Parallel Catalyst Synthesis Protocol for Accelerating Heterogeneous Olefin Polymerization Research
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
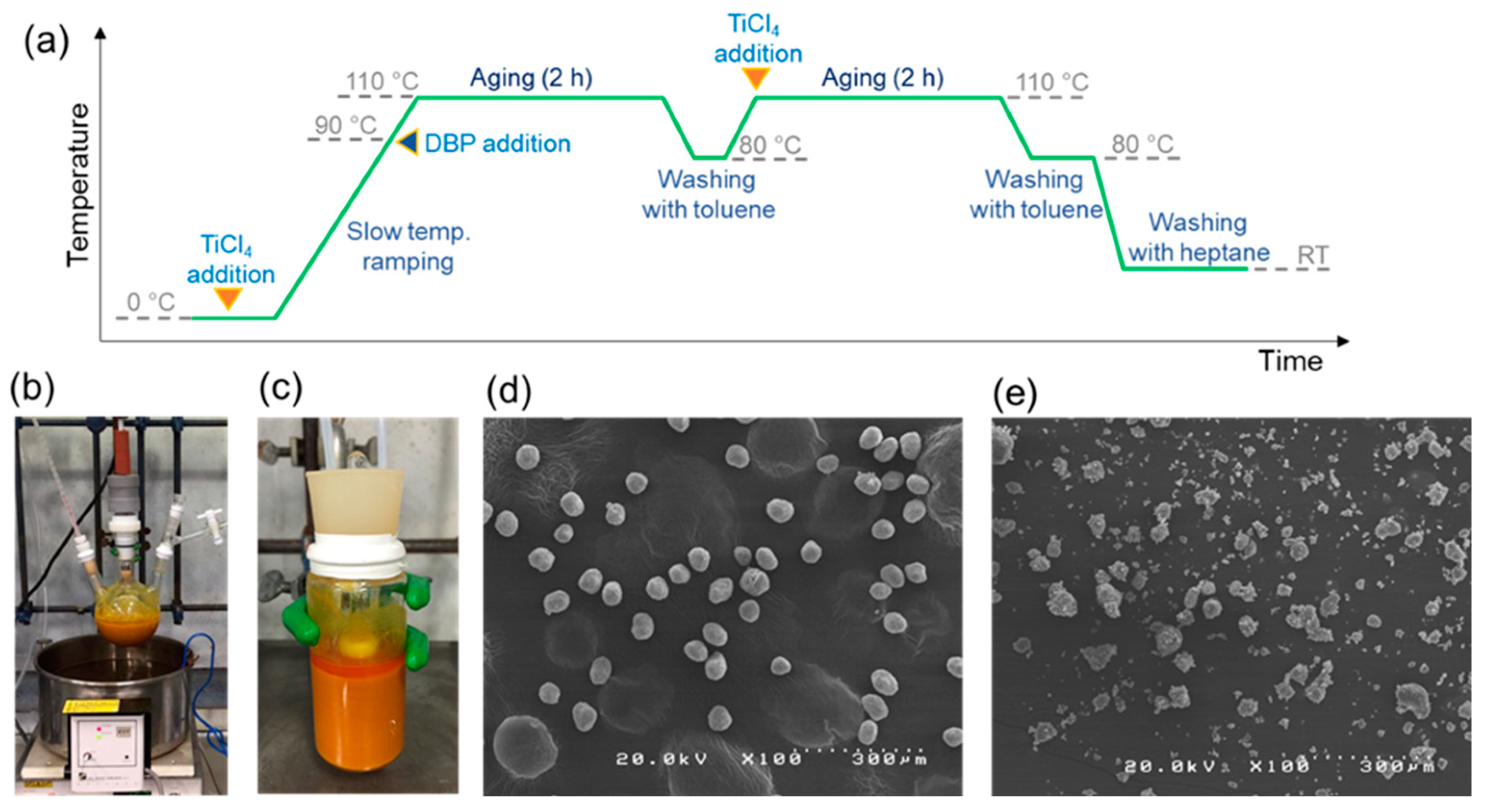
2.2. Synthesis of Magnesium Ethoxide as a Catalyst Precursor
2.3. Synthesis of Catalyst
2.4. Characterization
2.5. Polymerization Performance Evaluation
3. Results and Discussion
3.1. Setup and Protocol for Catalyst Synthesis
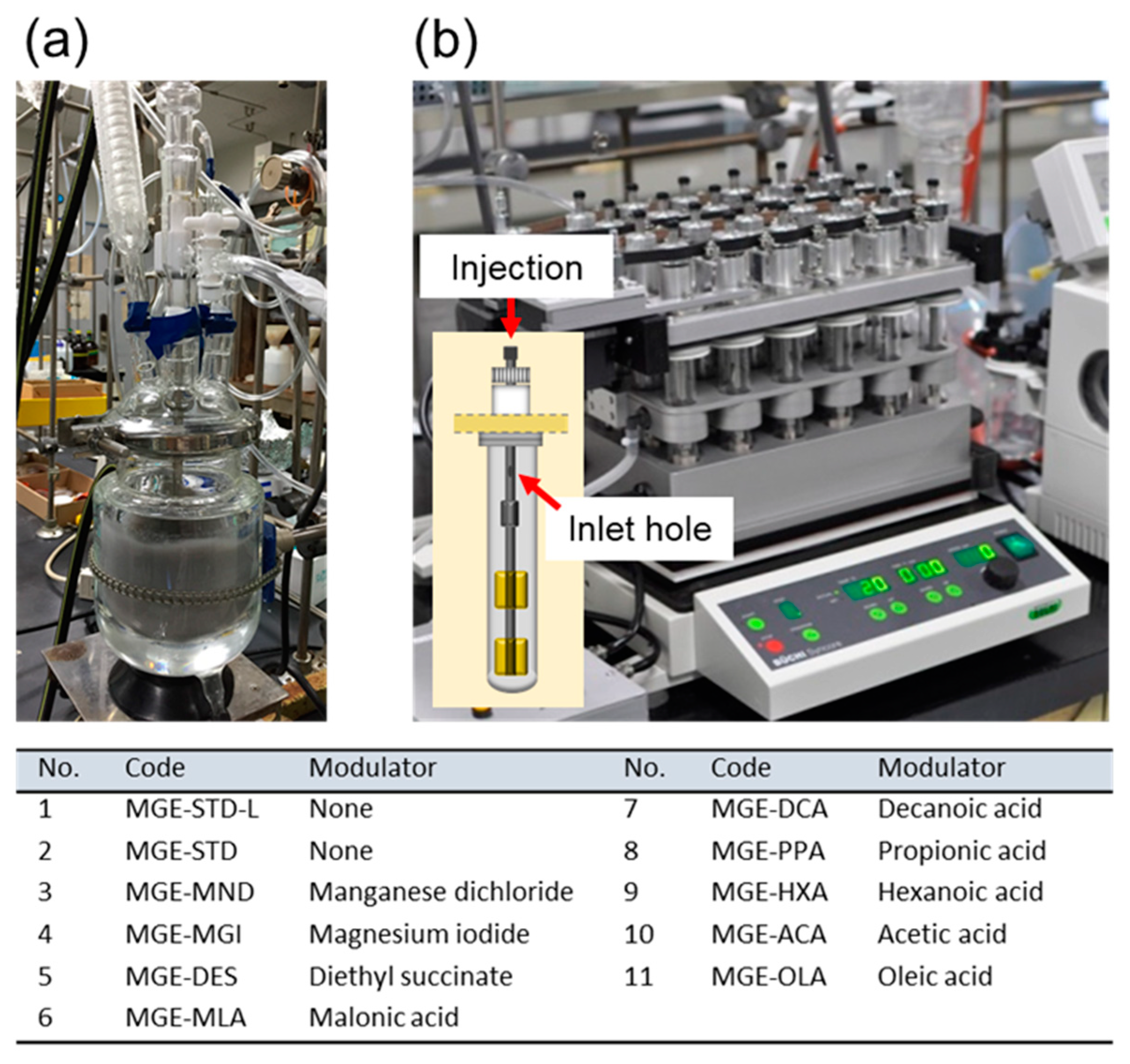
3.1.1. Downsizing
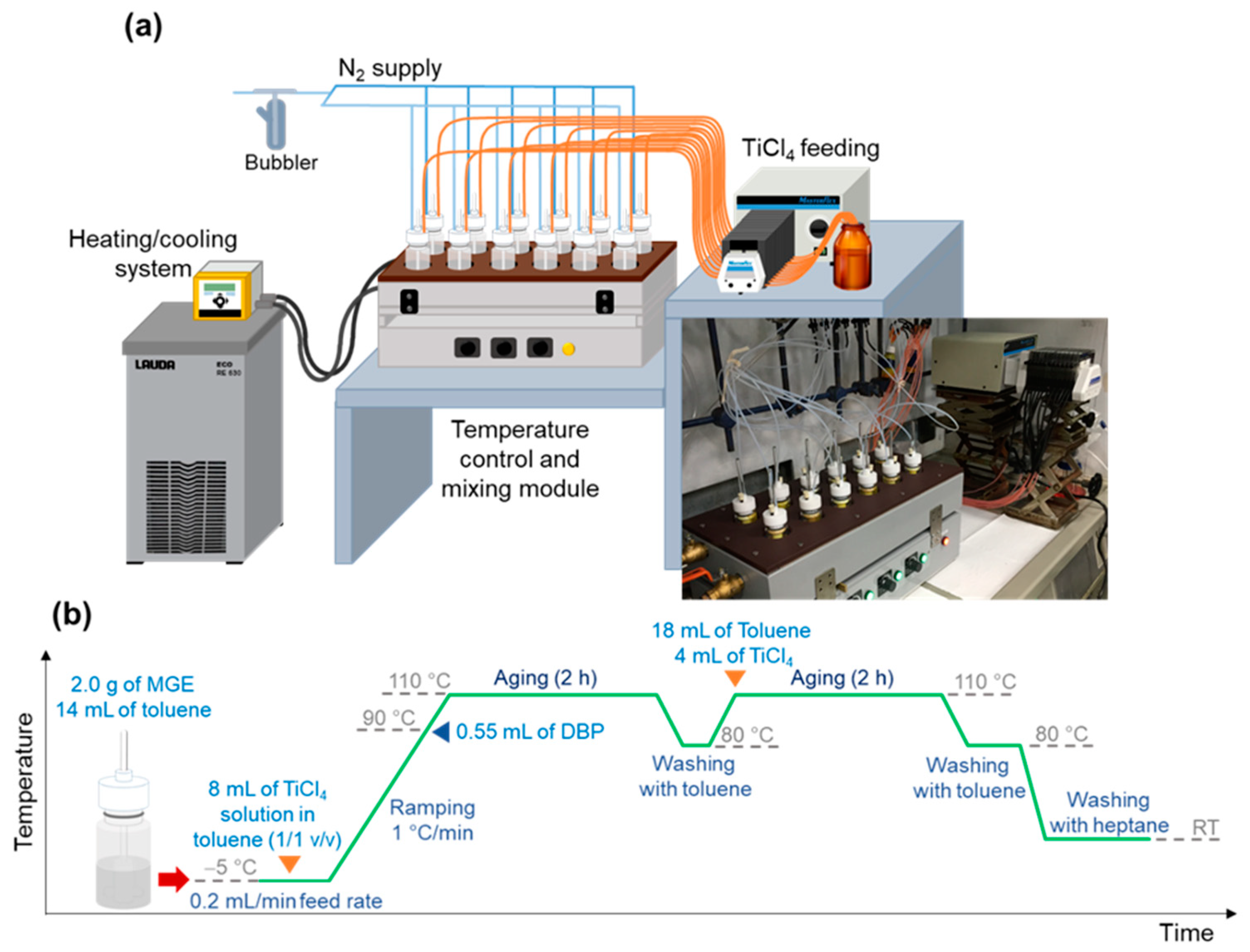
3.1.2. Parallelization
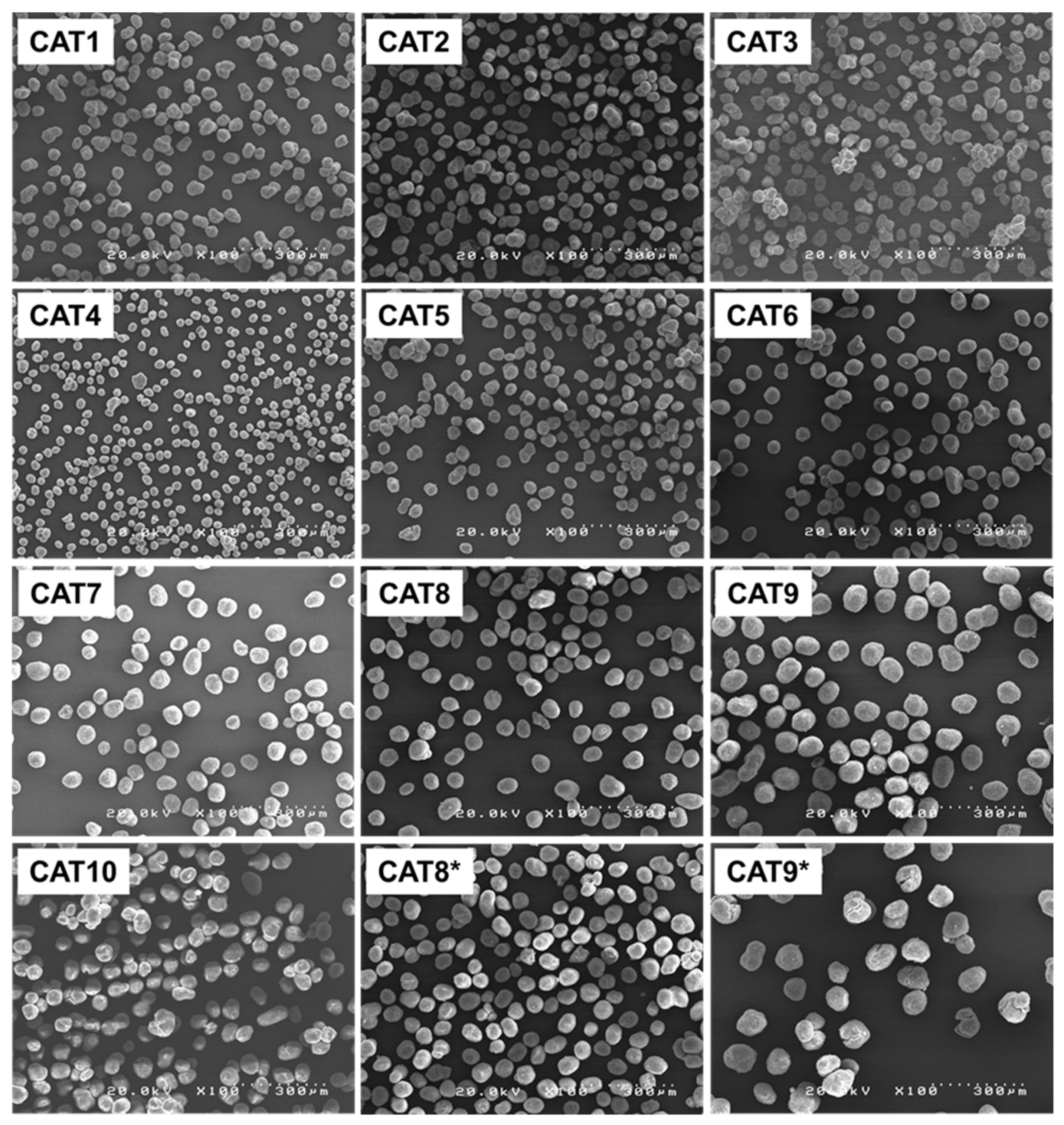
3.2. Generation of the Catalyst Library
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kissin, Y.V. Active centers in Ziegler–Natta catalysts: Formation kinetics and structure. J. Catal. 2012, 292, 188–200. [Google Scholar] [CrossRef]
- Correa, A.; Piemontesi, F.; Morini, G.; Cavallo, L. Key elements in the structure and function relationship of the MgCl2/TiCl4/Lewis base Ziegler−Natta catalytic system. Macromolecules 2007, 40, 9181–9189. [Google Scholar] [CrossRef]
- Bahri-Laleh, N.; Correa, A.; Mehdipour-Ataei, S.; Arabi, H.; Haghighi, M.N.; Zohuri, G.; Cavallo, L. Moving up and down the titanium oxidation state in Ziegler−Natta catalysis. Macromolecules 2011, 44, 778–783. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Feng, L.X.; Yang, S.L. Distribution of active centers on TiCl4/MgCl2 catalyst for olefin polymerization. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 3329–3335. [Google Scholar] [CrossRef]
