3D-Printed PLA Medical Devices: Physicochemical Changes and Biological Response after Sterilisation Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. PLA Precursor Material
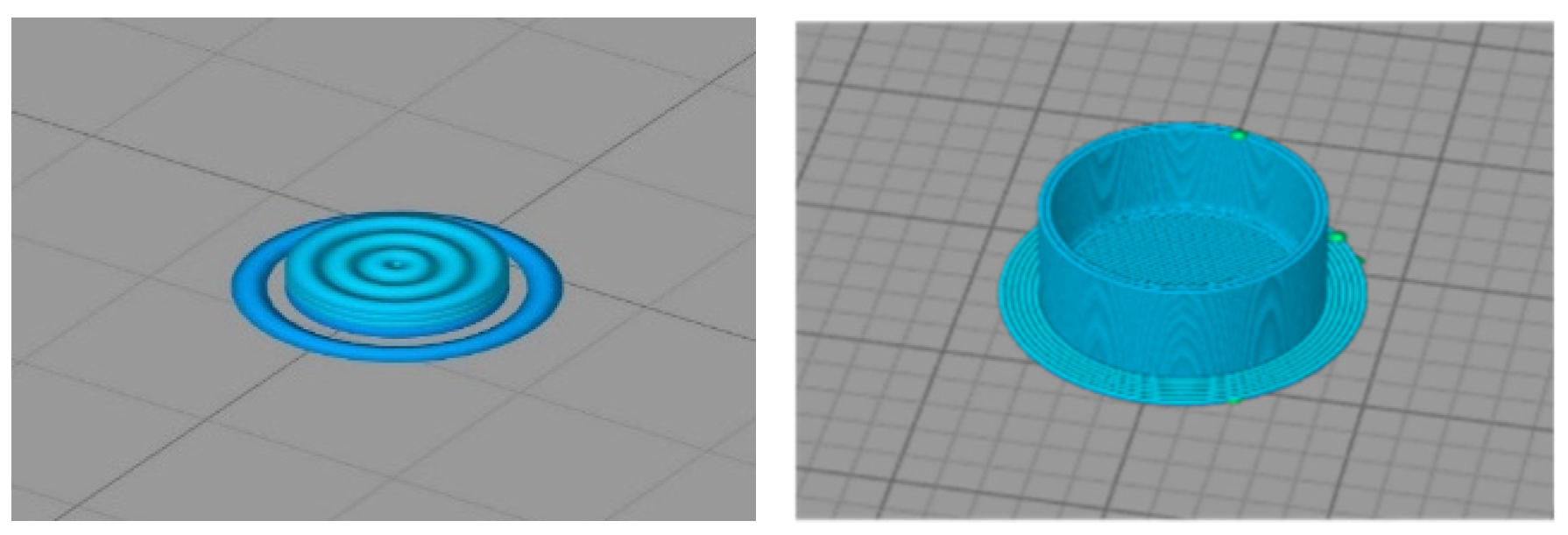
2.2. Obtaining 3D-Printed PLA
2.3. Sterilisation Techniques
- -
- Saturated steam (SS), carried out in an autoclave (Selecta Presoclave II 75, Cham, Switzerland) at Grupo de Novos Materiais (Vigo, Spain) operating at 121 °C and 2 bar for 20 min. The samples were sterilised in a surgical paper package.
- -
- Low temperature steam with 2% formaldehyde (LTSF), carried out in a Matachana steriliser (Matachana 130 LF, Matachana Group, Barcelona, Spain) at Povisa Hospital (Vigo, Spain). This steriliser complied with EN 14180:2014 and used a mixture of steam and 2% formaldehyde in thermodynamic equilibrium. Sterilisation was performed at 78 °C, with a standard duration of 153 min at full load and the samples were sterilised in a surgical paper package.
- -
- Gamma irradiation (GR), performed by Aragogamma S.L. (Barcelona, Spain) using a 60Co source irradiator at a dose level of between 25 and 35 kGy at room temperature, as per ISO 13485:2018.
- -
- Hydrogen peroxide gas plasma (HPGP), performed in a Sterrad NX steriliser (Advanced Sterilization Products, Irvine, CA, USA) at Hospital Universitario Lucus Augusti (Lugo, Spain). This was based on 59% aqueous hydrogen peroxide, which was concentrated to about 95% through removal of water from the peroxide solution before its evaporation and transfer to the chamber. PLA discs were conditioned in Tyvek packages compatible with the sterilisation process. The temperature during the sterilisation cycle was kept at between 45 °C and 55 °C in a standard cycle.
- -
- CO2 under critical conditions (SCCO) was carried out at the Biomaterials and Biomedical Technology lab (CBQF, Porto, Portugal). PLA discs were packed in sealed permeable plastic cartridges and placed in the reactor, to which hydrogen peroxide was added as an additive (300 ppm) to make sterilisation more effective. The optimised operating parameters for sterilisation were 40 °C temperature and 240 bar pressure with constant agitation of 600 rpm. Once pressurisation occurred, CO2 under critical conditions acted for 4 h.
