the numerous determining factors for optimal enzyme functioning in the synthesis and production of polymer materials. By altering the synthesis interaction, the optimal enzyme design and the ideal catalyst choice can both influence the polymer function and product profile. However, the enzyme–polymer synthesis scientific community still requires a friendly standardized methodology, biological and chemical engineering, as well as enhanced protein model-based methodologies to expedite the structural unification designs for the synthesis of enzyme-catalyzed polymers and/or other materials.
Besides the free, immobilized, or modified enzymes, several non-enzymatic one-step methods also exist that might have a significant effect on the synthesis of the polymer-coupled complexes. Although this paper is focused on the entire synthesis of enzyme polymers, some of the most recent breakthroughs in one-step organic/inorganic chemical synthesis were discussed as prospective directions to be modified and merged in enzyme systems. Green chemistry has successfully used enzyme-mediated polymer synthesis at low cost and under mild reaction conditions. In addition to the various polymerization reactions, existing polymers can also undergo modification reactions to develop new polymeric materials. However, compared to enzymatic polymerization, enzymatic modification has produced far fewer polymers so far. Enzymatic catalysis has been utilized for the synthesis of polymer conjugates, selectively functionalized polymers, the cross-linking of polymers, the modification of the surface characteristics of polymers, and other chemical modifications.
In addition to the added advantages for enzymes in the one-step process, there are some limitations to their use which include (1) enzyme catalysts have fewer types of substrates and reactions than do chemical catalysts; (2) the availability of enzymes and their cost; (3) although there is substantial turnover, responses seem to be slow. In many instances, the amount of catalyst is the issue, normally due to the catalytic active site contained in a single enzyme molecule. However, there is still opportunity for development given these drawbacks in terms of the large reaction rate and extremely high selectivity, a low amount of by-products, enzymes being renewable, and immobilized enzymes can be readily recovered and recycled, functional in organic and supercritical fluids, are renewable resources, and in most cases, products are biodegradable.
It is desired that using the one-step approach to perform green polymer chemistry can enable the establishment of a sustainable society in the future. Enzymes offer several prospects to expand our reach to a wide variety of polymers with roots in chemical and biosynthetic pathways due to their extensive selectivity and preference for the available substrate pool. We conclude by stating that generating materials via enzyme-wise synthesis of polymer partners can comprehensively shape the future generation of enzyme–polymer catalysis research.
Author Contributions
J.W.: conceptualization, supervision, fund acquisition, resources, writing—review and editing; S.Y.: resources, writing—review and editing; R.A.H.: conceptualization, fund acquisition, writing—original draft, writing—review and editing; X.Z.: writing—original draft; E.A.: writing—review and editing, tables. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Jiangsu Excellent Postdoctoral Program Fund (2022ZB681), the Jiangsu Agricultural Science and Technology Innovation Fund (CX(20)2029), and the Jiangsu University of Science and Technology Postdoctoral Research Fund (1062152103).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the support provided by the above fundings.
Conflicts of Interest
No potential conflict of interest was reported by the authors.
Abbreviations and Acronyms
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt |
| ACTs | artificial catalytic traid system |
| AHA | 6-azidohexanoic acid |
| APD | ambient pressure drying |
| AuNPs | gold nanoparticles |
| CaBDC | calcium-carboxylate based metal organic frameworks |
| CafAc | caffeic acid |
| co-PMA | co-propargyl methacrylate |
| co-EDMA | co-ethylene dimethacrylate |
| CALB | Candida antarctica Lipase B |
| CS-RGO | chitosan-reduced graphene oxide |
| CLEAs | cross-linked enzyme aggregates |
| dPG | dendritic polyglycerol |
| DEHP | degrading di(2-ethylhexyl) phthalate |
| e-CL | e-caprolactone |
| e-TCL | e-thiocaprolactone |
| EGCG | epigallocatechin gallate |
| GOD | glucose oxidase |
| GO | graphite oxide |
| HIPEs | high internal phase emulsions |
| HIPN | hybrid interpenetrating polymer network |
| HRP | horseradish peroxidase |
| LNA | local nucleic acid |
| Lyz | lysozyme |
| MOFs | metal organic frameworks |
| NBR | nitrile butadiene rubber |
| NIR | near infrared spectroscopy |
| NPs | nano particles |
| OPME | one-pot multi-enzyme |
| PCM | phase change materials |
| Pd | palladium |
| PEG | polyethylene glycol |
| PEG-g-PDMAEMA | poly(ethylene- glycol)-graft-poly(N,N-dimethylaminoethyl methacrylate) |
| PGBA | Poly(4-(β-glucosyloxy) butyl acrylate |
| PGEA | Poly(2-(β-glucosyloxy) ethyl acrylate |
| PGEMA | Poly(2-(β-glucosyloxy) ethyl methacrylate |
| pNIPAM | Poly-N-isopropylacrylamide |
| PVA | polyvinyl alcohol |
| RAFT | reversible addition-fragmentation chain transfer |
| SPD | supercritical drying |
| TBTA | tris-(benzyltriazolylmethyl) amine |
| TDMT | temperature directed morphology transformation |
| TEM | Transmission electron microscope |
| TMB | 3,5,3′,5′-tetrame- thylbenzidine |
| TOFA | tall oil fatty acids |
| UCST | upper critical solution temperature |
| VOxBG | hexacyanoferrate Berlin green analogue complex |
| Zn-DDVA | Zinc-5,5′-dehydrodivanillate |
| ZIF-8 | zeolitic imidazole framework-8 |
References
- Hall, M. Enzymatic strategies for asymmetric synthesis. RSC Chem. Biol. 2021, 2, 847–1302. [Google Scholar]
- Ku, S.H.; Ryu, J.; Hong, S.K.; Lee, H.; Park, C.B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef]