- Groppo, E.; Gallo, E.; Seenivasan, K.; Lomachenko, K.A.; Sommazzi, A.; Bordiga, S.; Glatzel, P.; van Silfhout, R.; Kachatkou, A.; Bras, W.; et al. XAS and XES techniques shed light on the dark side of Ziegler–Natta catalysts: Active-site generation. ChemCatChem 2015, 7, 1432–1437. [Google Scholar] [CrossRef]
- Ashuiev, A.; Humbert, M.; Norsic, S.; Blahut, J.; Gajan, D.; Searles, K.; Klose, D.; Lesage, A.; Pintacuda, G.; Raynaud, J.; et al. Spectroscopic signature and structure of the active sites in Ziegler–Natta polymerization catalysts revealed by electron paramagnetic resonance. J. Am. Chem. Soc. 2021, 143, 9791–9797. [Google Scholar] [CrossRef]
- McKenna, T.F.L.; Di Martino, A.; Weickert, G.; Soares, J.B.P. Particle growth during the polymerisation of olefins on supported catalysts, 1—Nascent polymer structures. Macromol. React. Eng. 2010, 4, 40–64. [Google Scholar] [CrossRef]
- McKenna, T.F.L. Growth and evolution of particle morphology: An experimental & modelling study. Macromol. Symp. 2007, 260, 65–73. [Google Scholar] [CrossRef]
- Nejad, M.H.; Ferrari, P.; Pennini, G.; Cecchin, G. Ethylene homo- and copolymerization over MgCl2-TiCl4 catalysts: Polymerization kinetics and polymer particle morphology. J. Appl. Polym. Sci. 2008, 108, 3388–3402. [Google Scholar] [CrossRef]
- Himanen, L.; Geurts, A.; Foster, A.S.; Rinke, P. Data-driven materials science: Status, challenges, and perspectives. Adv. Sci. 2019, 6, 1900808. [Google Scholar] [CrossRef]
