Exploitation of a New Nucleating Agent by Molecular Structure Modification of Aryl Phosphate and Its Effect on Application Properties of the Polypropylene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
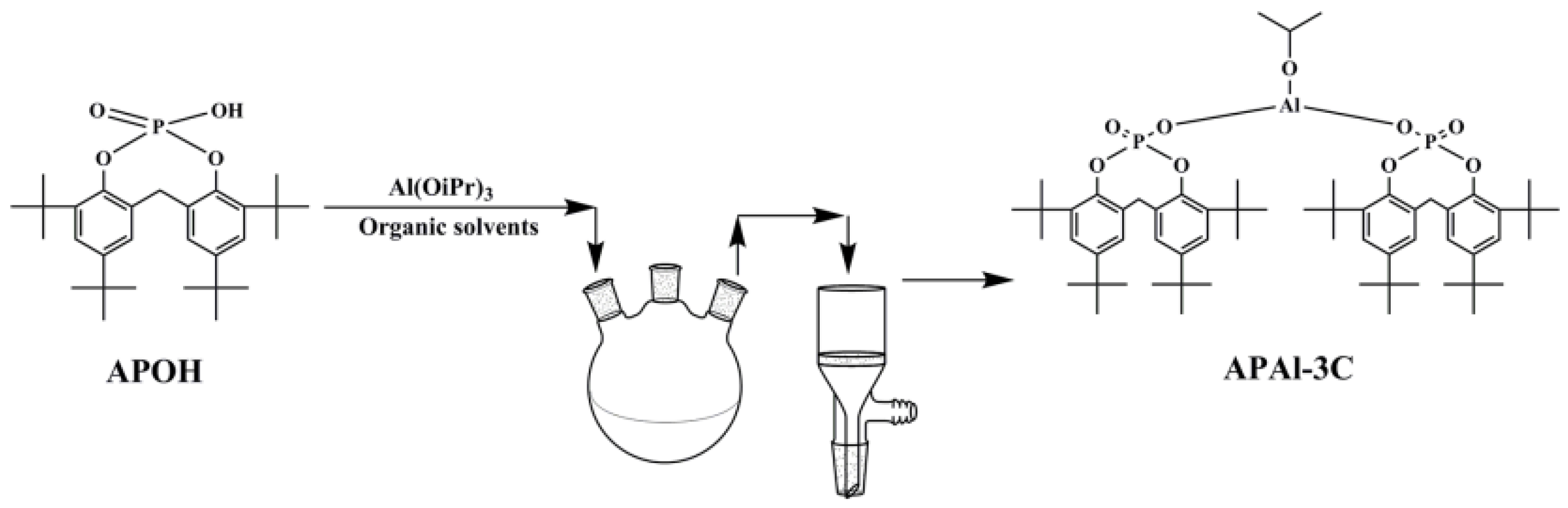
2.2. Preparation of APAl-3C
2.3. Preparation of the Nucleating Agent
2.4. Preparation of the PP Composites
2.5. Measurement and Characterization
3. Result and Discussion
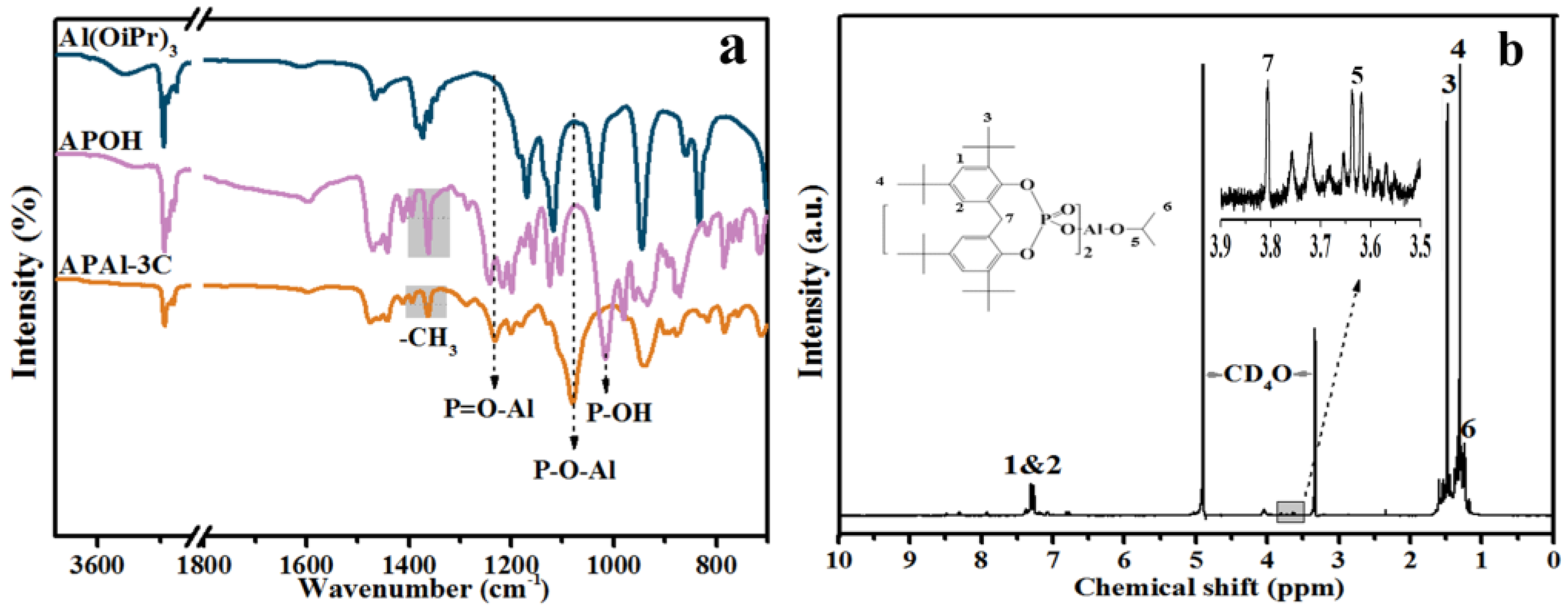
3.1. Characterization of APAl-3C
3.2. Non-Isothermal Crystallization Behavior of the PP Composites
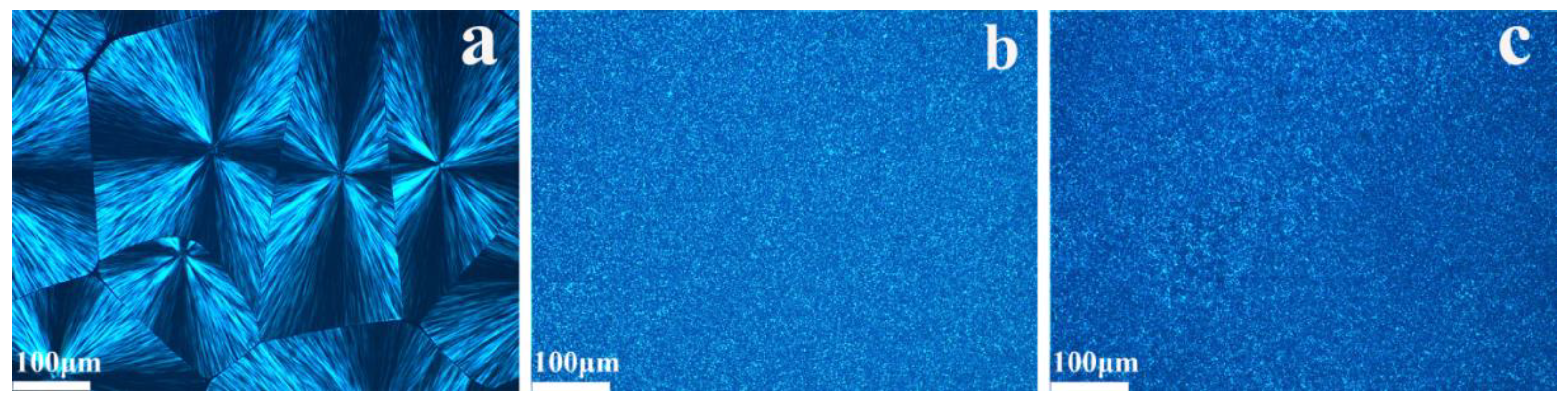
3.3. POM of the PP Composites
3.4. Mechanical Properties of the PP Composites
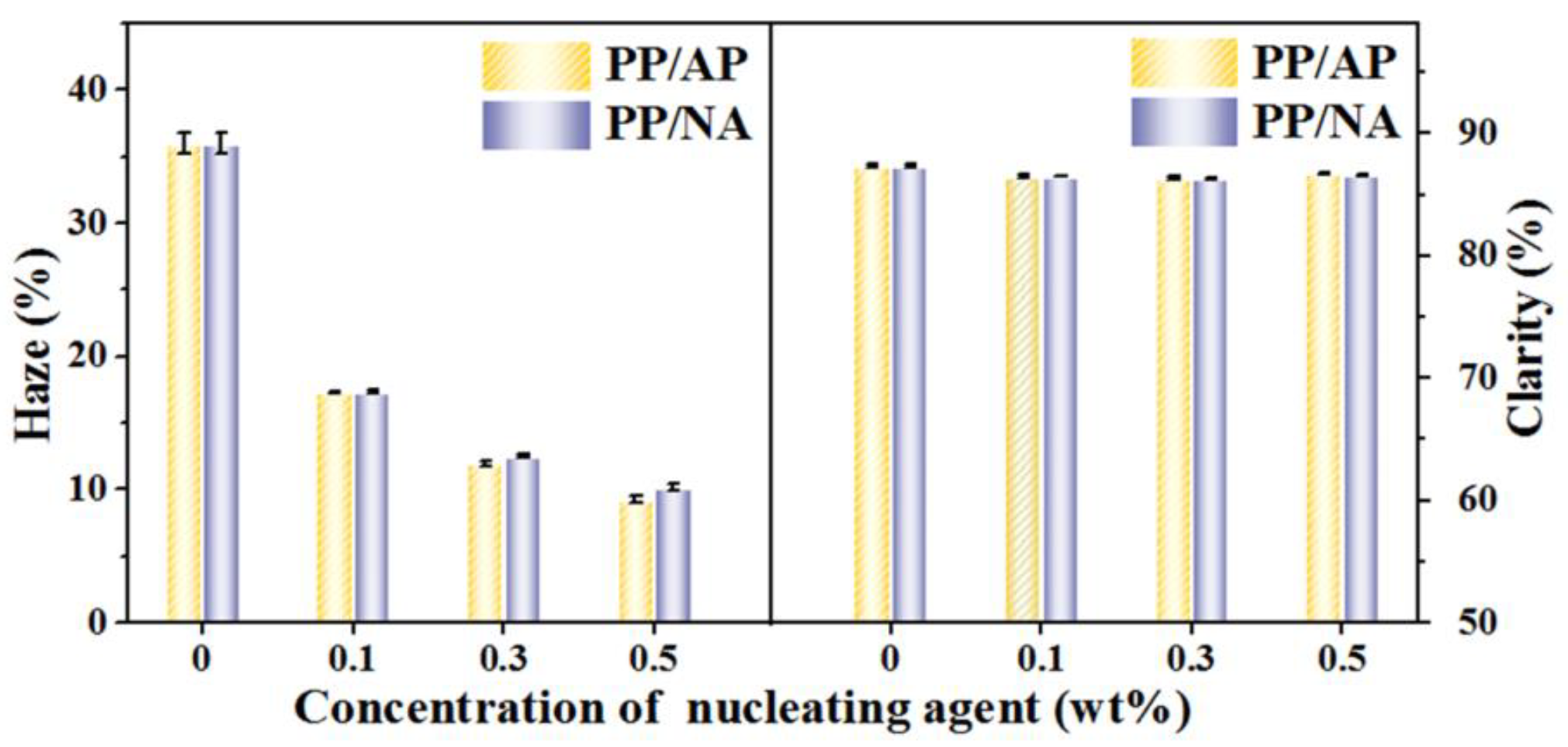
3.5. Optical Property of the PP Composites
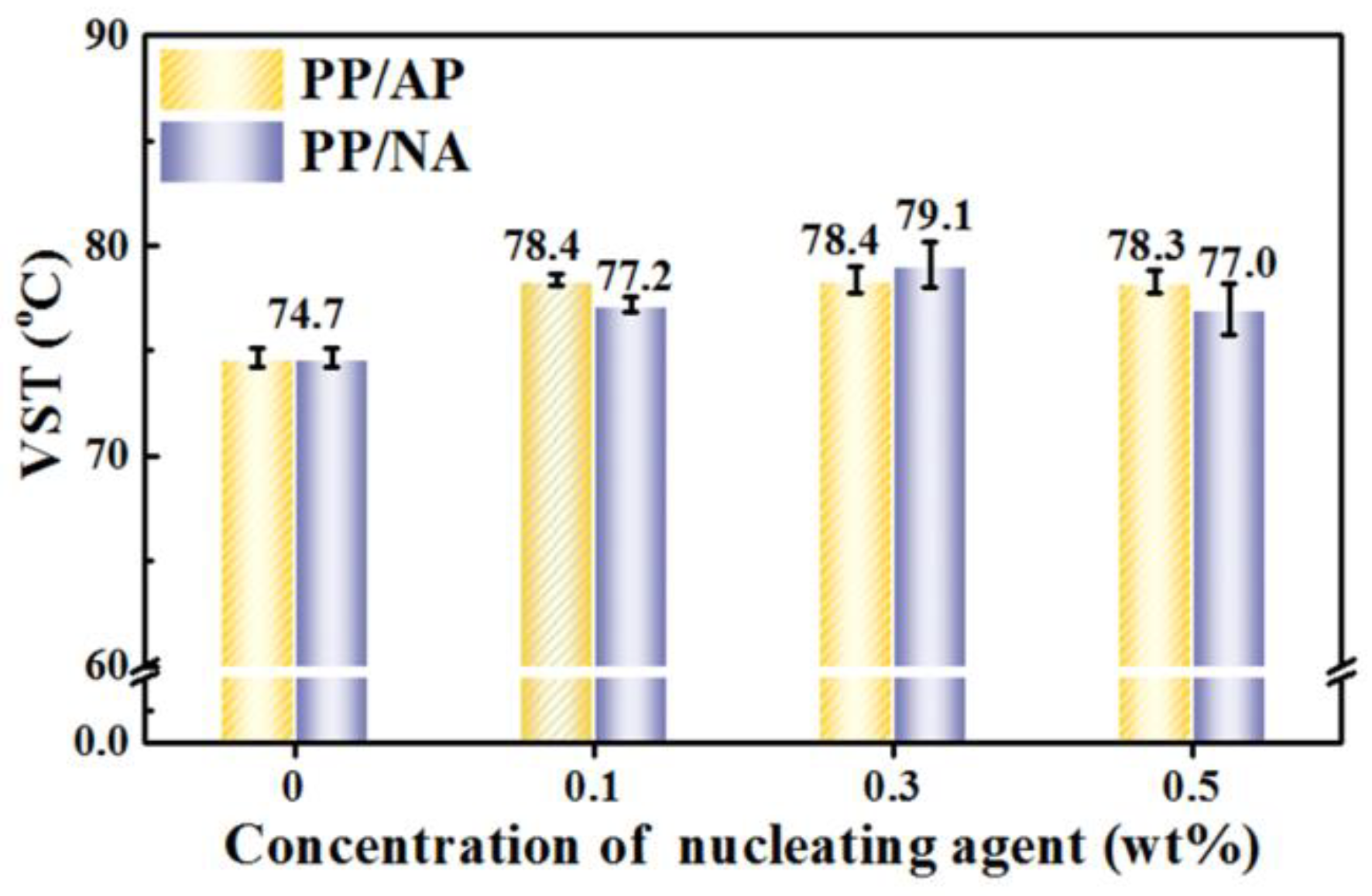
3.6. VST of the PP Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, G.; Xin, Z.; Yu, J.; Gui, Q.; Wang, S. Nucleating efficiency of organic phosphates in polypropylene. J. Macromol. Sci. B 2003, 42, 467–478. [Google Scholar] [CrossRef]
- Zsuzsanna, H.; Benjámin, G.; Alfréd, M.; Petar, D.; Markus, G.; Jozsef, V.; Bela, P. The role of solubility and critical temperatures for the efficiency of sorbitol clarifiers in polypropylene. RSC Adv. 2014, 4, 19737–19745. [Google Scholar]
- Wang, B.; Lin, F.H.; Li, X.Y.; Zhang, Z.W.; Xue, X.R.; Liu, S.X.; Ji, X.R.; Yu, Q.; Yuan, Z.Q.; Chen, X.D.; et al. Isothermal Crystallization and Rheology Properties of Isotactic Polypropylene/Bacterial Cellulose Composite. Polymers 2018, 10, 1284. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lin, F.H.; Li, X.Y.; Ji, X.R.; Liu, S.X.; Han, X.J.; Yuan, Z.Q.; Luo, J. Transcrystallization of Isotactic Polypropylene/Bacterial Cellulose Hamburger Composite. Polymers 2019, 11, 508. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Burke, U.; Ramalingam, A.K.; Lee, C.; Davis, A.C.; Cai, L.; Selim, H.; Fernandes, R.X.; Heufer, K.A.; Sarathy, S.M.; et al. Oxidation of 2-methylfuran and 2-methylfuran/n-heptane blends: An experimental and modeling study. Combust. Flame 2018, 196, 54–70. [Google Scholar] [CrossRef]