- Zhou, W.; Xiao, X.; Cai, M.; Yang, L. Polydopamine-coated, nitrogen-doped, hollow carbon–sulfur double-layered core–shell structure for improving lithium–sulfur batteries. Nano Lett. 2014, 14, 5250–5256. [Google Scholar] [CrossRef]
- Yang, H.-C.; Luo, J.; Lv, Y.; Shen, P.; Xu, Z.-K. Surface engineering of polymer membranes via mussel-inspired chemistry. J. Membr. Sci. 2015, 483, 42–59. [Google Scholar] [CrossRef]
- Borges, J.; Mano, J.F. Molecular interactions driving the layer-by-layer assembly of multilayers. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, M.-Q.; Chen, T.-T.; Zhang, H.; Hu, D.-F.; Wu, B.-H.; Ji, J.; Xu, Z.-K. Dopamine-triggered one-step polymerization and codeposition of acrylate monomers for functional coatings. ACS Appl. Mater. Interfaces 2017, 9, 34356–34366. [Google Scholar] [CrossRef]
- Wei, Q.; Haag, R. Universal polymer coatings and their representative biomedical applications. Mater. Horiz. 2015, 2, 567–577. [Google Scholar] [CrossRef]
- Heckmann, C.M.; Paradisi, F. Looking back: A short history of the discovery of enzymes and how they became powerful chemical tools. ChemCatChem 2020, 12, 6082–6102. [Google Scholar] [CrossRef]
- Williams, G.; Hall, M. Modern Biocatalysis: Advances towards Synthetic Biological Systems; Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- De Gonzalo, G.; Lavandera, I. Biocatalysis for Practitioners; Wiley: New York, NY, USA, 2021; pp. 1–509. [Google Scholar]
- Das, S.; Das, P.; Dowari, P.; Das, B.K.; Das, D. Bi-directional feedback controlled transience in Cucurbituril based tandem nanozyme. J. Colloid Interface Sci. 2022, 614, 172–180. [Google Scholar] [CrossRef]
- Truppo, M.D. Biocatalysis in the pharmaceutical industry: The need for speed. ACS Med. Chem. Lett. 2017, 8, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.P.; Brown, M.J.; Diaz-Rodriguez, A.; Lloyd, R.C.; Roiban, G.D. Biocatalysis: A pharma perspective. Adv. Synth. Catal. 2019, 361, 2421–2432. [Google Scholar] [CrossRef]
- Miao, Y.; Metzner, R.; Asano, Y. Kemp elimination catalyzed by naturally occurring aldoxime dehydratases. ChemBioChem 2017, 18, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Priestley, R.D. Rational design and fabrication of core–Shell nanoparticles through a one-step/pot strategy. J. Mater. Chem. A 2016, 4, 6680–6692. [Google Scholar] [CrossRef]
- Yang, P.; Xu, Y.; Chen, L.; Wang, X.; Zhang, Q. One-pot synthesis of monodisperse noble metal@ resorcinol-formaldehyde (M@ RF) and M@ carbon core–shell nanostructure and their catalytic applications. Langmuir 2015, 31, 11701–11708. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, Y.; Zhu, K.; Bian, K.; Wang, J.; Sun, X.; Gao, Y.; Luo, H.; Lu, L.; Liu, J. Ultrathin VO 2 nanosheets self-assembled into 3D micro/nano-structured hierarchical porous sponge-like micro-bundles for long-life and high-rate Li-ion batteries. J. Mater. Chem. A 2017, 5, 8307–8316. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, K.; Bian, K.; Xu, Y.; Zhang, F.; Zhang, W.; Huang, W.; Zhang, J. One-step and short-time synthesis of 3D NaV2O5 mesocrystal as anode materials of Na-Ion batteries. J. Power Sources 2018, 395, 158–162. [Google Scholar] [CrossRef]
- Liu, R.; Qu, F.; Guo, Y.; Yao, N.; Priestley, R.D. Au@ carbon yolk–Shell nanostructures via one-step core–shell–shell template. Chem. Commun. 2014, 50, 478–480. [Google Scholar] [CrossRef]
- Liu, R.; Yeh, Y.-W.; Tam, V.H.; Qu, F.; Yao, N.; Priestley, R.D. One-pot Stöber route yields template for Ag@ carbon yolk–Shell nanostructures. Chem. Commun. 2014, 50, 9056–9059. [Google Scholar] [CrossRef]
- He, Y.; Wang, B.; Hu, X.; Zhang, X.; Sun, L.; Priestley, R.D.; Liu, R. One-step constrained-volume synthesis of silver decorated polymer colloids with antimicrobial and sensing properties. Colloid Polym. Sci. 2017, 295, 521–527. [Google Scholar] [CrossRef]
- Liu, P.; Gao, H.; Chen, X.; Chen, D.; Lv, J.; Han, M.; Cheng, P.; Wang, G. In situ one-step construction of monolithic silica aerogel-based composite phase change materials for thermal protection. Compos. Part B Eng. 2020, 195, 108072. [Google Scholar] [CrossRef]
- Chen, D.-Z.; Qin, S.-Y.; Tsui, G.C.; Tang, C.-Y.; Ouyang, X.; Liu, J.-H.; Tang, J.-N.; Zuo, J.-D. Fabrication, morphology and thermal properties of octadecylamine-grafted graphene oxide-modified phase-change microcapsules for thermal energy storage. Compos. Part B Eng. 2019, 157, 239–247. [Google Scholar] [CrossRef]
- Li, G.; Hong, G.; Dong, D.; Song, W.; Zhang, X. Multiresponsive graphene-aerogel-directed phase-change smart fibers. Adv. Mater. 2018, 30, 1801754. [Google Scholar] [CrossRef] [PubMed]
- Aftab, W.; Huang, X.; Wu, W.; Liang, Z.; Mahmood, A.; Zou, R. Nanoconfined phase change materials for thermal energy applications. Energy Environ. Sci. 2018, 11, 1392–1424. [Google Scholar] [CrossRef]
- Feng, D.; Feng, Y.; Zang, Y.; Li, P.; Zhang, X. Phase change in modified metal organic frameworks MIL-101 (Cr): Mechanism on highly improved energy storage performance. Microporous Mesoporous Mater. 2019, 280, 124–132. [Google Scholar] [CrossRef]
- Ai, C.; Li, J.; Gong, G.; Zhao, X.; Liu, P. Preparation of hydrogenated nitrile-butadiene rubber (H-NBR) with controllable molecular weight with heterogeneous catalytic hydrogenation after degradation via olefin cross metathesis. React. Funct. Polym. 2018, 129, 53–57. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Zhang, Z.; Yang, H.; Nie, X. One-step Synthesis of End-Functionalized Hydrogenated Nitrile-Butadiene Rubber by Combining the Functional Metathesis with Hydrogenation. ChemistryOpen 2020, 9, 374–380. [Google Scholar] [CrossRef]
- Toyonaga, M.; Chammingkwan, P.; Terano, M.; Taniike, T. Well-defined polypropylene/polypropylene-grafted silica nanocomposites: Roles of number and molecular weight of grafted chains on mechanistic reinforcement. Polymers 2016, 8, 300. [Google Scholar] [CrossRef]