- Agrawal, A.; Choudhary, A. Perspective: Materials informatics and big data: Realization of the “fourth paradigm” of science in materials science. APL Mater. 2016, 4, 053208. [Google Scholar] [CrossRef]
- Pollice, R.; dos Passos Gomes, G.; Aldeghi, M.; Hickman, R.J.; Krenn, M.; Lavigne, C.; Lindner-D’Addario, M.; Nigam, A.; Ser, C.T.; Yao, Z.; et al. Data-driven strategies for accelerated materials design. Acc. Chem. Res. 2021, 54, 849–860. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, T.; Ju, W.; Shi, S. Materials discovery and design using machine learning. J. Mater. 2017, 3, 159–177. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Nhat, T.T.P.; Takimoto, K.; Thakur, A.; Nishimura, S.; Ohyama, J.; Miyazato, I.; Takahashi, L.; Fujima, J.; Takahashi, K.; et al. High-throughput experimentation and catalyst informatics for oxidative coupling of methane. ACS Catal. 2020, 10, 921–932. [Google Scholar] [CrossRef]
- Williams, T.; McCullough, K.; Lauterbach, J.A. Enabling catalyst discovery through machine learning and high-throughput experimentation. Chem. Mater. 2020, 32, 157–165. [Google Scholar] [CrossRef]
- Boussie, T.R.; Diamond, G.M.; Goh, C.; Hall, K.A.; LaPointe, A.M.; Leclerc, M.; Lund, C.; Murphy, V.; Shoemaker, J.A.W.; Tracht, U.; et al. A fully integrated high-throughput screening methodology for the discovery of new polyolefin catalysts: Discovery of a new class of high temperature single-site group (IV) copolymerization catalysts. J. Am. Chem. Soc. 2003, 125, 4306–4317. [Google Scholar] [CrossRef] [PubMed]
