Poly(lactic acid) and Nanocrystalline Cellulose Methacrylated Particles for Preparation of Cryogelated and 3D-Printed Scaffolds for Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells
2.3. Methods
2.3.1. PLA-Based Particle Formation
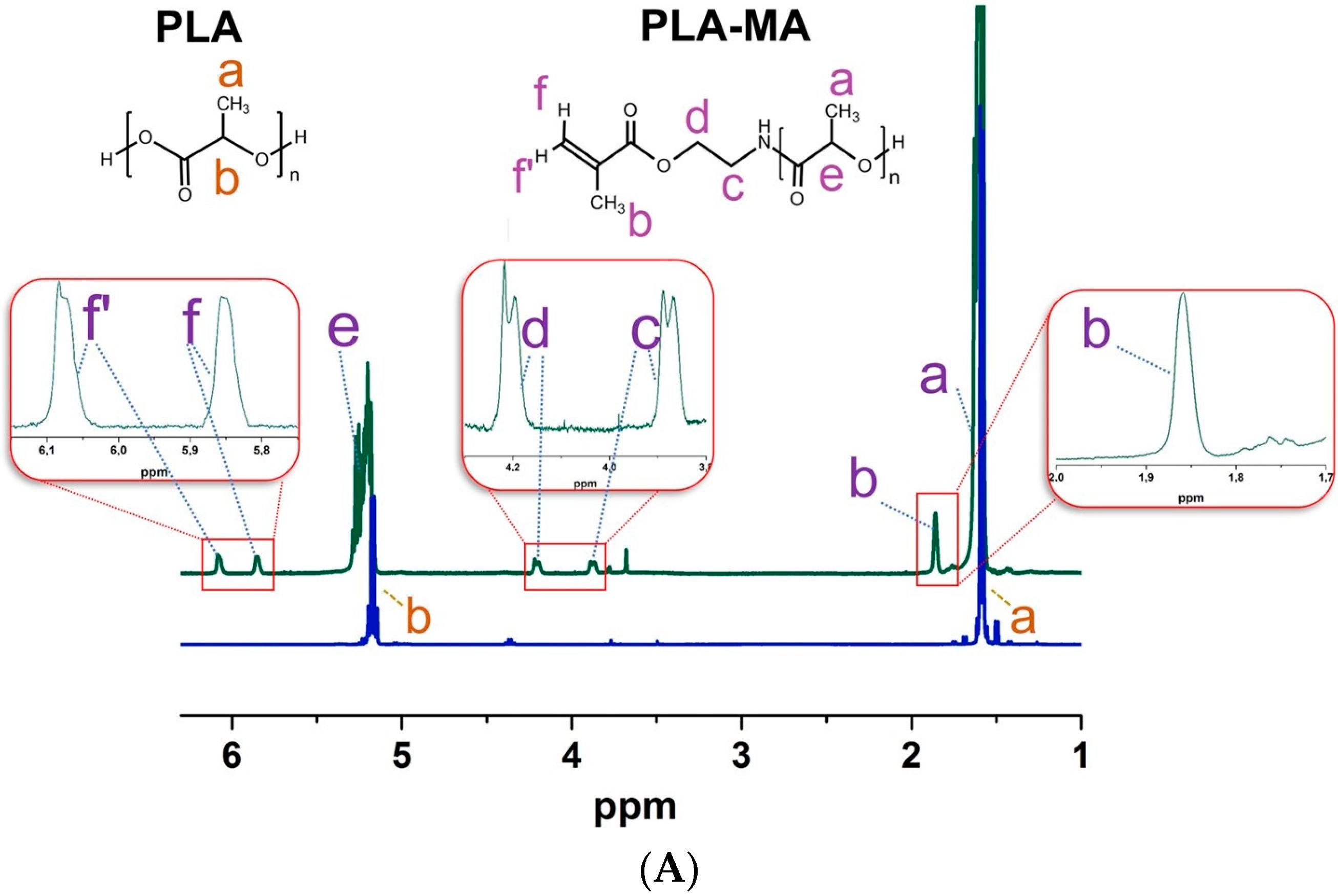
2.3.2. PLA Particle Modification with 2-Aminoethyl Methacrylate
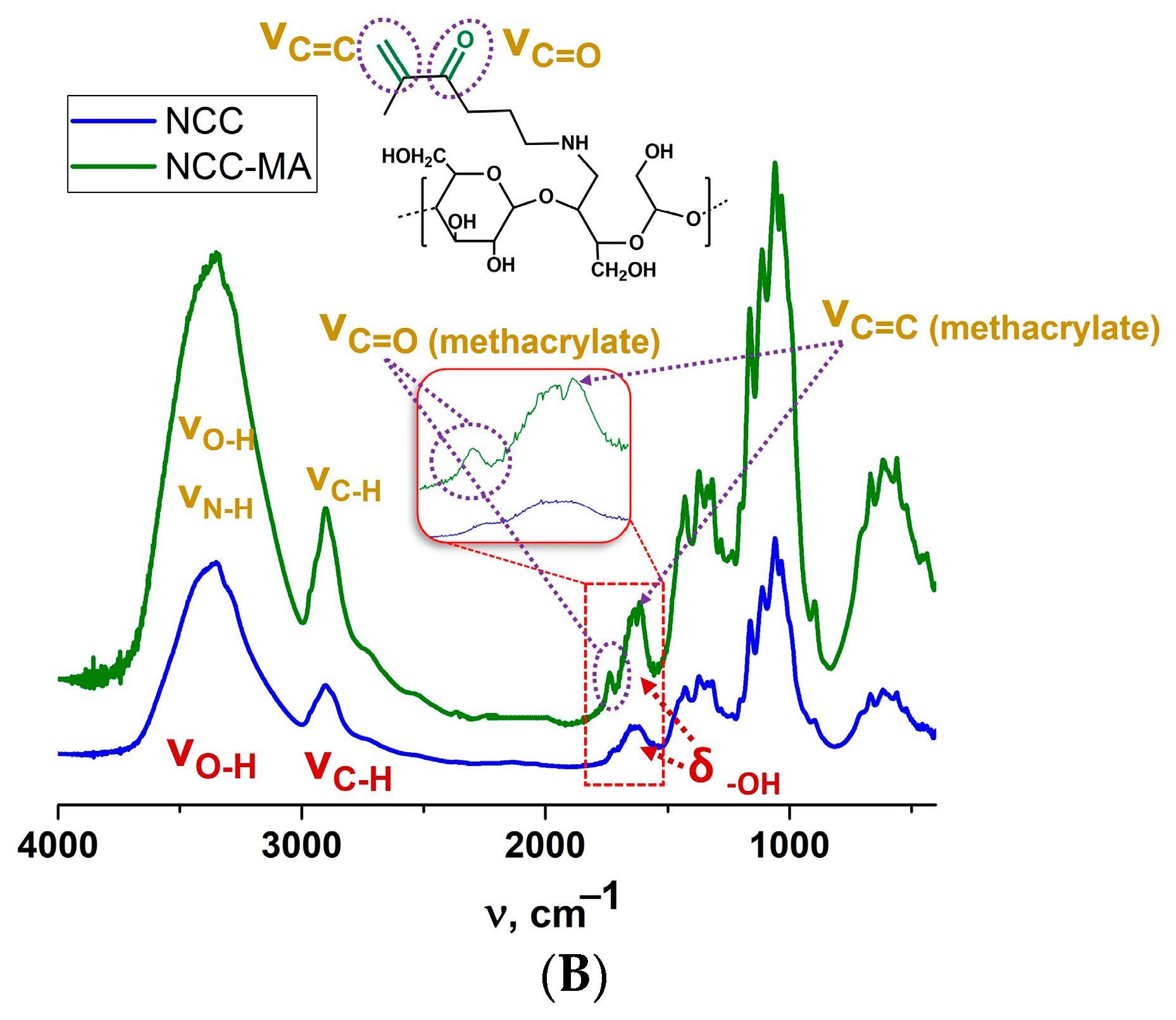
2.3.3. Nanocrystalline Cellulose Modification with AEMA
2.3.4. Particle Characterization
2.3.5. Rheological Measurements of Particle Cross-Linking
2.3.6. Cryogelation
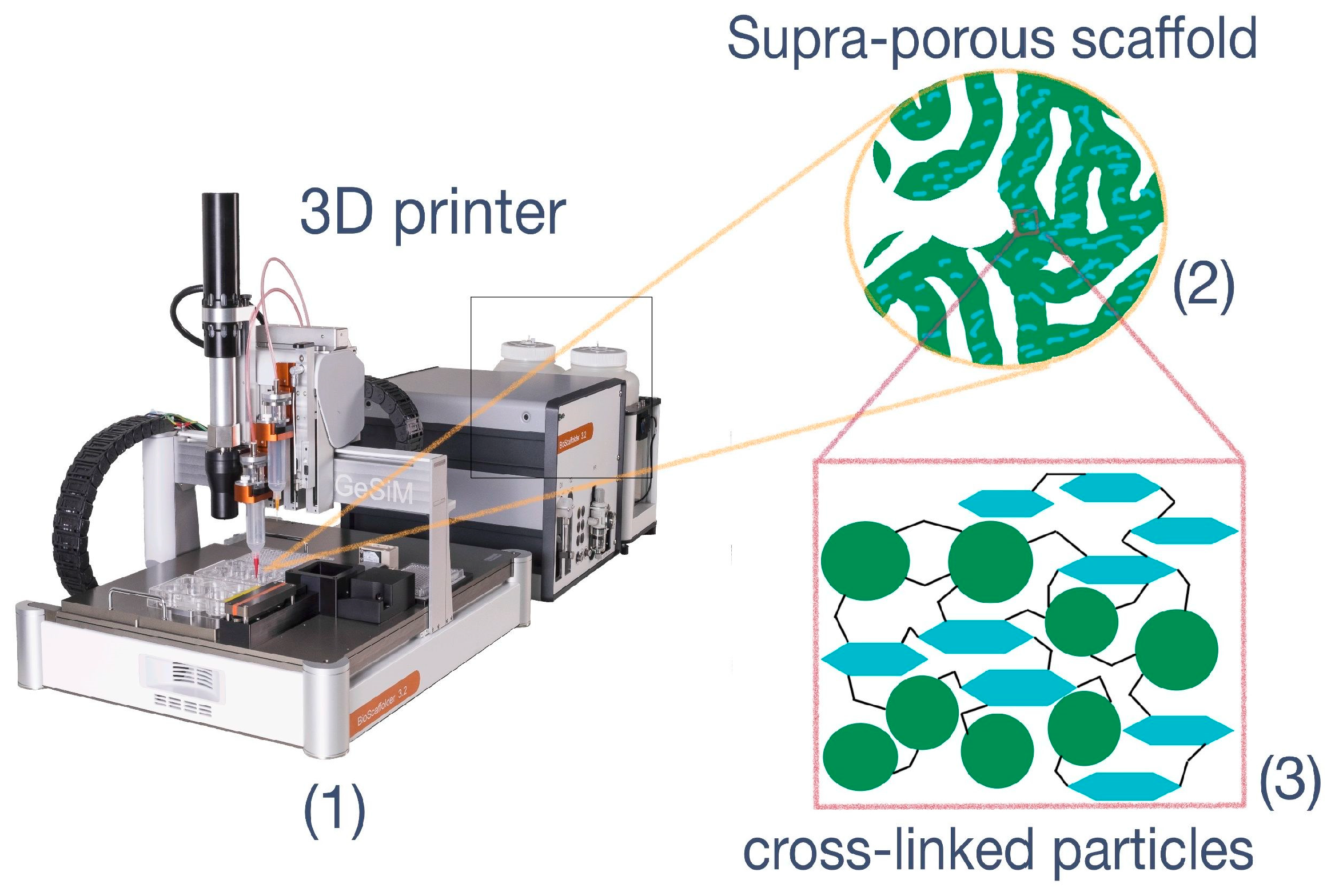
2.3.7. 3D Printing
2.3.8. Study of Mechanical Properties
2.3.9. Cell Culture Experiments
3. Results and Discussion
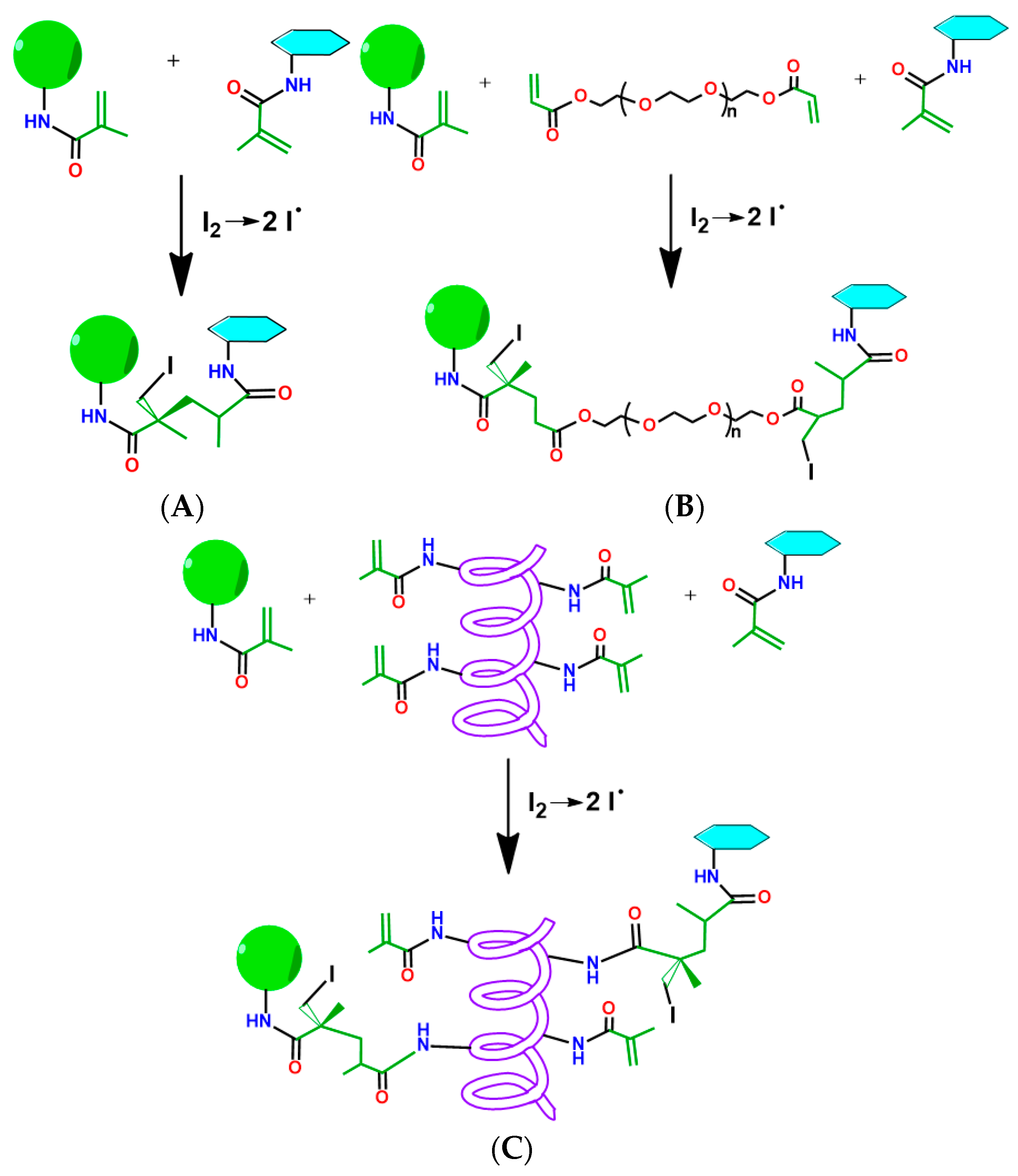
3.1. Preparation of Particles Capable of Participation in Free-Radical Polymerization-Mediated Cross-Linking
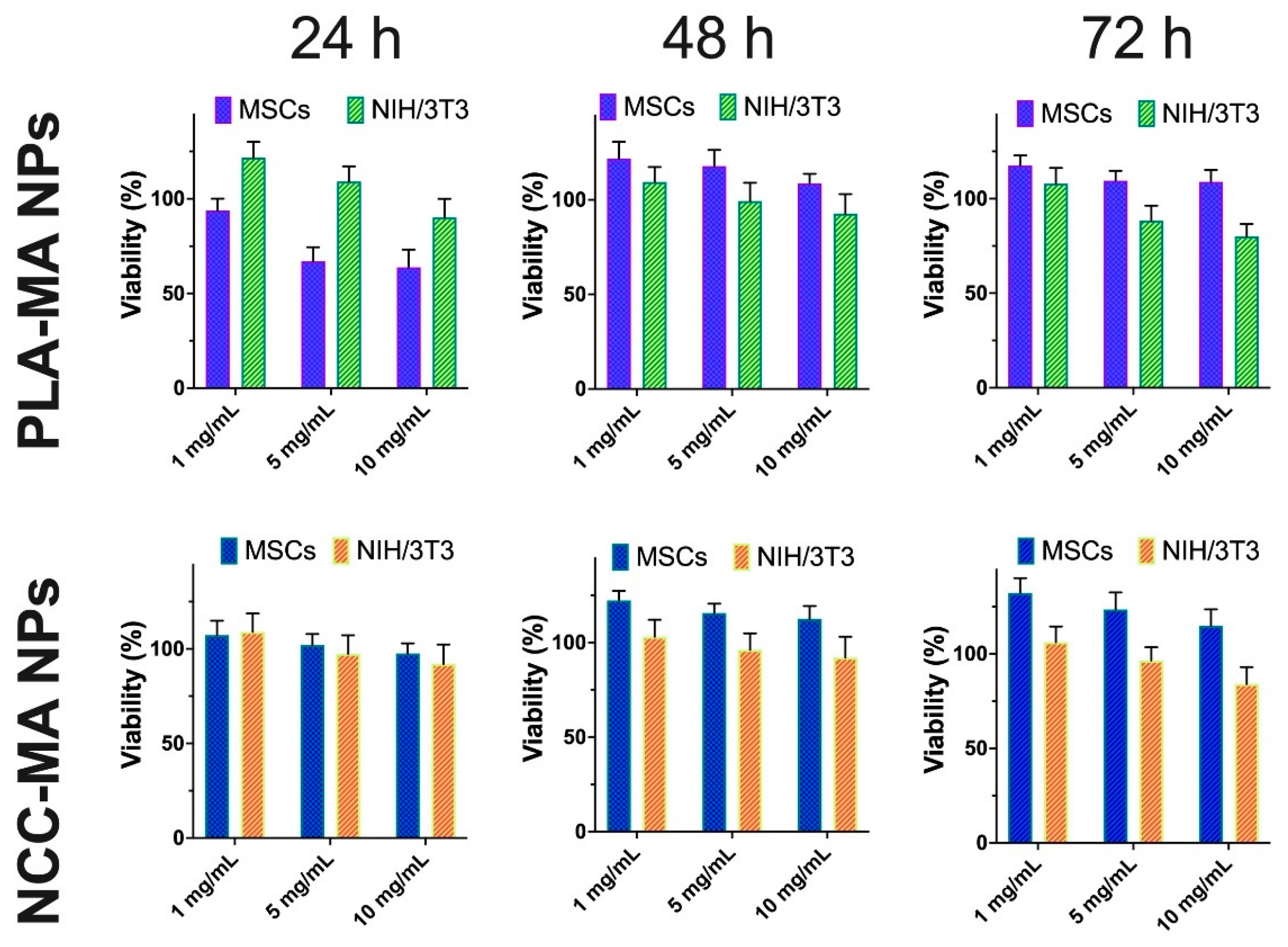
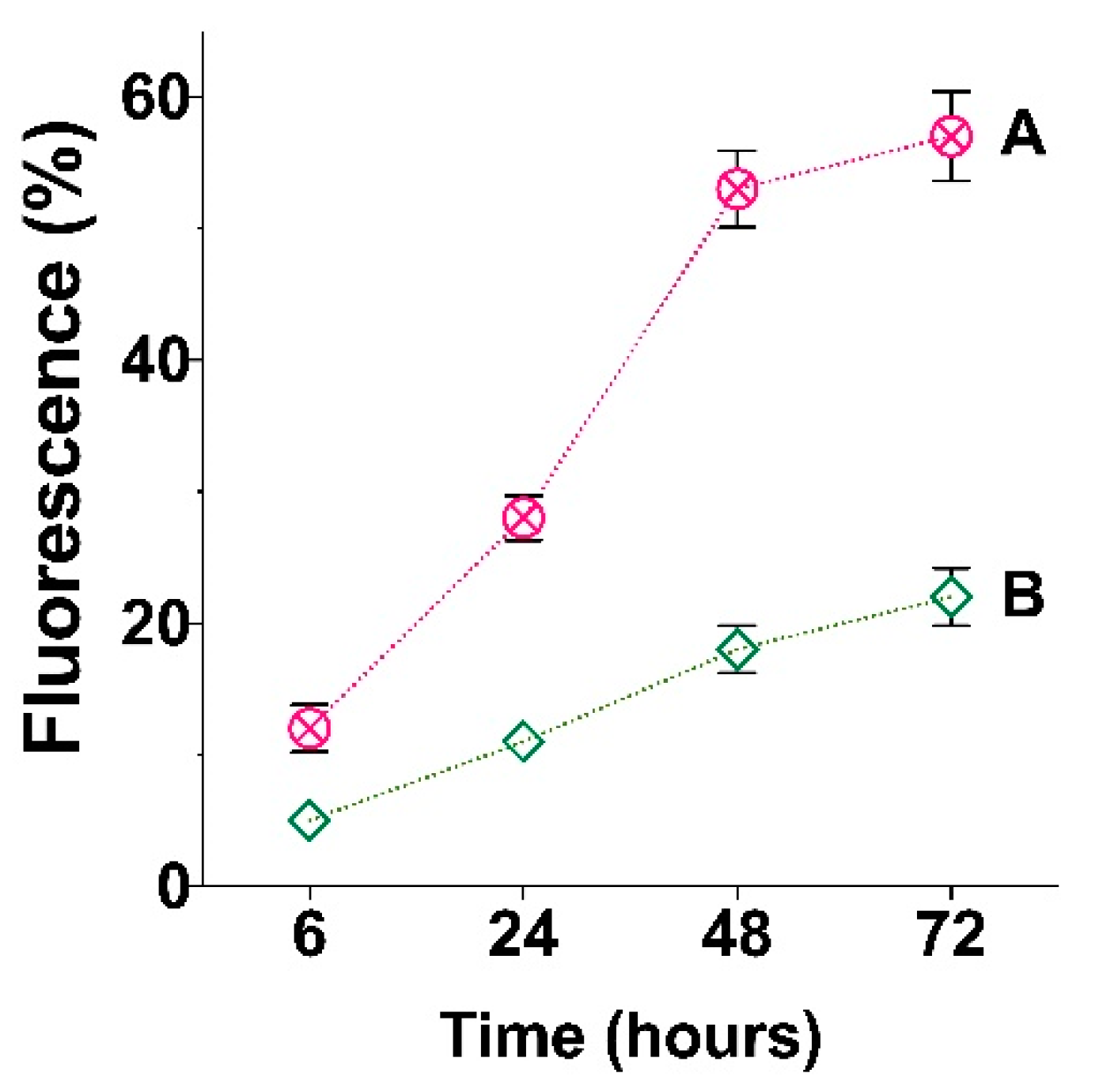
3.2. Effect of Particle Modification on Cells Viability
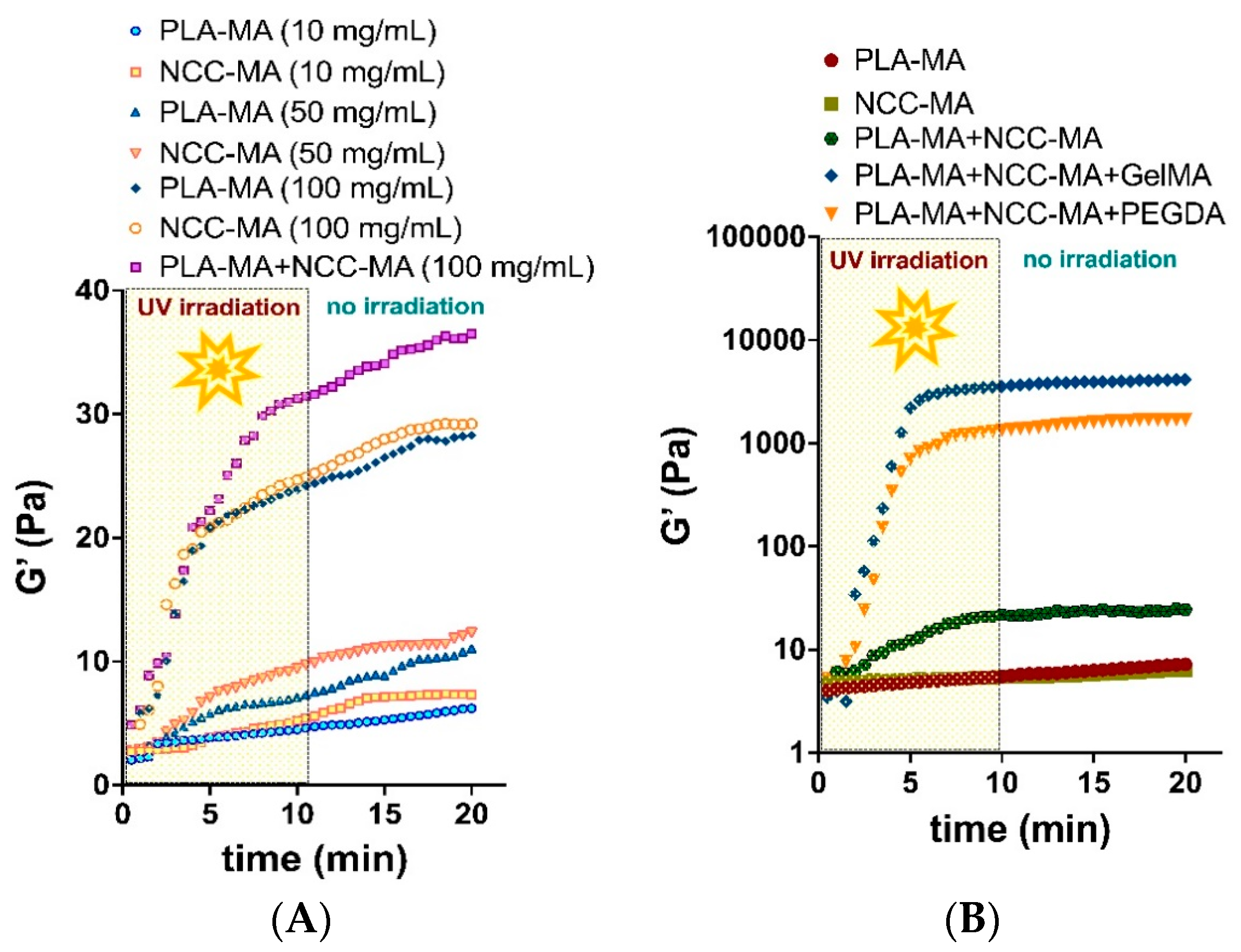
3.3. Rheological Studies of PLA and NCC Particle Cross-Linking
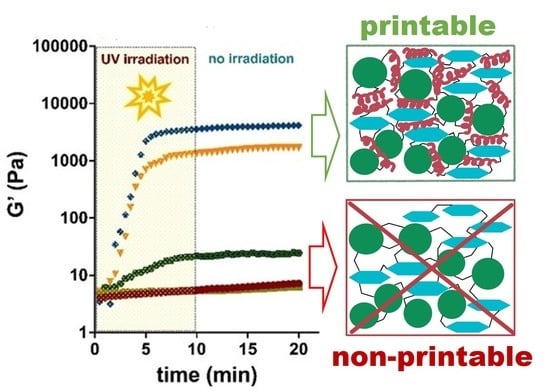
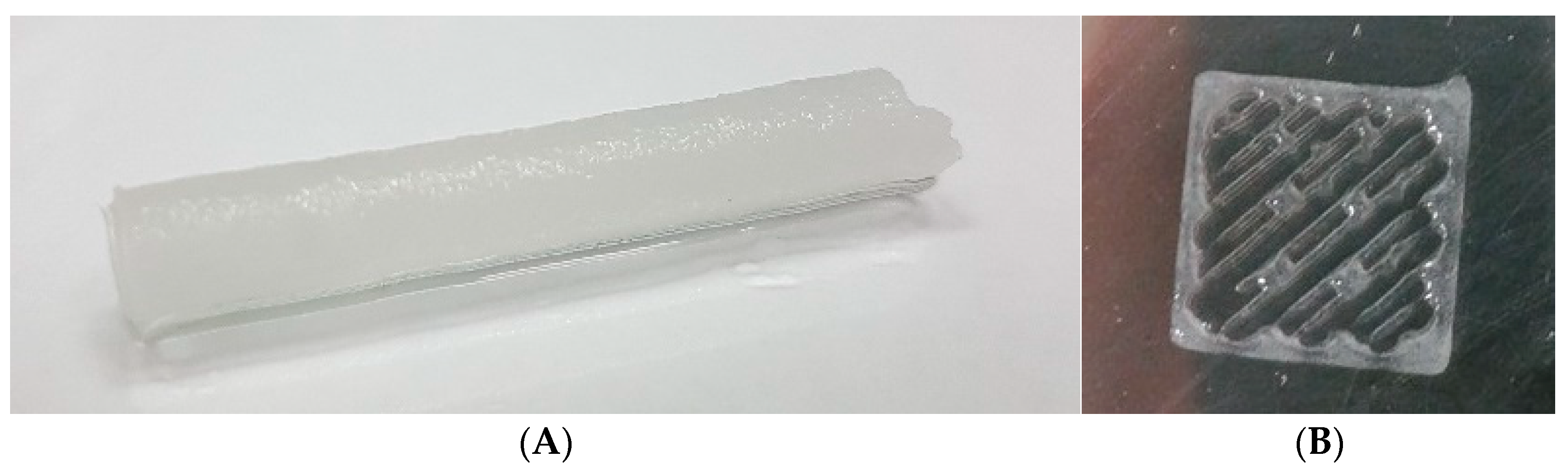
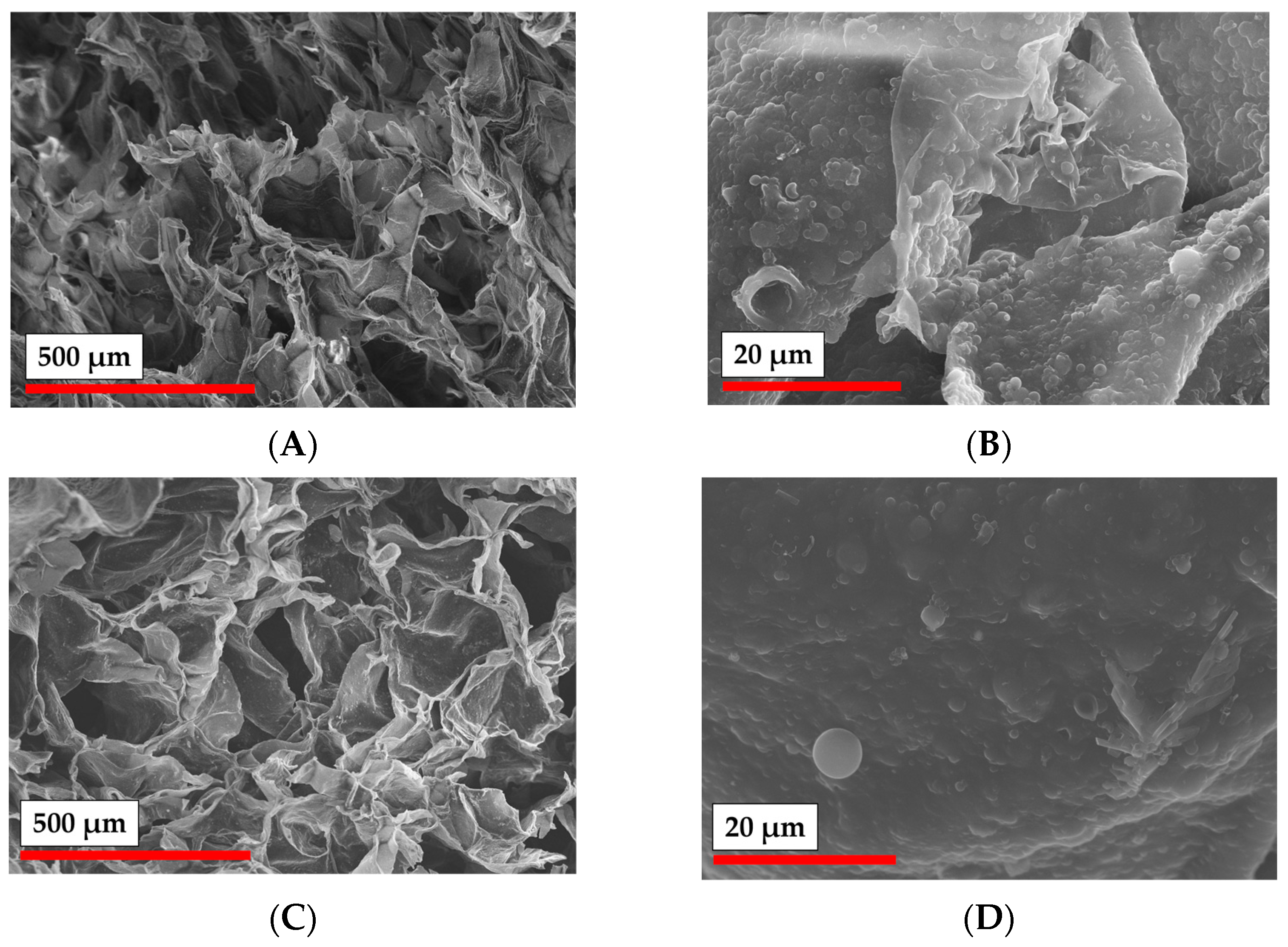
3.4. Cryogelation and 3D-Printing: Methods for Preparation of Particle-Based Scaffolds
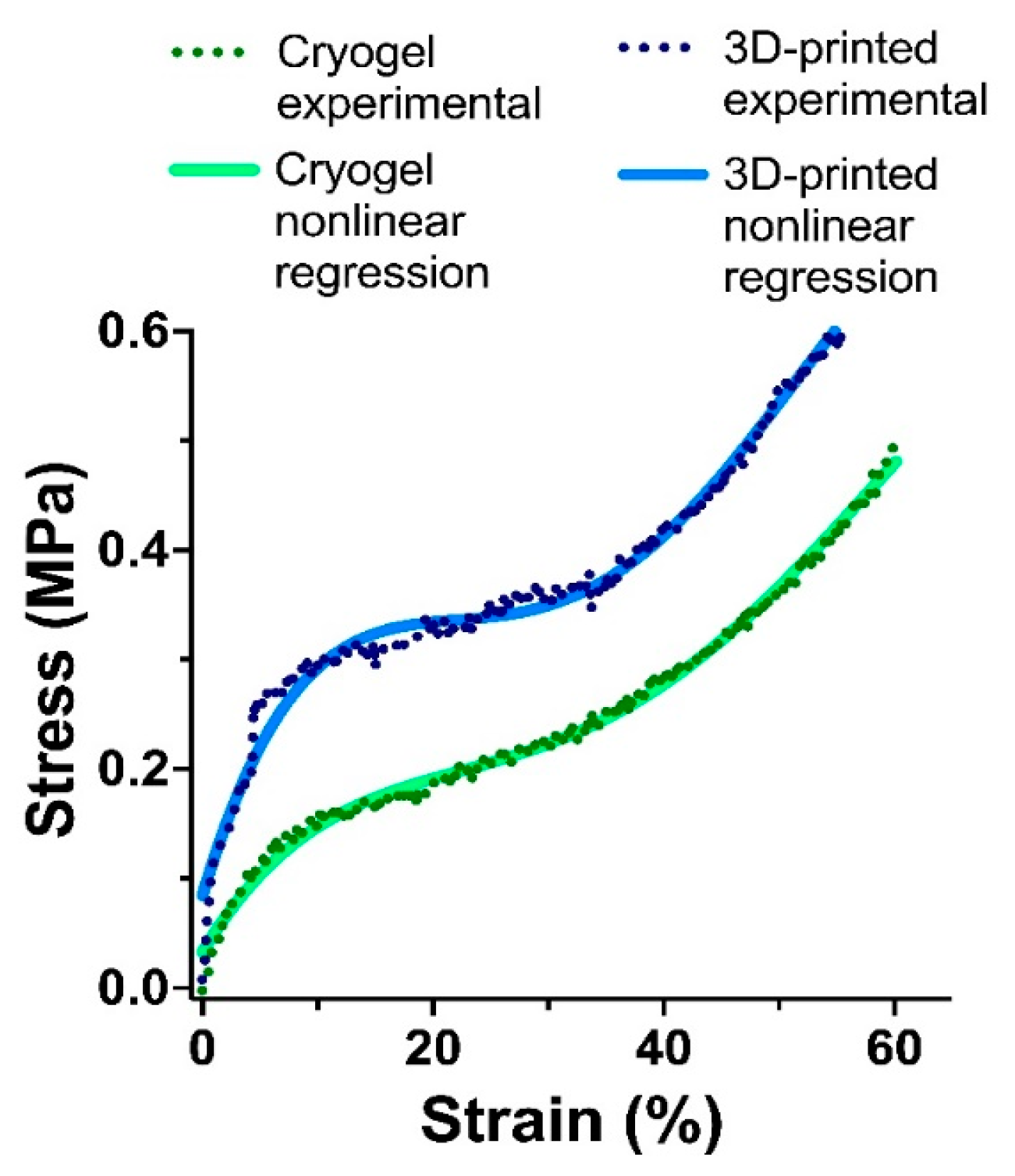
3.5. Mechanical Testing
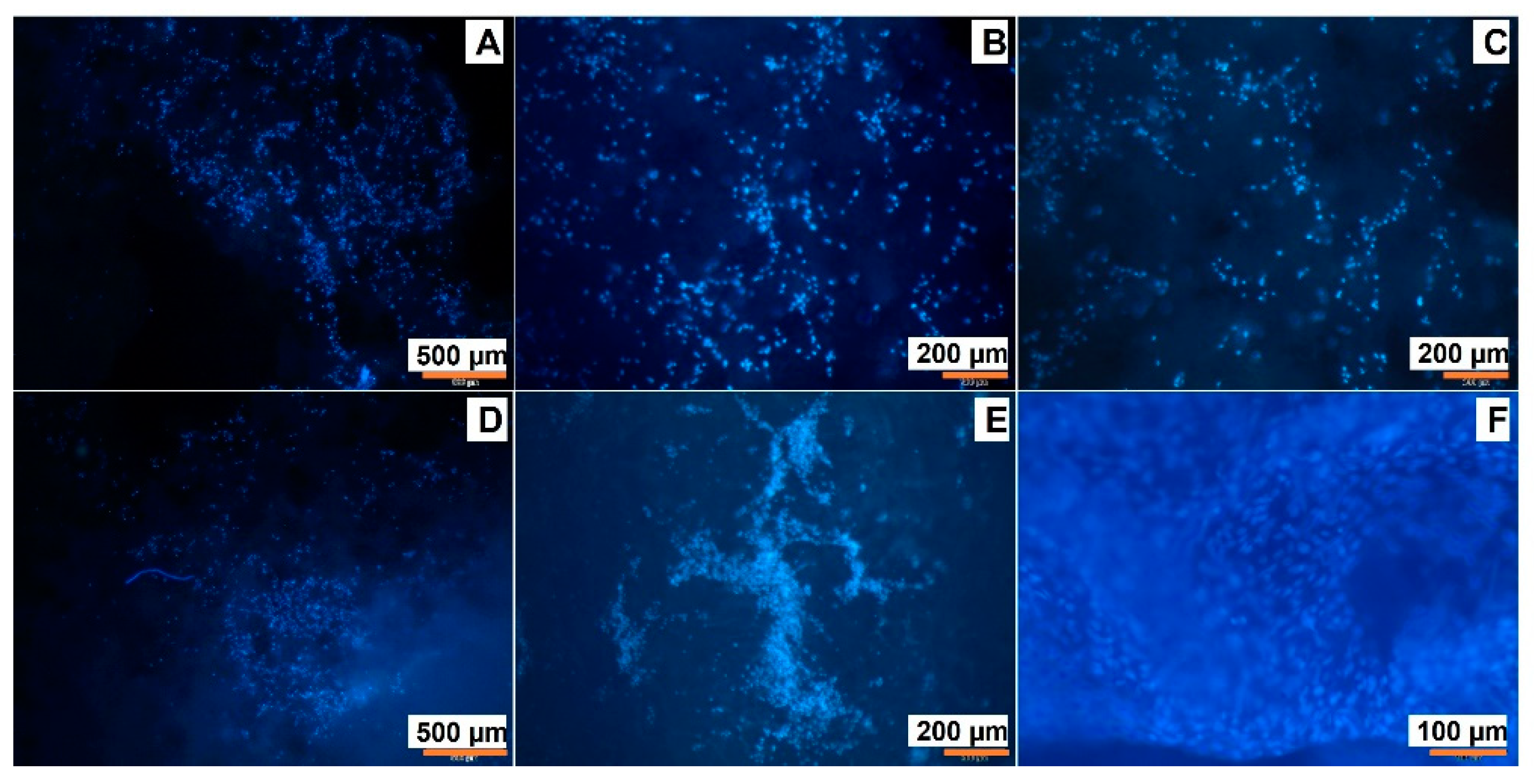
3.6. Cell Adhesion on Cryogelated and 3D Printed Matrices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Du, X.; Kim, I.; Ferguson, S.J. Scaffolds for bone-tissue engineering. Matter 2022, 5, 2722–2759. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.F.; Bhat, H.; Pathak, V. Prospects for In Vitro Cultured Meat – A Future Harvest. In Principles of Tissue Engineering, 4th ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 1663–1683. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J. Advances in tissue engineering. J. Pediatr. Surg. 2016, 51, 8–12. [Google Scholar] [CrossRef]
- Jafari, M.; Paknejad, Z.; Rad, M.R.; Motamedian, S.R.; Eghbal, M.J.; Nadjmi, N.; Khojasteh, A. Polymeric scaffolds in tissue engineering: A literature review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 431–459. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef]
- Cattalini, J.P.; Roether, J.; Hoppe, A.; Pishbin, F.; Haro Durand, L.; Gorustovich, A.; Boccaccini, A.R.; Lucangioli, S.; Mouriño, V. Nanocomposite scaffolds with tunable mechanical and degradation capabilities: Co-delivery of bioactive agents for bone tissue engineering. Biomed. Mater. 2016, 11, 065003. [Google Scholar] [CrossRef] [PubMed]
- Samorezov, J.E.; Alsberg, E. Spatial regulation of controlled bioactive factor delivery for bone tissue engineering. Adv. Drug Deliv. Rev. 2015, 84, 45–67. [Google Scholar] [CrossRef]
- Brown, B.N.; Badylak, S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 2014, 163, 268–285. [Google Scholar] [CrossRef]
- Huang, W.; Li, X.; Shi, X.; Lai, C. Microsphere based scaffolds for bone regenerative applications. Biomater. Sci. 2014, 2, 1145–1153. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Varshney, R.R.; Ren, L.; Gong, Y.; Wang, D.A. Microsphere-based drug releasing scaffolds for inducing osteogenesis of human mesenchymal stem cells in vitro. Eur. J. Pharm. Sci. 2010, 39, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.; Sheu, C.; Fong, Y.; Liao, H.-T.; Chen, J.