2.4. Physicochemical Characterisation
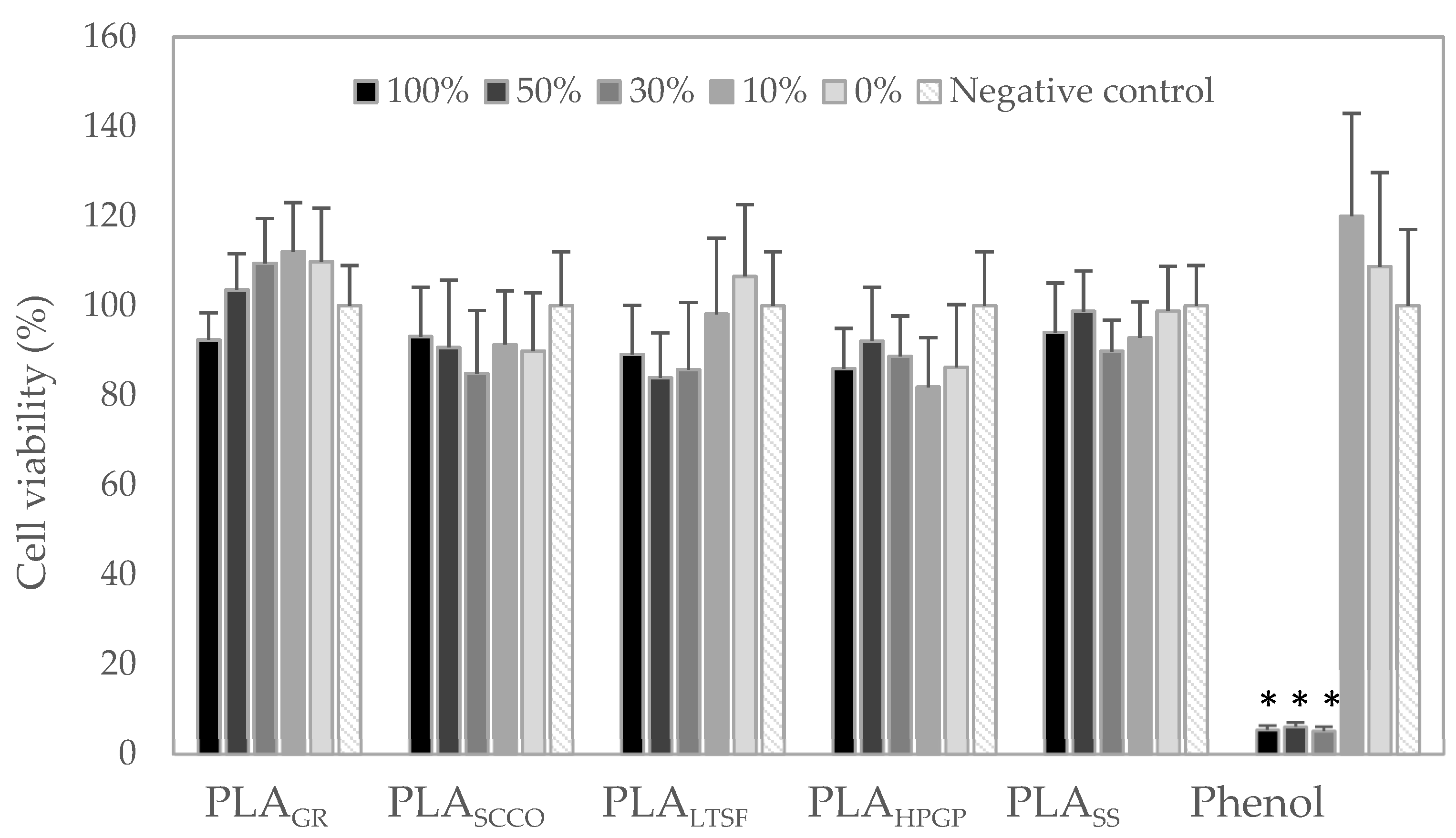
2.5. Biological Response In Vitro: Cytotoxicity Assay
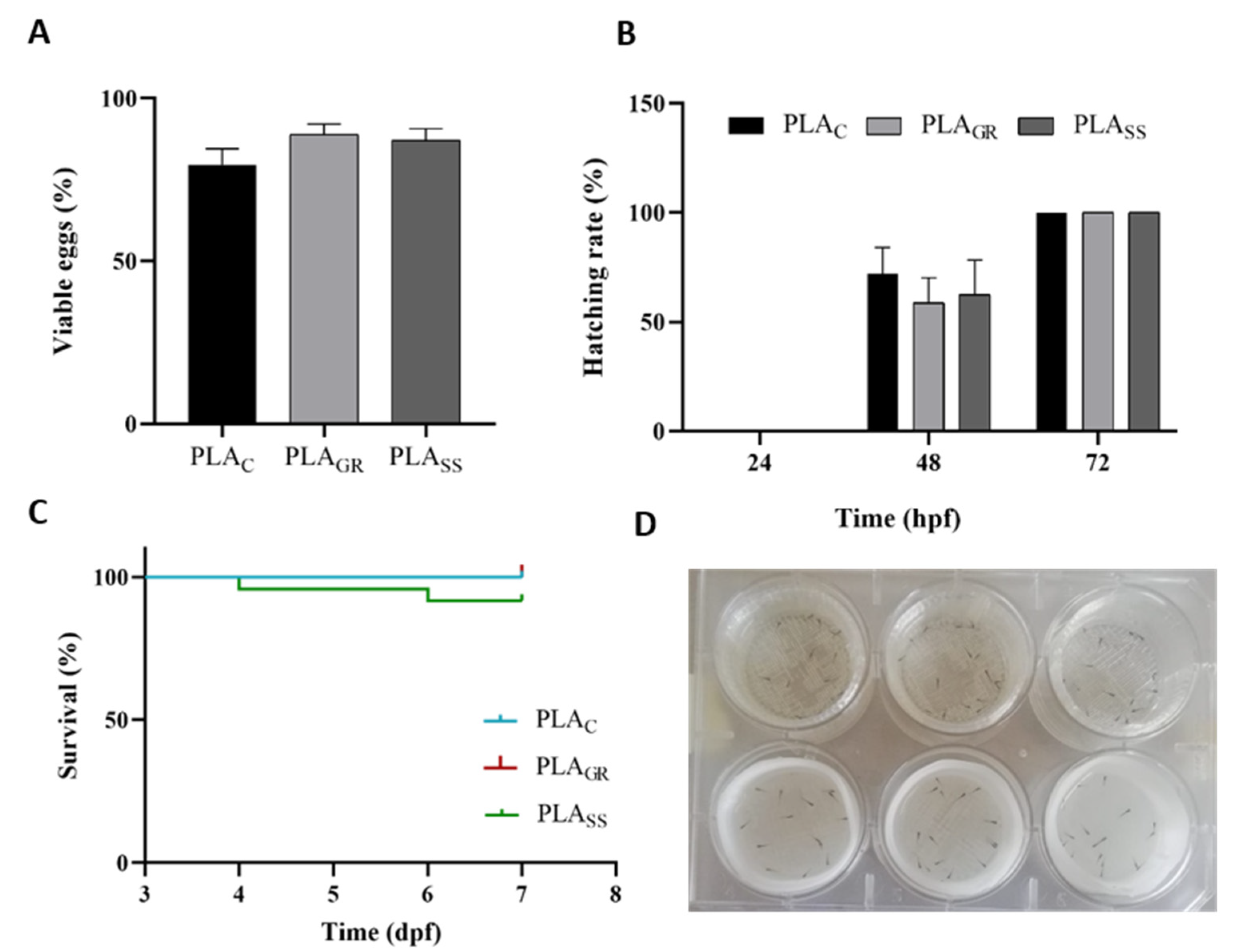
2.6. Biological Response In Vivo: Acute Toxicity Test in Zebrafish
2.7. Statistical Analysis
3. Results and Discussion
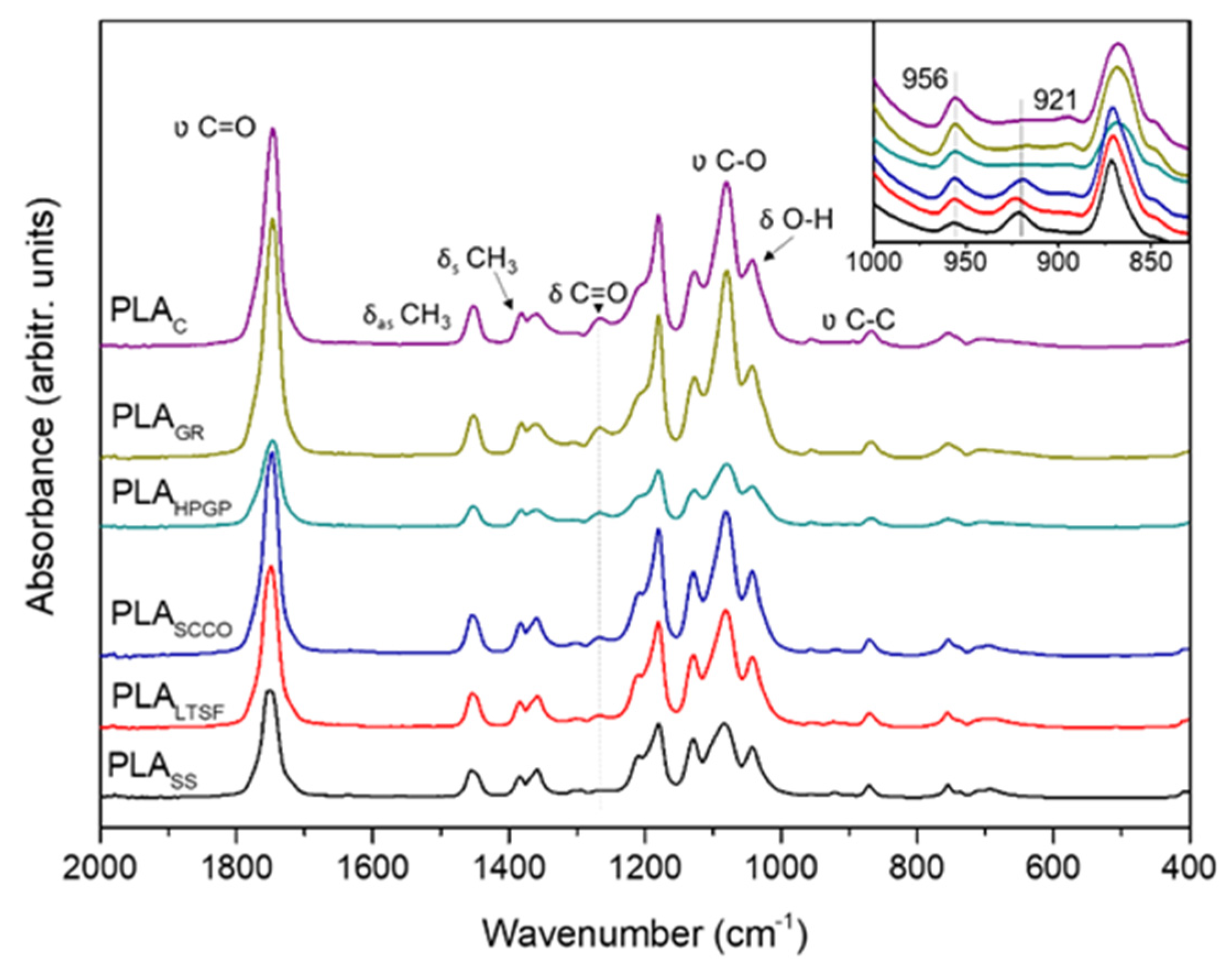
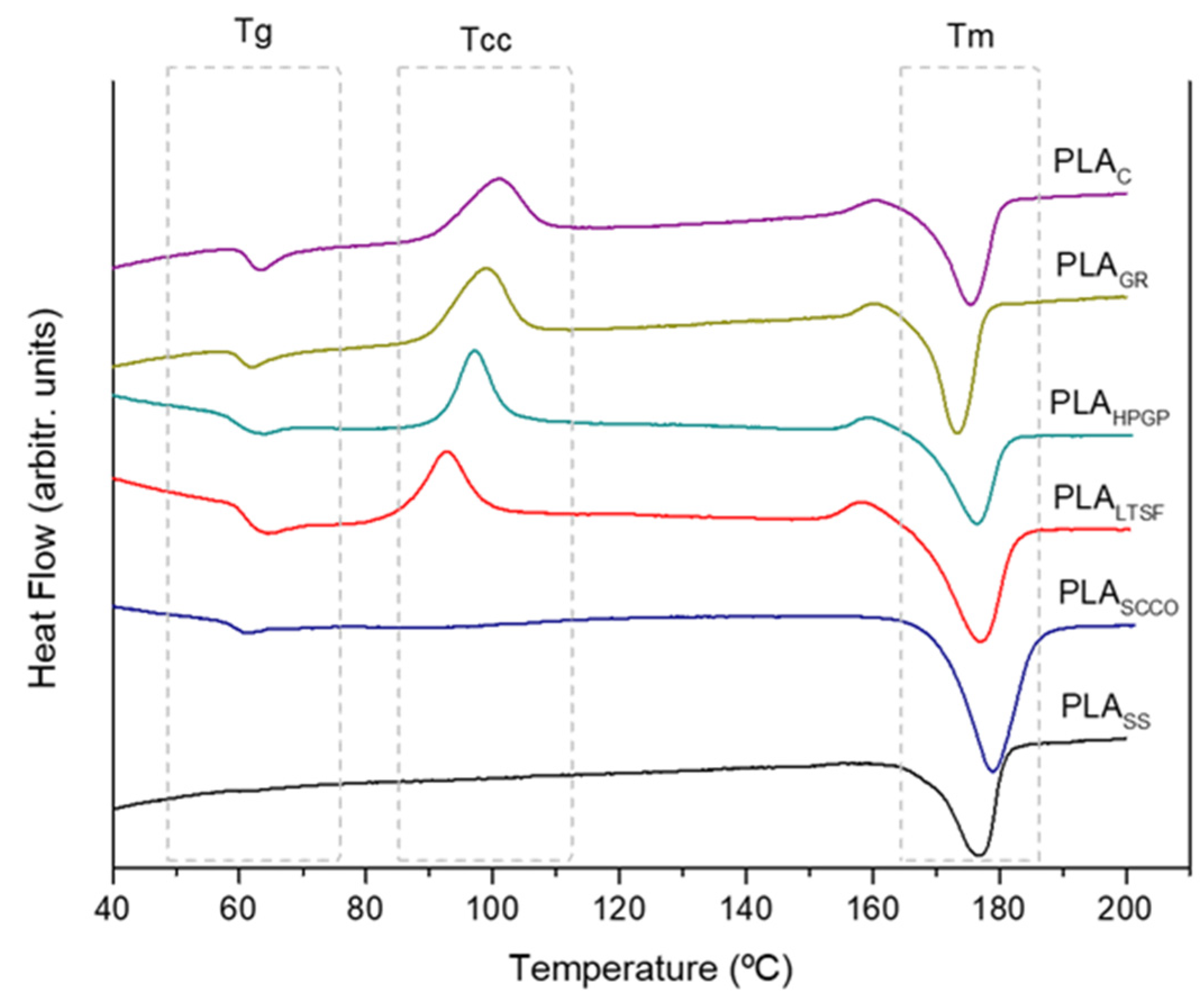
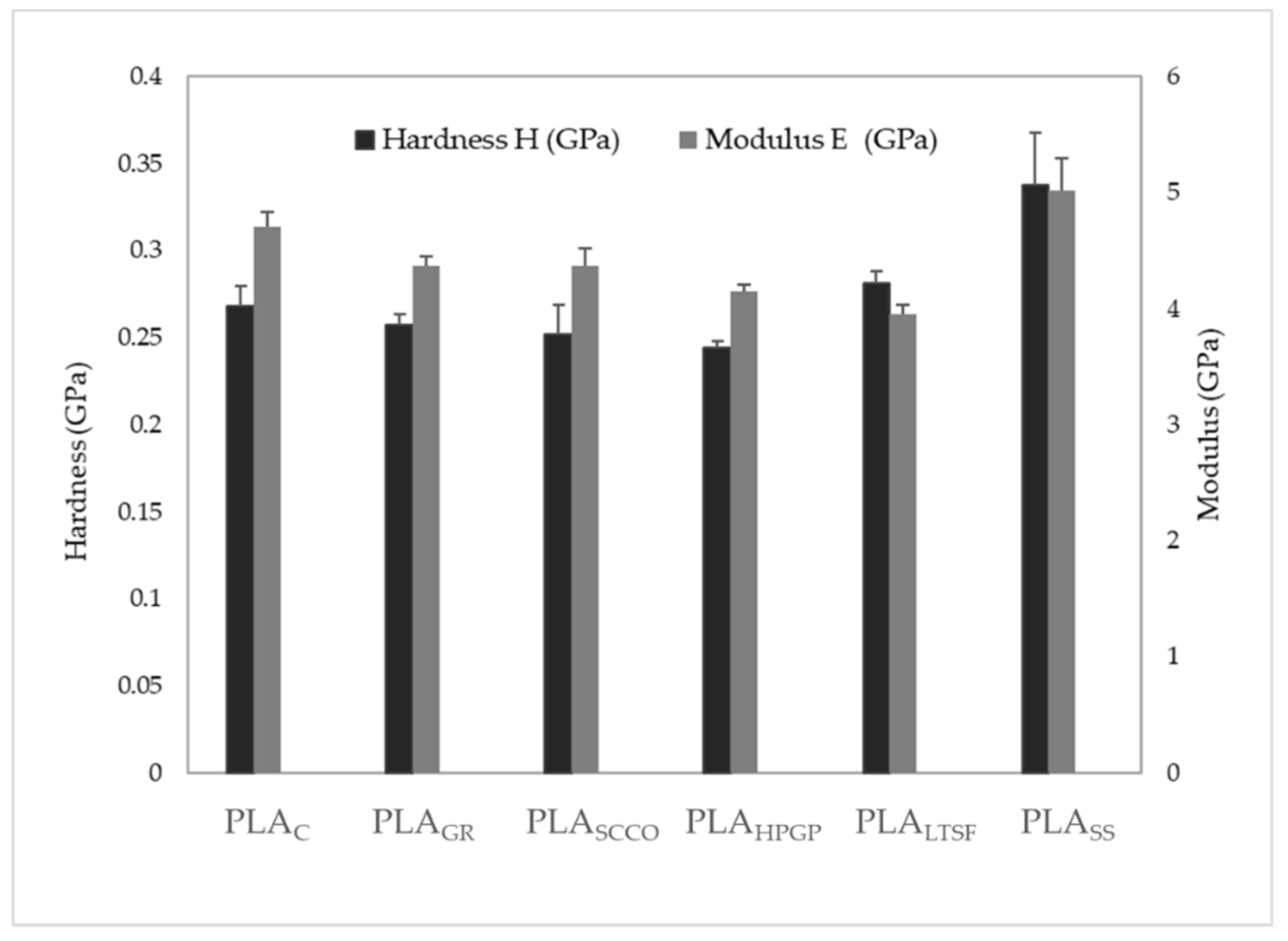
3.1. Physicochemical Characterisation
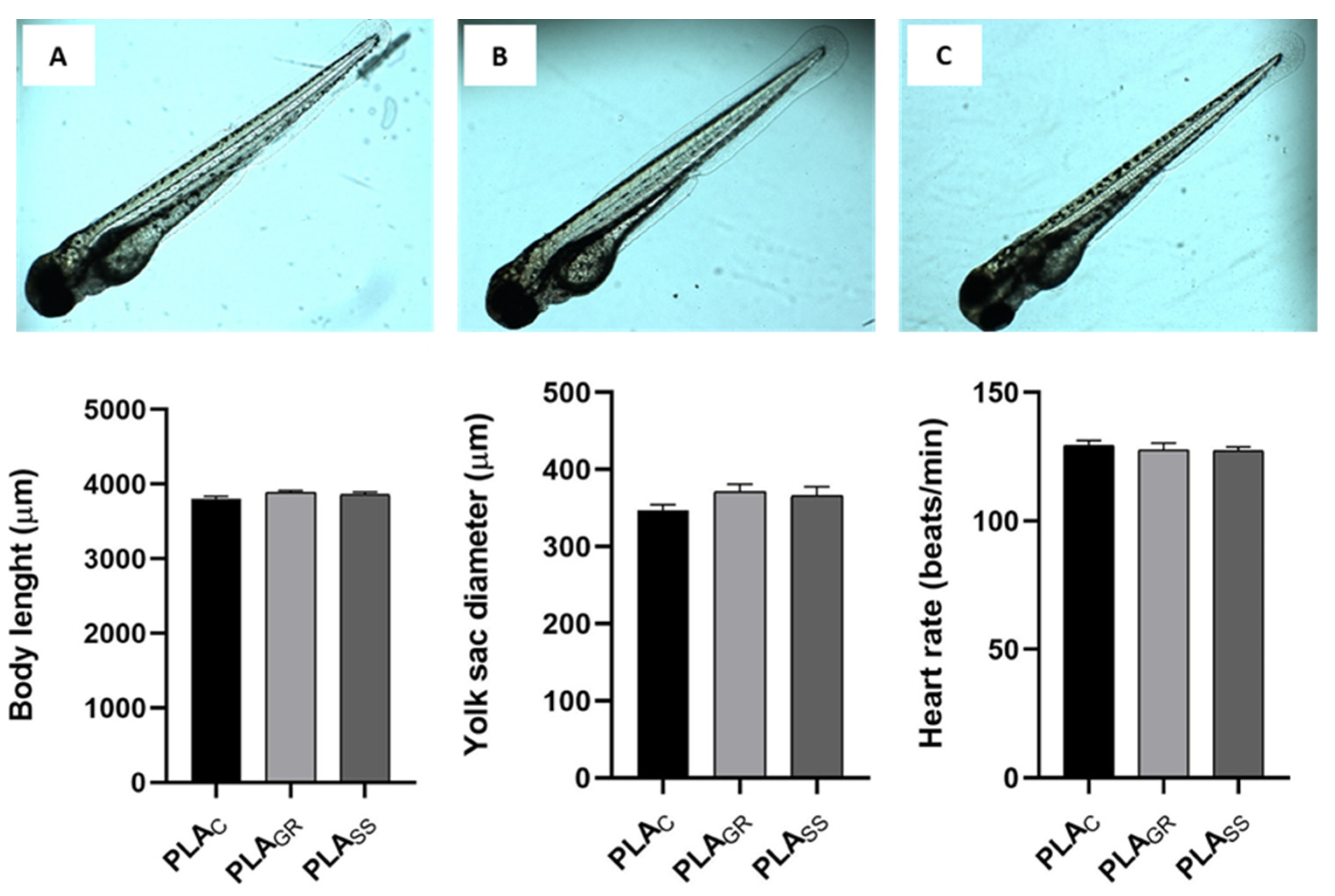
3.2. Biological Response: Cytotoxicity In Vitro and Acute Toxicity in Zebrafish Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cuiffo, M.A.; Snyder, J.; Elliott, A.M.; Romero, N.; Kannan, S.; Halada, G.P. Impact of the Fused Deposition (FDM) Printing Process on Polylactic Acid (PLA) Chemistry and Structure. Appl. Sci. 2017, 7, 579. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, Biodegradation and Excretion of Polylactic Acid (PLA) in Medical Implants and Theranostic Systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Marcatto, V.A.; Sant’Ana Pegorin, G.; Barbosa, G.F.; Herculano, R.D.; Guerra, N.B. 3D Printed-polylactic Acid Scaffolds Coated with Natural Rubber Latex for. Pdf. J. Appl. Polym. Sci. 2022, 139, 51728. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Pérez Davila, S.; González Rodríguez, L.; Chiussi, S.; Serra, J.; González, P. How to Sterilize Polylactic Acid Based Medical Devices? Polymers 2021, 13, 2115. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and Medical Applications of Polylactic Acid: A Review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Serra, T.; Planell, J.A.; Navarro, M. High-Resolution PLA-Based Composite Scaffolds via 3-D Printing Technology. Acta Biomater. 2013, 9, 5521–5530. [Google Scholar] [CrossRef]
