Advanced Polymeric Nanocomposite Membranes for Water and Wastewater Treatment: A Comprehensive Review
Abstract
:1. Introduction
2. Incorporation of NPs in TFNCs/MMMs
2.1. Metal/Metal-Oxide-Based Nanocomposites
2.1.1. Iron-Oxide-Based Nanocomposites
2.1.2. Silver/Zinc-Based Nanocomposites
2.1.3. Silica-Based Nanocomposites
2.1.4. Titania-Based Nanocomposites
| Membrane Type | Enhancement and Agglomeration Due to Modification | Reference |
|---|---|---|
| PSf UF membrane with PANI-coated TiO2 NPs and PEG as additives fabrication by PI process | Enhanced porosity, permeability, hydrophilicity, water uptake, antifouling property with a rejection of 68% and 53.78% for Pb2+ and Cd2+, respectively. Agglomeration @ 1.5 wt.% loading. | [204] |
| PSf-based PANI-coated TiO2 NPs-coated PA nanocomposite hollow fiber membrane | Enhanced hydrophilicity and antifouling property with a rejection of 81.5% and 96.5% for Reactive Black 5 and Reactive Orange 16. Agglomeration @ 1 wt.% loading. | [125] |
| TiO2 NPs incorporated into PSf UF membrane | Better porosity, hydrophilicity, and antifouling property. Tiny aggregates @ 2.0 wt.% loading | [124] |
| Addition of TiO2 NPs in a PVDF and sulfonated PES blend membrane fabrication by PI method | Enhanced hydrophilicity, antifouling, photo-bactericidal effect against E. coli, higher FRR (86.2%). NPs loading negative effect on pure water flux. Agglomeration ≥ 4 wt.%. | [197] |
| Addition of TiO2 NPs in microporous PES membrane | Enhanced hydrophilicity, mean pore size and permeation property, flux (3711 ), mechanical strength, thermal stability. Agglomeration @ 4–5 wt.% loading. | [110] |
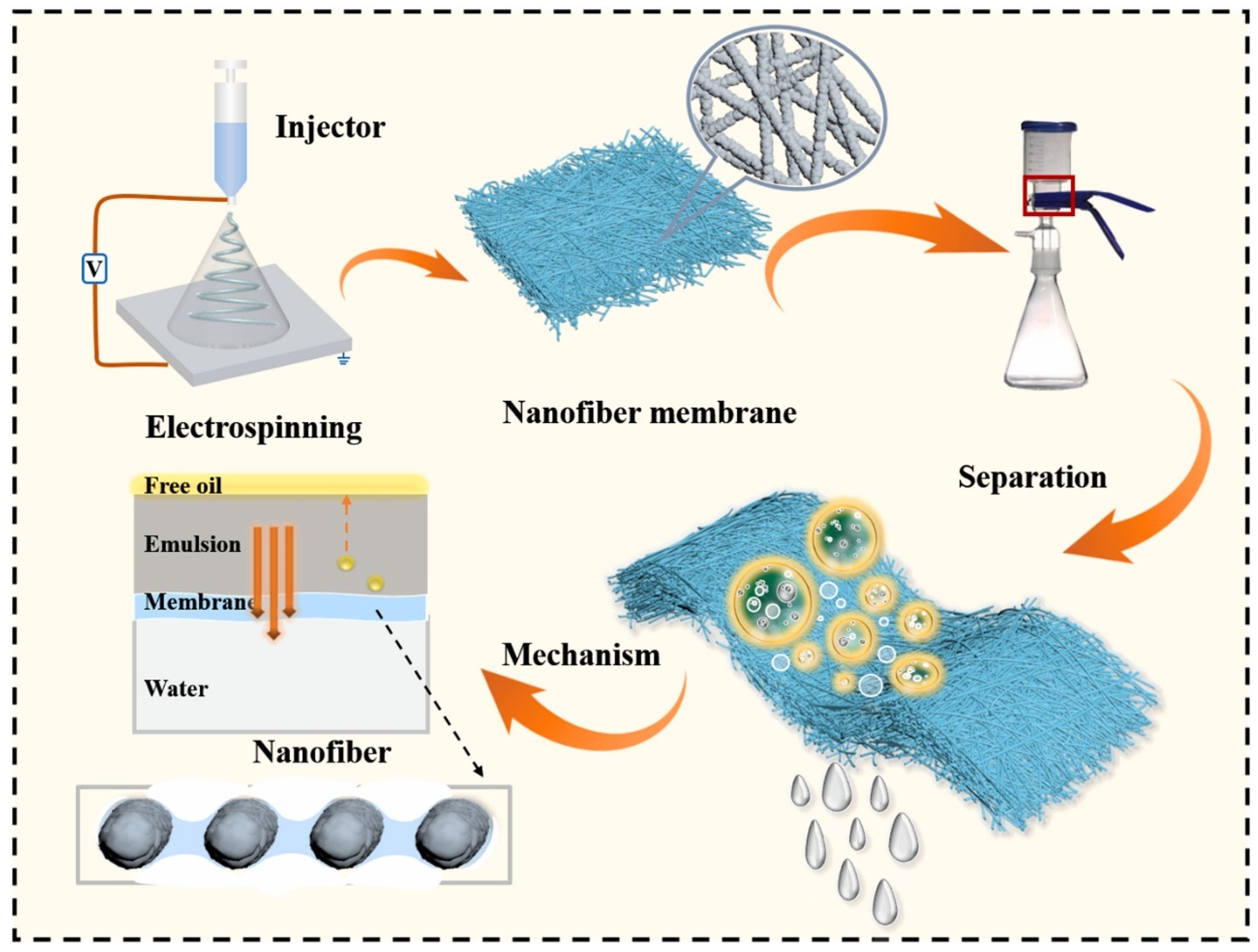
| Electrospun nanofibers from a blend of PVP, PVDF and TiO2 NPs (oil–water separation) | Enhanced hydrophilicity, mechanical strength, chemical stability, and antifouling property with high separation efficiency (98.4%) and FRR (95.68%) (Schematic shown in Figure 6) | [205] |
| PSf membrane using TiO2 nanorods forming flower-like structures used as additive | Enhanced hydrophilicity, high surface area, self-cleaning efficiency (68.8%), antifouling activity | [111] |
| L-cysteine-surface-modified TiO2 NPs incorporated in PES membrane by PI process | Enhanced water flux, direct red-16 (98%) and liquorice (90%) removal, hydrophilicity, antifouling. Agglomeration @ 1 wt.% loading | [140] |
2.2. Carbon-Nanostructure-Based Nanocomposites
2.2.1. Carbon-Nanotube-Based Nanocomposites
2.2.2. Graphene/Graphene Oxide (GO)/Reduced Graphene Oxide (rGO)-Based Nanocomposites
| Membrane | Application | Results (Compared to TFCs) | References |
|---|---|---|---|
| rGO/PVDF | MF (1 bar) | Enhanced water flux: 1024 ; Acetaminophen rejection: 72%; Triclosan rejection: 81%; Enhanced antifouling | [234] |
| TiO2/GO/PVDF | UF (1 bar) | Water flux: 487.8 ; BSA rejection: 92.5%; Enhanced photodegradation efficiency; Enhanced antifouling; Self-cleaning | [235] |
| TiO2@GO/PES | UF (1 bar) | Water flux: 109.8 ; BSA rejection: 99.1%; MB photodegradation rate: 95.1%; FFR: 86.1% | [236] |
| Ag@GO/PVDF | UF | Water flux: 491 ; Flux loss: 21%; Improved hydrophilicity (86.1 → 62.5°); mechanical strength (1.94 → 2.13 MPa); Enhanced antifouling due to GO | [237] |
| GO-ND/PVC | UF (2 bar) | Improved Water flux (0 → 0.15 wt.%): 200 → 440 ; BSA rejection: 95.08%; Flux recovery: 83.07%; Enhanced hydrophilic, antifouling, and mechanical strength | [238] |
| GO/PANI/PVDF | NF (1 bar) | Enhanced water flux (0 → 0.1% wt./v GO): 112 → 454 ; BSA rejection: 38.6 → 78.3%; Allura red: ~80 → 98%; Methyl orange: ~80 → 95%; Enhanced hyrophobicity; degradation temperature: 398 → 470 °C; Improved Tensile strength: 32 → 90 MPa, Enhanced antifouling | [239] |
| COOH-GO/PA | NF (10 bar) | Enhanced water flux (0 → 0.07% wt./v GO): 110.4 ; New Coccine (dye) rejection: 95.1%; NaCl rejection: 25%; Improved hydrophilicity and surface charge density | [240] |
| GO/PPS | NF (0.3 bar) | Enhanced flux: 325.65 ; Methyl blue rejection: ≥99%; Methylene blue rejection: ~99%; Rhodamine B (RhB) rejection: >99% | [241] |
| rGO-NH2/PA | NF (2 bar) | Enhanced water flux (0 → 50 rGO-NH2): 30.44 → 38.57 ; Salt rejection: NaCl: 26.9%, Na2SO4: 98.5%, MgSO4: 98.1%, CaCl2: 96.1%; Improved antifouling properties | [242] |
| Zeolite/GO/PVDF | RO (55 bar) | Enhanced water flux (GO:Zeolite: 0.07): 15.6→ 34.5 ; Enhanced salt rejection: 82.8 → 96.86%; Higher porosity; Improved hydrophilicity | [243] |
| GO/PSf | RO (55 bar) | Enhanced water flux (0 → 0.5 wt.% GO): 27.2 → 35.6 ; NaCl rejection: 98.8 → 99.2%; Higher porosity: 63 → 71.1%; Surface free energy: −91.63 → −108.68 (higher wettability); Enhanced tensile strength: 17.2 → 23.6 MPa | [244] |

2.2.3. Computational Studies
| Membrane Type | Computational Method Used | Modeling Results | Reference |
|---|---|---|---|
| CNT embedded in membrane | Quenched solid DFT to understand effect of foulants, moving particle semi-implicit method to understand implication of foulant (BSA) on velocity and pressure understand foulant (BSA) | Due to fouling, there was decrease in BET surface area (12.63 → 9.77 ), average pore size, and pore volume because of saturated mesoporous structure, foulant content increasing dead flow section, and membrane pressure | [255] |
| CNT and CNF incorporated in membrane | LAMMPS and OpenMM Ver. 7.5 package to study hydration and permeation with boron as antiscaling contaminant | Simulation demonstrated higher H2O diffusion (0.766 × 10−5 → 0.923 × 10−5 ) after incorporating CNT and CNF compared to pristine membrane, CNF enhanced water hydration and boron diffusion on the membrane, and CNT responsible for increased charge transfer to PA | [256] |
| MWCNT incorporated in PA membrane | LAMMPS to study interaction between membrane surface and foulant (BSA) | MWCNT-PA membrane exhibited superior antifouling compared to pristine due to enhanced hydrophilicity, smoother surface, and results in a stiffer PA structure that lowers structural conformity with BSA | [257] |
| PA and GO membranes | MD simulation to study the effect of presence of ions (Na+, Cl−) on BSA–membrane interaction | With increase in ionic strength, no changes were observed for protein-PA membrane while repulsion was observed between protein-GO membrane, PA showed attractive interaction with BSA while GO showed a repulsive one | [258] |
| CNT incorporated in membrane | LAMMPS to study effect of ions and nanomaterials on membrane fouling during crossflow measurements including natural organic matter (NOM) or alginate. | Low MW NOM interacts irreversibly with surface cavities of PA, high MW alginate either uncoil and spread on the surface or bind to foulant via ionic bridge due to Ca2+ ions, CNTs induce a stiffer and less rough surface, leading to low conformity to foulant interaction | [259] |
2.3. Zeolite-Based Nanocomposite
2.3.1. Naturally Available Zeolites
2.3.2. Synthetically Available Zeolites
Organic Dyes
Heavy Metals
| Nanocomposite Membrane Composition | Heavy Metal Ion (or Other Molecules/Ions) | Adsorption Capacity (mg g−1) (or Recovery/ Removal Rate b (%)) | References |
|---|---|---|---|
| Incorporation of NaX zeolite NPs into PSf membrane | Ni(II), Pb(II) | 122.0, 682.0 | [265,287] |
| Impregnation of zeolite and PVP in matrix of PSf | Cu(II) | 38 | [288] |
| Hybrid membrane made up of Ca-Activated zeolite, PVP, and PES blend | 70 b | [289] | |
| Fabricating ZIF-8 NPs into cellulose UF membranes | As(III), methylene blue | 97.7, 100 b | [71] |
| Mixing of zeolite into chitosan (CS) and poly(vinyl alcohol) PVA mixture via electrospinning | Cr(VI), Fe(III), Ni(II) | 8.84, 6.16, 1.77 | [290] |
| Mixture of Polycaprolactone and clay was electrospun | Cd(II), Cr(III), Cu(II), Pb(II) | 29.59, 27.23, 25.36, 32.88 | [291] |
| Mixture of PVA and clay was electrospun | 14.58, 17.36, 16.46, 16.50 | ||
| Integrating ZIF-8 NPs into PAN UF membrane | Congo Red, Pb(II), Cu(II) | 89, 92, 76 b | [286] |
| Embedding zeolite and PVP into PSf matrix | Cu(II) | 96.4 b | [292] |
| Blending of zeolite into CS and PVA mixture via casting | Cr(VI) | 450 | [293] |
| Incorporating NaX zeolite into PVA via electrospinning | Ni(II), Cd(II) | 342.8, 838.7 | [294] |
| Pd growth on electrospun mat of zeolite and poly-acrylonitrile-co-methyl acrylate using electroless plating | Ammonia nitrogen (NH4+-N) | 92 b | [295] |
| Deposition of microfine powdered zeolite on outer surface of PVDF fiber membrane | Total organic carbon, total nitrogen, NH4+-N | ~18, ~20, ~90 b | [296] |
Desalination
2.4. Biopolymer-Based Nanocomposites
2.4.1. Cellulose-Based Nanocomposites
2.4.2. Chitosan-Based Nanocomposites
3. Summary, Impact, and Future Scope
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Elimelech, M. The global challenge for adequate and safe water. J. Water Supply Res. Technol. 2006, 55, 3–10. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R.; et al. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Agboola, O.; Fayomi, O.S.I.; Ayodeji, A.; Ayeni, A.O.; Alagbe, E.E.; Sanni, S.E.; Okoro, E.E.; Moropeng, L.; Sadiku, R.; Kupolati, K.W.; et al. A review on polymer nanocomposites and their effective applications in membranes and adsorbents for water treatment and gas separation. Membranes 2021, 11, 139. [Google Scholar] [CrossRef]
- Beyene, H.D.; Ambaye, T.G. Application of sustainable nanocomposites for water purification process. In Sustainable Polymer Composites and Nanocomposites; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 387–412. [Google Scholar]
- Arman, N.Z.; Salmiati, S.; Aris, A.; Salim, M.R.; Nazifa, T.H.; Muhamad, M.S.; Marpongahtun, M. A review on emerging pollutants in the water environment: Existences, health effects and treatment processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, J.; Huang, C.H.; Hawkins, G.L.; Li, K.; Chen, Y.; Huang, Q. Occurrence of per- and polyfluoroalkyl substances in water: A review. Environ. Sci. Water Res. Technol. 2022, 8, 1136–1151. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef] [Green Version]
- Babich, R.; Craig, E.; Muscat, A.; Disney, J.; Farrell, A.; Silka, L.; Jayasundara, N. Defining drinking water metal contaminant mixture risk by coupling zebrafish behavioral analysis with citizen science. Sci. Rep. 2021, 11, 17303. [Google Scholar] [CrossRef]
- Acrylamide, O. National Primary Drinking Water Regulations. Kidney 2009, 2, 7. [Google Scholar]
- USEPA. Inorganic Contaminant Accumulation in Potable Water Distribution Systems; U.S. Environmental Protection Agency: Washington, DC, USA, 2006.