- Gebhardt, M.; Manolakis, I.; Chatterjee, A.; Kalinka, G.; Deubener, J.; Pfnür, H.; Chakraborty, S.; Meiners, D. Reducing the raw material usage for room temperature infusible and polymerisable thermoplastic CFRPs through reuse of recycled waste matrix material. Compos. Part B Eng. 2021, 216, 108877. [Google Scholar] [CrossRef]
- Yang, T.; Cheng, Y.; Wu, Y.; Yu, B.; Huang, T.; Yu, H. Enhanced crosslinking of polypropylene in γ-irradiation via copper(Ⅱ) doping. Radiat. Phys. Chem. 2022, 194, 110042. [Google Scholar] [CrossRef]
- Sukhonthamethirat, N.; Soongnern, V.; Okamoto, S.; Sakurai, S. Morphology and crystallization kinetics of g-resins and isotactic polypropylene. Polym. Eng. Sci. 2022, 62, 1775–1785. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, S.; Hua, Z.; Niu, H. Influence of isotactic polypropylene grafted with styryl-group on the polymer crystallization behavior. Polym. Test. 2022, 108, 107508–107517. [Google Scholar] [CrossRef]
- Navratilova, L. Long-chain branched polypropylene: Crystallization under high pressure and polymorphic composition. J. Therm. Anal. Calorim. 2021, 143, 3377–3383. [Google Scholar] [CrossRef]
- Luo, D.J.; Shao, H.J.; Wei, F.J.; Zhang, K.Z.; Qin, S.H. Morphology and isothermal crystallization kinetics of polypropylene/poly(ethylene-co-vinyl alcohol) blends. Int. Polym. Proc. 2019, 34, 195–208. [Google Scholar] [CrossRef]
- Chen, P.; Xu, M.; Li, X.M.; Han, Y.; Ding, J.; Lin, Y.; Liu, G.; Zhang, X.; Chen, L.; Tian, X. The influence of melt status and beta-nucleation agent distribution on the crystallization of isotactic polypropylene. CrystEngComm 2022, 24, 2429–2445. [Google Scholar] [CrossRef]
- Nosova, N.; Roiter, Y.; Samaryk, V.; Varvarenko, S.; Stetsyshyn, Y.; Minko, S.; Stamm, M.; Voronov, S. Polypropylene surface peroxidation with heterofunctional polyperoxides. Macromol. Symp. 2004, 210, 339–348. [Google Scholar] [CrossRef]