- Lerouge, S. Introduction to Sterilization: Definitions and Challenges. In Sterilisation of Biomaterials and Medical Devices; Lerouge, S., Simmons, A., Eds.; Woodhead Publishing Limited: Cambridge, MA, USA, 2012; pp. 1–19. [Google Scholar]
- Zhao, Y.; Zhu, B.; Wang, Y.; Liu, C.; Shen, C. Effect of Different Sterilization Methods on the Properties of Commercial Biodegradable Polyesters for Single-Use, Disposable Medical Devices. Mater. Sci. Eng. C 2019, 105, 110041. [Google Scholar] [CrossRef]
- Valente, T.A.M.; Silva, D.M.; Gomes, P.S.; Fernandes, M.H.; Santos, J.D.; Sencadas, V. Effect of Sterilization Methods on Electrospun Poly(Lactic Acid) (PLA) Fiber Alignment for Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 3241–3249. [Google Scholar] [CrossRef]
- Qiu, Q.Q.; Sun, W.Q.; Connor, J. Sterilization of Biomaterials of Synthetic and Biological Origin; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 4, ISBN 9780081006924. [Google Scholar]
- Aguado-Maestro, I.; De Frutos-Serna, M.; González-Nava, A.; Merino-De Santos, A.B.; García-Alonso, M. Are the Common Sterilization Methods Completely Effective for Our In-House 3D Printed Biomodels and Surgical Guides? Injury 2021, 52, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, K.; Kunishima, H.; Saga, T.; Harigae, H.; Imasaka, T.; Hirayama, Y.; Kaku, M. Residual Formaldehyde on Plastic Materials and Medical Equipment Following Low-Temperature Steam and Formaldehyde Sterilization. J. Hosp. Infect. 2005, 59, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, N.; Soares, G.C.; Santos-Rosales, V.; Concheiro, A.; Alvarez-Lorenzo, C.; García-González, C.A.; Oliveira, A.L. A New Era for Sterilization Based on Supercritical CO2 Technology. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 399–428. [Google Scholar] [CrossRef]