- Soriano, Á.; Gorri, D.; Urtiaga, A. Efficient treatment of perfluorohexanoic acid by nanofiltration followed by electrochemical degradation of the NF concentrate. Water Res. 2017, 112, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.; He, Y.; Jekel, M.; Reinhard, M.; Gin, K.Y.H. Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle. Environ. Int. 2014, 71, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Tsiourtis, N.X. Desalination and the environment. Desalination 2001, 141, 223–236. [Google Scholar] [CrossRef]
- National Research Council, Assembly of Life Sciences. Drinking Water and Health; National Academies Press: Washington, DC, USA, 1981; p. 311. [Google Scholar]
- National Research Council. Review of the Environmental Protection Agency’s Draft IRIS Assessment of Tetrachloroethylene; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Shelton, T.B. Interpreting Drinking Water Quality Analysis, What do the Number Mean? Cook College-Rutgers University: New Brunswick, NJ, USA, 1991. [Google Scholar]
- Fawell, J.K. The impact of inorganic chemicals on water quality and health. Ann. Ist. Super. Sanita 1993, 29, 293–303. [Google Scholar] [PubMed]
- Ashbolt, N.J. Microbial Contamination of Drinking Water and Human Health from Community Water Systems. Curr. Environ. Health Rep. 2015, 2, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Owoseni, M.C.; Olaniran, A.O.; Okoh, A.I. Chlorine Tolerance and Inactivation of Escherichia coli recovered from Wastewater Treatment Plants in the Eastern Cape, South Africa. Appl. Sci. 2017, 7, 810. [Google Scholar] [CrossRef]
- Canu, I.G.; Laurent, O.; Pires, N.; Laurier, D.; Dublineau, I. Health effects of naturally radioactive water ingestion: The need for enhanced studies. Environ. Health Perspect. 2011, 119, 1676–1680. [Google Scholar] [CrossRef] [Green Version]
- Castro-Muñoz, R.; Yáñez-Fernández, J.; Fíla, V. Phenolic compounds recovered from agro-food by-products using membrane technologies: An overview. Food Chem. 2016, 213, 753–762. [Google Scholar] [CrossRef]
- Buonomenna, M.G. Membrane processes for a sustainable industrial growth. RSC Adv. 2013, 3, 5694–5740. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Curcio, E.; Drioli, E. Process intensification in the textile industry: The role of membrane technology. J. Environ. Manag. 2004, 73, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.; Mohammad, A.W. Challenges and trends in membrane technology implementation for produced water treatment: A review. J. Water Process Eng. 2014, 4, 107–133. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z.V.P. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Shin, W.-K.; Lee, Y.-S.; Kim, D.-W. Hybrid composite membranes based on polyethylene separator and Al2O3 nanoparticles for lithium-ion batteries. J. Nanosci. Nanotechnol. 2013, 13, 3705–3710. [Google Scholar] [CrossRef]
- Li, X.H.; Yu, Y.F.; Meng, Y.Z. Novel Quaternized Poly(arylene ether sulfone)/Nano-ZrO2 Composite Anion Exchange Membranes for Alkaline Fuel Cells. ACS Appl. Mater. Interfaces 2013, 5, 1414–1422. [Google Scholar] [CrossRef]
- Karkhanechi, H.; Kazemian, H.; Nazockdast, H.; Mozdianfard, M.R.; Bidoki, S.M. Fabrication of Homogenous Polymer-Zeolite Nanocomposites as Mixed-Matrix Membranes for Gas Separation. Chem. Eng. Technol. 2012, 35, 885–892. [Google Scholar] [CrossRef]
- Rabajczyk, A.; Zielecka, M.; Cygańczuk, K.; Pastuszka, Ł.; Jurecki, L. Nanometals-containing polymeric membranes for purification processes. Materials 2021, 14, 513. [Google Scholar] [CrossRef]
- Wen, Y.; Yuan, J.; Ma, X.; Wang, S.; Liu, Y. Polymeric nanocomposite membranes for water treatment: A review. Environ. Chem. Lett. 2019, 17, 1539–1551. [Google Scholar] [CrossRef]
- Yin, K.; Chu, D.; Dong, X.; Wang, C.; Duan, J.A.; He, J. Femtosecond laser induced robust periodic nanoripple structured mesh for highly efficient oil-water separation. Nanoscale 2017, 9, 14229–14235. [Google Scholar] [CrossRef]
- Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.S.; Abdel-Hamid, S.M.S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Wankat, P.C. Separation Process Engineering; Pearson Education: London, UK, 2006. [Google Scholar]
- Pendergast, M.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Zahid, M.; Rashid, A.; Akram, S.; Rehan, Z.A.; Razzaq, W. A Comprehensive Review on Polymeric Nano-Composite Membranes for Water Treatment. J. Membr. Sci. Technol. 2018, 8, 179. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J. Nanoparticles in reverse osmosis membranes for desalination: A state of the art review. Desalination 2020, 475, 114171. [Google Scholar] [CrossRef]
- Ursino, C.; Castro-Muñoz, R.; Drioli, E.; Gzara, L.; Albeirutty, M.H.; Figoli, A. Progress of nanocomposite membranes for water treatment. Membranes 2018, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.T.; Hu, J.F.; Zhang, X.Q.; Yuan, D.D.; Duan, G.G.; Li, Y.W. Robust and multifunctional natural polyphenolic composites for water remediation. Mater. Horiz. 2022, 9, 2496–2517. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Labbez, C.; Fievet, P.; Szymczyk, A.; Vidonne, A.; Foissy, A.; Pagetti, J. Analysis of the salt retention of a titania membrane using the “DSPM” model: Effect of pH, salt concentration and nature. J. Membr. Sci. 2002, 208, 315–329. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Jain, H.; Garg, M.C. Fabrication of polymeric nanocomposite forward osmosis membranes for water desalination-A review. Environ. Technol. Innov. 2021, 23, 101561. [Google Scholar] [CrossRef]
- Ismail, A.F.; Padaki, M.; Hilal, N.; Matsuura, T.; Lau, W.J. Thin film composite membrane—Recent development and future potential. Desalination 2015, 356, 140–148. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.L.; Japip, S.; Zhang, Y.; Weber, M.; Maletzko, C.; Chung, T.-S. Emerging thin-film nanocomposite (TFN) membranes for reverse osmosis: A review. Water Res. 2020, 173, 115557. [Google Scholar] [CrossRef]
- Nambi Krishnan, J.; Venkatachalam, K.R.; Ghosh, O.; Jhaveri, K.; Palakodeti, A.; Nair, N. Review of Thin Film Nanocomposite Membranes and Their Applications in Desalination. Front. Chem. 2022, 10, 6. [Google Scholar] [CrossRef]
- Li, D.; Wang, H. Recent developments in reverse osmosis desalination membranes. J. Mater. Chem. 2010, 20, 4551–4566. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, S.; Peng, X.; Zhang, L.; Gao, C. Polyamide membranes with nanoscale Turing structures for water purification. Science 2018, 360, 518–521. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.S.; Lee, H.J. Nanostructured Materials for Water Purification: Adsorption of Heavy Metal Ions and Organic Dyes. Polymers 2022, 14, 2183. [Google Scholar] [CrossRef]
- Altaee, A.; Zaragoza, G.; van Tonningen, H.R. Comparison between Forward Osmosis-Reverse Osmosis and Reverse Osmosis processes for seawater desalination. Desalination 2014, 336, 50–57. [Google Scholar] [CrossRef]
- Al-Sakaji, B.A.K.; Al-Asheh, S.; Maraqa, M.A. A Review on the Development of an Integer System Coupling Forward Osmosis Membrane and Ultrasound Waves for Water Desalination Processes. Polymers 2022, 14, 2710. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Zinadini, S.; Vatanpour, V. A new approach to improve antifouling property of PVDF membrane using in situ polymerization of PAA functionalized TiO2 nanoparticles. J. Membr. Sci. 2011, 380, 155–162. [Google Scholar] [CrossRef]
- Sakarkar, S.; Muthukumaran, S.; Jegatheesan, V. Tailoring the Effects of Titanium Dioxide (TiO2) and Polyvinyl Alcohol (PVA) in the Separation and Antifouling Performance of Thin-Film Composite Polyvinylidene Fluoride (PVDF) Membrane. Membranes 2021, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Khalid, T.; Rehan, Z.A.; Javed, T.; Akram, S.; Rashid, A.; Mustafa, S.K.; Shabbir, R.; Mora-Poblete, F.; Asad, M.S.; et al. Fabrication and Characterization of Sulfonated Graphene Oxide (SGO) Doped PVDF Nanocomposite Membranes with Improved Anti-Biofouling Performance. Membranes 2021, 11, 749. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.; Vatanpour, V. A comprehensive study on the performance and antifouling enhancement of the PVDF mixed matrix membranes by embedding different nanoparticulates: Clay, functionalized carbon nanotube, SiO2 and TiO2. Sep. Purif. Technol. 2018, 197, 372–381. [Google Scholar] [CrossRef]
- Dehghankar, M.; Mohammadi, T.; Tavakolmoghadam, M.; Tofighy, M.A. Polyvinylidene Fluoride/Nanoclays (Cloisite 30B and Palygorskite) Mixed Matrix Membranes with Improved Performance and Antifouling Properties. Ind. Eng. Chem. Res. 2021, 60, 12078–12091. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Rahbari-Sisakht, M.; Ismail, A.F. A novel thin film composite forward osmosis membrane prepared from PSf-TiO2 nanocomposite substrate for water desalination. Chem. Eng. J. 2014, 237, 70–80. [Google Scholar] [CrossRef]
- Peyki, A.; Rahimpour, A.; Jahanshahi, M. Preparation and characterization of thin film composite reverse osmosis membranes incorporated with hydrophilic SiO2 nanoparticles. Desalination 2015, 368, 152–158. [Google Scholar] [CrossRef]
- Qian, X.J.; Wang, X.J.; Gao, X.L.; Cao, W.Q.; Gao, C.J. Effects of GO@CS core-shell nanomaterials loading positions on the properties of thin film nanocomposite membranes. J. Membr. Sci. 2021, 624, 119102. [Google Scholar] [CrossRef]
- Akther, N.; Phuntsho, S.; Chen, Y.; Ghaffour, N.; Shon, H.K. Recent advances in nanomaterial-modified polyamide thin-film composite membranes for forward osmosis processes. J. Membr. Sci. 2019, 584, 20–45. [Google Scholar] [CrossRef]
- Menge, H.G.; Huynh, N.D.; Cho, C.; Choi, D.; Park, Y.T. Designable functional polymer nanocomposites via layer-by-layer assembly for highly deformable power-boosted triboelectric nanogenerators. Compos. Part B Eng. 2022, 230, 109513. [Google Scholar] [CrossRef]
- Kong, L.C.; Wang, Y.; Andrews, C.B.; Zheng, C.M. One-step construction of hierarchical porous channels on electrospun MOF/polymer/graphene oxide composite nanofibers for effective arsenate removal from water. Chem. Eng. J. 2022, 435, 134830. [Google Scholar] [CrossRef]
- Kong, L.C.; Yan, Q.L.; Wang, Y.; Wang, Q.Y.; Andrews, C.B.; Zheng, C.M. Self-supported trimetallic NiZnLa nanosheets on hierarchical porous graphene oxide-polymer composite fibers for enhanced phosphate removal from water. J. Colloid Interface Sci. 2022, 628, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Li, X.; Zhang, M.; Xie, J.; Wang, X.L. Zeolitic Imidazole Framework/Graphene Oxide Hybrid Functionalized Poly(lactic acid) Electrospun Membranes: A Promising Environmentally Friendly Water Treatment Material. ACS Omega 2018, 3, 6860–6866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Q.L.; Zhou, X.F.; Deng, W.; Zheng, Z.; Liu, Z.P. Freestanding bacterial cellulose-graphene oxide composite membranes with high mechanical strength for selective ion permeation. Sci. Rep. 2016, 6, 33185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Z.W.; Shao, X.; Chen, Y.; Pan, J.M.; Qiu, F.X.; Zhang, T. Enhanced water permeability and rejection of As(III) in groundwater by nanochannels and active center formed in nanofibrillated celluloses UF membranes with ZIF-8. J. Membr. Sci. 2022, 646, 120255. [Google Scholar] [CrossRef]
- Krishnan, S.A.G.; Sasikumar, B.; Arthanareeswaran, G.; Laszlo, Z.; Santos, E.N.; Vereb, G.; Kertesz, S. Surface-initiated polymerization of PVDF membrane using amine and bismuth tungstate (BWO) modified MIL-100(Fe) nanofillers for pesticide photodegradation. Chemosphere 2022, 304, 135286. [Google Scholar] [CrossRef]
- Wang, H.T.; Zhao, X.W.; You, J.C.; Li, Y.J. Porous Nanocomposites with Monolayer Nano-SiO2 Coated Skeleton from Interfacial Nanoparticle-Anchored Cocontinuous Polymer Blends. ACS Appl. Polym. Mater. 2020, 2, 5735–5742. [Google Scholar] [CrossRef]
- Sahu, A.; Blackburn, K.; Durkin, K.; Eldred, T.B.; Johnson, B.R.; Sheikh, R.; Amburgey, J.E.; Poler, J.C. Green synthesis of nanoscale anion exchange resin for sustainable water purification. Environ. Sci. Water Res. Technol. 2018, 4, 1685–1694. [Google Scholar] [CrossRef]
- Sahu, A.; Alston, J.R.; Carlin, C.; Craps, M.; Davis, K.; Harrison, H.B.; Kongruengkit, T.; Manikonda, A.; Elmore, S.; Rollins, R.; et al. Fluorographite Nanoplatelets with Covalent Grafting of Anion-Exchange Resins for Water Purification. ACS Appl. Nano Mater. 2022, 5, 5709–5721. [Google Scholar] [CrossRef]
- Johnson, B.R.; Eldred, T.B.; Nguyen, A.T.; Payne, W.M.; Schmidt, E.E.; Alansari, A.Y.; Amburgey, J.E.; Poler, J.C. High-Capacity and Rapid Removal of Refractory NOM Using Nanoscale Anion Exchange Resin. ACS Appl. Mater. Interfaces 2016, 8, 18540–18549. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Amooghin, A.E.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Shahidi, K.; Mohammadi, T. Hydrogen separation and purification using crosslinkable PDMS/zeolite A nanoparticles mixed matrix membranes. Int. J. Hydrogen Energy 2012, 37, 14576–14589. [Google Scholar] [CrossRef]
- Lee, J.Y.; Qi, S.R.; Liu, X.; Li, Y.; Huo, F.W.; Tang, C.Y.Y. Synthesis and characterization of silica gel-polyacrylonitrile mixed matrix forward osmosis membranes based on layer-by-layer assembly. Sep. Purif. Technol. 2014, 124, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Mahdavi, H.; Karami, M. Cross-linked mixed matrix membranes made up of amine-functionalized silica and chloromethylated polysulfone for organic solvent nanofiltration applications. J. Environ. Chem. Eng. 2022, 10, 107145. [Google Scholar] [CrossRef]
- Wang, L.; Song, X.J.; Wang, T.; Wang, S.Z.; Wang, Z.N.; Gao, C.J. Fabrication and characterization of polyethersulfone/carbon nanotubes (PES/CNTs) based mixed matrix membranes (MMMs) for nanofiltration application. Appl. Surf. Sci. 2015, 330, 118–125. [Google Scholar] [CrossRef]
- Siddique, T.; Balu, R.; Mata, J.; Dutta, N.K.; Choudhury, N.R. Electrospun Composite Nanofiltration Membranes for Arsenic Removal. Polymers 2022, 14, 1980. [Google Scholar] [CrossRef]
- Srivastava, S.; Kotov, N.A. Composite Layer-by-Layer (LBL) Assembly with Inorganic Nanoparticles and Nanowires. Acc. Chem. Res. 2008, 41, 1831–1841. [Google Scholar] [CrossRef]
- Mohapatra, D.R.K. Nanomaterials. Available online: http://www.gcekjr.ac.in/pdf/lectures/2020/7166--_2nd%20Semester_ALL.pdf (accessed on 15 January 2023).