- Mendible, G.A.; Saleh, N.; Barry, C.; Johnston, S.P. Mechanical properties and crystallinity of polypropylene injection molded in polyjet and aluminum tooling. Rapid. Prototyp. J. 2022, 28, 686–694. [Google Scholar] [CrossRef]
- Cali, J.; Hu, W.; Kysor, E.; Kohlmann, O. Aging of polypropylene random copolymers studied by nmr relaxometry. Polymer 2021, 230, 124102. [Google Scholar] [CrossRef]
- Karimipour, A.; Brito, J.D. Influence of polypropylene fibres and silica fume on the mechanical and fracture properties of ultra-high-performance geopolymer concrete. Constr. Build. Mater. 2021, 283, 122753. [Google Scholar] [CrossRef]
- Shen, J.; Li, X.; Yan, X. Mechanical and acoustic properties of jute fiber-reinforced polypropylene composites. ACS Omega 2021, 6, 31154–31160. [Google Scholar] [CrossRef]
- Reshma, T.V.; Manjunatha, M.; Bharath, A.; Tangadagi, R.B.; Vengala, J.; Manjunatha, L.R. Influence of ZnO and TiO2 on mechanical and durability properties of concrete prepared with and without polypropylene fibers. Materialia 2021, 18, 101138. [Google Scholar] [CrossRef]
- Nair, S.T.; Poornima, V.P.; George, S.C.; Kalarikkal, N.; Thomas, S. Enhanced mechanical and thermal performance of multiwalled carbon nanotubes-filled polypropylene/natural rubber thermoplastic elastomers. New. J. Chem. 2021, 45, 4963–4976. [Google Scholar] [CrossRef]
- Patterson, A.K.; El-Qarra, L.H.; Smith, D.K. Chirality-directed hydrogel assembly and interactions with enantiomers of an active pharmaceutical ingredient. Chem. Commun. 2022, 58, 3941–3944. [Google Scholar] [CrossRef]
- Fan, K.; Wang, L.; Wei, W.; Wen, F.; Xu, Y.; Zhang, X.; Guan, X. Multifunctional self-healing eutectogels induced by supramolecular assembly for smart conductive materials, interface lubrication and dye adsorption. Chem. Eng. J. 2022, 441, 136026. [Google Scholar] [CrossRef]
- Kubwabo, C.; Fan, X.; Katuri, G.P.; Habibagahi, A.; Rasmussen, P.E. Occurrence of aryl and alkyl-aryl phosphates in canadian house dust. Emerg. Contam. 2021, 7, 149–159. [Google Scholar] [CrossRef]