- Soares, G.C.; Learmonth, D.A.; Vallejo, M.C.; Davila, S.P.; González, P.; Sousa, R.A.; Oliveira, A.L. Supercritical CO2 Technology: The next Standard Sterilization Technique? Mater. Sci. Eng. C 2019, 99, 520–540. [Google Scholar] [CrossRef] [PubMed]
- Dillow, A.K.; Dehghani, F.; Hrkach, J.S.; Foster, N.R.; Langer, R. Bacterial Inactivation by Using Near- and Supercritical Carbon Dioxide. Proc. Natl. Acad. Sci. USA 1999, 96, 10344–10348. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Campora, S.; Brucato, V.; Carfì Pavia, F.; Di Leonardo, E.R.; Ghersi, G.; Scialdone, O.; Galia, A. Sterilization of Macroscopic Poly(l-Lactic Acid) Porous Scaffolds with Dense Carbon Dioxide: Investigation of the Spatial Penetration of the Treatment and of Its Effect on the Properties of the Matrix. J. Supercrit. Fluids 2016, 111, 83–90. [Google Scholar] [CrossRef]
- Savaris, M.; dos Santos, V.; Brandalise, R.N. Influence of Different Sterilization Processes on the Properties of Commercial Poly(Lactic Acid). Mater. Sci. Eng. C 2016, 69, 661–667. [Google Scholar] [CrossRef]
- Nüsslein-Volhard, C.; Dahm, R. Zebrafish. A Practical Approach.; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio Rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Torres-Giner, S.; Gimeno-Alcañiz, J.V.; Ocio, M.J.; Lagaron, J.M. Optimization of Electrospun Polylactide-Based Ultrathin Fibers for Osteoconductibe Bone Scaffolds. J. Appl. Polym. Sci. 2011, 122, 914–925. [Google Scholar] [CrossRef]