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.Ó.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef]
- Rostam, A.B.; Peyravi, M.; Ghorbani, M.; Jahanshahi, M. Antibacterial surface modified of novel nanocomposite sulfonated polyethersulfone/polyrhodanine membrane. Appl. Surf. Sci. 2018, 427, 17–28. [Google Scholar] [CrossRef]
- Maggay, I.V.; Yeh, T.H.; Venault, A.; Hsu, C.H.; Dizon, G.V.; Chang, Y. Tuning the molecular design of random copolymers for enhancing the biofouling mitigation of membrane materials. J. Membr. Sci. 2019, 588, 117217. [Google Scholar] [CrossRef]
- Zoromba, M.S.; Ismail, M.I.M.; Bassyouni, M.I.; Abdel-Aziz, M.H.; Salah, N.; Alshahrie, A.; Memic, A. Fabrication and characterization of poly (aniline-co-o-anthranilic acid)/magnetite nanocomposites and their application in wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 121–130. [Google Scholar] [CrossRef]
- Chen, X.; Gao, X.; Fu, K.; Qiu, M.; Xiong, F.; Ding, D.; Cui, Z.; Wang, Z.; Fan, Y.; Drioli, E. Tubular hydrophobic ceramic membrane with asymmetric structure for water desalination via vacuum membrane distillation process. Desalination 2018, 443, 212–220. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; Fíla, V.; Drioli, E.; Figoli, A. Matrimid®5218 dense membrane for the separation of azeotropic MeOH-MTBE mixtures by pervaporation. Sep. Purif. Technol. 2018, 199, 27–36. [Google Scholar] [CrossRef]
- Wei, H.; Rodriguez, K.; Renneckar, S.; Vikesland, P.J. Environmental science and engineering applications of nanocellulose-based nanocomposites. Environ. Sci. Nano 2014, 1, 302–316. [Google Scholar] [CrossRef] [Green Version]
- Asatekin, A.; Menniti, A.; Kang, S.; Elimelech, M.; Morgenroth, E.; Mayes, A.M. Antifouling nanofiltration membranes for membrane bioreactors from self-assembling graft copolymers. J. Membr. Sci. 2006, 285, 81–89. [Google Scholar] [CrossRef]
- Shi, M.Y.; Zhu, J.; He, C.J. Durable antifouling polyvinylidene fluoride membrane via surface zwitterionicalization mediated by an amphiphilic copolymer. RSC Adv. 2016, 6, 114024–114036. [Google Scholar] [CrossRef]
- Bera, A.; Kumar, C.U.; Parui, P.; Jewrajka, S.K. Stimuli responsive and low fouling ultrafiltration membranes from blends of polyvinylidene fluoride and designed library of amphiphilic poly(methyl methacrylate) containing copolymers. J. Membr. Sci. 2015, 481, 137–147. [Google Scholar] [CrossRef]
- Lu, T.T.; Xu, X.X.; Liu, X.X.; Sun, T. Super hydrophilic PVDF based composite membrane for efficient separation of tetracycline. Chem. Eng. J. 2017, 308, 151–159. [Google Scholar] [CrossRef]
- Yuan, X.S.; Liu, W.; Zhu, W.Y.; Zhu, X.X. Enhancement in Flux and Antifouling Properties of Polyvinylidene Fluoride/Polycarbonate Blend Membranes for Water Environmental Improvement. ACS Omega 2020, 5, 30201–30209. [Google Scholar] [CrossRef]
- Shen, L.G.; Feng, S.S.; Li, J.X.; Chen, J.R.; Li, F.Q.; Lin, H.J.; Yu, G.Y. Surface modification of polyvinylidene fluoride (PVDF) membrane via radiation grafting: Novel mechanisms underlying the interesting enhanced membrane performance. Sci. Rep. 2017, 7, 2721. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.X.; Gu, L.; Wu, S.F.; Dong, G.X.; Qiao, X.B.; Zhang, K.; Lu, X.Y.; Wen, H.F.; Zhang, D.F. Hydrothermal carbon nanospheres assisted-fabrication of PVDF ultrafiltration membranes with improved hydrophilicity and antifouling performance. Sep. Purif. Technol. 2020, 247, 116889. [Google Scholar] [CrossRef]
- Sorribas, S.; Gorgojo, P.; Tellez, C.; Coronas, J.; Livingston, A.G. High Flux Thin Film Nanocomposite Membranes Based on Metal-Organic Frameworks for Organic Solvent Nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, O.; Sharifzadeh, E. Manipulation of the thermal/mechanical properties of the fiber/polymer interface in PA6/epoxy composite via uniform/un-uniform colloidal stamping of silica/hollow graphene oxide nanoparticles. Colloid Polym. Sci. 2022, 300, 1389–1404. [Google Scholar] [CrossRef]
- Zare, Y. Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties. Compos. Part A Appl. Sci. Manuf. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Zare, Y. The roles of nanoparticles accumulation and interphase properties in properties of polymer particulate nanocomposites by a multi-step methodology. Compos. Part A Appl. Sci. Manuf. 2016, 91, 127–132. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Peng, W.X.; Zare, Y.; Rhee, K.Y. Effects of Size and Aggregation/Agglomeration of Nanoparticles on the Interfacial/Interphase Properties and Tensile Strength of Polymer Nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef]
- Erdugan, B.M.; Demirel, E.; Suvaci, E. Preparation and characterization of polyvinyl chloride membranes decorated with designed novel zinc oxide particles for mitigating uncontrollable agglomeration. J. Environ. Chem. Eng. 2022, 10, 108388. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.Y.; Cao, D.P.; Zhang, L.Q.; Guo, Z.H. Nanoparticle Dispersion and Aggregation in Polymer Nanocomposites: Insights from Molecular Dynamics Simulation. Langmuir 2011, 27, 7926–7933. [Google Scholar] [CrossRef]
- Mousa, H.M.; Hamdy, M.; Yassin, M.A.; Seleman, M.M.E.; Abdel-Jaber, G.T. Characterization of nanofiber composite membrane for high water flux and antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129655. [Google Scholar] [CrossRef]
- Behm, N.; Brokaw, D.; Overson, C.; Peloquin, D.; Poler, J.C. High-throughput microwave synthesis and characterization of NiO nanoplates for supercapacitor devices. J. Mater. Sci. 2013, 48, 1711–1716. [Google Scholar] [CrossRef]
- Li, J.F.; Xu, Z.L.; Yang, H.; Yu, L.Y.; Liu, M. Effect of TiO2 nanoparticles on the surface morphology and performance of microporous PES membrane. Appl. Surf. Sci. 2009, 255, 4725–4732. [Google Scholar] [CrossRef]
- Azhar, F.H.; Harun, Z.; Alias, S.S.; Yunos, M.Z.; Ibrahim, S.A.; Abdullahi, T.; Ahmad, A.; Othman, M.H.D. Self-Cleaning antifouling performance based on the surface area of flower-like TiO2 as additive for PSf mixed matrix membrane. Macromol. Res. 2020, 28, 625–635. [Google Scholar] [CrossRef]
- Wang, H.S.; Qiao, X.L.; Chen, J.G.; Wang, X.J.; Ding, S.Y. Mechanisms of PVP in the preparation of silver nanoparticles. Mater. Chem. Phys. 2005, 94, 449–453. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Ng, L.Y.; Ang, W.L.; Chung, Y.T.; Rohani, R.; Mohammad, A.W. Enhancing Morphology and Separation Performance of Polyamide 6,6 Membranes By Minimal Incorporation of Silver Decorated Graphene Oxide Nanoparticles. Sci. Rep. 2019, 9, 1216. [Google Scholar] [CrossRef] [Green Version]
- Jeong, B.-H.; Hoek, E.M.V.; Yan, Y.; Subramani, A.; Huang, X.; Hurwitz, G.; Ghosh, A.K.; Jawor, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Membr. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Raval, H.D.; Mondal, M. Polymer-based nano-enhanced reverse osmosis membranes. In Advancement in Polymer-Based Membranes for Water Remediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 335–379. [Google Scholar]
- Olimattel, K.; Church, J.; Lee, W.H.; Chumbimuni-Torres, K.Y.; Zhai, L.; Sadmani, A.A. Enhanced fouling resistance and antimicrobial property of ultrafiltration membranes via polyelectrolyte-assisted silver phosphate nanoparticle immobilization. Membranes 2020, 10, 293. [Google Scholar] [CrossRef]
- Abounahia, N.; Qiblawey, H.; Zaidi, S.J. Progress for Co-Incorporation of Polydopamine and Nanoparticles for Improving Membranes Performance. Membranes 2022, 12, 675. [Google Scholar] [CrossRef]
- Zhang, S.; Acharya, D.P.; Tang, X.; Zheng, H.; Yang, G.; Ng, D.; Xie, Z. Dual Functions of a Au@ AgNP-Incorporated Nanocomposite Desalination Membrane with an Enhanced Antifouling Property and Fouling Detection Via Surface-Enhanced Raman Spectroscopy. ACS Appl. Mater. Interfaces 2021, 13, 46202–46212. [Google Scholar] [CrossRef]
- Shen, L.G.; Huang, Z.Y.; Liu, Y.; Li, R.J.; Xu, Y.H.; Jakaj, G.; Lin, H.J. Polymeric Membranes Incorporated With ZnO Nanoparticles for Membrane Fouling Mitigation: A Brief Review. Front. Chem. 2020, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Hasannasab, M.; Nourmohammadi, J.; Dehghan, M.M.; Ghaee, A. Immobilization of bromelain and ZnO nanoparticles on silk fibroin nanofibers as an antibacterial and anti-inflammatory burn dressing. Int. J. Pharm. 2021, 610, 121227. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Sabir, A.; Iqbal, S.S.; Felemban, B.F.; Riaz, T.; Bahadar, A.; Hossain, N.; Khan, R.U.; Inam, F. Novel antibacterial polyurethane and cellulose acetate mixed matrix membrane modified with functionalized TiO2 nanoparticles for water treatment applications. Chemosphere 2022, 301, 134711. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, K.; Arthanareeswaran, G.; Kumar, P.S.; Kweon, J. Fabrication of Zwitterion TiO2 Nanomaterial-Based Nanocomposite Membranes for Improved Antifouling and Antibacterial Properties and Hemocompatibility and Reduced Cytotoxicity. ACS Omega 2021, 6, 20279–20291. [Google Scholar] [CrossRef]
- Istirokhatun, T.; Lin, Y.; Wang, S.; Shen, Q.; Segawa, J.; Guan, K.; Matsuyama, H. Novel thin-film composite membrane with ultrathin surface mineralization layer engineered by electrostatic attraction induced In-situ assembling process for high-performance nanofiltration. Chem. Eng. J. 2021, 417, 127903. [Google Scholar] [CrossRef]
- Bidsorkhi, H.C.; Riazi, H.; Emadzadeh, D.; Ghanbari, M.; Matsuura, T.; Lau, W.; Ismail, A. Preparation and characterization of a novel highly hydrophilic and antifouling polysulfone/nanoporous TiO2 nanocomposite membrane. Nanotechnology 2016, 27, 415706. [Google Scholar] [CrossRef]
- Pereira, V.R.; Isloor, A.M.; Zulhairun, A.; Subramaniam, M.; Lau, W.; Ismail, A. Preparation of polysulfone-based PANI–TiO 2 nanocomposite hollow fiber membranes for industrial dye rejection applications. RSC Adv. 2016, 6, 99764–99773. [Google Scholar] [CrossRef]
- Kamarudin, D.; Hashim, N.A.; Ong, B.H.; Faried, M.; Suga, K.; Umakoshi, H.; Mahari, W.A.W. Alternative fouling analysis of PVDF UF membrane for surface water treatment: The credibility of silver nanoparticles. J. Membr. Sci. 2022, 661, 120865. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Semsarilar, M.; Fernandez-Pacheco, R.; Martinez, G.; Mallada, R.; Coelhoso, I.M.; Portugal, C.A.M.; Crespo, J.G.; Deratani, A.; Quemener, D. Nano-structured magneto-responsive membranes from block copolymers and iron oxide nanoparticles. Polym. Chem. 2017, 8, 605–614. [Google Scholar] [CrossRef]
- Gradinaru, L.M.; Vlad, S.; Ciobanu, R.C. The Development and Study of Some Composite Membranes Based on Polyurethanes and Iron Oxide Nanoparticles. Membranes 2022, 12, 1127. [Google Scholar] [CrossRef]
- Mosaffa, E.; Ghafuri, H.; Zand, H.R.E. Improvement on physical properties of polyethersulfone membranes modified by poly(1-vinylpyrrolidone) grafted magnetic Fe3O4@SiO2 nanoparticles. Appl. Organomet. Chem. 2019, 33, e4639. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Khadivi, M.A.; Astinchap, B.; Moradian, R. Fouling resistant mixed matrix polyethersulfone membranes blended with magnetic nanoparticles: Study of magnetic field induced casting. Sep. Purif. Technol. 2013, 109, 111–121. [Google Scholar] [CrossRef]
- Kusworo, T.D.; Dalanta, F.; Aryanti, N.; Othman, N.H. Intensifying separation and antifouling performance of PSf membrane incorporated by GO and ZnO nanoparticles for petroleum refinery wastewater treatment. J. Water Process Eng. 2021, 41, 102030. [Google Scholar] [CrossRef]
- Pawar, M.; Topcu Sendoğdular, S.; Gouma, P. A brief overview of TiO2 photocatalyst for organic dye remediation: Case study of reaction mechanisms involved in Ce-TiO2 photocatalysts system. J. Nanomater. 2018, 2018, 4923062. [Google Scholar] [CrossRef] [Green Version]
- Dalanta, F.; Kusworo, T.D.; Aryanti, N. Synthesis, characterization, and performance evaluation of UV light-driven Co-TiO2@SiO2 based photocatalytic nanohybrid polysulfone membrane for effective treatment of petroleum refinery wastewater. Appl. Catal. B Environ. 2022, 316, 121576. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, K.; Wang, K.; Xie, Z.; Ladewig, B.; Wang, H. Fabrication of polyethersulfone-mesoporous silica nanocomposite ultrafiltration membranes with antifouling properties. J. Membr. Sci. 2012, 423–424, 362–370. [Google Scholar] [CrossRef]
- Namvar-Mahboub, M.; Pakizeh, M. Development of a novel thin film composite membrane by interfacial polymerization on polyetherimide/modified SiO2 support for organic solvent nanofiltration. Sep. Purif. Technol. 2013, 119, 35–45. [Google Scholar] [CrossRef]
- Sun, Z.M.; Chen, H.; Ren, X.J.; Zhang, Z.G.; Guo, L.G.; Zhang, F.S.; Cheng, H.S. Preparation and application of zinc oxide/poly(m-phenylene isophthalamide) hybrid ultrafiltration membranes. J. Appl. Polym. Sci. 2019, 136, 47583. [Google Scholar] [CrossRef]
- Yang, Y.N.; Zhang, H.X.; Wang, P.; Zheng, Q.Z.; Li, J. The influence of nano-sized TiO2 fillers on the morphologies and properties of PSFUF membrane. J. Membr. Sci. 2007, 288, 231–238. [Google Scholar] [CrossRef]
- Zhang, Z.H.; An, Q.F.; Liu, T.; Zhou, Y.; Qian, J.W.; Gao, C.J. Fabrication and characterization of novel SiO2-PAMPS/PSF hybrid ultrafiltration membrane with high water flux. Desalination 2012, 297, 59–71. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Salehi, E.; Khadivi, M.A.; Moradian, R.; Astinchap, B. Novel polyethersulfone nanocomposite membrane prepared by PANI/Fe3O4 nanoparticles with enhanced performance for Cu(II) removal from water. J. Membr. Sci. 2012, 415, 250–259. [Google Scholar] [CrossRef]
- Gholami, F.; Zinatizadeh, A.A.; Zinadini, S.; Rittmann, B.E.; Torres, C.I. Enhanced antifouling and flux performances of a composite membrane via incorporating TiO2 functionalized with hydrophilic groups of L-cysteine for nanofiltration. Polym. Adv. Technol. 2022, 33, 1544–1560. [Google Scholar] [CrossRef]
