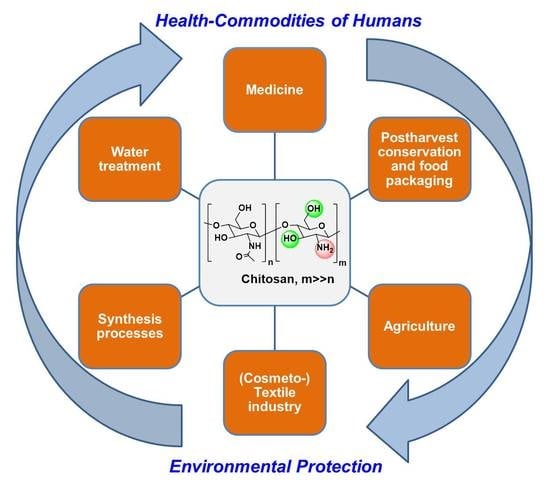
Chitosan as an Outstanding Polysaccharide Improving Health-Commodities of Humans and Environmental Protection
Abstract
1. Introduction
2. Antibacterial and Fungicide Power of Chitosan
3. Biomedical Application of Chitosan: Encapsulation of Active Molecules
3.1. Phytochemical Protection Using Chitosan: Nutraceutical Formulations
3.2. Chitosan in Synthetic Drug Encapsulation: Anticancer Drug Formulations
4. The Role of Chitosan in Food: Material to Extend the Shelf Life
4.1. Coatings from Chitosan for Fruits and Vegetables
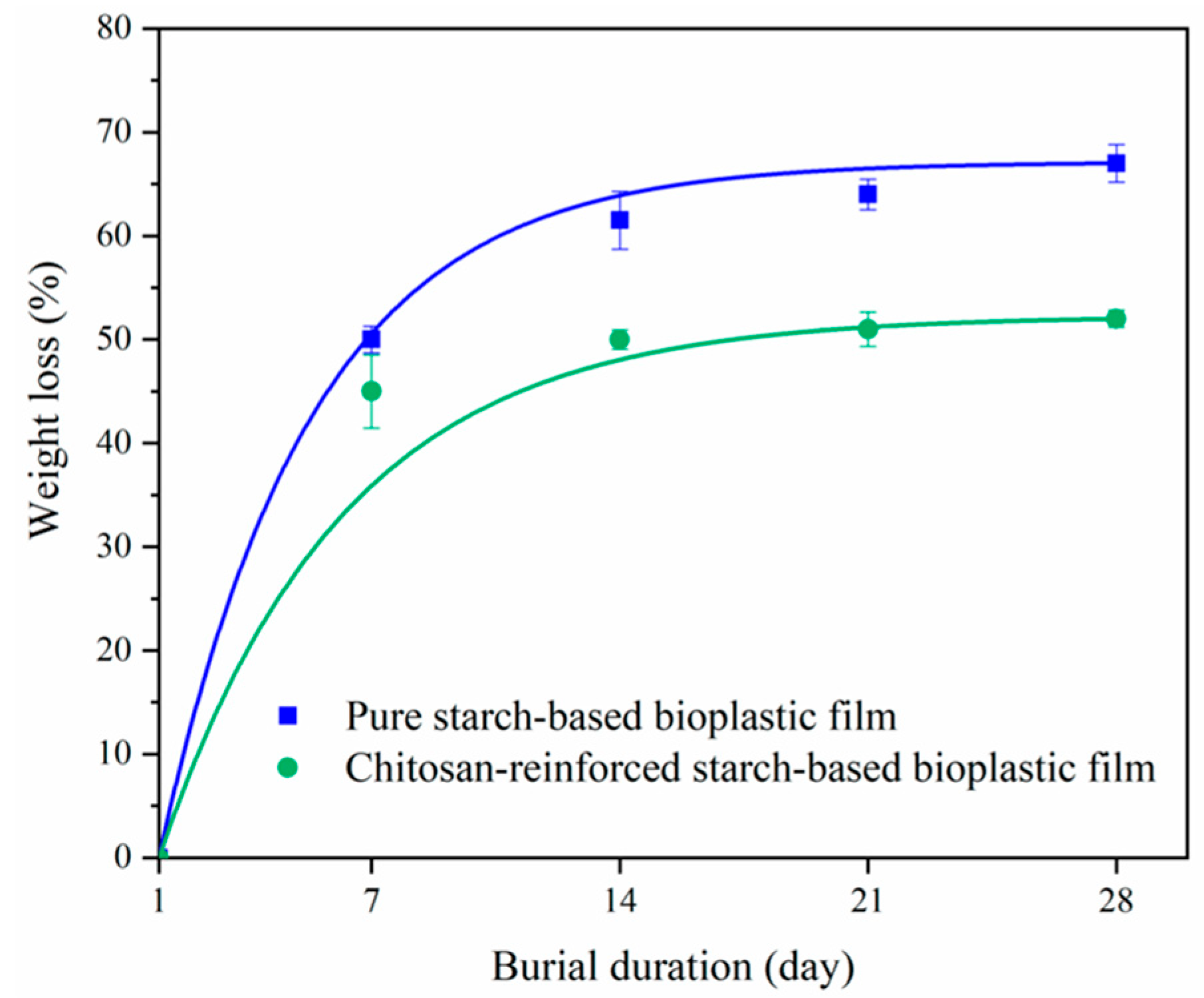
4.2. Biodegradable Plastics Containing Chitosan: Food Packaging
5. Agriculture: The Role of Chitosan in Plant Growth
6. Textile Industry: Development of (Cosmeto-)Textiles Containing Chitosan
7. Synthesis Processes: Chitosan in Catalytic Scaffolds
8. Water Treatment Using Chitosan: Flocculation
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Ding, Y.-F.; Wei, J.; Li, S.; Pan, Y.-T.; Wang, L.-H.; Wang, R. Host–Guest Interactions Initiated Supramolecular Chitosan Nanogels for Selective Intracellular Drug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 28665–28670. [Google Scholar] [CrossRef]
- Li, G.-B.; Wang, J.; Kong, X.-P. Coprecipitation-based synchronous pesticide encapsulation with chitosan for controlled spinosad release. Carbohydr. Polym. 2020, 249, 116865. [Google Scholar] [CrossRef]
- Liu, G.; Hu, M.; Du, X.; Qi, B.; Lu, K.; Zhou, S.; Xie, F.; Li, Y. Study on the interaction between succinylated soy protein isolate and chitosan and its utilization in the development of oil-in-water bilayer emulsions. Food Hydrocoll. 2022, 124, 107309. [Google Scholar] [CrossRef]
- Hu, Z.; Gänzle, M.G. Challenges and opportunities related to the use of chitosan as a food preservative. J. Appl. Microbiol. 2019, 126, 1318–1331. [Google Scholar] [CrossRef]
- Wang, B.; Bai, Z.; Jiang, H.; Prinsen, P.; Luque, R.; Zhao, S.; Xuan, J. Selective heavy metal removal and water purification by microfluidically-generated chitosan microspheres: Characteristics, modeling and application. J. Hazard. Mater. 2019, 364, 192–205. [Google Scholar] [CrossRef]
- Wongpreecha, J.; Polpanich, D.; Suteewong, T.; Kaewsaneha, C.; Tangboriboonrat, P. One-pot, large-scale green synthesis of silver nanoparticles-chitosan with enhanced antibacterial activity and low cytotoxicity. Carbohydr. Polym. 2018, 199, 641–648. [Google Scholar] [CrossRef]
- Lopes, P.P.; Tanabe, E.H.; Bertuol, D.A. Chitosan as biomaterial in drug delivery and tissue engineering. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 407–431. [Google Scholar]
- Kazemzadeh-Narbat, M.; Reid, M.; Brooks, M.S.-L.; Ghanem, A. Chitosan nanoparticles as adenosine carriers. J. Microencapsul. 2015, 32, 460–466. [Google Scholar] [CrossRef]
- Nallamuthu, I.; Devi, A.; Khanum, F. Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian J. Pharm. Sci. 2015, 10, 203–211. [Google Scholar] [CrossRef]
- Paul, W.; Shelma, R.; Sharma, C.P. Alginate Encapsulated Anacardic Acid-Chitosan Self Aggregated Nanoparticles for Intestinal Delivery of Protein Drugs. J. Nanopharm. Drug Deliv. 2013, 1, 82–91. [Google Scholar] [CrossRef]
- Chaiwong, N.; Phimolsiripol, Y.; Leelapornpisid, P.; Ruksiriwanich, W.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Sommano, S.R.; Leksawasdi, N.; Simirgiotis, M.J.; et al. Synergistics of Carboxymethyl Chitosan and Mangosteen Extract as Enhancing Moisturizing, Antioxidant, Antibacterial, and Deodorizing Properties in Emulsion Cream. Polymers 2022, 14, 178. [Google Scholar] [CrossRef]
- Agbovi, H.K.; Wilson, L.D. Design of amphoteric chitosan flocculants for phosphate and turbidity removal in wastewater. Carbohydr. Polym. 2018, 189, 360–370. [Google Scholar] [CrossRef]
- Nemati, M.; Bani, F.; Sepasi, T.; Zamiri, R.E.; Rasmi, Y.; Kahroba, H.; Rahbarghazi, R.; Sadeghi, M.R.; Wang, Y.; Zarebkohan, A.; et al. Unraveling the Effect of Breast Cancer Patients’ Plasma on the Targeting Ability of Folic Acid-Modified Chitosan Nanoparticles. Mol. Pharm. 2021, 18, 4341–4353. [Google Scholar] [CrossRef]
- Acemi, A. Chitosan versus plant growth regulators: A comparative analysis of their effects on in vitro development of Serapias vomeracea (Burm.f.) Briq. Plant Cell Tissue Organ Cult. 2020, 141, 327–338. [Google Scholar] [CrossRef]
- Ruelas-Leyva, J.P.; Contreras-Andrade, I.; Sarmiento-Sánchez, J.I.; Licea-Claveríe, A.; Jiménez-Lam, S.A.; Cristerna-Madrigal, Y.G.; Picos-Corrales, L.A. The Effectiveness of Moringa oleifera Seed Flour and Chitosan as Coagulant-Flocculants for Water Treatment. CLEAN—Soil Air Water 2017, 45, 1600339. [Google Scholar] [CrossRef]
- Osorio-Alvarado, C.E.; Ropero-Vega, J.L.; Farfán-García, A.E.; Flórez-Castillo, J.M. Immobilization Systems of Antimicrobial Peptide Ib−M1 in Polymeric Nanoparticles Based on Alginate and Chitosan. Polymers 2022, 14, 3149. [Google Scholar] [CrossRef]
- Tan, S.X.; Ong, H.C.; Andriyana, A.; Lim, S.; Pang, Y.L.; Kusumo, F.; Ngoh, G.C. Characterization and Parametric Study on Mechanical Properties Enhancement in Biodegradable Chitosan-Reinforced Starch-Based Bioplastic Film. Polymers 2022, 14, 278. [Google Scholar] [CrossRef]
- Li, J.; Ying, S.; Ren, H.; Dai, J.; Zhang, L.; Liang, L.; Wang, Q.; Shen, Q.; Shen, J.-W. Molecular dynamics study on the encapsulation and release of anti-cancer drug doxorubicin by chitosan. Int. J. Pharm. 2020, 580, 119241. [Google Scholar] [CrossRef]
- Rahbar, M.; Morsali, A.; Bozorgmehr, M.R.; Beyramabadi, S.A. Quantum chemical studies of chitosan nanoparticles as effective drug delivery systems for 5-fluorouracil anticancer drug. J. Mol. Liq. 2020, 302, 112495. [Google Scholar] [CrossRef]
- Vikas; Viswanadh, M.K.; Mehata, A.K.; Sharma, V.; Priya, V.; Varshney, N.; Mahto, S.K.; Muthu, M.S. Bioadhesive chitosan nanoparticles: Dual targeting and pharmacokinetic aspects for advanced lung cancer treatment. Carbohydr. Polym. 2021, 274, 118617. [Google Scholar] [CrossRef]