- Gui, Y.; Zheng, J.; Ye, X.; Han, D.; Xi, M.; Zhang, L. Preparation and performance of silica/SBR masterbatches with high silica loading by latex compounding method. Compos. Part B Eng. 2016, 85, 130–139. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Wang, D.; Ma, N.; Li, C.; Wang, Y. Surfactant-free emulsions with erasable triggered phase inversions. Langmuir 2016, 32, 11039–11042. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, Y.; Zhu, H.; Shi, G. SiO2-coated Fe3O4 nanoparticle/polyacrylonitrile beads for one-step lipase immobilization. ACS Appl. Nano Mater. 2021, 4, 7856–7869. [Google Scholar] [CrossRef]
- Zheng, X.; Ding, G.; Wang, H.; Cui, G.; Zhang, P. One-step hydrothermal synthesis of carbon dots-polymer composites with solid-state photoluminescence. Mater. Lett. 2019, 238, 22–25. [Google Scholar] [CrossRef]
- Wei, H.; Xia, Z.; Xia, D. One step synthesis of uniform SnO2 electrode by UV curing technology toward enhanced lithium-ion storage. ACS Appl. Mater. Interfaces 2017, 9, 7169–7176. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, S.M.; Sankaraiah, S.; Cheong, I.W.; Choi, S.W.; Kim, J.H. One-step synthesis of photoluminescent core/shell polystyrene/polythiophene particles. Macromol. Res. 2011, 19, 1114–1120. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, J.; Peng, J.; Li, J.; Zhai, M. One-step radiation synthesis of gel polymer electrolytes with high ionic conductivity for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 12393–12399. [Google Scholar] [CrossRef]
- Cesano, F.; Fenoglio, G.; Carlos, L.; Nisticò, R. One-step synthesis of magnetic chitosan polymer composite films. Appl. Surf. Sci. 2015, 345, 175–181. [Google Scholar] [CrossRef]
- Deng, H.; Zhu, M.; Jin, T.; Cheng, C.; Zheng, J.; Qian, Y. One-step synthesis of nitrogen, sulphur-codoped graphene as electrode material for supercapacitor with excellent cycling stability. Int. J. Electrochem. Sci 2020, 15, 16–25. [Google Scholar] [CrossRef]
- Su, Y.; Lin, H.; Zhang, S.; Yang, Z.; Yuan, T. One-step synthesis of novel renewable vegetable oil-based acrylate prepolymers and their application in UV-curable coatings. Polymers 2020, 12, 1165. [Google Scholar] [CrossRef]
- Korley, J.N.; Yazdi, S.; McHugh, K.; Kirk, J.; Anderson, J.; Putnam, D. One-step synthesis, biodegradation and biocompatibility of polyesters based on the metabolic synthon, dihydroxyacetone. Biomaterials 2016, 98, 41–52. [Google Scholar] [CrossRef]
- Kim, D.H.; Jang, W.; Choi, K.; Choi, J.S.; Pyun, J.; Lim, J.; Char, K.; Im, S.G. One-step vapor-phase synthesis of transparent high refractive index sulfur-containing polymers. Sci. Adv. 2020, 6, eabb5320. [Google Scholar] [CrossRef]
- Dong, Q.; He, Z.; Tang, X.; Zhang, Y.; Yang, L.; Huang, K.; Jiang, X.; Xiong, X. One-step synthesis of Mn3O4@ ZIF-67 on carbon cloth: As an effective non-enzymatic glucose sensor. Microchem. J. 2022, 175, 107203. [Google Scholar] [CrossRef]
- Jin, K.; Li, L.; Torkelson, J.M. Recyclable Crosslinked Polymer Networks via One-Step Controlled Radical Polymerization. Adv. Mater. 2016, 28, 6746–6750. [Google Scholar] [CrossRef]
- Kiratitanavit, W.; Bruno, F.F.; Kumar, J.; Nagarajan, R. Facile enzymatic preparation of fluorescent conjugated polymers of phenols and their application in sensing. J. Appl. Polym. Sci. 2018, 135, 46496. [Google Scholar] [CrossRef]
- Martrou, G.; Léonetti, M.; Gigmes, D.; Trimaille, T. One-step preparation of surface modified electrospun microfibers as suitable supports for protein immobilization. Polym. Chem. 2017, 8, 1790–1796. [Google Scholar] [CrossRef]
- Li, F.; Wang, C.; Guo, W. Multifunctional poly-N-isopropylacrylamide/DNAzyme microgels as highly efficient and recyclable catalysts for biosensing. Adv. Funct. Mater. 2018, 28, 1705876. [Google Scholar] [CrossRef]
- Porfirif, M.C.; Milatich, E.J.; Farruggia, B.M.; Romanini, D. Production of alpha-amylase from Aspergillus oryzae for several industrial applications in a single step. J. Chromatogr. B 2016, 1022, 87–92. [Google Scholar] [CrossRef]
- Wan, J.; Han, J.; Wang, Y.; Ni, L.; Wang, L.; Li, C. Switch on/off of cellulase activity based on synergetic polymer pair system. Biochem. Eng. J. 2017, 126, 1–7. [Google Scholar] [CrossRef]
- Morin, S.; Bockstal, L.; Jacquet, N.; Richel, A. One-step enzymatic grafting of ferulic acid with cellulose to enhance matrices–fibres compatibility in bio-composites. J. Mater. Sci. 2019, 54, 13314–13321. [Google Scholar] [CrossRef]
- Hanpanich, O.; Saito, K.; Shimada, N.; Maruyama, A. One-step isothermal RNA detection with LNA-modified MNAzymes chaperoned by cationic copolymer. Biosenso. Bioelectron. 2020, 165, 112383. [Google Scholar] [CrossRef]
- Nothling, M.D.; Ganesan, A.; Condic-Jurkic, K.; Pressly, E.; Davalos, A.; Gotrik, M.R.; Xiao, Z.; Khoshdel, E.; Hawker, C.J.; O’Mara, M.L. Simple design of an enzyme-inspired supported catalyst based on a catalytic triad. Chem 2017, 2, 732–745. [Google Scholar] [CrossRef]
- Baig, U.; Alam, M.F.; Sajid, M. One-pot synthesis of magnetite based polymeric-inorganic nanocomposite: Structural, morphological, spectroscopic and enzyme immobilization studies. Colloid Interface Sci. Commun. 2021, 40, 100337. [Google Scholar] [CrossRef]
- Pan, Y.; Li, H.; Lenertz, M.; Han, Y.; Ugrinov, A.; Kilin, D.; Chen, B.; Yang, Z. One-pot synthesis of enzyme@ metal–organic material (MOM) biocomposites for enzyme biocatalysis. Green Chem. 2021, 23, 4466–4476. [Google Scholar] [CrossRef]
- Kim, B.; Kim, S.-H.; Kim, K.; An, Y.-H.; So, K.-H.; Kim, B.-G.; Hwang, N. Enzyme-mediated one-pot synthesis of hydrogel with the polyphenol cross-linker for skin regeneration. Mater. Today Bio. 2020, 8, 100079. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhu, C.; Wang, S.; Liu, H.; Zhang, Y.; Guo, H.; Tao, L.; Wei, Y. One-pot synthesis of optically active polymer via concurrent cooperation of enzymatic resolution and living radical polymerization. Polym. Chem. 2013, 4, 264–267. [Google Scholar] [CrossRef]