- Murphy, V.; Bei, X.; Boussie, T.R.; Brümmer, O.; Diamond, G.M.; Goh, C.; Hall, K.A.; Lapointe, A.M.; Leclerc, M.; Longmire, J.M.; et al. High-throughput approaches for the discovery and optimization of new olefin polymerization catalysts. Chem. Rec. 2002, 2, 278–289. [Google Scholar] [CrossRef]
- Boussie, T.R.; Coutard, C.; Turner, H.; Murphy, V.; Powers, T.S. Solid-phase synthesis and encoding strategies for olefin polymerization catalyst libraries. Angew. Chem. Int. Ed. 1998, 37, 3272–3275. [Google Scholar] [CrossRef]
- Boussie, T.R.; Murphy, V.; Hall, K.A.; Coutard, C.; Dales, C.; Petro, M.; Carlson, E.; Turner, H.W.; Powers, T.S. Parallel solid-phase synthesis, screening, and encoding strategies for olefin-polymerization catalysts. Tetrahedron 1999, 55, 11699–11710. [Google Scholar] [CrossRef]
- Boussie, T.R.; Diamond, G.M.; Goh, C.; Hall, K.A.; LaPointe, A.M.; Leclerc, M.K.; Murphy, V.; Shoemaker, J.A.W.; Turner, H.; Rosen, R.K.; et al. Nonconventional catalysts for isotactic propene polymerization in solution developed by using high-throughput-screening technologies. Angew. Chem. Int. Ed. 2006, 45, 3278–3283. [Google Scholar] [CrossRef]