- Sirajudheen, P.; Poovathumkuzhi, N.C.; Vigneshwaran, S.; Chelaveettil, B.M.; Meenakshi, S. Applications of chitin and chitosan based biomaterials for the adsorptive removal of textile dyes from water—A comprehensive review. Carbohydr. Polym. 2021, 273, 118604. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wang, J.; Zhang, L.; Chen, Q.; Yue, W.; Ke, N.; Xie, H. Coumarin-Containing Light-Responsive Carboxymethyl Chitosan Micelles as Nanocarriers for Controlled Release of Pesticide. Polymers 2020, 12, 2268. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, M.; Espinosa, Y.G.; Goycoolea, F.M. Interaction Between Chitosan and Mucin: Fundamentals and Applications. Biomimetics 2019, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Rahat, I.; Imam, S.S.; Rizwanullah, M.; Alshehri, S.; Asif, M.; Kala, C.; Taleuzzaman, M. Thymoquinone-entrapped chitosan-modified nanoparticles: Formulation optimization to preclinical bioavailability assessments. Drug Deliv. 2021, 28, 973–984. [Google Scholar] [CrossRef]
- Lo, W.-H.; Deng, F.-S.; Chang, C.-J.; Lin, C.-H. Synergistic Antifungal Activity of Chitosan with Fluconazole against Candida albicans, Candida tropicalis, and Fluconazole-Resistant Strains. Molecules 2020, 25, 5114. [Google Scholar] [CrossRef]
- Rahman Bhuiyan, M.A.; Hossain, M.A.; Zakaria, M.; Islam, M.N.; Zulhash Uddin, M. Chitosan Coated Cotton Fiber: Physical and Antimicrobial Properties for Apparel Use. J. Polym. Environ. 2017, 25, 334–342. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef]
- Hu, L.; Zhu, B.; Zhang, L.; Yuan, H.; Zhao, Q.; Yan, Z. Chitosan–gold nanocomposite and its functionalized paper strips for reversible visual sensing and removal of trace Hg 2+ in practice. Analyst 2019, 144, 474–480. [Google Scholar] [CrossRef]
- Berillo, D.; Cundy, A. 3D-macroporous chitosan-based scaffolds with in situ formed Pd and Pt nanoparticles for nitrophenol reduction. Carbohydr. Polym. 2018, 192, 166–175. [Google Scholar] [CrossRef]
- Kou, S.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef]
- Tantala, J.; Thumanu, K.; Rachtanapun, C. An assessment of antibacterial mode of action of chitosan on Listeria innocua cells using real-time HATR-FTIR spectroscopy. Int. J. Biol. Macromol. 2019, 135, 386–393. [Google Scholar] [CrossRef]
- Garcia, L.G.S.; de Guedes, G.M.M.; da Silva, M.L.Q.; Castelo-Branco, D.S.C.M.; Sidrim, J.J.C.; de Cordeiro, R.A.; Rocha, M.F.G.; Vieira, R.S.; Brilhante, R.S.N. Effect of the molecular weight of chitosan on its antifungal activity against Candida spp. in planktonic cells and biofilm. Carbohydr. Polym. 2018, 195, 662–669. [Google Scholar] [CrossRef]
- Sun, Y.; Shang, L.; Xia, X.; Meng, D.; Ren, Y.; Zhang, J.; Yao, M.; Zhou, X.; Wang, Y. Cellular uptake of chitosan and its role in antifungal action against Penicillium expansum. Carbohydr. Polym. 2021, 269, 118349. [Google Scholar] [CrossRef]
- Hassan, M.A.; Omer, A.M.; Abbas, E.; Baset, W.M.A.; Tamer, T.M. Preparation, physicochemical characterization and antimicrobial activities of novel two phenolic chitosan Schiff base derivatives. Sci. Rep. 2018, 8, 11416. [Google Scholar] [CrossRef]
- Dias, A.M.; dos Santos Cabrera, M.P.; Lima, A.M.F.; Taboga, S.R.; Vilamaior, P.S.L.; Tiera, M.J.; de Oliveira Tiera, V.A. Insights on the antifungal activity of amphiphilic derivatives of diethylaminoethyl chitosan against Aspergillus flavus. Carbohydr. Polym. 2018, 196, 433–444. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Tan, W.; Wang, G.; Li, Q.; Dong, F.; Guo, Z. Antifungal activity of double Schiff bases of chitosan derivatives bearing active halogeno-benzenes. Int. J. Biol. Macromol. 2021, 179, 292–298. [Google Scholar] [CrossRef]
- Si, Z.; Hou, Z.; Vikhe, Y.S.; Thappeta, K.R.V.; Marimuthu, K.; De, P.P.; Ng, O.T.; Li, P.; Zhu, Y.; Pethe, K.; et al. Antimicrobial Effect of a Novel Chitosan Derivative and Its Synergistic Effect with Antibiotics. ACS Appl. Mater. Interfaces 2021, 13, 3237–3245. [Google Scholar] [CrossRef]
- Abdel-Razek, N. Antimicrobial activities of chitosan nanoparticles against pathogenic microorganisms in Nile tilapia, Oreochromis niloticus. Aquac. Int. 2019, 27, 1315–1330. [Google Scholar] [CrossRef]
- Alqahtani, F.; Aleanizy, F.; El Tahir, E.; Alhabib, H.; Alsaif, R.; Shazly, G.; AlQahtani, H.; Alsarra, I.; Mahdavi, J. Antibacterial Activity of Chitosan Nanoparticles Against Pathogenic N. gonorrhoea. Int. J. Nanomed. 2020, 15, 7877–7887. [Google Scholar] [CrossRef]
- Deng, P.; Chen, J.; Yao, L.; Zhang, P.; Zhou, J. Thymine-modified chitosan with broad-spectrum antimicrobial activities for wound healing. Carbohydr. Polym. 2021, 257, 117630. [Google Scholar] [CrossRef]
- Basit, H.M.; Mohd Amin, M.C.I.; Ng, S.-F.; Katas, H.; Shah, S.U.; Khan, N.R. Formulation and Evaluation of Microwave-Modified Chitosan-Curcumin Nanoparticles—A Promising Nanomaterials Platform for Skin Tissue Regeneration Applications Following Burn Wounds. Polymers 2020, 12, 2608. [Google Scholar] [CrossRef]
- Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Zafar, A.; Alsaidan, O.A.; Alruwaili, N.K.; Gilani, S.J.; Rizwanullah, M. Recent Advancement in Chitosan-Based Nanoparticles for Improved Oral Bioavailability and Bioactivity of Phytochemicals: Challenges and Perspectives. Polymers 2021, 13, 4036. [Google Scholar] [CrossRef]
- Maqsood, S.; Adiamo, O.; Ahmad, M.; Mudgil, P. Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients. Food Chem. 2020, 308, 125522. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Khezri, A.; Karimi, A.; Yazdian, F.; Jokar, M.; Mofradnia, S.R.; Rashedi, H.; Tavakoli, Z. Molecular dynamic of curcumin/chitosan interaction using a computational molecular approach: Emphasis on biofilm reduction. Int. J. Biol. Macromol. 2018, 114, 972–978. [Google Scholar] [CrossRef]
- Negi, A.; Kesari, K.K. Chitosan Nanoparticle Encapsulation of Antibacterial Essential Oils. Micromachines 2022, 13, 1265. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lin, Y.-S.; Wu, S.-J.; Mi, F.-L. Mutlifunctional nanoparticles prepared from arginine-modified chitosan and thiolated fucoidan for oral delivery of hydrophobic and hydrophilic drugs. Carbohydr. Polym. 2018, 193, 163–172. [Google Scholar] [CrossRef]
- Johari, M.A.; Azmi, A.S.; Jamaluddin, J.; Hasham, R.; Chee, C.F.; Ali, F. Comparison Study Between Encapsulation of Acalypha indica Linn Extracts with Chitosan-PCL and Chitosan-OA. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1192, 012007. [Google Scholar] [CrossRef]