- Farmakes, J.; Schuster, I.; Overby, A.; Alhalhooly, L.; Lenertz, M.; Li, Q.; Ugrinov, A.; Choi, Y.; Pan, Y.; Yang, Z. Enzyme immobilization on graphite oxide (GO) surface via one-pot synthesis of GO/metal–organic framework composites for large-substrate biocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 23119–23126. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, D.; Guo, Y.; Guo, Y.; Chen, Q. Simple one-pot preparation of chitosan-reduced graphene oxide-Au nanoparticles hybrids for glucose sensing. Sens. Actuators B Chem. 2015, 221, 265–272. [Google Scholar] [CrossRef]
- Movahedi, A.; Moth-Poulsen, K.; Eklöf, J.; Nydén, M.; Kann, N. One-pot synthesis of TBTA-functionalized coordinating polymers. React. Funct. Polym. 2014, 82, 1–8. [Google Scholar] [CrossRef]
- Ion, S.; Opris, C.; Cojocaru, B.; Tudorache, M.; Zgura, I.; Galca, A.C.; Bodescu, A.M.; Enache, M.; Maria, G.-M.; Parvulescu, V.I. One-pot enzymatic production of lignin-composites. Front. Chem. 2018, 6, 124. [Google Scholar] [CrossRef]
- Yu, R.; Hu, W.; Lin, G.; Xiao, Q.; Zheng, J.; Lin, Z. One-pot synthesis of polymer monolithic column by combination of free radical polymerization and azide–alkyne cycloaddition “click” reaction and its application in capillary liquid chromatography. RSC Adv. 2015, 5, 9828–9836. [Google Scholar] [CrossRef]
- Shen, X.; Yang, M.; Cui, C.; Cao, H. In situ immobilization of glucose oxidase and catalase in a hybrid interpenetrating polymer network by 3D bioprinting and its application. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 411–418. [Google Scholar] [CrossRef]
- Semenova, D.; Gernaey, K.; Morgan, B.; Silina, Y. Towards one-step design of tailored enzymatic nanobiosensors. Analyst 2020, 145, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Maniar, D.; Fodor, C.; Adi, I.K.; Woortman, A.J.; van Dijken, J.; Loos, K. Enzymatic synthesis and characterization of muconic acid-based unsaturated polymer systems. Polym. Int. 2021, 70, 555–563. [Google Scholar] [CrossRef]
- Nie, W.-C.; Dang, H.-C.; Wang, X.-L.; Song, F.; Wang, Y.-Z. One-step enzymatic synthesis of poly (p-dioxanone-co-butylene-co-succinate) copolyesters with well-defined structure and enhanced degradability. Polymer 2017, 111, 107–114. [Google Scholar] [CrossRef]
- Adharis, A.; Loos, K. Green synthesis of glycopolymers using an enzymatic approach. Macromol. Chem. Phys. 2019, 220, 1900219. [Google Scholar] [CrossRef]
- Wu, C.; Schwibbert, K.; Achazi, K.; Landsberger, P.; Gorbushina, A.; Haag, R. Active antibacterial and antifouling surface coating via a facile one-step enzymatic cross-linking. Biomacromolecules 2017, 18, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shu, H.; Wang, L.; Bashir, K.; Wang, Q.; Cui, X.; Li, X.; Luo, Z.; Chang, C.; Fu, Q. Facile one-step targeted immobilization of an enzyme based on silane emulsion self-assembled molecularly imprinted polymers for visual sensors. Analyst 2020, 145, 268–276. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, J.; Han, Q.; Hu, X.; Wang, D.; Zhang, X.; Yang, P. One-Step Assembly of a Biomimetic Biopolymer Coating for Particle Surface Engineering. Adv. Mater. 2018, 30, 1802851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, Y.; Lv, X.; Ding, Y.; Zhang, Y.; Jing, J.; Xu, C. One-step synthesis of uniform nanoparticles of porphyrin functionalized ceria with promising peroxidase mimetics for H2O2 and glucose colorimetric detection. Sens. Actuators B Chem. 2017, 240, 726–734. [Google Scholar] [CrossRef]
- Obst, F.; Simon, D.; Mehner, P.J.; Neubauer, J.W.; Beck, A.; Stroyuk, O.; Richter, A.; Voit, B.; Appelhans, D. One-step photostructuring of multiple hydrogel arrays for compartmentalized enzyme reactions in microfluidic devices. React. Chem. Eng. 2019, 4, 2141–2155. [Google Scholar] [CrossRef]
- Duchiron, S.W.; Pollet, E.; Givry, S.; Avérous, L. Enzymatic synthesis of poly (ε-caprolactone-co-ε-thiocaprolactone). Eur. Polym. J. 2017, 87, 147–158. [Google Scholar] [CrossRef]
- Tian, F.; Karboune, S.; Hill, A. Synthesis of fructooligosaccharides and oligolevans by the combined use of levansucrase and endo-inulinase in one-step bi-enzymatic system. Innov. Food Sci. Emerg. Technol. 2014, 22, 230–238. [Google Scholar] [CrossRef]
- Li, S.; Liu, D.; Wu, B.; Sun, H.; Liu, X.; Zhang, H.; Ding, N.; Wu, L. One-pot synthesis of a peroxidase-like nanozyme and its application in visual assay for tyrosinase activity. Talanta 2022, 239, 123088. [Google Scholar] [CrossRef]
- Fu, J.; Yang, Y.R.; Johnson-Buck, A.; Liu, M.; Liu, Y.; Walter, N.G.; Woodbury, N.W.; Yan, H. Multi-enzyme complexes on DNA scaffolds capable of substrate channelling with an artificial swinging arm. Nat. Nanotechnol. 2014, 9, 531–536. [Google Scholar] [CrossRef]
- Gdor, E.; Shemesh, S.; Magdassi, S.; Mandler, D. Multienzyme inkjet printed 2D arrays. ACS Appl. Mater. Interfaces 2015, 7, 17985–17992. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; Shen, L.; Qu, A.; Wang, R.; An, Y.; Shi, L. Artificial peroxidase/oxidase multiple enzyme system based on supramolecular hydrogel and its application as a biocatalyst for cascade reactions. ACS Appl. Mater. Interfaces 2015, 7, 16694–16705. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niu, D.; Li, P.; Wu, Q.; Bo, X.; Liu, B.; Bao, S.; Su, T.; Xu, H.; Wang, Q. Dual-enzyme-loaded multifunctional hybrid nanogel system for pathological responsive ultrasound imaging and T 2-weighted magnetic resonance imaging. ACS Nano 2015, 9, 5646–5656. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.C.; Tripathi, B.P.; Müller, M.; Stamm, M.; Ionov, L. Bienzymatic sequential reaction on microgel particles and their cofactor dependent applications. Biomacromolecules 2016, 17, 1610–1620. [Google Scholar] [CrossRef]
- Man, T.; Xu, C.; Liu, X.-Y.; Li, D.; Tsung, C.-K.; Pei, H.; Wan, Y.; Li, L. Hierarchically encapsulating enzymes with multi-shelled metal-organic frameworks for tandem biocatalytic reactions. Nat. Commun. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Mansur, A.A.; Mansur, H.S.; Carvalho, S.M. Engineered hybrid nanozyme catalyst cascade based on polysaccharide-enzyme-magnetic iron oxide nanostructures for potential application in cancer therapy. Catal. Today 2022, 388, 187–198. [Google Scholar] [CrossRef]