-P. Microsphere-Based Hierarchically Juxtapositioned Biphasic Scaffolds Prepared from Poly(Lactic-co-Glycolic Acid) and Nanohydroxyapatite for Osteochondral Tissue Engineering. Polymers 2016, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Son, J.S.; Kim, S.G.; Jin, S.C.; Piao, Z.G.; Lee, S.Y.; Oh, J.S.; Kim, C.S.; Kim, B.H.; Jeong, M.A. Development and structure of a novel barrier membrane composed of drug-loaded poly(lactic-co-glycolic acid) particles for guided bone regeneration. Biotechnol. Lett. 2012, 34, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Jin, Y.; Yi, K.; Ju, E.; Zhuo, C.; Wei, H.; Wen, X.; Wang, Y.; Li, M.; Tao, Y. Hemin particles-functionalized 3D printed scaffolds for combined photothermal and chemotherapy of osteosarcoma. Chem. Eng. J. 2021, 422, 129919. [Google Scholar] [CrossRef]
- Carter, P.; Bhattarai, N. Bioscaffolds: Fabrication and Performance. In Engineered Biomimicry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 161–188. ISBN 9780124159952. [Google Scholar]
- Rogers, Z.J.; Bencherif, S.A. Cryogelation and cryogels. Gels 2019, 5, 46. [Google Scholar] [CrossRef]
- Korzhikov-Vlakh, V.; Pepelanova, I. Biological, Natural, and Synthetic 3D Matrices. In Basic Concepts on 3D Cell Culture; Kasper, C., Egger, D., Lavrentieva, A., Eds.; Springer: Cham, Switzerland, 2021; pp. 79–104. ISBN 978-3-030-66748-1. [Google Scholar]
- Holländer, J.; Hakala, R.; Suominen, J.; Moritz, N.; Yliruusi, J.; Sandler, N. 3D printed UV light cured polydimethylsiloxane devices for drug delivery. Int. J. Pharm. 2018, 544, 433–442. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Emadi, R.; Valiani, A.; Kharaziha, M.; Poursamar, S.A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Three-dimensional printing constructs based on the chitosan for tissue regeneration: State of the art, developing directions and prospect trends. Materials 2020, 13, 2663. [Google Scholar] [CrossRef] [PubMed]