- Rainer, A.; Centola, M.; Spadaccio, C.; Gherardi, G.; Genovese, J.A.; Licoccia, S.; Trombetta, M. Comparative Study of Different Techniques for the Sterilization of Poly-L-Lactide Electrospun Microfibers: Effectiveness vs. Material Degradation. Int. J. Artif. Organs 2010, 33, 76–85. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, C.; Kong, W.; Wang, Y.; Cao, W.; Liu, C.; Shen, C. Memory Effect on the Crystallization Behavior of Poly(Lactic Acid) Probed by Infrared Spectroscopy. Eur. Polym. J. 2017, 91, 376–385. [Google Scholar] [CrossRef]
- Peniston, S.J.; Choi, S.J. Effect of Sterilization on the Physicochemical Properties of Molded Poly(L-Lactic Acid). J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 80, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Meaurio, E.; López-Rodríguez, N.; Sarasua, J.R. Infrared Spectrum of Poly(L-Lactide): Application to Crystallinity Studies. Macromolecules 2006, 39, 9291–9301. [Google Scholar] [CrossRef]
- Savaris, M.; Braga, G.L.; Dos Santos, V.; Carvalho, G.A.; Falavigna, A.; MacHado, D.C.; Viezzer, C.; Brandalise, R.N. Biocompatibility Assessment of Poly(Lactic Acid) Films after Sterilization with Ethylene Oxide in Histological Study in Vivo with Wistar Rats and Cellular Adhesion of Fibroblasts in Vitro. Int. J. Polym. Sci. 2017, 2017, 7158650. [Google Scholar] [CrossRef]
- Jalali, A.; Huneault, M.A.; Elkoun, S. Effect of Thermal History on Nucleation and Crystallization of Poly(Lactic Acid). J. Mater. Sci. 2016, 51, 7768–7779. [Google Scholar] [CrossRef]
- Wright-Charlesworth, D.D.; Miller, D.M.; Miskioglu, I.; King, J.A. Nanoindentation of Injection Molded PLA and Self-Reinforced Composite PLA after in Vitro Conditioning for Three Months. J. Biomed. Mater. Res. Part A 2005, 74, 388–396. [Google Scholar] [CrossRef]