- Yu, Y.; Mo, W.; Zhu, X.; Yu, X.; Sun, J.; Deng, F.; Jin, L.; Yin, H.; Zhu, L. Biodegradation of tricresyl phosphates isomers by a novel microbial consortium and the toxicity evaluation of its major products. Sci. Total Environ. 2022, 828, 154415–154423. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, J.; Yu, Y.; Bai, H.; Zhang, Z.; Huang, Y. Transparent MgO-Al2O3-SiO2 glass-ceramics prepared with ZrO2 and SnO2 as nucleating agents. J. Non-Cryst. Solids 2022, 588, 121585–121590. [Google Scholar] [CrossRef]
- Thamizhlarasan, A.; Meenarathi, B.; Parthasarathy, V.; Jancirani, A.; Anbarasan, R. Effect of nucleating agents on the non-isothermal crystallization and degradation kinetics of poly(ethylene terephthalate). Polym. Adv. Technol. 2021, 32, 766–778. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, P.; Wu, X.; Li, Y.; Xu, T. Modular and facile access to chiral α-aryl phosphates via dual nickel-and photoredox-catalyzed reductive cross-coupling. J. Am. Chem. Soc. 2022, 144, 3989–3997. [Google Scholar] [CrossRef]
- Bonesi, S.; Protti, S.; Fagnoni, M. Photohomolysis and photoheterolysis in aryl sulfonates and aryl phosphates. Chemistry 2021, 27, 6315–6323. [Google Scholar] [CrossRef]
- Qrareya, H.; Meazza, L.; Protti, S.; Fagnoni, M. Metal-free synthesis of biarenes via photoextrusion in di(tri)aryl phosphates. Beilstein J. Org. Chem. 2020, 16, 3008–3014. [Google Scholar] [CrossRef]
- Min, G.; Li, J.Q.; Zhuang, M.; Yang, Z.; Pan, G.Y.; Hao, Y. Modification of polypropylene with composite nucleating agents containing 2,2-methylene-bis-(4,6-di-t-butylphenylene) phosphate/attapulgite/silica nano-particles. Acta Polym. Sin. 2014, 8, 1124–1134. [Google Scholar]
- Zhang, Y.F.; He, B.; Hou, H.H.; Guo, L.H. Isothermal crystallization of isotactic polypropylene nucleated with a novel aromatic heterocyclic phosphate nucleating agent. J. Macromol. Sci. Part B 2017, 56, 811–820. [Google Scholar] [CrossRef]