- Aman, R.M.; Abu Hashim, I.I.; Meshali, M.M. Novel chitosan-based solid-lipid nanoparticles to enhance the bio-residence of the miraculous phytochemical “Apocynin”. Eur. J. Pharm. Sci. 2018, 124, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Anter, H.M.; Abu Hashim, I.I.; Awadin, W.; Meshali, M.M. Novel chitosan oligosaccharide-based nanoparticles for gastric mucosal administration of the phytochemical “apocynin”. Int. J. Nanomed. 2019, 14, 4911–4929. [Google Scholar] [CrossRef] [PubMed]
- Kamal, I.; Khedr, A.I.M.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Elshaarawy, R.F.M.; Saad, A.S. Chemotherapeutic and chemopreventive potentials of ρ-coumaric acid—Squid chitosan nanogel loaded with Syzygium aromaticum essential oil. Int. J. Biol. Macromol. 2021, 188, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carrasco, M.; Picos-Corrales, L.A.; Gutiérrez-Grijalva, E.P.; Angulo-Escalante, M.A.; Licea-Claverie, A.; Heredia, J.B. Loading and Release of Phenolic Compounds Present in Mexican Oregano (Lippia graveolens) in Different Chitosan Bio-Polymeric Cationic Matrixes. Polymers 2022, 14, 3609. [Google Scholar] [CrossRef]
- Moon, H.; Lertpatipanpong, P.; Hong, Y.; Kim, C.-T.; Baek, S.J. Nano-encapsulated quercetin by soluble soybean polysaccharide/chitosan enhances anti-cancer, anti-inflammation, and anti-oxidant activities. J. Funct. Foods 2021, 87, 104756. [Google Scholar] [CrossRef]
- Alzandi, A.A.; Naguib, D.M.; Abas, A.-S.M. Onion Extract Encapsulated on Nano Chitosan: A Promising Anticancer Agent. J. Gastrointest. Cancer 2022, 53, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Balan, P.; Indrakumar, J.; Murali, P.; Korrapati, P.S. Bi-faceted delivery of phytochemicals through chitosan nanoparticles impregnated nanofibers for cancer therapeutics. Int. J. Biol. Macromol. 2020, 142, 201–211. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Delfi, M.; Zarrabi, A.; Bigham, A.; Sharifi, E.; Rabiee, N.; Paiva-Santos, A.C.; Kumar, A.P.; Tan, S.C.; Hushmandi, K.; et al. Stimuli-responsive liposomal nanoformulations in cancer therapy: Pre-clinical & clinical approaches. J. Control. Release 2022, 351, 50–80. [Google Scholar] [CrossRef]
- Wang, G.; Li, R.; Parseh, B.; Du, G. Prospects and challenges of anticancer agents’ delivery via chitosan-based drug carriers to combat breast cancer: A review. Carbohydr. Polym. 2021, 268, 118192. [Google Scholar] [CrossRef]
- Knapp, J.P.; Kakish, J.E.; Bridle, B.W.; Speicher, D.J. Tumor Temperature: Friend or Foe of Virus-Based Cancer Immunotherapy. Biomedicines 2022, 10, 2024. [Google Scholar] [CrossRef]
- Marsili, L.; Dal Bo, M.; Berti, F.; Toffoli, G. Thermoresponsive Chitosan-Grafted-Poly(N-vinylcaprolactam) Microgels via Ionotropic Gelation for Oncological Applications. Pharmaceutics 2021, 13, 1654. [Google Scholar] [CrossRef] [PubMed]
- Braga, L.A.S.; Flauzino Junior, A.; González, M.E.L.; de Queiroz, A.A.A. Membranas termossensíveis baseadas em redes poliméricas semi-interpenetrantes de Quitosana e Poli(N-isopropilacrilamida). Res. Soc. Dev. 2019, 8, e3883748. [Google Scholar] [CrossRef]
- Chen, Y.; Song, G.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. Mechanically strong dual responsive nanocomposite double network hydrogel for controlled drug release of asprin. J. Mech. Behav. Biomed. Mater. 2018, 82, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Güngör, A.; Demir, D.; Bölgen, N.; Özdemir, T.; Genç, R. Dual stimuli-responsive chitosan grafted poly(NIPAM-co-AAc)/poly(vinyl alcohol) hydrogels for drug delivery applications. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 810–819. [Google Scholar] [CrossRef]
- Kiaee, G.; Mostafalu, P.; Samandari, M.; Sonkusale, S. A pH-Mediated Electronic Wound Dressing for Controlled Drug Delivery. Adv. Healthc. Mater. 2018, 7, 1800396. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Williams, G.R.; Wu, J.; Wu, J.; Zhang, X.; Chen, X.; Li, S.; Jiao, J.; Zhu, L.-M. A chitosan-based cascade-responsive drug delivery system for triple-negative breast cancer therapy. J. Nanobiotechnol. 2019, 17, 95. [Google Scholar] [CrossRef]
- Nivethaa, E.A.K.; Baskar, S.; Martin, C.A.; Ramya, J.R.; Stephen, A.; Narayanan, V.; Lakshmi, B.S.; Olga, V.F.-K.; Radhakrishnan, S.; Kalkura, S.N. A competent bidrug loaded water soluble chitosan derivative for the effective inhibition of breast cancer. Sci. Rep. 2020, 10, 3991. [Google Scholar] [CrossRef]
- Dubey, S.K.; Bhatt, T.; Agrawal, M.; Saha, R.N.; Saraf, S.; Saraf, S.; Alexander, A. Application of chitosan modified nanocarriers in breast cancer. Int. J. Biol. Macromol. 2022, 194, 521–538. [Google Scholar] [CrossRef]
- Xie, P.; Liu, P. Core-shell-corona chitosan-based micelles for tumor intracellular pH-triggered drug delivery: Improving performance by grafting polycation. Int. J. Biol. Macromol. 2019, 141, 161–170. [Google Scholar] [CrossRef]
- Almeida, A.; Castro, F.; Resende, C.; Lúcio, M.; Schwartz, S.; Sarmento, B. Oral delivery of camptothecin-loaded multifunctional chitosan-based micelles is effective in reduce colorectal cancer. J. Control. Release 2022, 349, 731–743. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Park, S.; Kim, S.-R.; Priya, V.V.; Wang, M.-H. Monoclonal Antibody Functionalized, and L-lysine α-Oxidase Loaded PEGylated-Chitosan Nanoparticle for HER2/Neu Targeted Breast Cancer Therapy. Pharmaceutics 2022, 14, 927. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Araújo, M.; Novoa-Carballal, R.; Andrade, F.; Gonçalves, H.; Reis, R.L.; Lúcio, M.; Schwartz, S.; Sarmento, B. Novel amphiphilic chitosan micelles as carriers for hydrophobic anticancer drugs. Mater. Sci. Eng. C 2020, 112, 110920. [Google Scholar] [CrossRef] [PubMed]
- Viswanadh, M.K.; Vikas; Jha, A.; Reddy Adena, S.K.; Mehata, A.K.; Priya, V.; Neogi, K.; Poddar, S.; Mahto, S.K.; Muthu, M.S. Formulation and in vivo efficacy study of cetuximab decorated targeted bioadhesive nanomedicine for non-small-cell lung cancer therapy. Nanomedicine 2020, 15, 2345–2367. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Jeevithan, E.; Hu, X.; Shin, S.; Wang, M.-H. Dual stimuli-responsive release of aptamer AS1411 decorated erlotinib loaded chitosan nanoparticles for non-small-cell lung carcinoma therapy. Carbohydr. Polym. 2020, 245, 116407. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Trends in Chitosan as a Primary Biopolymer for Functional Films and Coatings Manufacture for Food and Natural Products. Polymers 2021, 13, 767. [Google Scholar] [CrossRef]