- Xu, C.; Lei, C.; Wang, Y.; Yu, C. Dendritic mesoporous nanoparticles: Structure, synthesis and properties. Angew. Chem. 2022, 134, e202112752. [Google Scholar]
- Bhat, A.R.; Dongre, R.S.; Almalki, F.A.; Berredjem, M.; Aissaoui, M.; Touzani, R.; Hadda, T.B.; Akhter, M.S. Synthesis, biological activity and POM/DFT/docking analyses of annulated pyrano [2, 3-d] pyrimidine derivatives: Identification of antibacterial and antitumor pharmacophore sites. Bioorg. Chem. 2021, 106, 104480. [Google Scholar] [CrossRef]
- Sun, J.; Du, K.; Song, X.; Gao, Q.; Wu, H.; Ma, J.; Ji, P.; Feng, W. Specific immobilization of D-amino acid oxidase on hematin-functionalized support mimicking multi-enzyme catalysis. Green Chem. 2015, 17, 4465–4472. [Google Scholar] [CrossRef]
- Shangguan, L.; Wei, Y.; Liu, X.; Yu, J.; Liu, S. Confining a bi-enzyme inside the nanochannels of a porous aluminum oxide membrane for accelerating the enzymatic reactions. Chem. Commun. 2017, 53, 2673–2676. [Google Scholar] [CrossRef]
- Jia, F.; Narasimhan, B.; Mallapragada, S. Materials-based strategies for multi-enzyme immobilization and co-localization: A review. Biotechnol. Bioeng. 2014, 111, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Claaßen, C.; Gerlach, T.; Rother, D. Stimulus-responsive regulation of enzyme activity for one-step and multi-step syntheses. Adv. Synth. Catal. 2019, 361, 2387–2401. [Google Scholar] [CrossRef] [PubMed]
- Küchler, A.; Adamcik, J.; Mezzenga, R.; Schlüter, A.D.; Walde, P. Enzyme immobilization on silicate glass through simple adsorption of dendronized polymer–enzyme conjugates for localized enzymatic cascade reactions. RSC Adv. 2015, 5, 44530–44544. [Google Scholar] [CrossRef]
- Yang, Q.; Tan, L.; He, C.; Liu, B.; Xu, Y.; Zhu, Z.; Shao, Z.; Gong, B.; Shen, Y.-M. Redox-responsive micelles self-assembled from dynamic covalent block copolymers for intracellular drug delivery. Acta Biomater. 2015, 17, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zheng, W.; Han, J.; Wu, J.; Li, Y.; Mao, Y.; Wang, L.; Wang, Y. One-step immobilization of β-glucosidase in crude enzyme solution by recyclable UCST-responsive polymer with enhanced uniformly biocatalytic performance. Colloids Surf. B Biointerfaces 2022, 217, 112694. [Google Scholar] [CrossRef]
- Türkan, A.; Yılmaz, F.; Küçük, A.C.; Ozdemir, Y. One-pot two-step lipase-catalyzed synthesis of a, x-thiophene-capped poly (e-caprolactone) macromonomers and their use in electropolymerization. Polym. Bull. 2011, 67, 1483–1498. [Google Scholar] [CrossRef]
- Ricca, E.; Brucher, B.; Schrittwieser, J.H. Multi-enzymatic cascade reactions: Overview and perspectives. Adv. Synth. Catal. 2011, 353, 2239–2262. [Google Scholar] [CrossRef]
- Wu, F.; Minteer, S. Krebs cycle metabolon: Structural evidence of substrate channeling revealed by cross-linking and mass spectrometry. Angew. Chem. Int. Ed. 2015, 54, 1851–1854. [Google Scholar] [CrossRef]
- Wang, S.-Z.; Zhang, Y.-H.; Ren, H.; Wang, Y.-L.; Jiang, W.; Fang, B.-S. Strategies and perspectives of assembling multi-enzyme systems. Crit. Rev. Biotechnol. 2017, 37, 1024–1037. [Google Scholar] [CrossRef]
- Klermund, L.; Poschenrieder, S.T.; Castiglione, K. Biocatalysis in polymersomes: Improving multienzyme cascades with incompatible reaction steps by compartmentalization. ACS Catal. 2017, 7, 3900–3904. [Google Scholar] [CrossRef]
- Wang, X.; An, Z. Enzyme-initiated reversible addition− fragmentation chain transfer (RAFT) polymerization: Precision polymer synthesis via enzymatic catalysis. In Methods in Enzymology; Elsevier: San Diego, CA, USA, 2019; pp. 291–319. [Google Scholar]
- Lin, Y.; Wu, L.; Huang, Y.; Ren, J.; Qu, X. Positional assembly of hemin and gold nanoparticles in graphene–mesoporous silica nanohybrids for tandem catalysis. Chem. Sci. 2015, 6, 1272–1276. [Google Scholar] [CrossRef]
- Van Oers, M.; Rutjes, F.; Van Hest, J. Cascade reactions in nanoreactors. Curr. Opin. Biotechnol. 2014, 28, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Zhao, B.; Thayumanavan, S. Cascaded step-growth polymerization for functional polyamides with diverse architectures and stimuli responsive characteristics. ACS Macro Lett. 2019, 8, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Busto, E.; Simon, R.C.; Richter, N.; Kroutil, W. One-pot, two-module three-step cascade to transform phenol derivatives to enantiomerically pure (R)-or (S)-p-hydroxyphenyl lactic acids. ACS Catal. 2016, 6, 2393–2397. [Google Scholar] [CrossRef]
- Dennig, A.; Busto, E.; Kroutil, W.; Faber, K. Biocatalytic one-pot synthesis of L-tyrosine derivatives from monosubstituted benzenes, pyruvate, and ammonia. ACS Catal. 2015, 5, 7503–7506. [Google Scholar] [CrossRef]
- Yuan, B.; Huang, T.; Wang, X.; Ding, Y.; Jiang, L.; Zhang, Y.; Tang, J. Oxygen-Tolerant RAFT Polymerization Catalyzed by a Recyclable Biomimetic Mineralization Enhanced Biological Cascade System. Macromol. Rapid Commun. 2022, 43, 2100559. [Google Scholar] [CrossRef] [PubMed]
- Fischereder, E.-M.; Pressnitz, D.; Kroutil, W. Stereoselective Cascade to C3-methylated strictosidine derivatives employing transaminases and strictosidine synthases. ACS Catal. 2016, 6, 23–30. [Google Scholar] [CrossRef]
- France, S.P.; Hepworth, L.J.; Turner, N.J.; Flitsch, S.L. Constructing biocatalytic cascades: In vitro and in vivo approaches to de novo multi-enzyme pathways. ACS Catal. 2017, 7, 710–724. [Google Scholar] [CrossRef]
- Ciulla, M.G.; Zimmermann, S.; Kumar, K. Cascade reaction based synthetic strategies targeting biologically intriguing indole polycycles. Org. Biomol. Chem. 2019, 17, 413–431. [Google Scholar] [CrossRef]
- Köhler, V.; Wilson, Y.; Dürrenberger, M.; Ghislieri, D.; Churakova, E.; Quinto, T.; Knörr, L.; Häussinger, D.; Hollmann, F.; Turner, N. Synthetic cascades are enabled by combining biocatalysts with artificial metalloenzymes. Nat. Chem. 2013, 5, 93–99. [Google Scholar] [CrossRef]