- Qin, W.; Zhang, X.X.; Shao, L.; Xin, Z.; Ling, H.; Zhao, S. Failure mechanism of zinc adipate as a β-nucleating agent for polypropylene in the presence of calcium stearate. Polymer 2021, 215, 123374–123382. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Luo, X.X.; Zhu, L.; Yang, X.-J.; Chang, Y. Effects of α/β compound nucleating agents on mechanical properties and crystallization behaviors of isotactic polypropylene. J. Macromol. Sci. Part B 2012, 51, 2352–2360. [Google Scholar] [CrossRef]
- Kilic, A.; Shim, E.; Yeom, B.Y.; Pourdeyhimi, B. Effect of dmdbs (3:2,4-bis (3,4-dimethyldibenzylidene) sorbitol) and NA11 (sodium 2,2′-methylene-bis (4,6-di-tertbutylphenyl)-phosphate) on electret properties of polypropylene filaments. J. Appl. Polym. Sci. 2013, 130, 2068–2075. [Google Scholar] [CrossRef]
- Zhai, P.; Shi, C.; Zhao, S.; Liu, W.; Wang, W.; Yao, L. Thermal decomposition of ammonium perchlorate-based molecular perovskite from TG-DSC-FTIR-MS and ab initio molecular dynamics. RSC Adv. 2021, 11, 16388–16395. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Xu, C.; Gao, K.; Yang, H.; Liu, Y.; Fan, Z.; Liu, S.; Qin, Y.; Yu, H.; Li, P. Characterization of different salt forms of chitooligosaccharides and their effects on nitric oxide secretion by macrophages. Molecules 2021, 26, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Morth, C.M.; Rosqvist, G.; Chalov, S.R.; Efimov, V.; Jarsjo, J. Microbial sulfate reduction (MSE) as a nature-based solution (NBS) to mine drainage: Contrasting spatiotemporal conditions in northern europe. Water Resour. Res. 2022, 58, e2021WR031777. [Google Scholar] [CrossRef]
- Pieczka, A.; Ertl, A.; Gołębiowska, B.; Jeleń, P.; Kotowski, J.; Nejbert, K.; Stachowicz, M.; Giester, G. Crystal structure and Raman spectroscopic studies of OH stretching vibrations in Zn-rich fluor-elbaite. Am. Miner. 2020, 105, 1622–1630. [Google Scholar] [CrossRef]