- Gong, M.; Zhao, Q.; Dai, L.; Li, Y.; Jiang, T. Fabrication of Polylactic Acid/Hydroxyapatite/Graphene Oxide Composite and Their Thermal Stability, Hydrophobic and Mechanical Properties. J. Asian Ceram. Soc. 2017, 5, 160–168. [Google Scholar] [CrossRef]
- Izdebska-Podsiadły, J.; Dörsam, E. Storage stability of the oxygen plasma modified PLA film. Bull. Mater. Sci. 2021, 44, 79. [Google Scholar] [CrossRef]
- Weir, N.A.; Buchanan, F.J.; Orr, J.F.; Farrar, D.F.; Boyd, A. Processing, Annealing and Sterilisation of Poly-L-Lactide. Biomaterials 2004, 25, 3939–3949. [Google Scholar] [CrossRef]
- Manjunatha, B.; Park, S.H.; Kim, K.; Kundapur, R.R.; Lee, S.J. In Vivo Toxicity Evaluation of Pristine Graphene in Developing Zebrafish (Danio Rerio) Embryos. Environ. Sci. Pollut. Res. 2018, 25, 12821–12829. [Google Scholar] [CrossRef]
- Wang, Z.G.; Zhou, R.; Jiang, D.; Song, J.E.; Xu, Q.; Si, J.; Chen, Y.P.; Zhou, X.; Gan, L.; Li, J.Z.; et al. Toxicity of Graphene Quantum Dots in Zebrafish Embryo. Biomed. Environ. Sci. 2015, 28, 341–351. [Google Scholar] [CrossRef]
- MacDonald, N.P.; Zhu, F.; Hall, C.J.; Reboud, J.; Crosier, P.S.; Patton, E.E.; Wlodkowic, D.; Cooper, J.M. Assessment of Biocompatibility of 3D Printed Photopolymers Using Zebrafish Embryo Toxicity Assays. Lab Chip 2016, 16, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Oskui, S.M.; Diamante, G.; Liao, C.; Shi, W.; Gan, J.; Schlenk, D.; Grover, W.H. Assessing and Reducing the Toxicity of 3D-Printed Parts. Environ. Sci. Technol. Lett. 2016, 3, 1–6. [Google Scholar] [CrossRef]