- Turner, N.J.; O’reilly, E. Biocatalytic retrosynthesis. Nat. Chem. Biol. 2013, 9, 285–288. [Google Scholar] [CrossRef]
- Hepworth, L.J.; France, S.P.; Hussain, S.; Both, P.; Turner, N.J.; Flitsch, S.L. Enzyme cascades in whole cells for the synthesis of chiral cyclic amines. ACS Catal. 2017, 7, 2920–2925. [Google Scholar] [CrossRef]
- Nazor, J.; Liu, J.; Huisman, G. Enzyme evolution for industrial biocatalytic cascades. Curr. Opin. Biotechnol. 2021, 69, 182–190. [Google Scholar] [CrossRef]
- Nestl, B.M.; Hammer, S.C.; Nebel, B.A.; Hauer, B. New generation of biocatalysts for organic synthesis. Angew. Chem. Int. Ed. 2014, 53, 3070–3095. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Fernández-Fueyo, E.; Baraibar, A.G.; Ullrich, R.; Hofrichter, M.; Yanase, H.; Alcalde, M.; van Berkel, W.J.; Hollmann, F. Peroxygenase-catalyzed oxyfunctionalization reactions promoted by the complete oxidation of methanol. Angew. Chem. Int. Ed. 2016, 55, 798–801. [Google Scholar] [CrossRef]
- Toogood, H.S.; Scrutton, N.S. New developments in ‘ene’-reductase catalysed biological hydrogenations. Curr. Opin. Chem. Biol. 2014, 19, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Napora-Wijata, K.; Strohmeier, G.A.; Winkler, M. Biocatalytic reduction of carboxylic acids. Biotechnol. J. 2014, 9, 822–843. [Google Scholar] [CrossRef] [PubMed]
- Kourist, R. Biocatalysis in Organic Synthesis. Science of Synthesis; Wiley: Weinheim, Germany, 2015; pp. 12547–12729. [Google Scholar]
- Renata, H.; Wang, Z.J.; Arnold, F.H. Expanding the enzyme universe: Accessing non-natural reactions by mechanism-guided directed evolution. Angew. Chem. Int. Ed. 2015, 54, 3351–3367. [Google Scholar] [CrossRef]
- Otte, K.B.; Hauer, B. Enzyme engineering in the context of novel pathways and products. Curr. Opin. Biotechnol. 2015, 35, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.G.; Vögeli, B.; Quade, N.; Capitani, G.; Kiefer, P.; Vorholt, J.A.; Ebert, M.-O.; Erb, T.J. The use of ene adducts to study and engineer enoyl-thioester reductases. Nat. Chem. Biol. 2015, 11, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Giger, L.; Caner, S.; Obexer, R.; Kast, P.; Baker, D.; Ban, N.; Hilvert, D. Evolution of a designed retro-aldolase leads to complete active site remodeling. Nat. Chem. Biol. 2013, 9, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Röthlisberger, D.; Khersonsky, O.; Wollacott, A.M.; Jiang, L.; DeChancie, J.; Betker, J.; Gallaher, J.L.; Althoff, E.A.; Zanghellini, A.; Dym, O. Kemp elimination catalysts by computational enzyme design. Nature 2008, 453, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Dai, F.; Li, P.; Jin, X.; Huang, Y.; Nie, Z.; Yao, S. Label-free fluorescent enzymatic assay of citrate synthase by CoA–Au (I) co-ordination polymer and its application in a multi-enzyme logic gate cascade. Biosens. Bioelectron. 2016, 86, 1038–1046. [Google Scholar] [CrossRef]
- Chuaboon, L.; Wongnate, T.; Punthong, P.; Kiattisewee, C.; Lawan, N.; Hsu, C.Y.; Lin, C.H.; Bornscheuer, U.T.; Chaiyen, P. One-Pot Bioconversion of l-Arabinose to l-Ribulose in an Enzymatic Cascade. Angew. Chem. Int. Ed. 2019, 58, 2428–2432. [Google Scholar] [CrossRef] [PubMed]
- Sperl, J.M.; Sieber, V. Multienzyme Cascade Reactions—Status and Recent Advances. ACS Catal. 2018, 8, 2385–2396. [Google Scholar] [CrossRef]
- Muschiol, J.; Peters, C.; Oberleitner, N.; Mihovilovic, M.D.; Bornscheuer, U.T.; Rudroff, F. Cascade catalysis–strategies and challenges en route to preparative synthetic biology. Chem. Commun. 2015, 51, 5798–5811. [Google Scholar] [CrossRef] [PubMed]
- Schrittwieser, J.H.; Velikogne, S.; Hall, M.l.; Kroutil, W. Artificial biocatalytic linear cascades for preparation of organic molecules. Chem. Rev. 2018, 118, 270–348. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Wu, J.; Li, Z. Enantioselective cascade biocatalysis via epoxide hydrolysis and alcohol oxidation: One-pot synthesis of (R)-α-hydroxy ketones from meso-or racemic epoxides. ACS Catal. 2015, 5, 51–58. [Google Scholar] [CrossRef]
- Sahar, S.; Zeb, A.; Ling, C.; Raja, A.; Wang, G.; Ullah, N.; Lin, X.-M.; Xu, A.-W. A hybrid VO x incorporated hexacyanoferrate nanostructured hydrogel as a multienzyme mimetic via cascade reactions. ACS Nano 2020, 14, 3017–3031. [Google Scholar] [CrossRef]
- Cutlan, R.; De Rose, S.; Isupov, M.N.; Littlechild, J.A.; Harmer, N.J. Using enzyme cascades in biocatalysis: Highlight on transaminases and carboxylic acid reductases. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2020, 1868, 140322. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Finnigan, W.; Thomas, A.; Cromar, H.; Gough, B.; Snajdrova, R.; Adams, J.P.; Littlechild, J.A.; Harmer, N.J. Characterization of carboxylic acid reductases as enzymes in the toolbox for synthetic chemistry. ChemCatChem 2017, 9, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Ringborg, R.H.; Woodley, J. The application of reaction engineering to biocatalysis. React. Chem. Eng. 2016, 1, 10–22. [Google Scholar] [CrossRef]
- Domínguez de María, P.; Hollmann, F. On the (un) greenness of biocatalysis: Some challenging figures and some promising options. Front. Microbiol. 2015, 6, 1257. [Google Scholar] [CrossRef] [PubMed]
- Koeller, K.M.; Wong, C.-H. Enzymes for chemical synthesis. Nature 2001, 409, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Dannert, C.; Lopez-Gallego, F. A roadmap for biocatalysis–functional and spatial orchestration of enzyme cascades. Microb. Biotechnol. 2016, 9, 601–609. [Google Scholar] [CrossRef]
- Sicard, C. In-situ Enzyme Immobilization by Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2022, 62, e202213405. [Google Scholar]
- Tang, Q.; Li, Y.; Liu, W.; Li, B.; Jin, Y. One-step synthesis of biomimetic copper–cysteine nanoparticle with excellent laccase-like activity. J. Mater. Sci. 2022, 1–12. [Google Scholar] [CrossRef]