- Drygas, M.; Lejda, K.; Janik, J.F.; Stelmakh, S.; Palosz, B. Novel composite nitride nanoceramics from reaction-mixed nanocrystalline powders in the system aluminum nitride aln/gallium nitride gan/titanium nitride tin (Al:Ga:Ti = 1:1:1). Materials 2022, 15, 2200. [Google Scholar] [CrossRef]
- Al-Jumaili, M.; Hamed, A.S.; Akkurt, N.; Torun, L. Six-armed structures based on benzene ring, synthesis and characterization via sonogashira coupling. Indone. J. Chem. 2020, 20, 705. [Google Scholar] [CrossRef]
- Starovoytova, L.; SpěváEk, J.; Hanyková, L.; Ilavsky, M. 1H NMR study of thermotropic phase transitions in D2O solutions of poly(n-isopropylmethacrylamide)/poly(vinyl methyl ether) mixtures. Polymer 2004, 45, 5905–5911. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, M.P.; Dionysiou, D.D.; Yuan, B.; Fu, M.L. Interplay of bicarbonate and the oxygen-containing groups of carbon nanotubes dominated the metal-free activation of peroxymonosulfate. Chem. Eng. J. 2022, 430, 133102. [Google Scholar] [CrossRef]
- Tabak, M. Preparation and characterization of a novel activated carbon component via chemical activation of tea woody stem. J. Therm. Anal. Calorim. 2019, 138, 3885–3895. [Google Scholar] [CrossRef]
- Su, W.; Jiang, Y.; Zuo, X. Engineering nucleation/crystallization to intensify the enzymatic reactions and fermentation: A review. Chem. Eng. J. 2022, 431, 134186. [Google Scholar] [CrossRef]
- Gorbunova, M.A.; Komov, E.V.; Grunin, L.Y.; Ivanova, M.S.; Abukaev, A.F.; Imamutdinova, A.M. The effect of separation of blocks on the crystallization kinetics and phase composition of poly(butylene adipate) in multi-block thermoplastic polyurethanes. Phys. Chem. Chem. Phys. 2022, 24, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Mccreath, S.; Boinard, P.; Boinard, E.; Gritter, P.; Liggat, J.J. High clarity poly(caprolactone diol)-based polyurethane adhesives for polycarbonate lamination: Effect of isocyanate and chain-extender. Int. J. Adhes. Adhes. 2018, 86, 84–97. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Bezek, L.B.; Park, B.; Lee, K.-S. Use of nucleating agent NA11 in the preparation of polyvinylidene fluoride dual-layer hollow fiber membranes. Membranes 2023, 13, 75–84. [Google Scholar] [CrossRef]