- Zhu, F.; Friedrich, T.; Nugegoda, D.; Kaslin, J.; Wlodkowic, D. Assessment of the Biocompatibility of Three-Dimensional-Printed Polymers Using Multispecies Toxicity Tests. Biomicrofluidics 2015, 9, 061103. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Skommer, J.; Friedrich, T.; Kaslin, J.; Wlodkowic, D. 3D Printed Polymers Toxicity Profiling: A Caution for Biodevice Applications. Micro+ Nano Mater. Devices Syst. 2015, 9668, 96680Z. [Google Scholar] [CrossRef]



| Polylactic Acid 3D850 | Value |
|---|---|
| Material density | 1.24 g/cm3 |
| Tensile yield strength | 65.5 MPa |
| Flexural strength | 126 MPa |
| Flexural modulus | 4357 MPa |
| Heat distortion temperature | 144 °C |
| Extrusion temperature | 190–230 °C |
| Dual Extruder BCN3D+ Printer | Parameter Value |
|---|---|
| Nozzle | 0.4 mm |
| Nozzle temperature | 190–230 °C |
| Bed temperature | 45 °C |
| Infill density | 100% |
| Infill pattern | Concentric |
| Speed | 60 mm/s |
| Layer height | 0.2 mm |
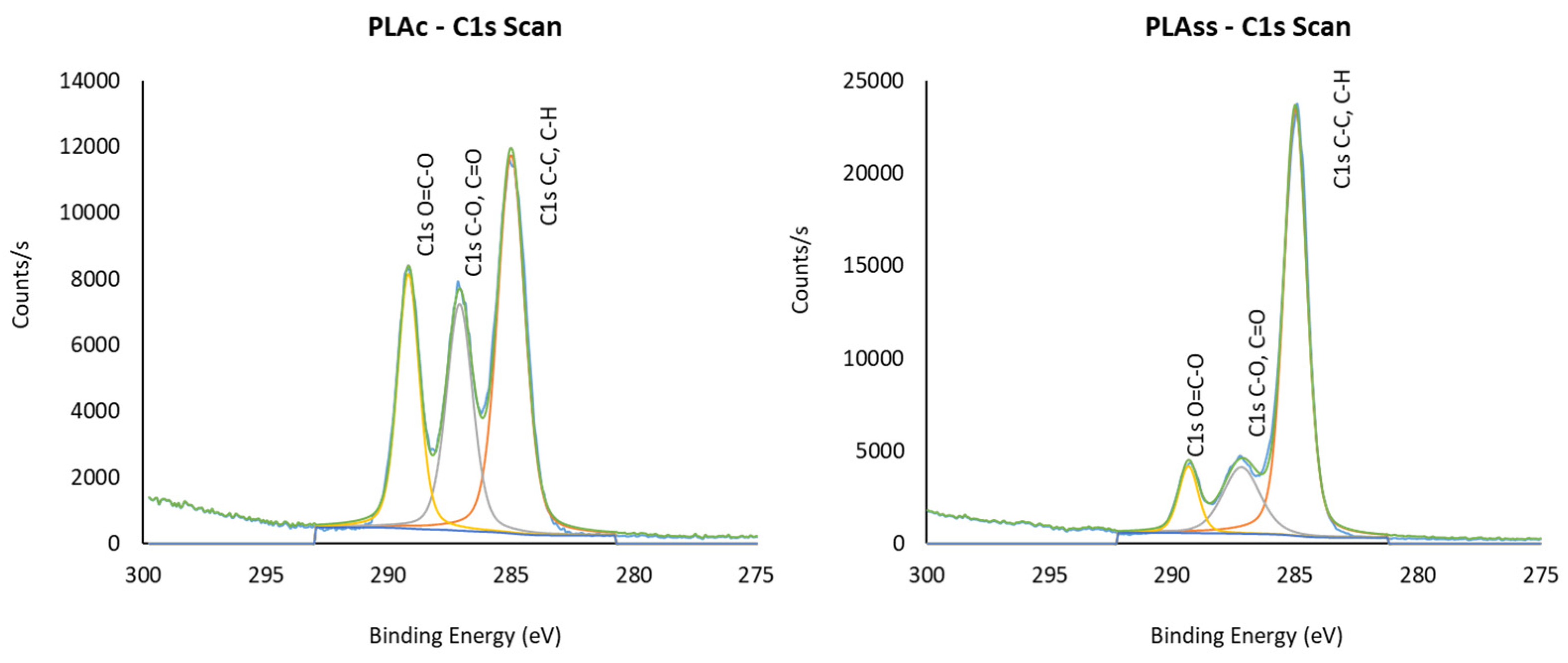
| Samples | C | O | Si | S | N | Na | I | Cl | O/C |
|---|---|---|---|---|---|---|---|---|---|
| PLAC | 66.24 | 33.42 | - | - | - | 0.34 | - | - | 0.50 |
| PLAGR | 68.65 | 31.35 | - | - | - | - | - | - | 0.46 |
| PLASCCO | 66.52 | 31.82 | - | - | 1.24 | 0.42 | - | - | 0.48 |
| PLALTSF | 73.78 | 21.20 | 3.34 | - | 1.07 | 0.61 | - | - | 0.29 |
| PLAHPGP | 70.92 | 24.73 | 1.85 | - | 1.11 | 0.93 | - | 0.47 | 0.35 |
| PLASS | 74.94 | 20.00 | 1.23 | 0.18 | 2.53 | 0.92 | 0.19 | - | 0.27 |
| C-H, C-C | C-OH, C-O-C | COOH, O=C-O | ||||
|---|---|---|---|---|---|---|
| BE | Rel.% | BE | Rel.% | BE | Rel.% | |
| PLAC | 285 | 47.12 | 287.10 | 27.35 | 289.18 | 25.54 |
| PLAGR | 285 | 54.45 | 287.12 | 23.95 | 289.20 | 21.61 |
| PLASCCO | 285 | 49.62 | 287.13 | 27.55 | 289.20 | 22.83 |
| PLALTSF | 285 | 72.64 | 287.18 | 17.32 | 289.41 | 10.04 |
| PLAHPGP | 285 | 67.36 | 287.20 | 18.75 | 289.35 | 13.89 |