- Bai, C.C.; Tang, M. Toxicological study of metal and metal oxide nanoparticles in zebrafish. J. Appl. Toxicol. 2020, 40, 37–63. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Sharma, N.; Maitra, S.S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Naikoo, G.A.; Arshad, F.; Almas, M.; Hassan, I.U.; Pedram, M.Z.; Aljabali, A.A.A.; Mishra, V.; Serrano-Aroca, A.; Birkett, M.; Charbe, N.B.; et al. 2D materials, synthesis, characterization and toxicity: A critical review. Chem.-Biol. Interact. 2022, 365, 110081. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M.; Aragaw, B.A. Iron-based nanoparticles in wastewater treatment: A review on synthesis methods, applications, and removal mechanisms. J. Saudi Chem. Soc. 2021, 25, 101280. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Semsarilar, M.; Nehache, S.; Cot, D.; Fernandez-Pacheco, R.; Martinez, G.; Mallada, R.; Deratani, A.; Quemener, D. Nanostructured Mixed Matrix Membranes from Supramolecular Assembly of Block Copolymer Nanoparticles and Iron Oxide Nanoparticles. Macromolecules 2016, 49, 7908–7916. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Semsarilar, M.; Quemener, D.; Fernández-Pacheco, R.; Martinez, G.; Coelhoso, I.M.; Nunes, S.P.; Crespo, J.G.; Mallada, R.; Portugal, C.A.M. Block Copolymer-Based Magnetic Mixed Matrix Membranes—Effect of Magnetic Field on Protein Permeation and Membrane Fouling. Membranes 2021, 11, 105. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, H.; Sharma, A.K.; Hong, W.G.; Shin, K.; Song, H.; Kim, H.Y.; Hong, Y.J. Recyclable aqueous metal adsorbent: Synthesis and Cu (II) sorption characteristics of ternary nanocomposites of Fe3O4 nanoparticles@ graphene–poly-N-phenylglycine nanofibers. J. Hazard. Mater. 2021, 401, 123283. [Google Scholar] [CrossRef]
- Nawi, N.S.M.; Lau, W.J.; Yusof, N.; Said, N.; Ismail, A.F. Enhancing water flux and antifouling properties of PES hollow fiber membranes via incorporation of surface-functionalized Fe3O4 nanoparticles. J. Chem. Technol. Biotechnol. 2022, 97, 1006–1020. [Google Scholar] [CrossRef]
- McDonogh, R.; Schaule, G.; Flemming, H.C. The permeability of biofouling layers on membranes. J. Membr. Sci. 1994, 87, 199–217. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, J.H.; Choo, K.H.; Lee, Y.S.; Lee, C.H. Hydrophilic modification of polypropylene microfiltration membranes by ozone-induced graft polymerization. J. Membr. Sci. 2000, 169, 269–276. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of water treatment membranes: A review of the underlying causes, monitoring techniques and control measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yunos, M.Z.; Harun, Z.; Basri, H.; Ismail, A.F. Studies on fouling by natural organic matter (NOM) on polysulfone membranes: Effect of polyethylene glycol (PEG). Desalination 2014, 333, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.L.; Etris, S.F. The development and functions of silver in water purification and disease control. Catal. Today 1997, 36, 107–114. [Google Scholar] [CrossRef]
- Khare, P.; Ramkumar, J.; Verma, N. Control of bacterial growth in water using novel laser-ablated metal-carbon-polymer nanocomposite-based microchannels. Chem. Eng. J. 2015, 276, 65–74. [Google Scholar] [CrossRef]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Sawai, J.; Shoji, S.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M.; Kojima, H. Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J. Ferment. Bioeng. 1998, 86, 521–522. [Google Scholar] [CrossRef]
- Jo, Y.J.; Choi, E.Y.; Choi, N.W.; Kim, C.K. Antibacterial and Hydrophilic Characteristics of Poly(ether sulfone) Composite Membranes Containing Zinc Oxide Nanoparticles Grafted with Hydrophilic Polymers. Ind. Eng. Chem. Res. 2016, 55, 7801–7809. [Google Scholar] [CrossRef]
- Hong, J.; He, Y. Polyvinylidene fluoride ultrafiltration membrane blended with nano-ZnO particle for photo-catalysis self-cleaning. Desalination 2014, 332, 67–75. [Google Scholar] [CrossRef]
- Otitoju, T.A.; Ahmad, A.L.; Ooi, B.S. Recent advances in hydrophilic modification and performance of polyethersulfone (PES) membrane via additive blending. RSC Adv. 2018, 8, 22710–22728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lu, M.; Zhu, J.; Tian, S. Wrapping DNA-gated mesoporous silica nanoparticles for quantitative monitoring of telomerase activity with glucometer readout. J. Mater. Chem. B 2014, 2, 5847–5853. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F.; Mohruni, A.S.; Mataram, A. Optimum parameters for treating coolant wastewater using PVDF-membrane. MATEC Web Conf. 2018, 156, 08011. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Zuo, X.; Bao, R.; Xu, X.; Wang, J.; Xu, J. Effect of SiO2 nanoparticle addition on the characteristics of a new organic–inorganic hybrid membrane. Polymer 2009, 50, 553–559. [Google Scholar] [CrossRef]
- Ali, M.E.A.; Shahat, A.; Ayoub, T.I.; Kamel, R.M. Fabrication of High Flux Polysulfone/Mesoporous Silica Nanocomposite Ultrafiltration Membranes for Industrial Wastewater Treatment. Biointerface Res. Appl. Chem. 2022, 12, 7556–7572. [Google Scholar] [CrossRef]
- Pi, H.; Wang, R.; Ren, B.; Zhang, X.; Wu, J. Facile fabrication of multi-structured SiO2@ PVDF-HFP nanofibrous membranes for enhanced copper ions adsorption. Polymers 2018, 10, 1385. [Google Scholar] [CrossRef] [Green Version]
- Teng, M.; Wang, H.; Li, F.; Zhang, B. Thioether-functionalized mesoporous fiber membranes: Sol–gel combined electrospun fabrication and their applications for Hg2+ removal. J. Colloid Interface Sci. 2011, 355, 23–28. [Google Scholar] [CrossRef]
- Keshtkar, A.R.; Tabatabaeefar, A.; Vaneghi, A.S.; Moosavian, M.A. Electrospun polyvinylpyrrolidone/silica/3-aminopropyltriethoxysilane composite nanofiber adsorbent: Preparation, characterization and its application for heavy metal ions removal from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1248–1258. [Google Scholar] [CrossRef]
- Xu, H.Y.; Liu, H.L.; Huang, Y.; Xiao, C.F. Three-dimensional structure design of tubular polyvinyl chloride hybrid nanofiber membranes for water-in-oil emulsion separation. J. Membr. Sci. 2021, 620, 118905. [Google Scholar] [CrossRef]
- Yin, J. Fabrication of a Modified Polyethersulfone Membrane with Anti-Fouling and Self-Cleaning Properties from SiO2-g-PHEMA NPs for Application in Oil/Water Separation. Polymers 2022, 14, 2169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Shan, L.B.; Tu, Z.Y.; Zhang, Y.H. Preparation and characterization of novel Ce-doped nonstoichiometric nanosilica/polysulfone composite membranes. Sep. Purif. Technol. 2008, 63, 207–212. [Google Scholar] [CrossRef]
- Yin, J.; Kim, E.S.; Yang, J.; Deng, B.L. Fabrication of a novel thin-film nanocomposite (TFN) membrane containing MCM-41 silica nanoparticles (NPs) for water purification. J. Membr. Sci. 2012, 423, 238–246. [Google Scholar] [CrossRef]
- Paidi, M.K.; Polisetti, V.; Damarla, K.; Singh, P.S.; Mandal, S.K.; Ray, P. 3D Natural Mesoporous Biosilica-Embedded Polysulfone Made Ultrafiltration Membranes for Application in Separation Technology. Polymers 2022, 14, 1750. [Google Scholar] [CrossRef]
- Tudu, B.K.; Sinhamahapatra, A.; Kumar, A. Surface modification of cotton fabric using TiO2 nanoparticles for self-cleaning, oil–water separation, antistain, anti-water absorption, and antibacterial properties. ACS Omega 2020, 5, 7850–7860. [Google Scholar] [CrossRef] [Green Version]
- Irshad, M.A.; Nawaz, R.; ur Rehman, M.Z.; Adrees, M.; Rizwan, M.; Ali, S.; Ahmad, S.; Tasleem, S. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 2021, 212, 111978. [Google Scholar] [CrossRef]
- Reghunath, S.; Pinheiro, D.; KR, S.D. A review of hierarchical nanostructures of TiO2: Advances and applications. Appl. Surf. Sci. Adv. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Sasi, S.; Chandran, A.; Sugunan, S.K.; Krishna, A.C.; Nair, P.R.; Peter, A.; Shaji, A.N.; Subramanian, K.R.; Pai, N.; Mathew, S. Flexible Nano-TiO2 Sheets Exhibiting Excellent Photocatalytic and Photovoltaic Properties by Controlled Silane Functionalization—Exploring the New Prospects of Wastewater Treatment and Flexible DSSCs. ACS Omega 2022, 7, 25094–25109. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Zhang, Y.; Zhao, S. Comparing the antifouling effects of activated carbon and TiO2 in ultrafiltration membrane development. J. Colloid Interface Sci. 2018, 515, 109–118. [Google Scholar] [CrossRef]
- Sienkiewicz, A.; Rokicka-Konieczna, P.; Wanag, A.; Kusiak-Nejman, E.; Morawski, A.W. Optimization of APTES/TiO2 nanomaterials modification conditions for antibacterial properties and photocatalytic activity. Desalination Water Treat. 2022, 256, 35–50. [Google Scholar] [CrossRef]
- Musial, J.; Mlynarczyk, D.T.; Stanisz, B.J. Photocatalytic degradation of sulfamethoxazole using TiO2-based materials–Perspectives for the development of a sustainable water treatment technology. Sci. Total Environ. 2022, 856, 159122. [Google Scholar] [CrossRef]
- Moon, Y.E.; Jung, G.; Yun, J.; Kim, H.I. Poly(vinyl alcohol)/poly(acrylic acid)/TiO2/graphene oxide nanocomposite hydrogels for pH-sensitive photocatalytic degradation of organic pollutants. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2013, 178, 1097–1103. [Google Scholar] [CrossRef]
- Pasini, S.M.; Valerio, A.; Yin, G.L.; Wang, J.F.; de Souza, S.; Hotza, D.; de Souza, A.A.U. An overview on nanostructured TiO2-containing fibers for photocatalytic degradation of organic pollutants in wastewater treatment. J. Water Process Eng. 2021, 40, 101827. [Google Scholar] [CrossRef]
- Bahal, M.; Kaur, N.; Sharotri, N.; Sud, D. Investigations on Amphoteric Chitosan/TiO2 Bionanocomposites for Application in Visible Light Induced Photocatalytic Degradation. Adv. Polym. Technol. 2019, 2019, 2345631. [Google Scholar] [CrossRef] [Green Version]
- Jumat, N.A.; Wai, P.S.; Ching, J.J.; Basirun, W.J. Synthesis of Polyaniline-TiO2 Nanocomposites and Their Application in Photocatalytic Degradation. Polym. Polym. Compos. 2017, 25, 507–514. [Google Scholar] [CrossRef]
- Wu, Y.F.; Zang, Y.; Xu, L.; Wang, J.J.; Jia, H.G.; Miao, F.J. Synthesis of functional conjugated microporous polymer/TiO2 nanocomposites and the mechanism of the photocatalytic degradation of organic pollutants. J. Mater. Sci. 2021, 56, 7936–7950. [Google Scholar] [CrossRef]
- Aoudjit, L.; Salazar, H.; Zioui, D.; Sebti, A.; Martins, P.M.; Lanceros-Mendez, S. Reusable Ag@ TiO2-based photocatalytic nanocomposite membranes for solar degradation of contaminants of emerging concern. Polymers 2021, 13, 3718. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, M.; Zhou, Y.; Yang, L.; Zhang, Y.; Wu, Z.; Liu, G.; Zheng, J. Preparation of Nano-TiO2-Modified PVDF Membranes with Enhanced Antifouling Behaviors via Phase Inversion: Implications of Nanoparticle Dispersion Status in Casting Solutions. Membranes 2022, 12, 386. [Google Scholar] [CrossRef]
- Kacprzynska-Golacka, J.; Lozynska, M.; Barszcz, W.; Sowa, S.; Wiecinski, P.; Woskowicz, E. Microfiltration Membranes Modified with Composition of Titanium Oxide and Silver Oxide by Magnetron Sputtering. Polymers 2021, 13, 141. [Google Scholar] [CrossRef]
- Fischer, K.; Grimm, M.; Meyers, J.; Dietrich, C.; Glaser, R.; Schulze, A. Photoactive microfiltration membranes via directed synthesis of TiO2 nanoparticles on the polymer surface for removal of drugs from water. J. Membr. Sci. 2015, 478, 49–57. [Google Scholar] [CrossRef]
- Pi, J.K.; Yang, H.C.; Wan, L.S.; Wu, J.; Xu, Z.K. Polypropylene microfiltration membranes modified with TiO2 nanoparticles for surface wettability and antifouling property. J. Membr. Sci. 2016, 500, 8–15. [Google Scholar] [CrossRef]
- Fischer, K.; Schulz, P.; Atanasov, I.; Latif, A.A.; Thomas, I.; Kuhnert, M.; Prager, A.; Griebel, J.; Schulze, A. Synthesis of High Crystalline TiO2 Nanoparticles on a Polymer Membrane to Degrade Pollutants from Water. Catalysts 2018, 8, 376. [Google Scholar] [CrossRef] [Green Version]
- Mahdhi, N.; Alsaiari, N.S.; Amari, A.; Chakhoum, M.A. Effect of TiO2 Nanoparticles on Capillary-Driven Flow in Water Nanofilters Based on Chitosan Cellulose and Polyvinylidene Fluoride Nanocomposites: A Theoretical Study. Polymers 2022, 14, 2908. [Google Scholar] [CrossRef] [PubMed]
- Sotto, A.; Boromand, A.; Balta, S.; Darvishmanash, S.; Kim, J.; Van der Bruggen, B. Nanofiltration membranes enhanced with TiO2 nanoparticles: A comprehensive study. Desalination Water Treat. 2011, 34, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Im, S.J.; Kim, J.H.; Kim, H.J.; Kim, J.P.; Min, B.R. Polyamide thin-film nanofiltration membranes containing TiO2 nanoparticles. Desalination 2008, 219, 48–56. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.; Zinadini, S.; Feyzi, M.; Bahnemann, D.W. A novel photocatalytic self-cleaning PES nanofiltration membrane incorporating triple metal-nonmetal doped TiO2 (K-B-N-TiO2) for post treatment of biologically treated palm oil mill effluent. React. Funct. Polym. 2018, 127, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Sotto, A.; Boromand, A.; Balta, S.; Kim, J.; Van der Bruggen, B. Doping of polyethersulfone nanofiltration membranes: Antifouling effect observed at ultralow concentrations of TiO2 nanoparticles. J. Mater. Chem. 2011, 21, 10311–10320. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Fakharian Torbati, S.; Alaei Shahmirzadi, M.A.; Tavangar, T. Fabrication, characterization, and performance evaluation of polyethersulfone/TiO2 nanocomposite ultrafiltration membranes for produced water treatment. Polym. Adv. Technol. 2018, 29, 2619–2631. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Rajaeian, B.; Rahimnejad, M. TiO2 entrapped nano-composite PVDF/SPES membranes: Preparation, characterization, antifouling and antibacterial properties. Desalination 2011, 278, 343–353. [Google Scholar] [CrossRef]