- Panchal, N.; Das, K.; Prabhakar, P.K.; Ghanghas, N. Edible Films and Coatings for Fruits and Vegetables: Composition, Functions, and Regulatory Aspects. In Edible Food Packaging; Springer Nature: Singapore, 2022; pp. 191–216. [Google Scholar]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and Incorporation of Functional Ingredients in Edible Films and Coatings. Food Bioprocess Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Singh, T.P.; Chatli, M.K.; Sahoo, J. Development of chitosan based edible films: Process optimization using response surface methodology. J. Food Sci. Technol. 2015, 52, 2530–2543. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha; Trajkovska Petkoska, A.; Khojah, E.; Sami, R.; Al-Mushhin, A.A.M. Chitosan Edible Films Enhanced with Pomegranate Peel Extract: Study on Physical, Biological, Thermal, and Barrier Properties. Materials 2021, 14, 3305. [Google Scholar] [CrossRef]
- Pavinatto, A.; de Almeida Mattos, A.V.; Malpass, A.C.G.; Okura, M.H.; Balogh, D.T.; Sanfelice, R.C. Coating with chitosan-based edible films for mechanical/biological protection of strawberries. Int. J. Biol. Macromol. 2020, 151, 1004–1011. [Google Scholar] [CrossRef]
- Adiletta, G.; Di Matteo, M.; Petriccione, M. Multifunctional Role of Chitosan Edible Coatings on Antioxidant Systems in Fruit Crops: A Review. Int. J. Mol. Sci. 2021, 22, 2633. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Mechanical and barrier properties of chitosan combined with other components as food packaging film. Environ. Chem. Lett. 2020, 18, 257–267. [Google Scholar] [CrossRef]
- Akter, N.; Khan, R.A.; Salmieri, S.; Sharmin, N.; Dussault, D.; Lacroix, M. Fabrication and mechanical characterization of biodegradable and synthetic polymeric films: Effect of gamma radiation. Radiat. Phys. Chem. 2012, 81, 995–998. [Google Scholar] [CrossRef]
- Sabaghi, M.; Maghsoudlou, Y.; Habibi, P. Enhancing structural properties and antioxidant activity of kefiran films by chitosan addition. Food Struct. 2015, 5, 66–71. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Homez-Jara, A.; Daza, L.D.; Aguirre, D.M.; Muñoz, J.A.; Solanilla, J.F.; Váquiro, H.A. Characterization of chitosan edible films obtained with various polymer concentrations and drying temperatures. Int. J. Biol. Macromol. 2018, 113, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, M.; Di Pierro, P.; Cammarota, M.; Dell’Olmo, E.; Arciello, A.; Porta, R. Development and properties of new chitosan-based films plasticized with spermidine and/or glycerol. Food Hydrocoll. 2019, 87, 245–252. [Google Scholar] [CrossRef]
- Reyes-Avalos, M.C.; Minjares-Fuentes, R.; Femenia, A.; Contreras-Esquivel, J.C.; Quintero-Ramos, A.; Esparza-Rivera, J.R.; Meza-Velázquez, J.A. Application of an Alginate–Chitosan Edible Film on Figs (Ficus carica): Effect on Bioactive Compounds and Antioxidant Capacity. Food Bioprocess Technol. 2019, 12, 499–511. [Google Scholar] [CrossRef]
- Khan, I.; Tango, C.N.; Chelliah, R.; Oh, D.-H. Development of antimicrobial edible coating based on modified chitosan for the improvement of strawberries shelf life. Food Sci. Biotechnol. 2019, 28, 1257–1264. [Google Scholar] [CrossRef]
- Salem, M.F.; Tayel, A.A.; Alzuaibr, F.M.; Bakr, R.A. Innovative Approach for Controlling Black Rot of Persimmon Fruits by Means of Nanobiotechnology from Nanochitosan and Rosmarinic Acid-Mediated Selenium Nanoparticles. Polymers 2022, 14, 2116. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef]
- Letcher, T.M. Plastic Waste and Recycling; Elsevier: London, UK, 2020; ISBN 9780128178805. [Google Scholar]
- Mukherjee, T.; Kao, N. PLA Based Biopolymer Reinforced with Natural Fibre: A Review. J. Polym. Environ. 2011, 19, 714–725. [Google Scholar] [CrossRef]
- Ruelas-Leyva, J.P.; Picos-Corrales, L.A. Lactides and Lactones Yielding Eco-Friendly and Biocompatible Polymers by Metal-Catalyzed Ring-Opening Polymerization. Mini. Rev. Org. Chem. 2021, 18, 680–689. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, S.; Sun, J.; Ren, Y.; Wang, S.; Wang, X.; Wang, W.; Li, H.; Fei, B.; Gu, X.; et al. A new strategy to prepare fully bio-based poly(lactic acid) composite with high flame retardancy, UV resistance, and rapid degradation in soil. Chem. Eng. J. 2022, 428, 131979. [Google Scholar] [CrossRef]
- Tábi, T.; Ageyeva, T.; Kovács, J.G. Improving the ductility and heat deflection temperature of injection molded Poly(lactic acid) products: A comprehensive review. Polym. Test. 2021, 101, 107282. [Google Scholar] [CrossRef]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Saad, G.R.; Sayed, A.M. Mechanical, thermal, and dielectric properties of poly(lactic acid)/chitosan nanocomposites. Polym. Eng. Sci. 2016, 56, 987–994. [Google Scholar] [CrossRef]
- Han, W.; Ren, J.; Xuan, H.; Ge, L. Controllable degradation rates, antibacterial, free-standing and highly transparent films based on polylactic acid and chitosan. Colloids Surf. A Physicochem. Eng. Asp. 2018, 541, 128–136. [Google Scholar] [CrossRef]
- Lizárraga-Laborín, L.L.; Quiroz-Castillo, J.M.; Encinas-Encinas, J.C.; Castillo-Ortega, M.M.; Burruel-Ibarra, S.E.; Romero-García, J.; Torres-Ochoa, J.A.; Cabrera-Germán, D.; Rodríguez-Félix, D.E. Accelerated weathering study of extruded polyethylene/poly (lactic acid)/chitosan films. Polym. Degrad. Stab. 2018, 155, 43–51. [Google Scholar] [CrossRef]
- Chang, S.-H.; Chen, Y.-J.; Tseng, H.-J.; Hsiao, H.-I.; Chai, H.-J.; Shang, K.-C.; Pan, C.-L.; Tsai, G.-J. Antibacterial Activity of Chitosan—Polylactate Fabricated Plastic Film and Its Application on the Preservation of Fish Fillet. Polymers 2021, 13, 696. [Google Scholar] [CrossRef] [PubMed]
- Fathima, P.E.; Panda, S.K.; Ashraf, P.M.; Varghese, T.O.; Bindu, J. Polylactic acid/chitosan films for packaging of Indian white prawn (Fenneropenaeus indicus). Int. J. Biol. Macromol. 2018, 117, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Torres-Huerta, A.M.; Palma-Ramírez, D.; Domínguez-Crespo, M.A.; Del Angel-López, D.; de la Fuente, D. Comparative assessment of miscibility and degradability on PET/PLA and PET/chitosan blends. Eur. Polym. J. 2014, 61, 285–299. [Google Scholar] [CrossRef]
- Environmental Protection Agency. EPA Reduces Burden, Promotes Innovation with Proposed Action on Chitosan. Available online: https://www.epa.gov/newsreleases/epa-reduces-burden-promotes-innovation-proposed-action-chitosan (accessed on 20 December 2022).