- Tian, J.; Coates, G.W. Development of a diversity-based approach for the discovery of stereoselective polymerization catalysts: Identification of a catalyst for the synthesis of syndiotactic polypropylene. Angew. Chem. 2000, 112, 3772–3775. [Google Scholar] [CrossRef]
- Adams, N.; Arts, H.J.; Bolton, P.D.; Cowell, D.; Dubberley, S.R.; Friederichs, N.; Grant, C.M.; Kranenburg, M.; Sealey, A.J.; Wang, B.; et al. Discovery and evaluation of highly active imidotitanium ethylene polymerisation catalysts using high throughput catalyst screening. Chem. Commun. 2004, 4, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Komon, Z.J.A.; Diamond, G.M.; Leclerc, M.K.; Murphy, V.; Okazaki, M.; Bazan, G.C. Triple tandem catalyst mixtures for the synthesis of polyethylenes with varying structures. J. Am. Chem. Soc. 2002, 124, 15280–15285. [Google Scholar] [CrossRef] [PubMed]
- Busico, V.; Pellecchia, R.; Cutillo, F.; Cipullo, R. High-throughput screening in olefin-polymerization catalysis: From serendipitous discovery towards rational understanding. Macromol. Rapid Commun. 2009, 20, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Busico, V.; Cipullo, R.; Mingione, A.; Rongo, L. Accelerating the research approach to Ziegler–Natta catalysts. Ind. Eng. Chem. Res. 2016, 55, 2686–2695. [Google Scholar] [CrossRef]
- Vittoria, A.; Urciuoli, G.; Costanzo, S.; Costanzo, S.; Tammaro, D.; Cannavacciuolo, F.D.; Pasquino, R.; Cipullo, R.; Auriemma, F.; Grizzuti, N.; et al. Extending the high-throughput experimentation (HTE) approach to catalytic olefin polymerizations: From catalysts to materials. Macromolecules 2022, 55, 5017–5026. [Google Scholar] [CrossRef]