- Bahri, S.; Homaei, A.; Mosaddegh, E. Zinc sulfide-chitosan hybrid nanoparticles as a robust surface for immobilization of Sillago sihama α-amylase. Colloids Surf. B Biointerfaces 2022, 218, 112754. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, J.; Xiao, W.; Chen, T.; Yi, C.; Xu, Z. Engineering of polystyrene-supported artificial catalytic triad constructed by nanoprecipitation for efficient ester hydrolysis in water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128902. [Google Scholar] [CrossRef]
- Shen, H.; Song, J.; Yang, Y.; Su, P.; Yang, Y. DNA-directed enzyme immobilization on Fe3O4 modified with nitrogen-doped graphene quantum dots as a highly efficient and stable multi-catalyst system. J. Mater. Sci. 2019, 54, 2535–2551. [Google Scholar] [CrossRef]
- Jia, Z.; Bobrin, V.A.; Truong, N.P.; Gillard, M.; Monteiro, M.J. Multifunctional nanoworms and nanorods through a one-step aqueous dispersion polymerization. J. Am. Chem. Soc. 2014, 136, 5824–5827. [Google Scholar] [CrossRef] [PubMed]
- Salgaonkar, M.; Nadar, S.S.; Rathod, V.K. Combi-metal organic framework (Combi-MOF) of α-amylase and glucoamylase for one pot starch hydrolysis. Int. J. Biol. Macromol. 2018, 113, 464–475. [Google Scholar] [CrossRef]
- Soh, N.; Kaneko, S.; Uozumi, K.; Ueda, T.; Kamada, K. Preparation of an enzyme/inorganic nanosheet/magnetic bead complex and its enzymatic activity. J. Mater. Sci. 2014, 49, 8010–8015. [Google Scholar] [CrossRef]
- Li, X.; Hu, X.; Qiao, Y.; Lu, T.; Bai, Y.; Xiong, J.; Li, X.; Gou, Q.; Ge, J. Enzyme-bimetallic hybrid catalyst for one-pot chemoenzymatic reactions. Chem. Eng. J. 2023, 452, 139356. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, X.; Rao, Y.; Lin, R.; Ge, L.; Yang, P.; Zhang, H.; Zhu, C.; Ying, H.; Zhuang, W. Stabilizing bienzymatic cascade catalysis via immobilization in ZIF-8/GO composites obtained by GO assisted co-growth. Colloids Surf. B Biointerfaces 2022, 217, 112585. [Google Scholar] [CrossRef]
- Oktay, B.; Demir, S.; Kayaman-Apohan, N. Immobilization of α-amylase onto poly (glycidyl methacrylate) grafted electrospun fibers by ATRP. Mater. Sci. Eng. C 2015, 50, 386–393. [Google Scholar] [CrossRef]
- Hooe, S.; Breger, J.; Dean, S.; Susumu, K.; Oh, E.; Walper, S.; Ellis, G.A.; Medintz, I.L. Benzaldehyde Lyase Kinetic Improvements, Potential Channeling to Alcohol Dehydrogenase, and Substrate Scope when Immobilized on Semiconductor Quantum Dots. ACS Appl. Nano Mater. 2022, 5, 10900–10911. [Google Scholar] [CrossRef]
- Ellis, G.A.; Klein, W.P.; Lasarte-Aragones, G.; Thakur, M.; Walper, S.A.; Medintz, I.L. Artificial multienzyme scaffolds: Pursuing in vitro substrate channeling with an overview of current progress. ACS Catal. 2019, 9, 10812–10869. [Google Scholar] [CrossRef]
- Liang, H.; Sun, S.; Zhou, Y.; Liu, Y. In-situ self-assembly of zinc/adenine hybrid nanomaterials for enzyme immobilization. Catalysts 2017, 7, 327. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, S.; Simmons, C.R.; Narayanan, R.P.; Zhang, F.; Aziz, A.-M.; Yan, H.; Stephanopoulos, N. Tunable nanoscale cages from self-assembling DNA and protein building blocks. ACS Nano 2019, 13, 3545–3554. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chai, Y.; Yuan, Y.; Yuan, R. Lattice-like DNA tetrahedron nanostructure as scaffold to locate GOx and HRP enzymes for highly efficient enzyme cascade reaction. ACS Appl. Mater. Interfaces 2019, 12, 2871–2877. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.D.; Löf, D.; Hvilsted, S.; Daugaard, A.E. Highly branched bio-based unsaturated polyesters by enzymatic polymerization. Polymers 2016, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, S.; Fu, X.; Liu, X.-w.; Wang, P.G.; Fang, J. Sequential one-pot multienzyme synthesis of hyaluronan and its derivative. Carbohydr. Polym. 2017, 178, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Tomaino, E.; Capecchi, E.; Piccinino, D.; Saladino, R. Lignin Nanoparticles Support Lipase-Tyrosinase Enzymatic Cascade in the Synthesis of Lipophilic Hydroxytyrosol Ester Derivatives. ChemCatChem 2022, 14, e202200380. [Google Scholar] [CrossRef]
- Naapuri, J.M.; Losada-Garcia, N.; Rothemann, R.A.; Pichardo, M.C.; Prechtl, M.H.; Palomo, J.M.; Deska, J. Cascade Catalysis Through Bifunctional Lipase Metal Biohybrids for the Synthesis of Enantioenriched O-Heterocycles from Allenes. ChemCatChem 2022, 14, e202200362. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wu, X.-L.; Zong, M.-H.; Lou, W.-Y. Construction of Zn-heptapeptide bionanozymes with intrinsic hydrolase-like activity for degradation of di (2-ethylhexyl) phthalate. J. Coll. Interface Sci. 2022, 622, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Dega, N.K.; Ganganboina, A.B.; Tran, H.L.; Kuncoro, E.P.; Doong, R.-a. BSA-stabilized manganese phosphate nanoflower with enhanced nanozyme activity for highly sensitive and rapid detection of glutathione. Talanta 2022, 237, 122957. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, Y.; Zhang, Q.; Zou, W.; Jin, L.; Guo, R. Arginine-rich peptide/platinum hybrid colloid nanoparticle cluster: A single nanozyme mimicking multi-enzymatic cascade systems in peroxisome. J. Coll. Interface Sci. 2021, 600, 37–48. [Google Scholar] [CrossRef]
- Edwards-Gayle, C.J.; Hamley, I.W. Self-assembly of bioactive peptides, peptide conjugates, and peptide mimetic materials. Org. Biomol. Chem. 2017, 15, 5867–5876. [Google Scholar] [CrossRef] [PubMed]
- Loubidi, M.; Lorion, M.; El Hakmaoui, A.; Bernard, P.; Akssira, M.; Guillaumet, G. One-step synthesis of 1H-Imidazo [1, 5-a] imidazole scaffolds and access to their polyheterocycles. Synthesis 2019, 51, 3973–3980. [Google Scholar] [CrossRef]
- Allijn, I.; du Preez, N.; Tasior, M.; Bansal, R.; Stamatialis, D. One-Step Fabrication of Porous Membrane-Based Scaffolds by Air-Water Interfacial Phase Separation: Opportunities for Engineered Tissues. Membranes 2022, 12, 453. [Google Scholar] [CrossRef]