- Yu, L.Y.; Shen, H.M.; Xu, Z.L. PVDF–TiO2 composite hollow fiber ultrafiltration membranes prepared by TiO2 sol–gel method and blending method. J. Appl. Polym. Sci. 2009, 113, 1763–1772. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Ghaemi, N.; Alizadeh, A.; Joshaghani, M. Influence of photo-induced superhydrophilicity of titanium dioxide nanoparticles on the anti-fouling performance of ultrafiltration membranes. Appl. Surf. Sci. 2011, 257, 6175–6180. [Google Scholar] [CrossRef]
- Razmjou, A.; Mansouri, J.; Chen, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Al Mayyahi, A. TiO2 Polyamide Thin Film Nanocomposite Reverses Osmosis Membrane for Water Desalination. Membranes 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Aassar, A. Improvement of reverse osmosis performance of polyamide thin-film composite membranes using TiO2 nanoparticles. Desalination Water Treat. 2015, 55, 2939–2950. [Google Scholar] [CrossRef]
- Gayed, H.M.; Abou El Fadl, F.I.; Maziad, N.A.; El-Aassar, A.H.M.; Abdel-Mottaleb, M.S.A. Surface modification of composite polyamide reverse osmosis membrane by irradiated chitosan and TiO2 nanoparticles. Desalination Water Treat. 2019, 160, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Pereira, V.R.; Isloor, A.M.; Al Ahmed, A.; Ismail, A. Preparation, characterization and the effect of PANI coated TiO2 nanocomposites on the performance of polysulfone ultrafiltration membranes. New J. Chem. 2015, 39, 703–712. [Google Scholar] [CrossRef]
- Du, C.X.; Wang, Z.H.; Liu, G.Y.; Wang, W.; Yu, D. One-step electrospinning PVDF/PVP-TiO2 hydrophilic nanofiber membrane with strong oil-water separation and anti-fouling property. Colloid Surf. A Physicochem. Eng. Asp. 2021, 624, 126790. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lin, S.; Jin, H.; Gao, S.; Zhu, Y.; Jin, J. Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat. Commun. 2018, 9, 2004. [Google Scholar] [CrossRef] [Green Version]
- Faghihian, H.; Atarodi, H.; Kooravand, M. Synthesis, treatment, and application of a novel carbon nanostructure for removal of fluoride from aqueous solution. Desalination Water Treat. 2015, 54, 2432–2440. [Google Scholar] [CrossRef]
- Chen, F.; Jin, X.; Jia, D.; Cao, Y.; Duan, H.; Long, M. Efficient treament of organic pollutants over CdS/graphene composites photocatalysts. Appl. Surf. Sci. 2020, 504, 144422. [Google Scholar] [CrossRef]
- Wanda, E.M.; Mamba, B.B.; Msagati, T.A. Comparative analysis of performance of fabricated nitrogen-doped carbon-nanotubes, silicon/germanium dioxide embedded polyethersulfone membranes for removal of emerging micropollutants from water. Phys. Chem. Earth Parts A/B/C 2022, 127, 103164. [Google Scholar] [CrossRef]
- An, S.; Joshi, B.N.; Lee, J.-G.; Lee, M.W.; Kim, Y.I.; Kim, M.-w.; Jo, H.S.; Yoon, S.S. A comprehensive review on wettability, desalination, and purification using graphene-based materials at water interfaces. Catal. Today 2017, 295, 14–25. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A.; Ng, B. Directional alignment of carbon nanotubes in polymer matrices: Contemporary approaches and future advances. Compos. Part A Appl. Sci. Manuf. 2014, 56, 103–126. [Google Scholar] [CrossRef]
- Alshammari, B.A.; Wilkinson, A.N.; AlOtaibi, B.M.; Alotibi, M.F. Influence of Carbon Micro-and Nano-Fillers on the Viscoelastic Properties of Polyethylene Terephthalate. Polymers 2022, 14, 2440. [Google Scholar] [CrossRef]
- Al Sheheri, S.Z.; Al-Amshany, Z.M.; Al Sulami, Q.A.; Tashkandi, N.Y.; Hussein, M.A.; El-Shishtawy, R.M. The preparation of carbon nanofillers and their role on the performance of variable polymer nanocomposites. Des. Monomers Polym. 2019, 22, 8–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, C.H.; Baek, Y.; Lee, C.; Kim, S.O.; Kim, S.; Lee, S.; Kim, S.-H.; Bae, S.S.; Park, J.; Yoon, J. Carbon nanotube-based membranes: Fabrication and application to desalination. J. Ind. Eng. Chem. 2012, 18, 1551–1559. [Google Scholar] [CrossRef]
- Ezat, G.S.; Kelly, A.L.; Youseffi, M.; Coates, P.D. Tensile, rheological and morphological characterizations of multi-walled carbon nanotube/polypropylene composites prepared by microinjection and compression molding. Int. Polym. Process. 2022, 37, 45–53. [Google Scholar] [CrossRef]
- Luna, C.B.B.; da Silva Barbosa Ferreira, E.; Siqueira, D.D.; Araújo, E.M.; do Nascimento, E.P.; Medeiros, E.S.; de Mélo, T.J.A. Electrical nanocomposites of PA6/ABS/ABS-MA reinforced with carbon nanotubes (MWCNTf) for antistatic packaging. Polym. Compos. 2022, 43, 3639–3658. [Google Scholar] [CrossRef]
- Wu, S.; Li, K.; Shi, W.; Cai, J. Chitosan/polyvinylpyrrolidone/polyvinyl alcohol/carbon nanotubes dual layers nanofibrous membrane constructed by electrospinning-electrospray for water purification. Carbohydr. Polym. 2022, 294, 119756. [Google Scholar] [CrossRef]
- Shawky, H.A.; Chae, S.-R.; Lin, S.; Wiesner, M.R. Synthesis and characterization of a carbon nanotube/polymer nanocomposite membrane for water treatment. Desalination 2011, 272, 46–50. [Google Scholar] [CrossRef]
- Dumee, L.; Lee, J.; Sears, K.; Tardy, B.; Duke, M.; Gray, S. Fabrication of thin film composite poly(amide)-carbon-nanotube supported membranes for enhanced performance in osmotically driven desalination systems. J. Membr. Sci. 2013, 427, 422–430. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, M.Y.; Lee, H.D.; Roh, J.S.; Kim, H.W.; Park, H.B. Highly porous carbon nanotube/polysulfone nanocomposite supports for high-flux polyamide reverse osmosis membranes. J. Membr. Sci. 2017, 539, 441–450. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Ma, X.D.; Xu, H.J.; Shi, Z.X.; Yin, J.; Jiang, X.S. Selective Adsorption and Separation through Molecular Filtration by Hyperbranched Poly(ether amine)/Carbon Nanotube Ultrathin Membranes. Langmuir 2016, 32, 13073–13083. [Google Scholar] [CrossRef]
- Perreault, F.; de Faria, A.F.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef]
- O’Hern, S.C.; Boutilier, M.S.; Idrobo, J.-C.; Song, Y.; Kong, J.; Laoui, T.; Atieh, M.; Karnik, R. Selective ionic transport through tunable subnanometer pores in single-layer graphene membranes. Nano Lett. 2014, 14, 1234–1241. [Google Scholar] [CrossRef] [Green Version]
- Compton, O.C.; Nguyen, S.T. Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.B.; Li, J.H. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Majumder, P.; Gangopadhyay, R. Evolution of graphene oxide (GO)-based nanohybrid materials with diverse compositions: An overview. RSC Adv. 2022, 12, 5686–5719. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, J.; Lu, T.; Tang, G.; Wu, S.; Ma, W.; Huang, C. Robust, functionalized reduced graphene-based nanofibrous membrane for contaminated water purification. Chem. Eng. J. 2021, 404, 126347. [Google Scholar] [CrossRef]
- Najafabadi, H.H.; Irani, M.; Rad, L.R.; Haratameh, A.H.; Haririan, I. Removal of Cu2+, Pb2+ and Cr6+ from aqueous solutions using a chitosan/graphene oxide composite nanofibrous adsorbent. RSC Adv. 2015, 5, 16532–16539. [Google Scholar] [CrossRef]
- Najafabadi, H.H.; Irani, M.; Rad, L.R.; Haratameh, A.H.; Haririan, I. Correction: Removal of Cu2+, Pb2+ and Cr6+ from aqueous solutions using a chitosan/graphene oxide composite nanofibrous adsorbent (vol 5, pg 16532, 2015). RSC Adv. 2015, 5, 22390. [Google Scholar] [CrossRef]
- Kim, S.; Lin, X.C.; Ou, R.W.; Liu, H.Y.; Zhang, X.W.; Simon, G.P.; Easton, C.D.; Wang, H.T. Highly crosslinked, chlorine tolerant polymer network entwined graphene oxide membrane for water desalination. J. Mater. Chem. A 2017, 5, 1533–1540. [Google Scholar] [CrossRef]
- Wang, Z.; Sahadevan, R.; Yeh, C.N.; Menkhaus, T.J.; Huang, J.X.; Fong, H. Hot-pressed polymer nanofiber supported graphene membrane for high-performance nanofiltration. Nanotechnology 2017, 28, 325602. [Google Scholar] [CrossRef]
- Ganesh, B.M.; Isloor, A.M.; Ismail, A.F. Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 2013, 313, 199–207. [Google Scholar] [CrossRef]
- Jin, T.H.; Peydayesh, M.; Mezzenga, R. Membrane-based technologies for per- and poly-fluoroalkyl substances (PFASs) removal from water: Removal mechanisms, applications, challenges and perspectives. Environ. Int. 2021, 157, 106876. [Google Scholar] [CrossRef]
- Wang, Y.F.; Shang, Y.X.; Gao, Z.X.; Qi, Y.C.; Li, M.Y.; Men, Y.; Huang, H.O. Modulation of reduced graphene oxide membrane for low-fouling wastewater filtration: Membrane structure, wastewater property, and DFT calculation. J. Clean. Prod. 2021, 321, 128982. [Google Scholar] [CrossRef]
- Xu, Z.W.; Wu, T.F.; Shi, J.; Teng, K.Y.; Wang, W.; Ma, M.J.; Li, J.; Qian, X.M.; Li, C.Y.; Fan, J.T. Photocatalytic antifouling PVDF ultrafiltration membranes based on synergy of graphene oxide and TiO2 for water treatment. J. Membr. Sci. 2016, 520, 281–293. [Google Scholar] [CrossRef]
- Ding, C.K.; Qin, X.W.; Tian, Y.Y.; Cheng, B.W. PES membrane surface modification via layer-by-layer self-assembly of GO@TiO2 for improved photocatalytic performance. J. Membr. Sci. 2022, 659, 120789. [Google Scholar] [CrossRef]
- Fan, Y.F.; Quan, X.; Zhao, H.M.; Chen, S.; Yu, H.T.; Zhang, Y.B.; Zhang, Q. Poly(vinylidene fluoride) hollow-fiber membranes containing silver/graphene oxide dope with excellent filtration performance. J. Appl. Polym. Sci. 2017, 134, 44713. [Google Scholar] [CrossRef]
- Khakpour, S.; Jafarzadeh, Y.; Yegani, R. Incorporation of graphene oxide/nanodiamond nanocomposite into PVC ultrafiltration membranes. Chem. Eng. Res. Des. 2019, 152, 60–70. [Google Scholar] [CrossRef]
- Nawaz, H.; Umar, M.; Ullah, A.; Razzaq, H.; Zia, K.M.; Liu, X.Q. Polyvinylidene fluoride nanocomposite super hydrophilic membrane integrated with Polyaniline-Graphene oxide nano fillers for treatment of textile effluents. J. Hazard. Mater. 2021, 403, 123587. [Google Scholar] [CrossRef]
- Zhang, H.J.; Li, B.; Pan, J.F.; Qi, Y.W.; Shen, J.N.; Gao, C.J.; Van der Bruggen, B. Carboxyl-functionalized graphene oxide polyamide nanofiltration membrane for desalination of dye solutions containing monovalent salt. J. Membr. Sci. 2017, 539, 128–137. [Google Scholar] [CrossRef]
- Gao, Y.; Su, K.M.; Wang, X.T.; Li, Z.H. A metal-nano GO frameworks/PPS membrane with super water flux and high dyes interception. J. Membr. Sci. 2019, 574, 55–64. [Google Scholar] [CrossRef]
- Li, X.P.; Zhao, C.W.; Yang, M.; Yang, B.; Hou, D.Y.; Wang, T. Reduced graphene oxide-NH2 modified low pressure nanofiltration composite hollow fiber membranes with improved water flux and antifouling capabilities. Appl. Surf. Sci. 2017, 419, 418–428. [Google Scholar] [CrossRef]
- Hassan, F.; Mushtaq, R.; Saghar, S.; Younas, U.; Pervaiz, M.; muteb Aljuwayid, A.; Habila, M.A.; Sillanpaa, M. Fabrication of graphene-oxide and zeolite loaded polyvinylidene fluoride reverse osmosis membrane for saltwater remediation. Chemosphere 2022, 307, 136012. [Google Scholar] [CrossRef]
- Bhoje, R.; Ghosh, A.K.; Nemade, P.R. Development of Performance-Enhanced Graphene Oxide-Based Nanostructured Thin-Film Composite Seawater Reverse Osmosis Membranes. ACS Appl. Polym. Mater. 2022, 4, 2149–2159. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, Z.; Zhou, C.; Liao, M.; Sun, C. Molecular dynamics simulation and DFT calculations on the oil-water mixture separation by single-walled carbon nanotubes. Appl. Surf. Sci. 2020, 523, 146446. [Google Scholar] [CrossRef]
- Altundal, O.F.; Haslak, Z.P.; Keskin, S. Combined GCMC, MD, and DFT Approach for Unlocking the Performances of COFs for Methane Purification. Ind. Eng. Chem. Res. 2021, 60, 12999–13012. [Google Scholar] [CrossRef]
- Veclani, D.; Tolazzi, M.; Melchior, A. Molecular interpretation of pharmaceuticals’ adsorption on carbon nanomaterials: Theory meets experiments. Processes 2020, 8, 642. [Google Scholar] [CrossRef]
- Zhan, C.; Aydin, F.; Schwegler, E.; Noy, A.; Pham, T.A. Understanding cation selectivity in carbon nanopores with hybrid first-principles/continuum simulations: Implications for water desalination and separation technologies. ACS Appl. Nano Mater. 2020, 3, 9740–9748. [Google Scholar] [CrossRef]
- Yang, D.C.; Tian, D.X.; Cheng, C.; Liu, Y.; Zhao, Z.B.; Liu, Y.; Bao, Y.M.; Xue, C. Carbon nanotube arrays hybrid membrane with excellent separation performance and conductivity. J. Membr. Sci. 2021, 620, 118874. [Google Scholar] [CrossRef]
- Bisignano, F.; Mattia, D.; De Luca, G. Selectivity-permeability optimization of functionalised CNT-polymer membranes for water treatment: A modeling study. Sep. Purif. Technol. 2015, 146, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Ha, J.U.; Kim, M.; Lee, J.; Choe, S.; Cheong, I.W.; Shim, S.E. A novel synthesis of polymer brush on multiwall carbon nanotubes bearing terminal monomeric unit. J. Polym. Sci. Pol. Chem. 2006, 44, 6394–6401. [Google Scholar] [CrossRef]
- Li, J.H.; Shao, X.S.; Zhou, Q.; Li, M.Z.; Zhang, Q.Q. The double effects of silver nanoparticles on the PVDF membrane: Surface hydrophilicity and antifouling performance. Appl. Surf. Sci. 2013, 265, 663–670. [Google Scholar] [CrossRef]
- Ouyang, Q.; Gui, Q.L.; Liu, C.; Zhang, J.X.; Chen, X.N. A novel strategy for making adsorptive membranes with high-capacity and excellent antifouling performance. Chem. Eng. J. 2023, 451, 138596. [Google Scholar] [CrossRef]
- Sahu, A.; Sheikh, R.; Poler, J.C. Green sonochemical synthesis, kinetics and functionalization of nanoscale anion exchange resins and their performance as water purification membranes. Ultrason. Sonochem. 2020, 67, 105163. [Google Scholar] [CrossRef]
- Fajardo-Diaz, J.L.; Morelos-Gomez, A.; Cruz-Silva, R.; Matsumoto, A.; Ueno, Y.; Takeuchi, N.; Kitamura, K.; Miyakawa, H.; Tejima, S.; Takeuchi, K.; et al. Antifouling performance of spiral wound type module made of carbon nanotubes/polyamide composite RO membrane for seawater desalination. Desalination 2022, 523, 115445. [Google Scholar] [CrossRef]