- Li, K.; Xing, R.; Liu, S.; Li, P. Chitin and Chitosan Fragments Responsible for Plant Elicitor and Growth Stimulator. J. Agric. Food Chem. 2020, 68, 12203–12211. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Fernandez, M.; Marhuenda-Egea, F.C.; Lopez-Moya, F.; Arnao, M.B.; Cabrera-Escribano, F.; Nueda, M.J.; Gunsé, B.; Lopez-Llorca, L.V. Chitosan Induces Plant Hormones and Defenses in Tomato Root Exudates. Front. Plant Sci. 2020, 11, 572087. [Google Scholar] [CrossRef]
- De Vega, D.; Holden, N.; Hedley, P.E.; Morris, J.; Luna, E.; Newton, A. Chitosan primes plant defence mechanisms against Botrytis cinerea, including expression of Avr9/Cf-9 rapidly elicited genes. Plant. Cell Environ. 2021, 44, 290–303. [Google Scholar] [CrossRef]
- Limpanavech, P.; Chaiyasuta, S.; Vongpromek, R.; Pichyangkura, R.; Khunwasi, C.; Chadchawan, S.; Lotrakul, P.; Bunjongrat, R.; Chaidee, A.; Bangyeekhun, T. Chitosan effects on floral production, gene expression, and anatomical changes in the Dendrobium orchid. Sci. Hortic. 2008, 116, 65–72. [Google Scholar] [CrossRef]
- Nge, K.L.; Nwe, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan as a growth stimulator in orchid tissue culture. Plant Sci. 2006, 170, 1185–1190. [Google Scholar] [CrossRef]
- Salachna, P.; Łopusiewicz, Ł. Chitosan Oligosaccharide Lactate Increases Productivity and Quality of Baby Leaf Red Perilla. Agronomy 2022, 12, 1182. [Google Scholar] [CrossRef]
- Asgari-Targhi, G.; Iranbakhsh, A.; Ardebili, Z.O. Potential benefits and phytotoxicity of bulk and nano-chitosan on the growth, morphogenesis, physiology, and micropropagation of Capsicum annuum. Plant Physiol. Biochem. 2018, 127, 393–402. [Google Scholar] [CrossRef]
- Colman, S.L.; Salcedo, M.F.; Mansilla, A.Y.; Iglesias, M.J.; Fiol, D.F.; Martín-Saldaña, S.; Alvarez, V.A.; Chevalier, A.A.; Casalongué, C.A. Chitosan microparticles improve tomato seedling biomass and modulate hormonal, redox and defense pathways. Plant Physiol. Biochem. 2019, 143, 203–211. [Google Scholar] [CrossRef]
- Ahmad, B.; Khan, M.M.A.; Jahan, A.; Shabbir, A.; Jaleel, H. Increased production of valuable secondary products in plants by leaf applied radiation-processed polysaccharides. Int. J. Biol. Macromol. 2020, 164, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Fooladi vanda, G.; Shabani, L.; Razavizadeh, R. Chitosan enhances rosmarinic acid production in shoot cultures of Melissa officinalis L. through the induction of methyl jasmonate. Bot. Stud. 2019, 60, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, K.; Xing, R.; Liu, S.; Chen, X.; Yang, H.; Li, P. miRNA and mRNA Expression Profiles Reveal Insight into Chitosan-Mediated Regulation of Plant Growth. J. Agric. Food Chem. 2018, 66, 3810–3822. [Google Scholar] [CrossRef] [PubMed]
- Rabêlo, V.M.; Magalhães, P.C.; Bressanin, L.A.; Carvalho, D.T.; dos Reis, C.O.; Karam, D.; Doriguetto, A.C.; dos Santos, M.H.; dos Santos Filho, P.R.S.; de Souza, T.C. The foliar application of a mixture of semisynthetic chitosan derivatives induces tolerance to water deficit in maize, improving the antioxidant system and increasing photosynthesis and grain yield. Sci. Rep. 2019, 9, 8164. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Khawar, K.M.; Camara, M.C.; Carvalho, L.B.; Fraceto, L.F.; Morsi, R.E.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Ullah, H.; et al. Chitosan-based delivery systems for plants: A brief overview of recent advances and future directions. Int. J. Biol. Macromol. 2020, 154, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Madzokere, T.C.; Murombo, L.T.; Chiririwa, H. Nano-based slow releasing fertilizers for enhanced agricultural productivity. Mater. Today Proc. 2021, 45, 3709–3715. [Google Scholar] [CrossRef]
- Antunes, J.C.; Moreira, I.P.; Gomes, F.; Cunha, F.; Henriques, M.; Fangueiro, R. Recent Trends in Protective Textiles against Biological Threats: A Focus on Biological Warfare Agents. Polymers 2022, 14, 1599. [Google Scholar] [CrossRef] [PubMed]
- Sakkara, S.; Santosh, M.S.; Reddy, N. Chitosan Fibers. In Handbook of Fibrous Materials; Wiley: Hoboken, NJ, USA, 2020; pp. 125–156. [Google Scholar]
- Elamri, A.; Zdiri, K.; Hamdaoui, M.; Harzallah, O. Chitosan: A biopolymer for textile processes and products. Text. Res. J. 2022, 1, 1. [Google Scholar] [CrossRef]
- Shahid-ul-Islam; Shahid, M.; Mohammad, F. Green Chemistry Approaches to Develop Antimicrobial Textiles Based on Sustainable Biopolymers—A Review. Ind. Eng. Chem. Res. 2013, 52, 5245–5260. [Google Scholar] [CrossRef]
- Kuznik, I.; Kruppke, I.; Cherif, C. Pure Chitosan-Based Fibers Manufactured by a Wet Spinning Lab-Scale Process Using Ionic Liquids. Polymers 2022, 14, 477. [Google Scholar] [CrossRef]
- Qamar, S.A.; Ashiq, M.; Jahangeer, M.; Riasat, A.; Bilal, M. Chitosan-based hybrid materials as adsorbents for textile dyes–A review. Case Stud. Chem. Environ. Eng. 2020, 2, 100021. [Google Scholar] [CrossRef]
- Zhou, B.C.-E.; Kan, C.; Sun, C.; Du, J.; Xu, C. A Review of Chitosan Textile Applications. AATCC J. Res. 2019, 6, 8–14. [Google Scholar] [CrossRef]
- Lim, S.-H.; Hudson, S.M. Review of Chitosan and Its Derivatives as Antimicrobial Agents and Their Uses as Textile Chemicals. J. Macromol. Sci. Part C Polym. Rev. 2003, 43, 223–269. [Google Scholar] [CrossRef]
- Stegmaier, T.; Wunderlich, W.; Hager, T.; Siddique, A.B.; Sarsour, J.; Planck, H. Chitosan—A Sizing Agent in Fabric Production—Development and Ecological Evaluation. CLEAN—Soil Air Water 2008, 36, 279–286. [Google Scholar] [CrossRef]
- Türkoğlu, G.C.; Sarıışık, A.M.; Karavana, S.Y. Development of textile-based sodium alginate and chitosan hydrogel dressings. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 916–925. [Google Scholar] [CrossRef]
- Flinčec Grgac, S.; Tarbuk, A.; Dekanić, T.; Sujka, W.; Draczyński, Z. The Chitosan Implementation into Cotton and Polyester/Cotton Blend Fabrics. Materials 2020, 13, 1616. [Google Scholar] [CrossRef]
- Sadeghi-Kiakhani, M.; Safapour, S. Eco-friendly dyeing of treated wool fabrics with reactive dyes using chitosanpoly(propylene imine)dendreimer hybrid. Clean Technol. Environ. Policy 2015, 17, 1019–1027. [Google Scholar] [CrossRef]
- Pan, H.; Zhao, T.; Xu, L.; Shen, Y.; Wang, L.; Ding, Y. Preparation of novel chitosan derivatives and applications in functional finishing of textiles. Int. J. Biol. Macromol. 2020, 153, 971–976. [Google Scholar] [CrossRef]