- Nava-Medina, I.B.; Gold, K.A.; Cooper, S.M.; Robinson, K.; Jain, A.; Cheng, Z.; Gaharwar, A.K. Self-Oscillating 3D Printed Hydrogel Shapes. Adv. Mater. Technol. 2021, 6, 2100418. [Google Scholar] [CrossRef]
- Kuss, M.A.; Wu, S.; Wang, Y.; Untrauer, J.B.; Li, W.; Lim, J.Y.; Duan, B. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1788–1798. [Google Scholar] [CrossRef]
- Liu, J.; Yan, C. 3D Printing of Scaffolds for Tissue Engineering. In 3D Printing; InTech: London, UK, 2018; Volume 11, p. 13. ISBN 0000957720. [Google Scholar]
- Auras, R.; Lim, L.-T.; Selke, S.E.M.; Tsuji, H. (Eds.) Poly(lactic acid): Synthesis, Structures, Properties, Processing, and Application; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; ISBN 9780470293669. [Google Scholar]
- Ghalia, M.A.; Dahman, Y. Biodegradable poly(lactic acid)-based scaffolds: Synthesis and biomedical applications. J. Polym. Res. 2017, 24, 74. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Jung, F.; Braune, S. Thrombogenicity and hemocompatibility of biomaterials. Biointerphases 2016, 11, 029601. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Gervaso, F.; Scalera, F.; Padmanabhan, S.K.; Madaghiele, M.; Montagna, F.; Sannino, A.; Licciulli, A.; Maffezzoli, A. Highly loaded hydroxyapatite microsphere/PLA porous scaffolds obtained by fused deposition modelling. Ceram. Int. 2019, 45, 2803–2810. [Google Scholar] [CrossRef]