- Fajardo-Diaz, J.L.; Morelos-Gomez, A.; Cruz-Silva, R.; Ishii, K.; Yasuike, T.; Kawakatsu, T.; Yamanaka, A.; Tejima, S.; Izu, K.; Saito, S.; et al. Low-pressure reverse osmosis membrane made of cellulose nanofiber and carbon nanotube polyamide nano-nanocomposite for high purity water production. Chem. Eng. J. 2022, 448, 137359. [Google Scholar] [CrossRef]
- Takizawa, Y.; Inukai, S.; Araki, T.; Cruz-Silva, R.; Uemura, N.; Morelos-Gomez, A.; Ortiz-Medina, J.; Tejima, S.; Takeuchi, K.; Kawaguchi, T.; et al. Antiorganic Fouling and Low-Protein Adhesion on Reverse-Osmosis Membranes Made of Carbon Nanotubes and Polyamide Nanocomposite. ACS Appl. Mater. Interfaces 2017, 9, 32192–32201. [Google Scholar] [CrossRef]
- Tiwari, S.; Gogoi, A.; Reddy, K.A. Effect of an ionic environment on membrane fouling: A molecular dynamics study. Phys. Chem. Chem. Phys. 2021, 23, 5001–5011. [Google Scholar] [CrossRef]
- Cruz-Silva, R.; Takizawa, Y.; Nakaruk, A.; Katouda, M.; Yamanaka, A.; Ortiz-Medina, J.; Morelos-Gomez, A.; Tejima, S.; Obata, M.; Takeuchi, K.; et al. New Insights in the Natural Organic Matter Fouling Mechanism of Polyamide and Nanocomposite Multiwalled Carbon Nanotubes-Polyamide Membranes. Environ. Sci. Technol. 2019, 53, 6255–6263. [Google Scholar] [CrossRef]
- Far, R.M.; Van der Bruggen, B.; Verliefde, A.; Cornelissen, E. A review of zeolite materials used in membranes for water purification: History, applications, challenges and future trends. J. Chem. Technol. Biotechnol. 2022, 97, 575–596. [Google Scholar] [CrossRef]
- Rahman, R.O.A.; El-Kamash, A.M.; Hung, Y.-T. Applications of Nano-Zeolite in Wastewater Treatment: An Overview. Water 2022, 14, 137. [Google Scholar] [CrossRef]
- Sodha, V.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Bandyopadhyay, R.; Sridewi, N. Comprehensive Review on Zeolite-Based Nanocomposites for Treatment of Effluents from Wastewater. Nanomaterials 2022, 12, 3199. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.L.; Huang, E.; Li, Y.H.; Hung, H.T.; Jiang, J.H.; Liu, T.C.; Fang, J.N.; Chen, H.F. Raman Spectroscopic Characteristics of Zeolite Group Minerals. Minerals 2021, 11, 167. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Golembiewski, R.; Trykowski, G.; Buszewski, B. Heterogeneity and hierarchy of clinoptilolite porosity. J. Phys. Chem. Solids 2010, 71, 1269–1277. [Google Scholar] [CrossRef]
- Nasir, A.M.; Goh, P.S.; Abdullah, M.S.; Ng, B.C.; Ismail, A.F. Adsorptive nanocomposite membranes for heavy metal remediation: Recent progresses and challenges. Chemosphere 2019, 232, 96–112. [Google Scholar] [CrossRef]
- Kraljević Pavelić, S.; Simović Medica, J.; Gumbarević, D.; Filošević, A.; Pržulj, N.; Pavelić, K. Critical review on zeolite clinoptilolite safety and medical applications in vivo. Front. Pharmacol. 2018, 9, 1350. [Google Scholar] [CrossRef]
- Casadella, A.; Kuntke, P.; Schaetzle, O.; Loos, K. Clinoptilolite-based mixed matrix membranes for the selective recovery of potassium and ammonium. Water Res. 2016, 90, 62–70. [Google Scholar] [CrossRef]
- Noack, M.; Kolsch, P.; Seefeld, V.; Toussaint, P.; Georgi, G.; Caro, J. Influence of the Si/Al-ratio on the permeation properties of MFI-membranes. Microporous Mesoporous Mater. 2005, 79, 329–337. [Google Scholar] [CrossRef]
- Kazemimoghadam, M. New nanopore zeolite membranes for water treatment. Desalination 2010, 251, 176–180. [Google Scholar] [CrossRef]
- Cho, C.H.; Oh, K.Y.; Kim, S.K.; Yeo, J.G.; Sharma, P. Pervaporative seawater desalination using NaA zeolite membrane: Mechanisms of high water flux and high salt rejection. J. Membr. Sci. 2011, 371, 226–238. [Google Scholar] [CrossRef]
- Zhu, B.; Zou, L.D.; Doherty, C.M.; Hill, A.J.; Lin, Y.S.; Hu, X.R.; Wang, H.T.; Duke, M. Investigation of the effects of ion and water interaction on structure and chemistry of silicalite MFI type zeolite for its potential use as a seawater desalination membrane. J. Mater. Chem. 2010, 20, 4675–4683. [Google Scholar] [CrossRef]
- Ivkovic, S.; Deutsch, U.; Silberbach, A.; Walraph, E.; Mannel, M. Dietary supplementation with the tribomechanically activated zeolite clinoptilolite in immunodeficiency: Effects on the immune system. Adv. Ther. 2004, 21, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, H.; Kazemi, R.; Ghasemian, N.; Shokri, E. Effect of Transmembrane Pressure on Antifouling Properties of PVC/Clinoptilolite Ultrafiltration Nanocomposite Membranes. Chem. Eng. Technol. 2022, 45, 1192–1200. [Google Scholar] [CrossRef]
- An, W.; Zhou, X.; Liu, X.; Chai, P.W.; Kuznicki, T.; Kuznicki, S.M. Natural zeolite clinoptilolite-phosphate composite Membranes for water desalination by pervaporation. J. Membr. Sci. 2014, 470, 431–438. [Google Scholar] [CrossRef]
- Maghami, M.; Abdelrasoul, A. Zeolites-mixed-matrix nanofiltration membranes for the next generation of water purification. In Nanofiltration; IntechOpen: London, UK, 2018. [Google Scholar]
- Zhang, Y.A.; Zhang, Z.L.; Han, H.J.; Zhang, M.; Wang, H.Y.; Song, H.; Chen, Y.G. Effective removal of organic dyes using the ultrasonic-assisted hydrothermal synthesis of NaP zeolite doping Cu or Fe in Fenton-like oxidation systems. Sep. Purif. Technol. 2022, 299, 121767. [Google Scholar] [CrossRef]
- Nazir, M.A.; Najam, T.; Shahzad, K.; Wattoo, M.A.; Hussain, T.; Tufail, M.K.; Shah, S.S.A.; u rRehman, A. Heterointerface engineering of water stable ZIF-8@ZIF-67: Adsorption of rhodamine B from water. Surf. Interfaces 2022, 34, 102324. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Lee, J.; Nandi, D.; Parameswaranpillai, J.; Pant, B.; Siengchin, S. Efficient Removal of Organic Dye from Aqueous Solution Using Hierarchical Zeolite-Based Biomembrane: Isotherm, Kinetics, Thermodynamics and Recycling Studies. Catalysts 2022, 12, 886. [Google Scholar] [CrossRef]
- Song, Y.; Seo, J.Y.; Kim, H.; Beak, K.Y. Structural control of cellulose nanofibrous composite membrane with metal organic framework (ZIF-8) for highly selective removal of cationic dye. Carbohydr. Polym. 2019, 222, 115018. [Google Scholar] [CrossRef]
- Gowriboy, N.; Kalaivizhi, R.; Ganesh, M.R.; Aswathy, K.A. Development of thin film polymer nanocomposite membrane (ZIF-8@PSf/ CS) for removal of textile pollutant and evaluating the effect of water samples on human monocytic cell lines (THP-1) using flow cytometer. J. Clean. Prod. 2022, 377, 134399. [Google Scholar] [CrossRef]
- Kim, S.G.; Hyeon, D.H.; Chun, J.H.; Chun, B.H.; Kim, S.H. Nanocomposite poly(arylene ether sulfone) reverse osmosis membrane containing functional zeolite nanoparticles for seawater desalination. J. Membr. Sci. 2013, 443, 10–18. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Neolaka, Y.A.B.; Supriyanto, G.; Kusuma, H.S. Adsorption performance of Cr(VI)-imprinted poly(4-VP-co-MMA) supported on activated Indonesia (Ende-Flores) natural zeolite structure for Cr(VI) removal from aqueous solution. J. Environ. Chem. Eng. 2018, 6, 3436–3443. [Google Scholar] [CrossRef]
- Modi, A.; Bellare, J. Zeolitic imidazolate framework-67/carboxylated graphene oxide nanosheets incorporated polyethersulfone hollow fiber membranes for removal of toxic heavy metals from contaminated water. Sep. Purif. Technol. 2020, 249, 117160. [Google Scholar] [CrossRef]
- Qiu, M.; He, C.J. Efficient removal of heavy metal ions by forward osmosis membrane with a polydopamine modified zeolitic imidazolate framework incorporated selective layer. J. Hazard. Mater. 2019, 367, 339–347. [Google Scholar] [CrossRef]
- Li, M.; Luo, J.W.; Lu, J.J.; Shang, W.T.; Mu, J.L.; Sun, F.Y.; Dong, Z.J.; Li, X.Y. A novel nanofibrous PAN ultrafiltration membrane embedded with ZIF-8 nanoparticles for effective removal of Congo red, Pb(II), and Cu(II) in industrial wastewater treatment. Chemosphere 2022, 304, 135285. [Google Scholar] [CrossRef]
- Yurekli, Y. Removal of heavy metals in wastewater by using zeolite nano-particles impregnated polysulfone membranes. J. Hazard. Mater. 2016, 309, 53–64. [Google Scholar] [CrossRef]
- Abd Hamid, S.; Shahadat, M.; Ballinger, B.; Azha, S.F.; Ismail, S.; Ali, S.W.; Ahammad, S.Z. Role of clay-based membrane for removal of copper from aqueous solution. J. Saudi Chem. Soc. 2020, 24, 785–798. [Google Scholar] [CrossRef]
- Hermassi, M.; Valderrama, C.; Gibert, O.; Moreno, N.; Font, O.; Querol, X.; Batis, N.H.; Cortina, J.L. Integration of Powdered Ca-Activated Zeolites in a Hybrid Sorption-Membrane Ultrafiltration Process for Phosphate Recovery. Ind. Eng. Chem. Res. 2016, 55, 6204–6212. [Google Scholar] [CrossRef]
- Habiba, U.; Afifi, A.M.; Salleh, A.; Ang, B.C. Chitosan/(polyvinyl alcohol)/zeolite electrospun composite nanofibrous membrane for adsorption of Cr6+, Fe3+ and Ni2+. J. Hazard. Mater. 2017, 322, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Roque-Ruiz, J.H.; Cabrera-Ontiveros, E.A.; Torres-Perez, J.; Reyes-Lopez, S.Y. Preparation of PCL/Clay and PVA/Clay Electrospun Fibers for Cadmium (Cd+2), Chromium (Cr+3), Copper (Cu+2) and Lead (Pb+2) Removal from Water. Water Air Soil Pollut. 2016, 227, 286. [Google Scholar] [CrossRef]
- Abd Hamid, S.; Shahadat, M.; Ismail, S. Zeolite-polysulfone-based adsorptive membrane for removal of metal pollutants. Chem. Pap. 2021, 75, 4479–4492. [Google Scholar] [CrossRef]
- Habiba, U.; Siddique, T.A.; Joo, T.C.; Salleh, A.; Ang, B.C.; Afifi, A.M. Synthesis of chitosan/polyvinyl alcohol/zeolite composite for removal of methyl orange, Congo red and chromium(VI) by flocculation/adsorption. Carbohydr. Polym. 2017, 157, 1568–1576. [Google Scholar] [CrossRef]
- Rad, L.R.; Momeni, A.; Ghazani, B.F.; Irani, M.; Mahmoudi, M.; Noghreh, B. Removal of Ni2+ and Cd2+ ions from aqueous solutions using electrospun PVA/zeolite nanofibrous adsorbent. Chem. Eng. J. 2014, 256, 119–127. [Google Scholar] [CrossRef]
- Choi, J.; Chan, S.; Yip, G.; Joo, H.; Yang, H.; Ko, F.K. Palladium-Zeolite nanofiber as an effective recyclable catalyst membrane for water treatment. Water Res. 2016, 101, 46–54. [Google Scholar] [CrossRef]
- Song, D.; Zhang, W.J.; Cheng, W.; Jia, B.H.; Wang, P.P.; Sun, Z.Q.; Ma, J.; Zhai, X.D.; Qi, J.Y.; Liu, C.H. Micro fine particles deposition on gravity-driven ultrafiltration membrane to modify the surface properties and biofilm compositions: Water quality improvement and biofouling mitigation. Chem. Eng. J. 2020, 393, 123270. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wei, J.; She, Q.H.; Pacheco, F.; Tang, C.Y.Y. Microscopic Characterization of FO/PRO Membranes—A Comparative Study of CLSM, TEM and SEM. Environ. Sci. Technol. 2012, 46, 9995–10003. [Google Scholar] [CrossRef]
- Ma, N.; Wei, J.; Qi, S.R.; Zhao, Y.; Gao, Y.B.; Tang, C.Y.Y. Nanocomposite substrates for controlling internal concentration polarization in forward osmosis membranes. J. Membr. Sci. 2013, 441, 54–62. [Google Scholar] [CrossRef]
- Cay-Durgun, P.; McCloskey, C.; Konecny, J.; Khosravi, A.; Lind, M.L. Evaluation of thin film nanocomposite reverse osmosis membranes for long-term brackish water desalination performance. Desalination 2017, 404, 304–312. [Google Scholar] [CrossRef]
- Loeb, S.; Titelman, L.; Korngold, E.; Freiman, J. Effect of porous support fabric on osmosis through a Loeb-Sourirajan type asymmetric membrane. J. Membr. Sci. 1997, 129, 243–249. [Google Scholar] [CrossRef]
- Tang, C.Y.Y.; She, Q.H.; Lay, W.C.L.; Wang, R.; Fane, A.G. Coupled effects of internal concentration polarization and fouling on flux behavior of forward osmosis membranes during humic acid filtration. J. Membr. Sci. 2010, 354, 123–133. [Google Scholar] [CrossRef]
- Zou, S.; Gu, Y.S.; Xiao, D.Z.; Tang, C.Y.Y. The role of physical and chemical parameters on forward osmosis membrane fouling during algae separation. J. Membr. Sci. 2011, 366, 356–362. [Google Scholar] [CrossRef]
- Xiao, D.Z.; Tang, C.Y.Y.; Zhang, J.S.; Lay, W.C.L.; Wang, R.; Fane, A.G. Modeling salt accumulation in osmotic membrane bioreactors: Implications for FO membrane selection and system operation. J. Membr. Sci. 2011, 366, 314–324. [Google Scholar] [CrossRef]
- Jin, X.; Tang, C.Y.; Gu, Y.S.; She, Q.H.; Qi, S.R. Boric Acid Permeation in Forward Osmosis Membrane Processes: Modeling, Experiments, and Implications. Environ. Sci. Technol. 2011, 45, 2323–2330. [Google Scholar] [CrossRef]
- Ma, N.; Wei, J.; Liao, R.H.; Tang, C.Y.Y. Zeolite-polyamide thin film nanocomposite membranes: Towards enhanced performance for forward osmosis. J. Membr. Sci. 2012, 405, 149–157. [Google Scholar] [CrossRef]
- Salehi, T.M.; Peyravi, M.; Jahanshahi, M.; Lau, W.J.; Rad, A.S. Impacts of zeolite nanoparticles on substrate properties of thin film nanocomposite membranes for engineered osmosis. J. Nanopart. Res. 2018, 20, 113. [Google Scholar] [CrossRef]
- Lejarazu-Larranaga, A.; Landaburu-Aguirre, J.; Senan-Salinas, J.; Ortiz, J.M.; Molina, S. Thin Film Composite Polyamide Reverse Osmosis Membrane Technology towards a Circular Economy. Membranes 2022, 12, 864. [Google Scholar] [CrossRef]
- Xie, W.Y.; He, F.; Wang, B.F.; Chung, T.S.; Jeyaseelan, K.; Armugam, A.; Tong, Y.W. An aquaporin-based vesicle-embedded polymeric membrane for low energy water filtration. J. Mater. Chem. A 2013, 1, 7592–7600. [Google Scholar] [CrossRef]
- Prezelus, F.; Chabni, D.; Barna, L.; Guigui, C.; Remigy, J.C. A metrics-based approach to preparing sustainable membranes: Application to ultrafiltration. Green Chem. 2019, 21, 4457–4469. [Google Scholar] [CrossRef] [Green Version]
- Yi, T.; Zhao, H.; Mo, Q.; Pan, D.; Liu, Y.; Huang, L.; Xu, H.; Hu, B.; Song, H. From Cellulose to Cellulose Nanofibrils—A Comprehensive Review of the Preparation and Modification of Cellulose Nanofibrils. Materials 2020, 13, 5062. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, P.J.; Zhou, J.P.; Zhang, L.N. Structure and solution properties of cyanoethyl celluloses synthesized in LiOH/urea aqueous solution. Cellulose 2012, 19, 161–169. [Google Scholar] [CrossRef]
- Liu, C.; Baumann, H. Exclusive and complete introduction of amino groups and their N-sulfo and N-carboxymethyl groups into the 6-position of cellulose without the use of protecting groups. Carbohydr. Res. 2002, 337, 1297–1307. [Google Scholar] [CrossRef]