- Yip, J.; Yuen, C.-W.M.; Kan, C.-W.; Cheung, H.-C.; Leung, H.-M.P.; Cheuk, K.; Cheng, S.-Y.; Liu, L. Chitosan/Clotrimazole microcapsules for Tinea pedis treatment: In-vitro antifungal and cytotoxicity study. J. Text. Inst. 2015, 106, 641–647. [Google Scholar] [CrossRef]
- Yuen, C.-W.M.; Yip, J.; Liu, L.; Cheuk, K.; Kan, C.-W.; Cheung, H.-C.; Cheng, S.-Y. Chitosan microcapsules loaded with either miconazole nitrate or clotrimazole, prepared via emulsion technique. Carbohydr. Polym. 2012, 89, 795–801. [Google Scholar] [CrossRef]
- García-Serna, J.; Piñero-Hernanz, R.; Durán-Martín, D. Inspirational perspectives and principles on the use of catalysts to create sustainability. Catal. Today 2022, 387, 237–243. [Google Scholar] [CrossRef]
- Zaera, F. Molecular approaches to heterogeneous catalysis. Coord. Chem. Rev. 2021, 448, 214179. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.; Setiabudi, H.D.; Nanda, S.; Vo, D.-V.N. Advanced synthesis strategies of mesoporous SBA-15 supported catalysts for catalytic reforming applications: A state-of-the-art review. Appl. Catal. A Gen. 2018, 559, 57–74. [Google Scholar] [CrossRef]
- Ruelas-Leyva, J.P.; Maldonado-Garcia, L.F.; Talavera-Lopez, A.; Santos-López, I.A.; Picos-Corrales, L.A.; Santolalla-Vargas, C.E.; Torres, S.A.G.; Fuentes, G.A. A Comprehensive Study of Coke Deposits on a Pt-Sn/SBA-16 Catalyst during the Dehydrogenation of Propane. Catalysts 2021, 11, 128. [Google Scholar] [CrossRef]
- Boominathan, T.; Sivaramakrishna, A. Recent Advances in the Synthesis, Properties, and Applications of Modified Chitosan Derivatives: Challenges and Opportunities. Top. Curr. Chem. 2021, 379, 19. [Google Scholar] [CrossRef]
- Takeshita, S.; Zhao, S.; Malfait, W.J.; Koebel, M.M. Chemistry of Chitosan Aerogels: Three-Dimensional Pore Control for Tailored Applications. Angew. Chemie Int. Ed. 2021, 60, 9828–9851. [Google Scholar] [CrossRef]
- Bergamonti, L.; Bergonzi, C.; Graiff, C.; Lottici, P.P.; Bettini, R.; Elviri, L. 3D printed chitosan scaffolds: A new TiO2 support for the photocatalytic degradation of amoxicillin in water. Water Res. 2019, 163, 114841. [Google Scholar] [CrossRef]
- Wu, X.; Luo, N.; Xie, S.; Zhang, H.; Zhang, Q.; Wang, F.; Wang, Y. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem. Soc. Rev. 2020, 49, 6198–6223. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; Jin, D.; Jiao, G.; Yang, X.; Liu, K.; Zhou, J.; Sun, R. Copper oxide functionalized chitosan hybrid hydrogels for highly efficient photocatalytic-reforming of biomass-based monosaccharides to lactic acid. Appl. Catal. B Environ. 2021, 291, 120123. [Google Scholar] [CrossRef]
- Rostami, N.; Dekamin, M.G.; Valiey, E.; Fanimoghadam, H. Chitosan-EDTA-Cellulose network as a green, recyclable and multifunctional biopolymeric organocatalyst for the one-pot synthesis of 2-amino-4H-pyran derivatives. Sci. Rep. 2022, 12, 8642. [Google Scholar] [CrossRef]
- Celaya, C.A.; Méndez-Galván, M.; Castro-Ocampo, O.; Torres-Martínez, L.M.; Luévano-Hipólito, E.; Díaz de León, J.N.; Lara-García, H.A.; Díaz, G.; Muñiz, J. Exploring the CO2 conversion into hydrocarbons via a photocatalytic process onto M-doped titanate nanotubes (M = Ni and Cu). Fuel 2022, 324, 124440. [Google Scholar] [CrossRef]
- De la Rosa-Priego, F.A.; Gutierrez-López, E.D.; Zepeda, T.A.; Acosta-Alejandro, M.; Venezia, A.M.; Fuentes-Moyado, S.; Pawelec, B.; Díaz-de-León, J.N. Enhanced CO2 Hydrogenation to C2+ Hydrocarbons over Mesoporous x%Fe2O3–Al2O3 Catalysts. Ind. Eng. Chem. Res. 2021, 60, 18660–18671. [Google Scholar] [CrossRef]
- Witoon, T.; Permsirivanich, T.; Donphai, W.; Jaree, A.; Chareonpanich, M. CO2 hydrogenation to methanol over Cu/ZnO nanocatalysts prepared via a chitosan-assisted co-precipitation method. Fuel Process. Technol. 2013, 116, 72–78. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A.; et al. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Show, P.-L. A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Fourmentin, M.; Ribeiro, A.R.L.; Noutsopoulos, C.; Mapelli, F.; Fenyvesi, É.; Vieira, M.G.A.; Picos-Corrales, L.A.; Moreno-Piraján, J.C.; et al. Removal of emerging contaminants from wastewater using advanced treatments. A review. Environ. Chem. Lett. 2022, 20, 1333–1375. [Google Scholar] [CrossRef]
- Rodríguez, A.G.P.; López, M.I.R.; Casillas, Á.D.; León, J.A.A.; Banik, S.D. Impact of pesticides in karst groundwater. Review of recent trends in Yucatan, Mexico. Groundw. Sustain. Dev. 2018, 7, 20–29. [Google Scholar] [CrossRef]
- Castresana, G.P.; Roldán, E.C.; García Suastegui, W.A.; Morán Perales, J.L.; Cruz Montalvo, A.; Silva, A.H. Evaluation of Health Risks due to Heavy Metals in a Rural Population Exposed to Atoyac River Pollution in Puebla, Mexico. Water 2019, 11, 277. [Google Scholar] [CrossRef]
- Iber, B.T.; Okomoda, V.T.; Rozaimah, S.A.; Kasan, N.A. Eco-friendly approaches to aquaculture wastewater treatment: Assessment of natural coagulants vis-a-vis chitosan. Bioresour. Technol. Reports 2021, 15, 100702. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Morin-Crini, N.; Fourmentin, M.; Zemmouri, H.; do Carmo Nascimento, I.O.; Queiroz, L.M.; Tadza, M.Y.M.; Picos-Corrales, L.A.; Pei, H.; Wilson, L.D.; et al. Chitosan for direct bioflocculation of wastewater. Environ. Chem. Lett. 2019, 17, 1603–1621. [Google Scholar] [CrossRef]
- Picos-Corrales, L.A.; Sarmiento-Sánchez, J.I.; Ruelas-Leyva, J.P.; Crini, G.; Hermosillo-Ochoa, E.; Gutierrez-Montes, J.A. Environment-Friendly Approach toward the Treatment of Raw Agricultural Wastewater and River Water via Flocculation Using Chitosan and Bean Straw Flour as Bioflocculants. ACS Omega 2020, 5, 3943–3951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jing, R.; He, S.; Qian, J.; Zhang, K.; Ma, G.; Chang, X.; Zhang, M.; Li, Y. Coagulation of low temperature and low turbidity water: Adjusting basicity of polyaluminum chloride (PAC) and using chitosan as coagulant aid. Sep. Purif. Technol. 2018, 206, 131–139. [Google Scholar] [CrossRef]
- Hou, T.; Du, H.; Yang, Z.; Tian, Z.; Shen, S.; Shi, Y.; Yang, W.; Zhang, L. Flocculation of different types of combined contaminants of antibiotics and heavy metals by thermo-responsive flocculants with various architectures. Sep. Purif. Technol. 2019, 223, 123–132. [Google Scholar] [CrossRef]