- Vittoria, A.; Meppelder, A.; Friederichs, N.; Busico, V.; Cipullo, R. Demystifying Ziegler–Natta catalysts: The origin of stereoselectivity. ACS Catal. 2017, 7, 4509–4518. [Google Scholar] [CrossRef]
- Ehm, C.; Mingione, A.; Vittoria, A.; Zaccaria, F.; Cipullo, R.; Busico, V. High-throughput experimentation in olefin polymerization catalysis: Facing the challenges of miniaturization. Ind. Eng. Chem. Res. 2020, 59, 13940–13947. [Google Scholar] [CrossRef]
- Ehm, C.; Vittoria, A.; Goryunov, G.P.; Kulyabin, P.S.; Budzelaar, P.H.M.; Voskoboynikov, A.Z.; Busico, V.; Uborsky, D.V.; Cipullo, R. Connection of stereoselectivity, regioselectivity, and molecular weight capability in rac-R′2Si(2-Me-4-R-indenyl)2ZrCl2 type catalysts. Macromolecules 2018, 51, 8073–8083. [Google Scholar] [CrossRef]
- Ehm, C.; Vittoria, A.; Goryunov, P.G.; Izmer, V.V.; Kononovich, S.D.; Samsonov, V.O.; Di Girolamo, R.; Budzelaar, H.M.P.; Voskoboynikov, Z.A.; Busico, V.; et al. An integrated high throughput experimentation/predictive QSAR modeling approach to ansa-zirconocene catalysts for isotactic polypropylene. Polymers 2020, 12, 1005. [Google Scholar] [CrossRef]