- Rowland, S.P.; Bullock, A.L.; Cirino, V.O.; Roberts, E.J.; Hoiness, D.E.; Wade, C.P.; Brannan, M.A.F.; Janssen, H.J.; Pittman, P.F. The Relative Reactivities of the Hydroxyl Groups of Cotton Cellulose—A Progress Report1. Text. Res. J. 1967, 37, 1020–1030. [Google Scholar] [CrossRef]
- Lavagna, L.; Nistico, R.; Musso, S.; Pavese, M. Hydrophobic cellulose ester as a sustainable material for simple and efficient water purification processes from fatty oils contamination. Wood Sci. Technol. 2019, 53, 249–261. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef]
- Du, H.S.; Parit, M.; Liu, K.; Zhang, M.M.; Jiang, Z.H.; Huang, T.S.; Zhang, X.Y.; Si, C.L. Engineering cellulose nanopaper with water resistant, antibacterial, and improved barrier properties by impregnation of chitosan and the followed halogenation. Carbohydr. Polym. 2021, 270, 118372. [Google Scholar] [CrossRef]
- Ghanadpour, M.; Carosio, F.; Larsson, P.T.; Wagberg, L. Phosphorylated Cellulose Nanofibrils: A Renewable Nanomaterial for the Preparation of Intrinsically Flame-Retardant Materials. Biomacromolecules 2015, 16, 3399–3410. [Google Scholar] [CrossRef]
- Wang, N.N.; Qiu, Y.Y.; Hu, K.M.; Huang, C.J.; Xiang, J.S.; Li, H.; Tang, J.F.; Wang, J.Q.; Xiao, T.F. One-step synthesis of cake-like biosorbents from plant biomass for the effective removal and recovery heavy metals: Effect of plant species and roles of xanthation. Chemosphere 2021, 266, 129129. [Google Scholar] [CrossRef]
- Fiol, N.; Vasquez, M.G.; Pereira, M.; Tarres, Q.; Mutje, P.; Delgado-Aguilar, M. TEMPO-oxidized cellulose nanofibers as potential Cu(II) adsorbent for wastewater treatment. Cellulose 2019, 26, 903–916. [Google Scholar] [CrossRef]
- De Nino, A.; Tallarida, M.A.; Algieri, V.; Olivito, F.; Costanzo, P.; De Filpo, G.; Maiuolo, L. Sulfonated Cellulose-Based Magnetic Composite as Useful Media for Water Remediation from Amine Pollutants. Appl. Sci. 2020, 10, 8155. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Saber-Samandari, S.; Heydaripour, S.; Abdouss, M. Novel carboxymethyl cellulose based nanocomposite membrane: Synthesis, characterization and application in water treatment. J. Environ. Manag. 2016, 166, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.L.; Yao, Z.L.; Wang, X.C.; Crombeen, M.; Sweeney, D.G.; Tam, K.C. Cellulose-based materials in wastewater treatment of petroleum industry. Green Energy Environ. 2020, 5, 37–49. [Google Scholar] [CrossRef]
- Chen, H.; Chi, K.; Cao, R.J.; Sharma, S.K.; Bokhari, S.M.Q.; Johnson, K.I.; Li, D.N.; Sharma, P.R.; Hsiao, B.S. Nitro-oxidation process for fabrication of efficient bioadsorbent from lignocellulosic biomass by combined liquid-gas phase treatment. Carbohydr. Polym. Technol. Appl. 2022, 3, 100219. [Google Scholar] [CrossRef]
- Ateia, M.; Attia, M.F.; Maroli, A.; Tharayil, N.; Alexis, F.; Whitehead, D.C.; Karanfil, T. Rapid Removal of Poly- and Perfluorinated Alkyl Substances by Poly(ethylenimine)-Functionalized Cellulose Microcrystals at Environmentally Relevant Conditions. Environ. Sci. Technol. Lett. 2018, 5, 764–769. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhao, J.Q.; Cheng, L.; Lu, C.H.; Wang, Y.R.; He, X.; Zhang, W. Acrylic acid grafted and acrylic acid/sodium humate grafted bamboo cellulose nanofibers for Cu2+ adsorption. RSC Adv. 2014, 4, 55195–55201. [Google Scholar] [CrossRef]
- Saito, N.; Shimizu, Y.; Takai, M.; Hayashi, J. Super absorbent materials prepared from lignocellulosic materials by phosphorylation.5. crystalline-structure and water absorbency. Mokuzai Gakkaishi 1994, 40, 1200–1207. [Google Scholar]
- Muratore, F.; Barbosa, S.E.; Rincon, E.; Garcia, A.; Martini, R.E.; Serrano, L. Microwave-assisted cellulose grafting for food packaging. Techno-economic comparative with other curing technologies. J. Wood Chem. Technol. 2020, 40, 408–420. [Google Scholar] [CrossRef]
- Liu, Y.S.; Nie, P.; Yu, F.C. Enhanced adsorption of sulfonamides by a novel carboxymethyl cellulose and chitosan-based composite with sulfonated graphene oxide. Bioresour. Technol. 2021, 320, 124373. [Google Scholar] [CrossRef]
- Hadid, M.; Noukrati, H.; Ben Youcef, H.; Barroug, A.; Sehaqui, H. Phosphorylated cellulose for water purification: A promising material with outstanding adsorption capacity towards methylene blue. Cellulose 2021, 28, 7893–7908. [Google Scholar] [CrossRef]
- Dufresne, A. Cellulose nanomaterials as green nanoreinforcements for polymer nanocomposites. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2018, 376, 20170040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonoobi, M.; Ashori, A.; Siracusa, V. Characterization and properties of polyethersulfone/modified cellulose nanocrystals nanocomposite membranes. Polym. Test 2019, 76, 333–339. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Tam, K.C. Cellulose nanomaterials: Promising sustainable nanomaterials for application in water/wastewater treatment processes. Environ. Sci. Nano 2018, 5, 623–658. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L.N. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Xu, C.Y.; Chen, W.S.; Gao, H.P.; Xie, X.; Chen, Y.S. Cellulose nanocrystal/silver (CNC/Ag) thin-film nanocomposite nanofiltration membranes with multifunctional properties. Environ. Sci. Nano 2020, 7, 803–816. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Lindstrom, T.; Hsiao, B.S. Nanocellulose-Enabled Membranes for Water Purification: Perspectives. Adv. Sustain. Syst. 2020, 4, 1900114. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, Y.H.; Wang, R.J.; Chen, K.; Zhou, N.Y.; Yang, Q.F.; Shi, J.Y. Triple-functional lignocellulose/chitosan/Ag@TiO2 nanocomposite membrane for simultaneous sterilization, oil/water emulsion separation, and organic pollutant removal. J. Environ. Chem. Eng. 2021, 9, 106728. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Yang, L.; Yang, F.Y.; Bai, W.J.; Zhang, X.Q.; Li, H.T.; Duan, G.G.; Xu, Y.T.; Li, Y.W. A bioinspired antibacterial and photothermal membrane for stable and durable clean water remediation. Mater. Horiz. 2023, 10, 268–276. [Google Scholar] [CrossRef]
- Sharma, A.; Anjana; Rana, H.; Goswami, S. A Comprehensive Review on the Heavy Metal Removal for Water Remediation by the Application of Lignocellulosic Biomass-Derived Nanocellulose. J. Polym. Environ. 2022, 30, 1–18. [Google Scholar] [CrossRef]
- Salama, A.; Abouzeid, R.; Leong, W.S.; Jeevanandam, J.; Samyn, P.; Dufresne, A.; Bechelany, M.; Barhoum, A. Nanocellulose-Based Materials for Water Treatment: Adsorption, Photocatalytic Degradation, Disinfection, Antifouling, and Nanofiltration. Nanomaterials 2021, 11, 3008. [Google Scholar] [CrossRef]
- Nazri, A.I.; Ahmad, A.L.; Hussin, M.H. Microcrystalline Cellulose-Blended Polyethersulfone Membranes for Enhanced Water Permeability and Humic Acid Removal. Membranes 2021, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.T.; Pham, T.D.; Verheyen, D.; Nguyen, M.K.; Pham, T.T.; Zhu, J.Y.; Van der Bruggen, B. Fabrication of thin film nanocomposite nanofiltration membrane incorporated with cellulose nanocrystals for removal of Cu(II) and Pb(II). Chem. Eng. Sci. 2020, 228, 115998. [Google Scholar] [CrossRef]
- Liu, P.; Milletto, C.; Monti, S.; Zhu, C.T.; Mathew, A.P. Design of ultrathin hybrid membranes with improved retention efficiency of molecular dyes. RSC Adv. 2019, 9, 28657–28669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.Y.; Yoon, K.; Rong, L.X.; Shokralla, M.; Kopot, A.; Wang, X.; Fang, D.F.; Hsiao, B.S.; Chu, B. Thin-Film Nanofibrous Composite Ultrafiltration Membranes Based on Polyvinyl Alcohol Barrier Layer Containing Directional Water Channels. Ind. Eng. Chem. Res. 2010, 49, 11978–11984. [Google Scholar] [CrossRef]
- Bai, L.M.; Wu, H.Y.; Ding, J.W.; Ding, A.; Zhang, X.Y.; Ren, N.Q.; Li, G.B.; Liang, H. Cellulose nanocrystal-blended polyethersulfone membranes for enhanced removal of natural organic matter and alleviation of membrane fouling. Chem. Eng. J. 2020, 382, 122919. [Google Scholar] [CrossRef]
- Ding, Z.D.; Zhong, L.L.; Wang, X.; Zhang, L.P. Effect of lignin-cellulose nanofibrils on the hydrophilicity and mechanical properties of polyethersulfone ultrafiltration membranes. High Perform. Polym. 2016, 28, 1192–1200. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z.V.P. Nanocomposite membranes. Desalination Water Treat. 2016, 57, 26803–26819. [Google Scholar] [CrossRef]
- Aliabadi, M.; Irani, M.; Ismaeili, J.; Piri, H.; Parnian, M.J. Electrospun nanofiber membrane of PEO/Chitosan for the adsorption of nickel, cadmium, lead and copper ions from aqueous solution. Chem. Eng. J. 2013, 220, 237–243. [Google Scholar] [CrossRef]
- Aliabadi, M.; Irani, M.; Ismaeili, J.; Najafzadeh, S. Design and evaluation of chitosan/hydroxyapatite composite nanofiber membrane for the removal of heavy metal ions from aqueous solution. J. Taiwan Inst. Chem. Eng. 2014, 45, 518–526. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N.; Mitropoulos, A.C. Chitosan adsorbents for dye removal: A review. Polym. Int. 2017, 66, 1800–1811. [Google Scholar] [CrossRef]
- Kurita, K.; Ichikawa, H.; Ishizeki, S.; Fujisaki, H.; Iwakura, Y. Studies on chitin.8. modification reaction of chitin in highly swollen state with aromatic cyclic carboxylic-acid anhydrides. Makromol. Chem. Macromol. Chem. Phys. 1982, 183, 1161–1169. [Google Scholar] [CrossRef]
- Zhou, L.M.; Xu, J.P.; Liang, X.Z.; Liu, Z.R. Adsorption of platinum(IV) and palladium(II) from aqueous solution by magnetic cross-linking chitosan nanoparticles modified with ethylenediamine. J. Hazard. Mater. 2010, 182, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.; Oldinski, R.; Ma, H.Y.; Bryers, J.D.; Zhang, M.Q. Chitosan-based nanofibrous membranes for antibacterial filter applications. Carbohydr. Polym. 2013, 92, 254–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankararamakrishnan, N.; Dixit, A.; Iyengar, L.; Sanghi, R. Removal of hexavalent chromium using a novel cross linked xanthated chitosan. Bioresour. Technol. 2006, 97, 2377–2382. [Google Scholar] [CrossRef]
- Jagtap, S.; Yenkie, M.K.; Das, S.; Rayalu, S. Synthesis and characterization of lanthanum impregnated chitosan flakes for fluoride removal in water. Desalination 2011, 273, 267–275. [Google Scholar] [CrossRef]
- Sivakami, M.S.; Gomathi, T.; Venkatesan, J.; Jeong, H.S.; Kim, S.K.; Sudha, P.N. Preparation and characterization of nano chitosan for treatment wastewaters. Int. J. Biol. Macromol. 2013, 57, 204–212. [Google Scholar] [CrossRef]
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of chitosan membranes using glutaraldehyde: Effect on ion permeability and water absorption. J. Membr. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Pakdel, P.M.; Peighambardoust, S.J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Jiang, R.; Xiao, L. Adsorption of an anionic azo dye by chitosan/kaolin/gamma-Fe2O3 composites. Appl. Clay Sci. 2010, 48, 522–526. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.P.; Grahn, M.; Mouzon, J.; Oksman, K. Nanoporous membranes with cellulose nanocrystals as functional entity in chitosan: Removal of dyes from water. Carbohydr. Polym. 2014, 112, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Gharbani, P.; Mehrizad, A. Preparation and characterization of graphitic carbon nitrides/ polyvinylidene fluoride adsorptive membrane modified with chitosan for Rhodamine B dye removal from water: Adsorption isotherms, kinetics and thermodynamics. Carbohydr. Polym. 2022, 277, 118860. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.X.; Jin, Y.L.; Sun, Z.F.; Ren, F.; Pei, L.; Ren, P.G. Facile synthesis of chitosan-based acid-resistant composite films for efficient selective adsorption properties towards anionic dyes. Carbohydr. Polym. 2021, 254, 117473. [Google Scholar] [CrossRef]
- Montaser, A.S.; Wassel, A.R.; Al-Shaye’a, O.N. Synthesis, characterization and antimicrobial activity of Schiff bases from chitosan and salicylaldehyde/TiO2 nanocomposite membrane. Int. J. Biol. Macromol. 2019, 124, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, H.L.; Yuan, X.; Ding, W.J.; Li, Y.; Wang, J.K. Separation of oil-water emulsion and adsorption of Cu(II) on a chitosan-cellulose acetate-TiO2 based membrane. Chemosphere 2019, 235, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Siddique, T.A.; Lee, J.J.L.; Joo, T.C.; Ang, B.C.; Afifi, A.M. Adsorption study of methyl orange by chitosan/polyvinyl alcohol/zeolite electrospun composite nanofibrous membrane. Carbohydr. Polym. 2018, 191, 79–85. [Google Scholar] [CrossRef]
- Hegab, H.M.; Wimalasiri, Y.; Ginic-Markovic, M.; Zou, L. Improving the fouling resistance of brackish water membranes via surface modification with graphene oxide functionalized chitosan. Desalination 2015, 365, 99–107. [Google Scholar] [CrossRef]
- Tang, W.J.; Meng, Y.Y.; Yang, B.; He, D.Y.; Li, Y.; Li, B.J.; Shi, Z.M.; Zhao, C.W. Preparation of hollow-fiber nanofiltration membranes of high performance for effective removal of PFOA and high resistance to BSA fouling. J. Environ. Sci. 2022, 122, 14–24. [Google Scholar] [CrossRef]
- Boonya-atichart, A.; Boontanon, S.K.; Boontanon, N. Study of hybrid membrane filtration and photocatalysis for removal of perfluorooctanoic acid (PFOA) in groundwater. Water Sci. Technol. 2018, 2017, 561–569. [Google Scholar] [CrossRef]
- Wang, J.X.; Wang, L.; Xu, C.Q.; Zhi, R.; Miao, R.; Liang, T.; Yue, X.L.; Lv, Y.T.; Liu, T.T. Perfluorooctane sulfonate and perfluorobutane sulfonate removal from water by nanofiltration membrane: The roles of solute concentration, ionic strength, and macromolecular organic foulants. Chem. Eng. J. 2018, 332, 787–797. [Google Scholar] [CrossRef]