- Wang, M.; Feng, L.; You, X.; Zheng, H. Effect of fine structure of chitosan-based flocculants on the flocculation of bentonite and humic acid: Evaluation and modeling. Chemosphere 2021, 264, 128525. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lee, S.J.; Jin, Y.J.; Jeon, Y.; Lee, S.J.; Kim, Y.; Kwon, G.; Hwang, D.Y.; Seo, S. Phenolic-modified cationic polymers as coagulants for microplastic removal. J. Ind. Eng. Chem. 2022; in press. [Google Scholar] [CrossRef]
- Divakaran, R.; Sivasankara Pillai, V.N. Flocculation of river silt using chitosan. Water Res. 2002, 36, 2414–2418. [Google Scholar] [CrossRef]
- Hermosillo-Ochoa, E.; Picos-Corrales, L.A.; Licea-Claverie, A. Eco-friendly flocculants from chitosan grafted with PNVCL and PAAc: Hybrid materials with enhanced removal properties for water remediation. Sep. Purif. Technol. 2021, 258, 118052. [Google Scholar] [CrossRef]
- Wu, P.; Yi, J.; Feng, L.; Li, X.; Chen, Y.; Liu, Z.; Tian, S.; Li, S.; Khan, S.; Sun, Y. Microwave assisted preparation and characterization of a chitosan based flocculant for the application and evaluation of sludge flocculation and dewatering. Int. J. Biol. Macromol. 2020, 155, 708–720. [Google Scholar] [CrossRef]
- Vedula, S.S.; Yadav, G.D. Chitosan-based membranes preparation and applications: Challenges and opportunities. J. Indian Chem. Soc. 2021, 98, 100017. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Lotfy, T.M.R.; Shawir, S.M.S. Preparation and antibacterial activity of chitosan-silver nanoparticles for application in preservation of minced meat. Bull. Natl. Res. Cent. 2019, 43, 83. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Bousserrhine, N.; Alphonse, V.; Le Floch, F.; Charif Mechiche, Y.; Menidjel, I.; Carbonnier, B. Chitosan based self-assembled nanocapsules as antibacterial agent. Colloids Surf. B Biointerfaces 2019, 181, 158–165. [Google Scholar] [CrossRef]
- Khalil, M.; Hashmi, U.; Riaz, R.; Rukh Abbas, S. Chitosan coated liposomes (CCL) containing triamcinolone acetonide for sustained delivery: A potential topical treatment for posterior segment diseases. Int. J. Biol. Macromol. 2020, 143, 483–491. [Google Scholar] [CrossRef]
- Kathle, P.K.; Gautam, N.; Kesavan, K. Tamoxifen citrate loaded chitosan-gellan nanocapsules for breast cancer therapy: Development, characterisation and in-vitro cell viability study. J. Microencapsul. 2018, 35, 292–300. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Tajali Ardakani, M.; Hajipour Verdom, B.; Bagheri, A.; Mohammad-Beigi, H.; Aliakbari, F.; Salehpour, Z.; Alipour, M.; Afrouz, S.; Bardania, H. Chitosan nanoparticles containing Physalis alkekengi-L extract: Preparation, optimization and their antioxidant activity. Bull. Mater. Sci. 2019, 42, 131. [Google Scholar] [CrossRef]
- Palacio, J.; Monsalve, Y.; Ramírez-Rodríguez, F.; López, B. Study of encapsulation of polyphenols on succinyl-chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 57, 101610. [Google Scholar] [CrossRef]
- Al-Moghazy, M.; El-sayed, H.S.; Salama, H.H.; Nada, A.A. Edible packaging coating of encapsulated thyme essential oil in liposomal chitosan emulsions to improve the shelf life of Karish cheese. Food Biosci. 2021, 43, 101230. [Google Scholar] [CrossRef]
- Coyotl-Pérez, W.A.; Rubio-Rosas, E.; Morales-Rabanales, Q.N.; Ramírez-García, S.A.; Pacheco-Hernández, Y.; Pérez-España, V.H.; Romero-Arenas, O.; Villa-Ruano, N. Improving the Shelf Life of Avocado Fruit against Clonostachys rosea with Chitosan Hybrid Films Containing Thyme Essential Oil. Polymers 2022, 14, 2050. [Google Scholar] [CrossRef]
- Jiménez-Regalado, E.J.; Caicedo, C.; Fonseca-García, A.; Rivera-Vallejo, C.C.; Aguirre-Loredo, R.Y. Preparation and Physicochemical Properties of Modified Corn Starch—Chitosan Biodegradable Films. Polymers 2021, 13, 4431. [Google Scholar] [CrossRef]
- Liang, W.; Liu, X.; Zheng, J.; Zhao, W.; Su, C.; Ge, X.; Shen, H.; Zhang, X.; Lu, Y.; Muratkhan, M.; et al. Insight into crosslinked chitosan/soy protein isolate /PVA plastics by revealing its structure, physicochemical properties, and biodegradability. Ind. Crop. Prod. 2022, 187, 115548. [Google Scholar] [CrossRef]
- Vosoughi, N.; Gomarian, M.; Ghasemi Pirbalouti, A.; Khaghani, S.; Malekpoor, F. Essential oil composition and total phenolic, flavonoid contents, and antioxidant activity of sage (Salvia officinalis L.) extract under chitosan application and irrigation frequencies. Ind. Crop. Prod. 2018, 117, 366–374. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, J.; Garamus, V.M.; Li, N.; Zou, A. Preparation of an Environmentally Friendly Formulation of the Insecticide Nicotine Hydrochloride through Encapsulation in Chitosan/Tripolyphosphate Nanoparticles. J. Agric. Food Chem. 2018, 66, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Abid, S.; Azam, A.; Rehman, A. Synthesis of alpha-tocopherol encapsulated chitosan nano-assemblies and their impregnation on cellulosic fabric for potential antibacterial and antioxidant cosmetotextiles. Cellulose 2020, 27, 1717–1731. [Google Scholar] [CrossRef]
- Rehan, M.; El-Naggar, M.E.; Mashaly, H.M.; Wilken, R. Nanocomposites based on chitosan/silver/clay for durable multi-functional properties of cotton fabrics. Carbohydr. Polym. 2018, 182, 29–41. [Google Scholar] [CrossRef]
- Alkabli, J.; Rizk, M.A.; Elshaarawy, R.F.M.; El-Sayed, W.N. Ionic chitosan Schiff bases supported Pd(II) and Ru(II) complexes; production, characterization, and catalytic performance in Suzuki cross-coupling reactions. Int. J. Biol. Macromol. 2021, 184, 454–462. [Google Scholar] [CrossRef]
- Tzereme, A.; Christodoulou, E.; Kyzas, G.; Kostoglou, M.; Bikiaris, D.; Lambropoulou, D. Chitosan Grafted Adsorbents for Diclofenac Pharmaceutical Compound Removal from Single-Component Aqueous Solutions and Mixtures. Polymers 2019, 11, 497. [Google Scholar] [CrossRef]
- Crini, G. Chitin and Chitosan; Elsevier: London, UK, 2022; ISBN 9780323961196. [Google Scholar]
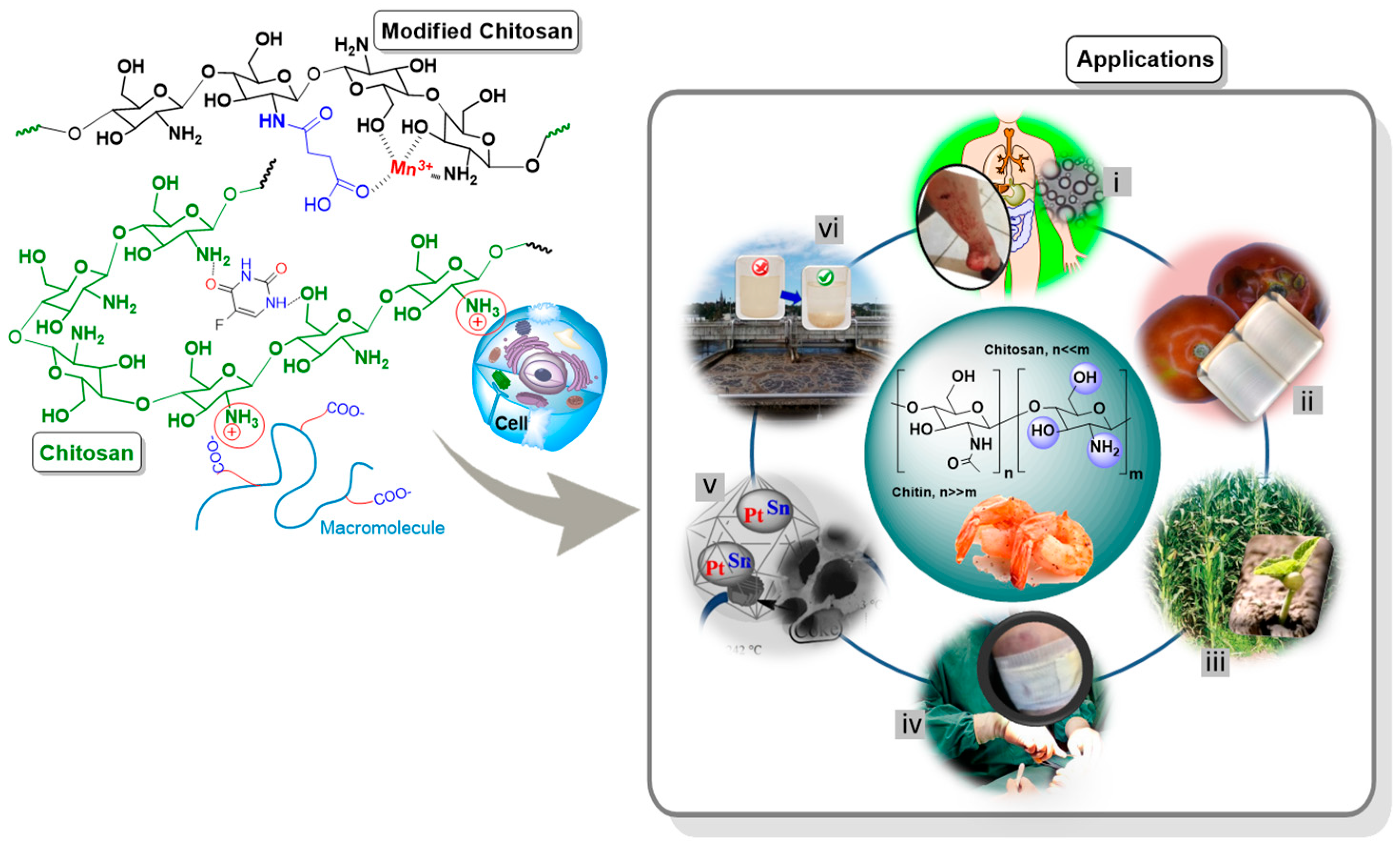





| Material/System | Purpose | Result | Ref. |
|---|---|---|---|
| Chitosan-silver nanoparticles (Ch-AgNPs) | Bacterial growth inhibition in food | Escherichia coli was more susceptible to Ch-AgNPs than Salmonella typhimurium. In vivo antibacterial activity against Escherichia coli revealed excellent activity compared with single chitosan | [167] |
| Hollow nanoparticles from chitosan and alginate | Bacterial growth inhibition | Flexible capsules inhibited microbial growth more strongly than rigid particles. The inhibitory effect was from 18.6% to 34.9% for Staphylococcus aureus and from 23.7% to 40% for Escherichia coli | [168] |