- Thakur, V.K.; Vennerberg, D.; Kessler, M.R. Green aqueous surface modification of polypropylene for novel polymer nanocomposites. Acs Appl. Mater. Inter. 2014, 6, 9349–9356. [Google Scholar] [CrossRef]
- Joshi, J.; Lehman, R.; Hall, G.S. Insight into the molecular arrangement of high-density polyethylene polymer chains in blends of polystyrene/highdensity polyethylene from differential scanning calorimetry and raman techniques. Appl. Spectrosc. 2006, 60, 483–489. [Google Scholar] [CrossRef]
- Sowinski, P.; Piorkowska, E.; Boyer, S.; Haudin, J.M.; Zapala, K. The role of nucleating agents in high-pressure-induced gamma crystallization in isotactic polypropylene. Colloid. Polym. Sci. 2015, 293, 665–675. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Luo, K.; Huang, Y. Translation and rotation of spherulites during the crystallization of isotactic polypropylene with reduced chain entanglements. Macromolecules 2011, 44, 2844–2851. [Google Scholar] [CrossRef]
- Zhao, D.; Gimenez-Pinto, V.; Jimenez, A.M.; Zhao, L.; Jestin, J.; Kumar, S.K.; Kuei, B.; Gomez, E.D.; Prasad, A.S.; Schadler, L.S.; et al. Tunable multiscale nanoparticle ordering by polymer crystallization. ACS Central Sci. 2017, 3, 751–758. [Google Scholar] [CrossRef] [PubMed]




| Samples | PP/g | APAl-3C-12Li/g | NA-21/g |
|---|---|---|---|
| PP | 1000 | 0 | 0 |
| PP/AP-1 | 999 | 1 | 0 |
| PP/AP-2 | 997 | 3 | 0 |
| PP/AP-3 | 995 | 5 | 0 |
| PP/NA-1 | 999 | 0 | 1 |
| PP/NA-2 | 997 | 0 | 3 |
| PP/NA-3 | 995 | 0 | 5 |
| Samples | Tc,p (°C) | ΔTc,p (°C) | ΔHc (J/g) | Samples | Tc,p (°C) | ΔTc,p (°C) | ΔHc (J/g) |
|---|---|---|---|---|---|---|---|
| PP | 107.6 | - | 105.6 | - | - | - | - |
| PP/AP-1 | 116.7 | 9.1 | 99.1 | PP/NA-1 | 116.5 | 8.9 | 105.0 |
| PP/AP-2 | 118.5 | 10.9 | 105.0 | PP/NA-2 | 118.3 | 10.7 | 102.0 |
| PP/AP-3 | 118.8 | 11.2 | 110.4 | PP/NA-3 | 118.9 | 11.3 | 93.9 |