- Boo, C.; Wang, Y.K.; Zucker, I.; Choo, Y.; Osuji, C.O.; Elimelech, M. High Performance Nanofiltration Membrane for Effective Removal of Perfluoroalkyl Substances at High Water Recovery. Environ. Sci. Technol. 2018, 52, 7279–7288. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, Y.X.; Liu, G.X.; Zhao, C.W. Preparation, characterization and performance of poly(m-phenylene isophthalamide)/organically modified montmorillonite nanocomposite membranes in removal of perfluorooctane sulfonate. J. Environ. Sci. 2016, 46, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.D.; Yu, M.; Li, Y.T.; Niu, B.H.; Qin, F.H.; Yan, N.; Xu, Y.Y.; Zheng, Y. MoS2 nanoflowers decorated natural fiber-derived hollow carbon microtubes for boosting perfluorooctanoic acid degradation. Colloid Surf. A Physicochem. Eng. Asp. 2022, 642, 128670. [Google Scholar] [CrossRef]
- El Meragawi, S.; Akbari, A.; Hernandez, S.; Mirshekarloo, M.S.; Bhattacharyya, D.; Tanksale, A.; Majumder, M. Enhanced permselective separation of per-fluorooctanoic acid in graphene oxide membranes by a simple PEI modification. J. Mater. Chem. A 2020, 8, 24800–24811. [Google Scholar] [CrossRef]
- Dai, Y.R.; Niu, J.F.; Yin, L.F.; Xu, J.J.; Sun, K. Enhanced sorption of perfluorooctane sulfonate (PFOS) on carbon nanotube-filled electrospun nanofibrous membranes. Chemosphere 2013, 93, 1593–1599. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Ahmed, B.; Mehnaz, T.; Mehejabin, F.; Maliat, D.; Hoang, A.T.; Shafiullah, G.M. Nanomaterials as a sustainable choice for treating wastewater. Environ. Res. 2022, 214, 113807. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.X.; Liu, Y.L.; Zheng, J.F.; Wang, X.M.; Xia, S.J.; Van der Bruggen, B. A critical review on thin-film nanocomposite membranes enabled by nanomaterials incorporated in different positions and with diverse dimensions: Performance comparison and mechanisms. J. Membr. Sci. 2022, 661, 120952. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites-A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Wu, S.L.; Liu, F.Q.; Yang, H.C.; Darling, S.B. Recent progress in molecular engineering to tailor organic-inorganic interfaces in composite membranes. Mol. Syst. Des. Eng. 2020, 5, 433–444. [Google Scholar] [CrossRef]
- Zargar, M.; Hartanto, Y.; Jin, B.; Dai, S. Polyethylenimine modified silica nanoparticles enhance interfacial interactions and desalination performance of thin film nanocomposite membranes. J. Membr. Sci. 2017, 541, 19–28. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, H.C.; Liang, H.Q.; Wan, L.S.; Xu, Z.K. Novel nanofiltration membrane with ultrathin zirconia film as selective layer. J. Membr. Sci. 2016, 500, 265–271. [Google Scholar] [CrossRef]
- Hanemann, T.; Szabo, D.V. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Rajaeian, B.; Rahimpour, A.; Tade, M.O.; Liu, S.M. Fabrication and characterization of polyamide thin film nanocomposite (TFN) nanofiltration membrane impregnated with TiO2 nanoparticles. Desalination 2013, 313, 176–188. [Google Scholar] [CrossRef]
- Li, G.; Lv, L.; Fan, H.T.; Ma, J.Y.; Li, Y.Q.; Wan, Y.; Zhao, X.S. Effect of the agglomeration of TiO2 nanoparticles on their photocatalytic performance in the aqueous phase. J. Colloid Interface Sci. 2010, 348, 342–347. [Google Scholar] [CrossRef]
- Yang, R.; Aubrecht, K.B.; Ma, H.Y.; Wang, R.; Grubbs, R.B.; Hsiao, B.S.; Chu, B. Thiol-modified cellulose nanofibrous composite membranes for chromium (VI) and lead (II) adsorption. Polymer 2014, 55, 1167–1176. [Google Scholar] [CrossRef]
- Tavakol, I.; Hadadpour, S.; Shabani, Z.; Tofighy, M.A.; Mohammadi, T.; Sahebi, S. Synthesis of novel thin film composite (TFC) forward osmosis (FO) membranes incorporated with carboxylated carbon nanofibers (CNFs). J. Environ. Chem. Eng. 2020, 8, 104614. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, P.; Xu, R.M.; Wang, Z.W.; Song, W.L.; Wang, X.H. Porous graphene oxide surface-coated thin-film composite membrane for simultaneously increasing permeation performance and organic-fouling migration capacities. J. Membr. Sci. 2022, 661, 120942. [Google Scholar] [CrossRef]
- Kumar, N.; Fosso-Kankeu, E.; Ray, S.S. Achieving Controllable MoS2 Nanostructures with Increased Interlayer Spacing for Efficient Removal of Pb(II) from Aquatic Systems. ACS Appl. Mater. Interfaces 2019, 11, 19141–19155. [Google Scholar] [CrossRef]
- Das, R.; Giri, S.; Abia, A.L.K.; Dhonge, B.; Maity, A. Removal of Noble Metal Ions (Ag+) by Mercapto Group-Containing Polypyrrole Matrix and Reusability of Its Waste Material in Environmental Applications. ACS Sustain. Chem. Eng. 2017, 5, 2711–2724. [Google Scholar] [CrossRef]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Das, C.; Sen, S.; Singh, T.; Ghosh, T.; Paul, S.S.; Kim, T.W.; Jeon, S.; Maiti, D.K.; Im, J.; Biswas, G. Green Synthesis, Characterization and Application of Natural Product Coated Magnetite Nanoparticles for Wastewater Treatment. Nanomaterials 2020, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Mukherjee, D.; Deb, N.; Swarnakar, S.; Banerjee, S. Application of green synthesized ZnO nanoparticle coated ceramic ultrafiltration membrane for remediation of pharmaceutical components from synthetic water: Reusability assay of treated water on seed germination. J. Environ. Chem. Eng. 2020, 8, 103803. [Google Scholar] [CrossRef]
- Mehta, K.P. Application of Nano Technology in Waste Water Treatment. In Climate Change and Water Security; Springer: Berlin/Heidelberg, Germany, 2022; pp. 423–432. [Google Scholar]
- Mamah, S.C.; Goh, P.S.; Ismail, A.F.; Suzaimi, N.D.; Yogarathinam, L.T.; Raji, Y.O.; El-badawy, T.H. Recent development in modification of polysulfone membrane for water treatment application. J. Water Process Eng. 2021, 40, 101835. [Google Scholar] [CrossRef]
- Kabir, E.; Kumar, V.; Kim, K.-H.; Yip, A.C.; Sohn, J. Environmental impacts of nanomaterials. J. Environ. Manag. 2018, 225, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Etim, U.J.; Bai, P.; Yan, Z. Nanotechnology applications in petroleum refining. In Nanotechnology in Oil and Gas Industries; Springer: Berlin/Heidelberg, Germany, 2018; pp. 37–65. [Google Scholar]
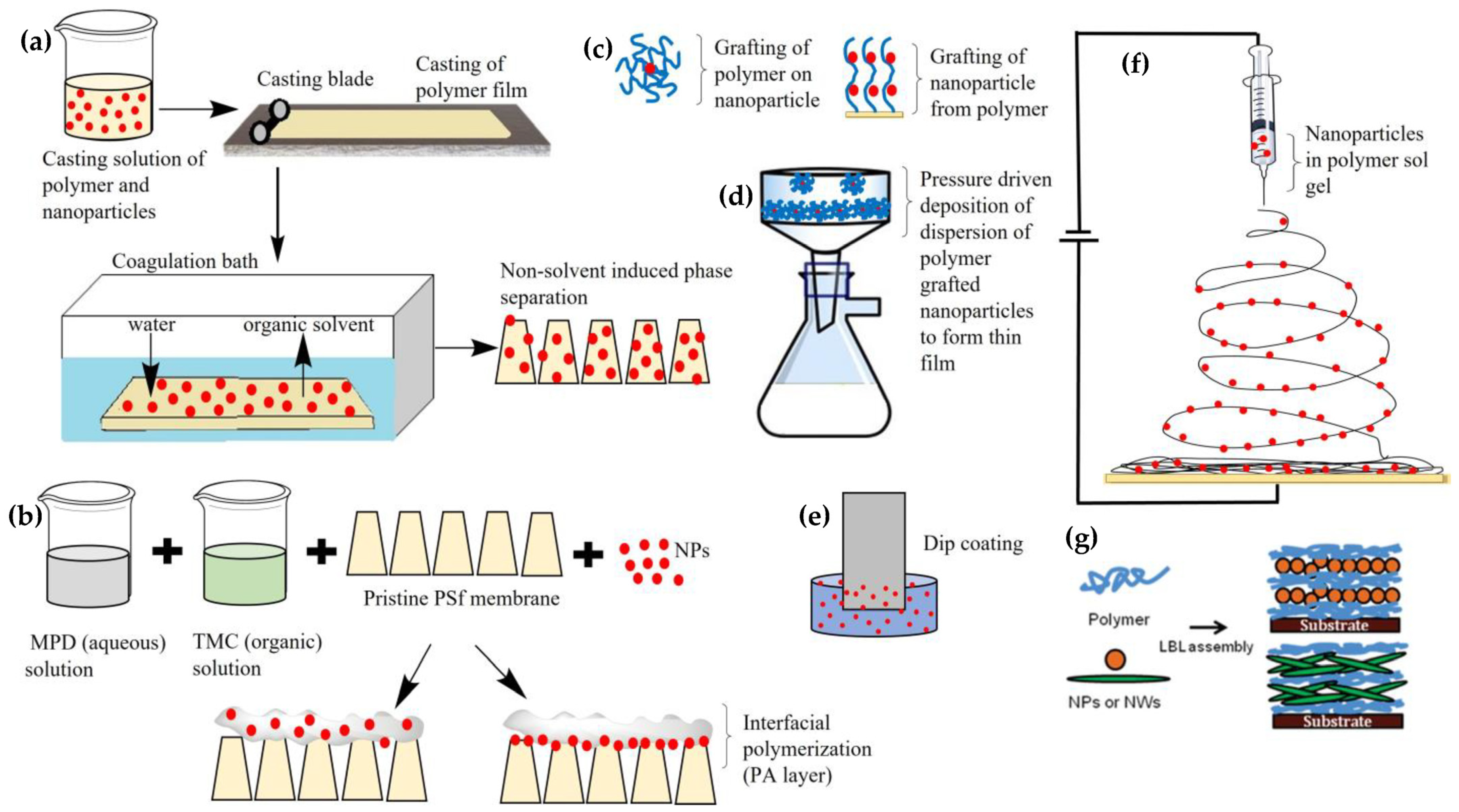
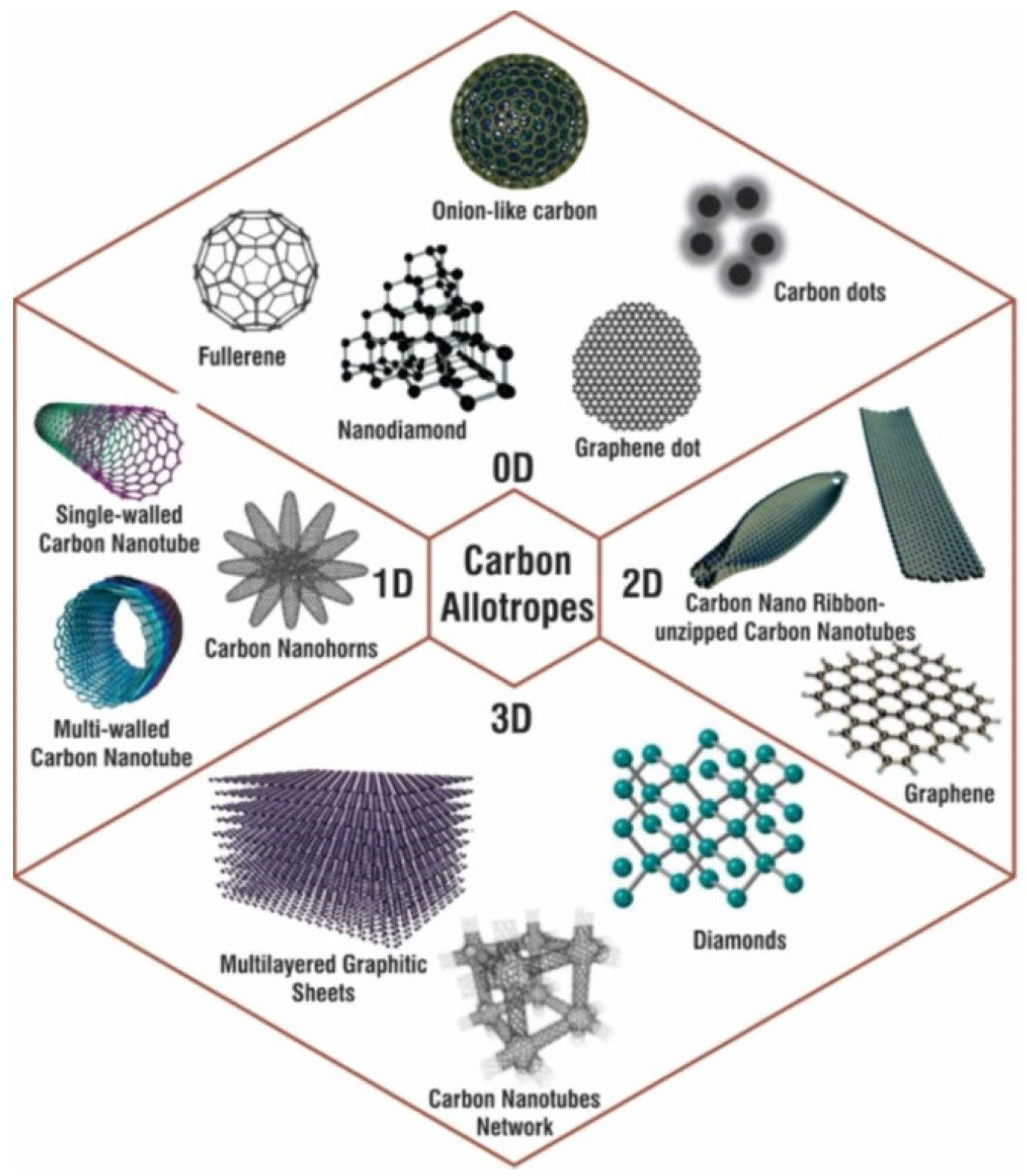





| Contaminants | Generation Source | Impact on Human Health or Ecology | Examples and Their Maximum Concentration Levels (Parts per Billion (ppb)) |
|---|---|---|---|
| Organic | Pesticides, pharmaceuticals, natural organic matter, disinfection byproducts, endocrine disrupting chemicals, hormones and steroids, personal care products, flame retardants, plasticizers [6,10] | Mutagenicity, carcinogenicity [17], bladder cancer, developmental issues, increased birth defects [18] | Dibromochloropropane—0.2, simazine—4 [9], Dioxin (2,3,7,8-TCDD)—0.00003, Hexachlorocyclopentadiene—50, Hexachlorobenzene—1 [19] |
| Inorganic (acids, salts, heavy metals) | Byproduct of metal mining, smelting, fossil fuel combustion, mineral deposits, anerobic groundwater, soil erosion | Toxic effect on aquatic flora and fauna, catharsis, congenital malformation, increased cancer risk, cardiovascular effects [20] | Arsenic—10, cadmium—5, lead —15 [11], Mercury—2, cyanide —200 [13] |
| Microbial (bacteria, virus, algae, protozoa) | Human and animal fecal wastes, Fertilizer, livestock, sewage | Typhoid, cholera, diarrhea, damage to liver, skin, nervous system, stomach cramps [21] | E. coli—0 [22] |
| Perfluoroalkylated compounds | Firefighting foams, lubricants, coating additives, cookware, food packaging, textile industry, paper packaging [8] | Adversely affect growth, birth weight, fertility disorders, early menopause, thyroid malfunction, and carcinogenesis [7] | Perfluorooctane sulfonate, perflourooctanoic acid—0.07 (both individually and combined) [14] |
| Radioactive substances | Mining and processing of radioactive minerals | DNA damage, osteosarcoma incidence, leukemia, stomach cancer, urinary cancer, biomarkers of renal (tubular) damage [23] | Uranium (U)—30 [11,13] |
| Membrane Type | Enhancements Due to Modification | Reference |
|---|---|---|
| Tubular hollow nanofiber PVC membrane with dispersed hydrophobic nano-SiO2 for water in oil emulsion separation | High permeation flux, thermal, and hydrophobic stability, outstanding lipophilicity and superhydrophobicity | [168] |
| MSNs (~500 nm) incorporated in presence of PVP in PSf UF membrane | Enhanced hydrophilicity, methylene blue (MB) rejection (84.7%), but decreased water permeability with increase of MSNs wt.% | [164] |
| PES-MSNs nanocomposite UF membranes | Higher thermal stability, hydrophilicity, porosity, antifouling, and water uptake properties. Properties deteriorate at highest (4 wt.%) loading due to agglomeration | [134] |
| Silica NPs grafted onto PHEMA on PES membrane (PES)/SiO2-g-PHEMA carboxyl-modified fluorocarbon surfactant functionalized PEG segment: fPEG-COOH; Grafting fPEG-COOH onto surface of the PES/SiO2-g-PHEMA forming amphiphilic porous membrane | Higher oil–water flux, flux recovery ratio, lower flux decline ratio, antifouling, and self-cleaning properties | [169] |
| Composite membrane of Ce-doped nanosilica dispersed in PSf prepared by sol–gel process for oil–water separation | Higher tensile strength, hydrophilicity, and antifouling property, >98% oil retention rate | [170] |
| Porous MCM-41 silica NPs and nonporous silica incorporated into PA thin-film layer via IP process with PSf support at the bottom | Higher surface hydrophilicity, water flux/permeability compared to nonporous structure, enhanced salt rejections (NaCl (98.1%) and Na2SO4 (98.6%)) | [171] |
| Incorporation of fumed silica NPs functionalized with APTES into chloromethylated PSf matrix using vapor induced phase inversion and NIPS processes | High water permeance (0.46 ) and high percentage removal of contaminants (reactive red (99.99%), direct yellow (99.94%), methyl green (99.80%), rhodamine B (99.79%), crystal violet (98.69%). Negative impact on mechanical and selectivity for 3 and 4 loading due to agglomeration. | [80] |
| Nanocomposite Membrane | Operating/Working Conditions | Results | References | |
|---|---|---|---|---|
| Incorporation of NaY zeolite NPs into the PA layer on porous PSf TFNC membrane | RO tests: 500 NaCl feed solution under 2.5 bar | 0.1% (wt./v) loaded | 0.4% (wt./v) loaded | [305] |
| Enhanced water permeability: (4.0 × 10−12 → 7.1 × 10−12 ), reduction of salt rejection (95.6 → 77.6%), exacerbation in B/A (9.74 → 61.1 kPa) | Decreased water permeability (4.13 × 10−12 ), improved salt rejection (90.5%), improvement in B/A (22.2 kPa) | |||
| FO tests: Both FS and DS at 500 mL min−1 cross flow rate FS: 10 mM NaCl or DI DS: 0.5, 1.0 or 2.0 M NaCl | S value (782 ± 160 µm) comparable to Hydration Technology Inc. FO membranes For DS: 1.0 M NaCl, FS: 10 mM NaCl and 0.1% (wt./v) loaded TFNC combination: ~50% enhanced water flux in AL-DS (30.7 ), ~50% enhanced water flux in AL-FS (14.6 ) For DS: 1.0 M NaCl), FS: DI water and 0.2% (wt./v) loaded TFNC combination: ~100% enhanced solute flux in AL-FS, >100% enhanced solute flux in AL-DS | |||
| Incorporation of 0.30 wt.% LTA zeolite NPs in PA layer on PSf TFNC membrane | Long term test (3000 h) under 200 psi | Enhanced water permeance (3.7 → 5.3 ), enhanced salt rejection: (97.4 → 97.9%), improved contact angle before test (62.1 → 95.2°), improved contact angle after test (44.0 → 50.8°) | [299] | |
| Incorporation of NaY zeolite NPs in the PA layer on porous PSf TFNC membrane | Optimal compatibility at 0.5 wt.% loading | Lower S value (0.34 mm) compared to conventional TFNC FO membranes (0.96 mm), enhanced water permeability (128 → 461 ), enhanced hydrophilicity (contact angle, 53 → 50°) | [298] | |
| FO tests: Both FS and DS at 500 cross flow rate FS: 10 mM NaCl or DI DS: 0.5, 1.0 or 2.0 M NaCl | For DS: 0.5 M NaCl, FS: DI and 0.5 % (wt./v) loaded TFNC, >100% enhanced water flux in AL-DS (43 ), >100% enhanced water flux in AL-FS (21 ) For DS (2.0 M NaCl), FS (DI water), and 0.5 % (wt./v) loaded TFNC, highest FO water flux reported under similar conditions (86 ) | |||
| Incorporation of surface-modified clinoptilolite into PSf substrate by phase inversion method and coating of PA layer on top | Optimal compatibility at 0.4 wt.% loading | Enhanced surface porosity (80 → 85.4%), better water permeability (118.2 → 185.3 ), lower S value (0.78 → 0.48 mm), enhanced hydrophilicity (contact angle, 71.45 → 57.24°) (surface of clinoptilolite modified with hexadecyl trimethyl ammonium bromide to enhance hydrophilicity) | [306] | |
| RO tests: 20 mM NaCl aqueous solution at 2.5 bar | For 0.4 wt.% loading, enhanced water permeability (1.93 → 2.74 ), exacerbation in B/A value (9.86 → 13.99 kPa), slightly reduced salt rejection (96.2% → 94.7%) | |||
| FO tests: FS: 10 mM NaCl DS: 0.5 or 2.0 M NaCl | FO performance for 10 mM NaCl as FS and 2 M NaCl as DS in AL-DS orientation (for 0.4 wt.% loading): ~50 % enhanced water flux in AL-DS (33.1 ), >50% enhanced water flux in AL-FS (~24.1 ), >100% enhanced solute flux in AL-FS (~15 ), ~100% enhanced solute flux in AL-DS (~20 ) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahu, A.; Dosi, R.; Kwiatkowski, C.; Schmal, S.; Poler, J.C. Advanced Polymeric Nanocomposite Membranes for Water and Wastewater Treatment: A Comprehensive Review. Polymers 2023, 15, 540. https://doi.org/10.3390/polym15030540
Sahu A, Dosi R, Kwiatkowski C, Schmal S, Poler JC. Advanced Polymeric Nanocomposite Membranes for Water and Wastewater Treatment: A Comprehensive Review. Polymers. 2023; 15(3):540. https://doi.org/10.3390/polym15030540
Chicago/Turabian StyleSahu, Abhispa, Raghav Dosi, Carly Kwiatkowski, Stephen Schmal, and Jordan C. Poler. 2023. "Advanced Polymeric Nanocomposite Membranes for Water and Wastewater Treatment: A Comprehensive Review" Polymers 15, no. 3: 540. https://doi.org/10.3390/polym15030540
APA StyleSahu, A., Dosi, R., Kwiatkowski, C., Schmal, S., & Poler, J. C. (2023). Advanced Polymeric Nanocomposite Membranes for Water and Wastewater Treatment: A Comprehensive Review. Polymers, 15(3), 540. https://doi.org/10.3390/polym15030540