- Hu, X.; He, J.; Yong, X.; Lu, J.; Xiao, J.; Liao, Y.; Li, Q.; Xiong, C. Biodegradable poly (lactic acid-co-trimethylene carbonate)/chitosan microsphere scaffold with shape-memory effect for bone tissue engineering. Colloids Surf. B Biointerfaces 2020, 195, 111218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Li, M.G.; Cooper, D.; Chen, X.B. Development of novel hybrid poly(l-lactide)/chitosan scaffolds using the rapid freeze prototyping technique. Biofabrication 2011, 3, 034105. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Zhang, R.; He, H.; Jin, T.; Zhang, J. Unique morphology in polylactide/graphene oxide nanocomposites. J. Macromol. Sci. Part B Phys. 2015, 54, 45–57. [Google Scholar] [CrossRef]
- Murizan, N.I.S.; Mustafa, N.S.; Ngadiman, N.H.A.; Mohd Yusof, N.; Idris, A. Review on Nanocrystalline Cellulose in Bone Tissue Engineering Applications. Polymers 2020, 12, 2818. [Google Scholar] [CrossRef]
- Averianov, I.; Stepanova, M.; Solomakha, O.; Gofman, I.; Serdobintsev, M.; Blum, N.; Kaftuirev, A.; Baulin, I.; Nashchekina, J.; Lavrentieva, A.; et al. 3D-Printed composite scaffolds based on poly(ε-caprolactone) filled with poly(glutamic acid)-modified cellulose nanocrystals for improved bone tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2422–2437. [Google Scholar] [CrossRef]
- Stepanova, M.; Korzhikova-Vlakh, E. Modification of Cellulose Micro- and Nanomaterials to Improve Properties of Aliphatic Polyesters/Cellulose Composites: A Review. Polymers 2022, 14, 1477. [Google Scholar] [CrossRef]
- Wang, H.; Leeuwenburgh, S.C.G.; Li, Y.; Jansen, J.A. The Use of Micro- and Nanospheres as Functional Components for Bone Tissue Regeneration. Tissue Eng. Part B. Rev. 2012, 18, 24–39. [Google Scholar] [CrossRef]
- Gupta, V.; Khan, Y.; Berkland, C.J.; Laurencin, C.T.; Detamore, M.S. Microsphere-Based Scaffolds in Regenerative Engineering. Annu. Rev. Biomed. Eng. 2017, 19, 135–161. [Google Scholar] [CrossRef]