| PLASS | 285 | 72.06 | 287.20 | 18.29 | 289.36 | 9.66 |
| Tg (°C) | Tcc (°C) | Tm (°C) | Xc (%) | |
|---|---|---|---|---|
| PLAC | 63.2 | 100.7 | 175.5 | 18.5 |
| PLAGR | 61.9 | 98.6 | 173.3 | 15.1 |
| PLAHPGP | 59.1 | 97.1 | 176.4 | 17.4 |
| PLALTSF | 66.6 | 92.9 | 176.9 | 29.0 |
| PLASCCO | 61.1 | 99.4 | 178.9 | 32.6 |
| PLASS | - | - | 176.8 | 39.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Davila, S.; González-Rodríguez, L.; Lama, R.; López-Álvarez, M.; Oliveira, A.L.; Serra, J.; Novoa, B.; Figueras, A.; González, P. 3D-Printed PLA Medical Devices: Physicochemical Changes and Biological Response after Sterilisation Treatments. Polymers 2022, 14, 4117. https://doi.org/10.3390/polym14194117
Pérez-Davila S, González-Rodríguez L, Lama R, López-Álvarez M, Oliveira AL, Serra J, Novoa B, Figueras A, González P. 3D-Printed PLA Medical Devices: Physicochemical Changes and Biological Response after Sterilisation Treatments. Polymers. 2022; 14(19):4117. https://doi.org/10.3390/polym14194117
Chicago/Turabian StylePérez-Davila, Sara, Laura González-Rodríguez, Raquel Lama, Miriam López-Álvarez, Ana Leite Oliveira, Julia Serra, Beatriz Novoa, Antonio Figueras, and Pío González. 2022. "3D-Printed PLA Medical Devices: Physicochemical Changes and Biological Response after Sterilisation Treatments" Polymers 14, no. 19: 4117. https://doi.org/10.3390/polym14194117
APA StylePérez-Davila, S., González-Rodríguez, L., Lama, R., López-Álvarez, M., Oliveira, A. L., Serra, J., Novoa, B., Figueras, A., & González, P. (2022). 3D-Printed PLA Medical Devices: Physicochemical Changes and Biological Response after Sterilisation Treatments. Polymers, 14(19), 4117. https://doi.org/10.3390/polym14194117