| Samples | Tm,p (°C) | ΔTm,p (°C) | ΔHm (J/g) | Samples | Tm,p (°C) | ΔTm,p (°C) | ΔHm (J/g) |
|---|---|---|---|---|---|---|---|
| PP | 146.7 | - | 92.3 | - | - | - | - |
| PP/AP-1 | 148.7 | 2.0 | 98.2 | PP/NA-1 | 149.1 | 2.4 | 105.9 |
| PP/AP-2 | 149.8 | 3.1 | 106.7 | PP/NA-2 | 149.3 | 2.6 | 99.7 |
| PP/AP-3 | 150.1 | 3.4 | 111.6 | PP/NA-3 | 148.6 | 1.9 | 96.4 |
| Samples | Tensile Strength (MPa) | Flexural Modulus (MPa) | Impact Strength (kJ/m2) |
|---|---|---|---|
| PP | 25.9 | 952.7 | 4.0 |
| PP/AP-1 | 28.3 | 1058.4 | 5.1 |
| PP/AP-2 | 28.2 | 1084.1 | 4.9 |
| PP/AP-3 | 28.2 | 1105.8 | 4.8 |
| PP/NA-1 | 28.1 | 1068.2 | 5.0 |
| PP/NA-2 | 28.2 | 1089.8 | 4.7 |
| PP/NA-3 | 28.5 | 1108.5 | 4.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, F.; Zhang, M.; Zhao, T.; Zhang, Y.; Ning, D.; Cui, W.; Li, Y.; Chen, X.; Luo, J. Exploitation of a New Nucleating Agent by Molecular Structure Modification of Aryl Phosphate and Its Effect on Application Properties of the Polypropylene. Polymers 2023, 15, 4730. https://doi.org/10.3390/polym15244730
Lin F, Zhang M, Zhao T, Zhang Y, Ning D, Cui W, Li Y, Chen X, Luo J. Exploitation of a New Nucleating Agent by Molecular Structure Modification of Aryl Phosphate and Its Effect on Application Properties of the Polypropylene. Polymers. 2023; 15(24):4730. https://doi.org/10.3390/polym15244730
Chicago/Turabian StyleLin, Fuhua, Mi Zhang, Tianjiao Zhao, Yanli Zhang, Dingyi Ning, Wenju Cui, Yingchun Li, Xinde Chen, and Jun Luo. 2023. "Exploitation of a New Nucleating Agent by Molecular Structure Modification of Aryl Phosphate and Its Effect on Application Properties of the Polypropylene" Polymers 15, no. 24: 4730. https://doi.org/10.3390/polym15244730
APA StyleLin, F., Zhang, M., Zhao, T., Zhang, Y., Ning, D., Cui, W., Li, Y., Chen, X., & Luo, J. (2023). Exploitation of a New Nucleating Agent by Molecular Structure Modification of Aryl Phosphate and Its Effect on Application Properties of the Polypropylene. Polymers, 15(24), 4730. https://doi.org/10.3390/polym15244730