- Jiang, T.; Nukavarapu, S.P.; Deng, M.; Jabbarzadeh, E.; Kofron, M.D.; Doty, S.B.; Abdel-Fattah, W.I.; Laurencin, C.T. Chitosan-poly(lactide-co-glycolide) microsphere-based scaffolds for bone tissue engineering: In vitro degradation and in vivo bone regeneration studies. Acta Biomater. 2010, 6, 3457–3470. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Yun, Y.; Park, K. PLA micro- and nano-particles. Adv. Drug Deliv. Rev. 2016, 107, 176–191. [Google Scholar] [CrossRef]
- Luciani, A.; Coccoli, V.; Orsi, S.; Ambrosio, L.; Netti, P.A. PCL microspheres based functional scaffolds by bottom-up approach with predefined microstructural properties and release profiles. Biomaterials 2008, 29, 4800–4807. [Google Scholar] [CrossRef] [PubMed]
- Nukavarapu, S.P.; Kumbar, S.G.; Brown, J.L.; Krogman, N.R.; Weikel, A.L.; Hindenlang, M.D.; Nair, L.S.; Allcock, H.R.; Laurencin, C.T. Polyphosphazene/Nano-Hydroxyapatite Composite Microsphere Scaffolds for Bone Tissue Engineering. Biomacromolecules 2008, 9, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Wang, M.; Zhou, W.Y.; Cheung, W.L.; Li, Z.Y.; Lu, W.W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010, 6, 4495–4505. [Google Scholar] [CrossRef] [PubMed]
- Masutani, K.; Kimura, Y. Chapter 1. PLA Synthesis. From the Monomer to the Polymer. In Poly(lactic acid) Science and Technology: Processing, Properties, Additives and Applications; RSC Publishing: Oxford, UK, 2014; pp. 1–36. [Google Scholar]
- Pepelanova, I.; Kruppa, K.; Scheper, T.; Lavrentieva, A. Gelatin-Methacryloyl (GelMA) Hydrogels with Defined Degree of Functionalization as a Versatile Toolkit for 3D Cell Culture and Extrusion Bioprinting. Bioengineering 2018, 5, 55. [Google Scholar] [CrossRef]