- Song, R.; Yu, H.; Huang, H.; Chen, Y. Controlled One-Pot Synthesis of Multiple Heterocyclic Scaffolds Based on an Amphiphilic Claisen-Schmidt Reaction Intermediate. ChemistrySelect 2019, 4, 14021–14026. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kodama, S.; Nishimura, R.; Nomoto, A.; Ueshima, M.; Ogawa, A. One-Pot Construction of Diverse β-Lactam Scaffolds via the Green Oxidation of Amines and Its Application to the Diastereoselective Synthesis of β-Amino Acids. J. Org. Chem. 2021, 86, 11571–11582. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Abetxuko, A.; Sánchez-deAlcázar, D.; Muñumer, P.; Beloqui, A. Tunable polymeric scaffolds for enzyme immobilization. Front. Bioeng. Biotechnol. 2020, 8, 830. [Google Scholar] [CrossRef]
- Wong, J.X.; Ogura, K.; Chen, S.; Rehm, B.H. Bioengineered polyhydroxyalkanoates as immobilized enzyme scaffolds for industrial applications. Front. Bioeng. Biotechnol. 2020, 8, 156. [Google Scholar] [CrossRef]
- Lu, D.; Cardiel, J.; Cao, G.; Shen, A.Q. Nanoporous Scaffold with Immobilized Enzymes during Flow-Induced Gelation for Sensitive H2O2 Biosensing. Adv. Mater. 2010, 22, 2809–2813. [Google Scholar] [CrossRef]
- Steier, A.; Schmieg, B.; Irtel von Brenndorff, Y.; Meier, M.; Nirschl, H.; Franzreb, M.; Lahann, J. Enzyme scaffolds with hierarchically defined properties via 3D jet writing. Macromol. Biosci. 2020, 20, 2000154. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Y.; Lin, Y.-H.; Lee, A.K.-X.; Kuo, T.-Y.; Tsai, C.-H.; Shie, M.-Y. Combined Effects of Polydopamine-Assisted Copper Immobilization on 3D-Printed Porous Ti6Al4V Scaffold for Angiogenic and Osteogenic Bone Regeneration. Cells 2022, 11, 2824. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; Stenzel, M.H. All wrapped up: Stabilization of enzymes within single enzyme nanoparticles. J. Am. Chem. Soc. 2019, 141, 2754–2769. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Sundarrajan, S.; Venugopal, J.R.; Mukherjee, S.; Ramakrishna, S. Advances in polymeric systems for tissue engineering and biomedical applications. Macromol. Biosci. 2012, 12, 286–311. [Google Scholar] [CrossRef]
- Teuschl, A.H.; Neutsch, L.; Monforte, X.; Rünzler, D.; Van Griensven, M.; Gabor, F.; Redl, H. Enhanced cell adhesion on silk fibroin via lectin surface modification. Acta Biomater. 2014, 10, 2506–2517. [Google Scholar] [CrossRef] [PubMed]
- Karahaliloğlu, Z.; Demirbilek, M.; Şam, M.; Erol-Demirbilek, M.; Sağlam, N.; Denkbaş, E.B. Plasma polymerization-modified bacterial polyhydroxybutyrate nanofibrillar scaffolds. J. Appl. Polym. Sci. 2013, 128, 1904–1912. [Google Scholar] [CrossRef]
- Jurgens, W.J.; Kroeze, R.J.; Bank, R.A.; Ritt, M.J.; Helder, M.N. Rapid attachment of adipose stromal cells on resorbable polymeric scaffolds facilitates the one-step surgical procedure for cartilage and bone tissue engineering purposes. J. Orthop. Res. 2011, 29, 853–860. [Google Scholar] [CrossRef]
- Khojasteh, A.; Behnia, H.; Naghdi, N.; Esmaeelinejad, M.; Alikhassy, Z.; Stevens, M. Effects of different growth factors and carriers on bone regeneration: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e405–e423. [Google Scholar] [CrossRef]
- Motamedian, S.R.; Hosseinpour, S.; Ahsaie, M.G.; Khojasteh, A. Smart scaffolds in bone tissue engineering: A systematic review of literature. World J. Stem Cells 2015, 7, 657. [Google Scholar] [CrossRef]
- Jafari, M.; Paknejad, Z.; Rad, M.R.; Motamedian, S.R.; Eghbal, M.J.; Nadjmi, N.; Khojasteh, A. Polymeric scaffolds in tissue engineering: A literature review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 431–459. [Google Scholar] [CrossRef] [PubMed]
- Barradas, A.M.; Lachmann, K.; Hlawacek, G.; Frielink, C.; Truckenmoller, R.; Boerman, O.C.; Van Gastel, R.; Garritsen, H.; Thomas, M.; Moroni, L. Surface modifications by gas plasma control osteogenic differentiation of MC3T3-E1 cells. Acta Biomater. 2012, 8, 2969–2977. [Google Scholar] [CrossRef]
- Calori, I.R.; Braga, G.; de Jesus, P.d.C.C.; Bi, H.; Tedesco, A.C. Polymer scaffolds as drug delivery systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Theodore, N.; Hlubek, R.; Danielson, J.; Neff, K.; Vaickus, L.; Ulich, T.R.; Ropper, A.E. First human implantation of a bioresorbable polymer scaffold for acute traumatic spinal cord injury: A clinical pilot study for safety and feasibility. Neurosurgery 2016, 79, E305–E312. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Kuang, X.; Deng, J.; Wang, Y.C.; Wang, A.C.; Ding, W.; Lai, Y.C.; Chen, J.; Wang, P.; Lin, Z. Shape memory polymers for body motion energy harvesting and self-powered mechanosensing. Adv. Mater. 2018, 30, 1705195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, J.; Yang, G.; Zhou, Y.; Gao, W.; Wu, G.; Li, X.; Mao, C.; Sheng, T.; Zhou, M. Mechanical properties and degradation of drug eluted bioresorbable vascular scaffolds prepared by three-dimensional printing technology. J. Biomater. Sci. Polym. Ed. 2019, 30, 547–560. [Google Scholar] [CrossRef]
- Baumann, B.; Jungst, T.; Stichler, S.; Feineis, S.; Wiltschka, O.; Kuhlmann, M.; Lindén, M.; Groll, J. Control of nanoparticle release kinetics from 3D printed hydrogel scaffolds. Angew. Chem. Int. Ed. 2017, 56, 4623–4628. [Google Scholar] [CrossRef]
- Ardeshirzadeh, B.; Anaraki, N.A.; Irani, M.; Rad, L.R.; Shamshiri, S. Controlled release of doxorubicin from electrospun PEO/chitosan/graphene oxide nanocomposite nanofibrous scaffolds. Mater. Sci. Eng. C 2015, 48, 384–390. [Google Scholar] [CrossRef]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regener. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef] [PubMed]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold techniques and designs in tissue engineering functions and purposes: A review. Adv. Mater. Sci. Eng. 2019, 2019. [Google Scholar] [CrossRef]
- Koyyada, A.; Orsu, P. Recent advancements and associated challenges of scaffold fabrication techniques in tissue engineering applications. Regener. Eng. Transl. Med. 2021, 7, 147–159. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardio. Sci. Pract. 2013, 38–64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).