| Conventional liposomes-triamcinolone acetonide coated with chitosan | Topical drug delivery system | High encapsulation efficiency (74%), suitable particle size of 176 nm, high positive surface charge (+41.1 mV, high stability), increased retention time, and maximum drug release of around 73% | [169] |
| Chitosan-gellan nanocapsules containing tamoxifen citrate | Drug encapsulation for breast cancer therapy | Spherical shape with particle size = 242 nm and zeta potential = 39 mV (value usually associated with high stability), providing sustained drug release and increased cytotoxicity against breast cancer cells (∼90%) | [170] |
| Chitosan nanoparticles containing Physalis alkekengi-L extract | Phytochemicals encapsulation: antioxidant compounds | Suitable particle size = 196 nm, zeta potential around 8 mV, and high percentage of encapsulated extract, close to 95%; resulting in improved stability and antioxidant activity of the P. alkekengi-L extract | [171] |
| Succinyl-chitosan nanoparticles | Phytochemicals encapsulation: antioxidant compounds | Encapsulation efficiency of 88%, 65%, and 27% for gallic acid, epigallocatechin-3-gallate, and propyl gallate, respectively. Encapsulation process governed by both the ability to form hydrogen bonds and the size of the guest molecules | [172] |
| Liposomal chitosan emulsions containing thyme essential oil | Phytochemicals encapsulation in edible coating | Emulsions were stable over 2 months at 4 °C. The Karish cheese preserved with the edible coating showed antimicrobial activity over 4 weeks, thus the shelf life of the product was extended | [173] |
| Chitosan-thyme essential oil films | Film containing in food packaging | Excellent antifungal activity against Clonostachys rosea. Conservation of fruit firmness, nutritional composition, and nutraceutical content, resulting in improved shelf life of Hass avocadoes | [174] |
| Corn starch–chitosan | Biodegradable film as packaging for food | Chitosan interacts effectively with starch, improving tensile strength, thermal stability, hydrophobicity, water adsorption capacity, and the gas barrier of starch films | [175] |
| Cross-linked chitosan/soybean protein isolate/polyvinyl alcohol | Hybrid plastic for packaging | Excellent compatibility of chitosan and soybean protein reducing the plastic surface roughness and enhancing mechanical properties, yielding superior water resistance compared to pure PVA. Hybrid plastic with desirable degradability | [176] |
| Chitosan | Foliar application | Reduced adverse effects of limited irrigation on essential oil yield, improved essential oil content, and positive influence on the amount of secondary metabolites. The antioxidant activity of sage (Salvia officinalis L.) was increased | [177] |
| Chitosan-tripolyphosphate nanoparticles containing nicotine hydrochloride | Insecticide encapsulation | Encapsulation efficiency of 55%, physicochemical stability (45 days) with particle size around 300 nm, and zeta potential close to 50 mV. Less than 20% of the insecticide was released within 24 h. The 24 h mortality of the formulation was 95% (against Musca domestica) | [178] |
| Chitosan-spinosad formulation | Insecticide encapsulation | High encapsulation efficiency (60%). Long sustained-release time (>18 days) and high cumulative release (>80%). Outstanding UV shielding ability of chitosan protecting spinosad from photodegradation | [3] |
| Emulsions from chitosan and alpha-tocopherol | Impregnation of cellulosic fabric for cosmetotextiles | Treated fabric with a slight decrease in absorbency and tensile strength, and good antibacterial (against Escherichia coli and Staphylococcus aureus) and antioxidant activities (36.78 unit g−1) | [179] |
| Nanocomposites based on chitosan/silver/clay | Treatment for cotton fabrics | Uniform morphology, high strength, flame retardant, high water absorption, high antimicrobial activity (against Escherichia coli and Staphylococcus aureus, >98%), controlled release of Lavender oil (odor retention even after 3 months), and UV protection | [180] |
| Scaffolds (imidazolium-vanillyl-chitosan Schiff bases (IVCSSBs)) for supporting Pd(II) | Catalytic systems | Heterogeneous catalyst with high catalytic activity (up to 99%) and stability in the reaction medium. Reusable materials with comparable catalytic activity after five operation runs. Excellent selectivity toward the Suzuki cross-coupling reaction | [181] |
| Cross-linked carboxyl-grafted chitosan derivatives | Wastewater treatment | Higher diclofenac removal (92.8%) using chitosan grafted with trans-aconitic acid, compared to succinic anhydride (80.9%) and maleic anhydride (66.2%) as grafting agents. Higher removal for diclofenac from a mixture with salicylic acid, ibuprofen, and ketoprofen | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picos-Corrales, L.A.; Morales-Burgos, A.M.; Ruelas-Leyva, J.P.; Crini, G.; García-Armenta, E.; Jimenez-Lam, S.A.; Ayón-Reyna, L.E.; Rocha-Alonzo, F.; Calderón-Zamora, L.; Osuna-Martínez, U.; et al. Chitosan as an Outstanding Polysaccharide Improving Health-Commodities of Humans and Environmental Protection. Polymers 2023, 15, 526. https://doi.org/10.3390/polym15030526
Picos-Corrales LA, Morales-Burgos AM, Ruelas-Leyva JP, Crini G, García-Armenta E, Jimenez-Lam SA, Ayón-Reyna LE, Rocha-Alonzo F, Calderón-Zamora L, Osuna-Martínez U, et al. Chitosan as an Outstanding Polysaccharide Improving Health-Commodities of Humans and Environmental Protection. Polymers. 2023; 15(3):526. https://doi.org/10.3390/polym15030526
Chicago/Turabian StylePicos-Corrales, Lorenzo A., Ana M. Morales-Burgos, Jose P. Ruelas-Leyva, Grégorio Crini, Evangelina García-Armenta, Sergio A. Jimenez-Lam, Lidia E. Ayón-Reyna, Fernando Rocha-Alonzo, Loranda Calderón-Zamora, Ulises Osuna-Martínez, and et al. 2023. "Chitosan as an Outstanding Polysaccharide Improving Health-Commodities of Humans and Environmental Protection" Polymers 15, no. 3: 526. https://doi.org/10.3390/polym15030526
APA StylePicos-Corrales, L. A., Morales-Burgos, A. M., Ruelas-Leyva, J. P., Crini, G., García-Armenta, E., Jimenez-Lam, S. A., Ayón-Reyna, L. E., Rocha-Alonzo, F., Calderón-Zamora, L., Osuna-Martínez, U., Calderón-Castro, A., De-Paz-Arroyo, G., & Inzunza-Camacho, L. N. (2023). Chitosan as an Outstanding Polysaccharide Improving Health-Commodities of Humans and Environmental Protection. Polymers, 15(3), 526. https://doi.org/10.3390/polym15030526