- Kritchenkov, I.S.; Zhukovsky, D.D.; Mohamed, A.; Korzhikov-Vlakh, V.A.; Tennikova, T.B.; Lavrentieva, A.; Scheper, T.; Pavlovskiy, V.V.; Porsev, V.V.; Evarestov, R.A.; et al. Functionalized Pt(II) and Ir(III) NIR Emitters and Their Covalent Conjugates with Polymer-Based Nanocarriers. Bioconjug. Chem. 2020, 31, 1327–1343. [Google Scholar] [CrossRef]
- Korzhikov-Vlakh, V.; Averianov, I.; Sinitsyna, E.; Nashchekina, Y.; Polyakov, D.; Guryanov, I.; Lavrentieva, A.; Raddatz, L.; Korzhikova-Vlakh, E.; Scheper, T.; et al. Novel pathway for efficient covalent modification of polyester materials of different design to prepare biomimetic surfaces. Polymers 2018, 10, 1299. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012045. [Google Scholar] [CrossRef]
- Le Troedec, M.; Sedan, D.; Peyratout, C.; Bonnet, J.P.; Smith, A.; Guinebretiere, R.; Gloaguen, V.; Krausz, P. Influence of various chemical treatments on the composition and structure of hemp fibres. Compos. Part A Appl. Sci. Manuf. 2008, 39, 514–522. [Google Scholar] [CrossRef]
- Li, W.; Yue, J.; Liu, S. Preparation of nanocrystalline cellulose via ultrasound and its reinforcement capability for poly(vinyl alcohol) composites. Ultrason. Sonochem. 2012, 19, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Dijkstra, P.J.; Poot, A.A.; Grijpma, D.W. Hybrid Hydrogels Based on Methacrylate-Functionalized Gelatin (GelMA) and Synthetic Polymers. Biomed. Mater. Devices 2022. [Google Scholar] [CrossRef]
- Lavrentieva, A.; Fleischhammer, T.; Enders, A.; Pirmahboub, H.; Bahnemann, J.; Pepelanova, I. Fabrication of Stiffness Gradients of GelMA Hydrogels Using a 3D Printed Micromixer. Macromol. Biosci. 2020, 20, 2000107. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef]

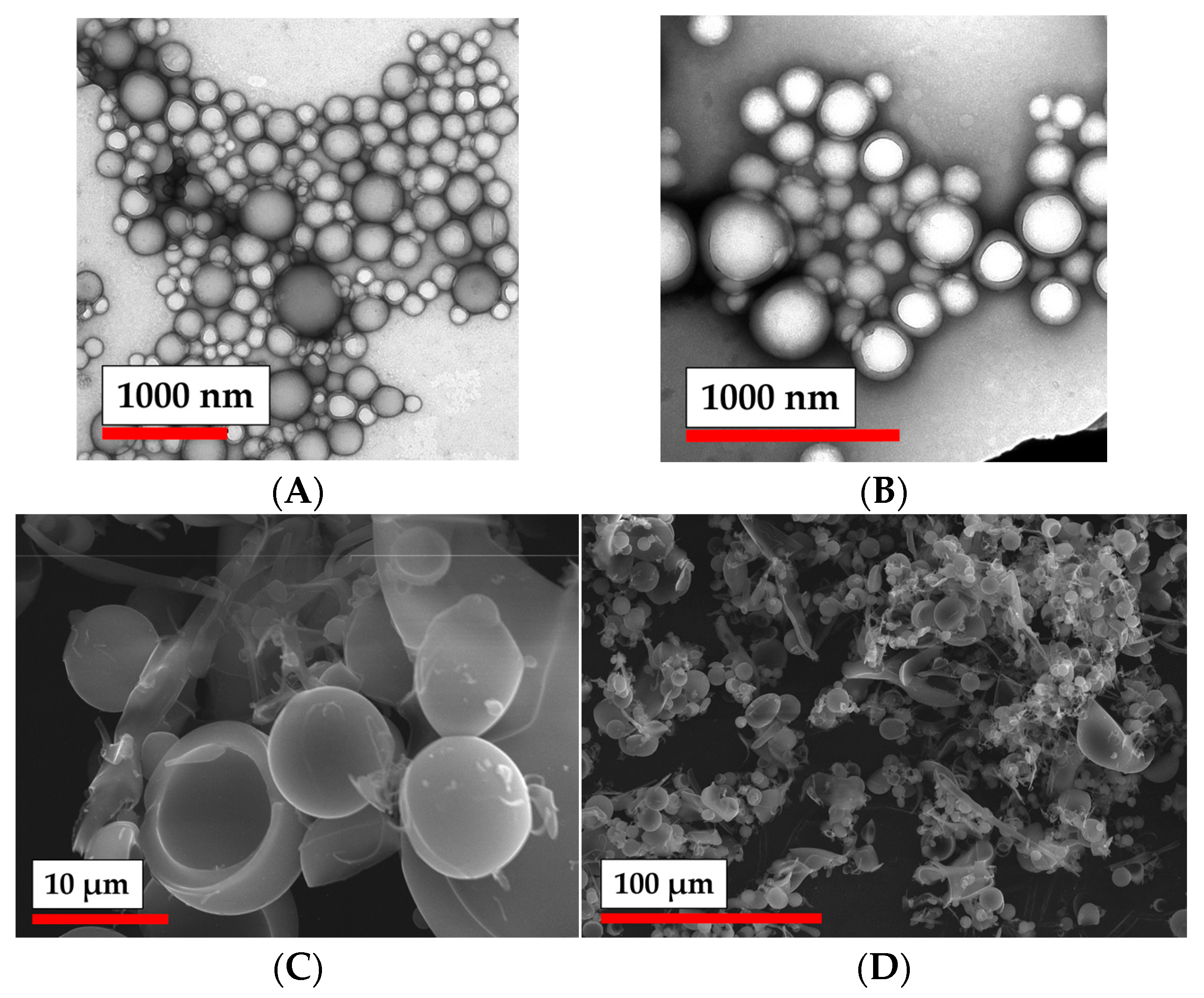
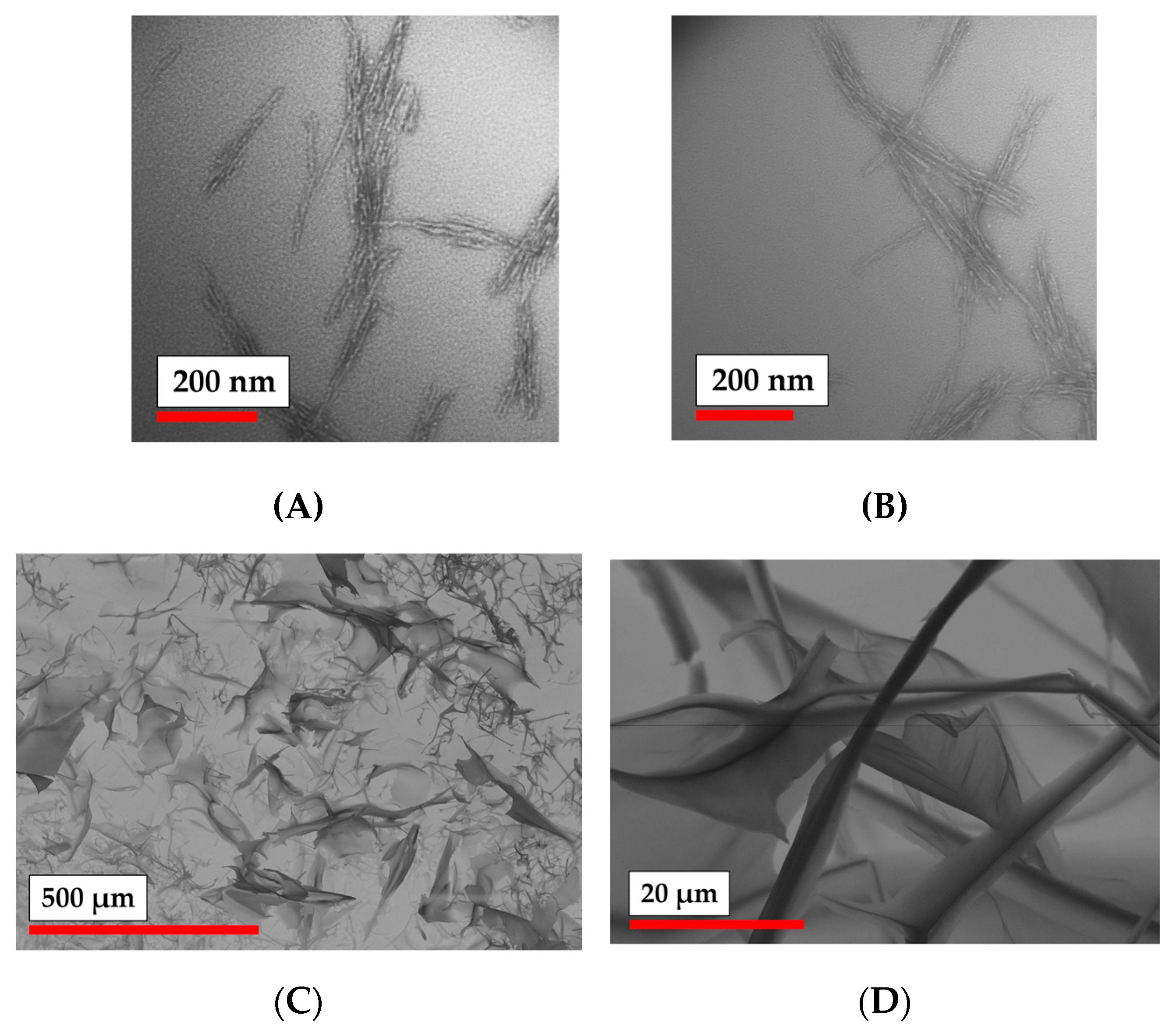

| Sample # | DH (DLS), nm | ζ-Potential, mV | D (TEM), nm | D (NTA), nm |
|---|---|---|---|---|
| PLA MPs | 258 ± 74 | −45 ± 7 | 210 ± 70 | 263 ± 85 |
| PLA-MA MPs | 347 ± 129 | −33 ± 8 | 290 ± 120 | 443 ± 181 |
| NCC | 95 ± 18 | −39 ± 11 | not determined | 121 ± 30 |
| NCC-MA | 110 ± 24 | −36 ± 5 | not determined | 182 ± 51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonovich, M.; Korzhikov-Vlakh, V.; Lavrentieva, A.; Pepelanova, I.; Korzhikova-Vlakh, E.; Tennikova, T. Poly(lactic acid) and Nanocrystalline Cellulose Methacrylated Particles for Preparation of Cryogelated and 3D-Printed Scaffolds for Tissue Engineering. Polymers 2023, 15, 651. https://doi.org/10.3390/polym15030651
Leonovich M, Korzhikov-Vlakh V, Lavrentieva A, Pepelanova I, Korzhikova-Vlakh E, Tennikova T. Poly(lactic acid) and Nanocrystalline Cellulose Methacrylated Particles for Preparation of Cryogelated and 3D-Printed Scaffolds for Tissue Engineering. Polymers. 2023; 15(3):651. https://doi.org/10.3390/polym15030651
Chicago/Turabian StyleLeonovich, Mariia, Viktor Korzhikov-Vlakh, Antonina Lavrentieva, Iliyana Pepelanova, Evgenia Korzhikova-Vlakh, and Tatiana Tennikova. 2023. "Poly(lactic acid) and Nanocrystalline Cellulose Methacrylated Particles for Preparation of Cryogelated and 3D-Printed Scaffolds for Tissue Engineering" Polymers 15, no. 3: 651. https://doi.org/10.3390/polym15030651
APA StyleLeonovich, M., Korzhikov-Vlakh, V., Lavrentieva, A., Pepelanova, I., Korzhikova-Vlakh, E., & Tennikova, T. (2023). Poly(lactic acid) and Nanocrystalline Cellulose Methacrylated Particles for Preparation of Cryogelated and 3D-Printed Scaffolds for Tissue Engineering. Polymers, 15(3), 651. https://doi.org/10.3390/polym15030651