- Ehm, C.; Vittoria, A.; Goryunov, G.P.; Izmer, V.V.; Kononovich, D.S.; Samsonov, O.V.; Budzelaar, P.H.M.; Voskoboynikov, A.Z.; Busico, V.; Uborsky, D.V.; et al. On the limits of tuning comonomer affinity of ‘Spaleck-type’ ansa-zirconocenes in ethene/1-hexene copolymerization: A high-throughput experimentation/QSAR approach. Dalton Trans. 2020, 49, 10162–10172. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.J.; Gibson, V.C.; Green, S.M.; Maddox, P.J. Discovery of a new family of chromium ethylene polymerisation catalysts using high throughput screening methodology. Chem. Commun. 2002, 10, 1038–1039. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Funako, T.; Chammingkwan, P.; Thakur, A.; Matta, A.; Terano, M.; Taniike, T. Structure-performance relationship of Mg(OEt)2-based Ziegler-Natta catalysts. J. Catal. 2020, 389, 525–532. [Google Scholar] [CrossRef]
- Chammingkwan, P.; Wannaborworn, M.; Mai, L.T.T.; Terano, M.; Taniike, T.; Phiriyawirut, P. Particle engineering of magnesium ethoxide-based Ziegler-Natta catalyst through post-modification of magnesium ethoxide. Appl. Catal. A Gen. 2021, 626, 118337. [Google Scholar] [CrossRef]
- Chammingkwan, P.; Terano, M.; Taniike, T. High-throughput synthesis of support materials for olefin polymerization catalyst. ACS Comb. Sci. 2017, 19, 331–342. [Google Scholar] [CrossRef]
- Thakur, A.; Chammingkwan, P.; Wada, T.; Onishi, R.; Kamimura, W.; Seenivasan, K.; Terano, M.; Taniike, T. Solution-state NMR study of organic components of industrial Ziegler-Natta catalysts: Effect of by-products on catalyst performance. Appl. Catal. A Gen. 2021, 611, 117971. [Google Scholar] [CrossRef]
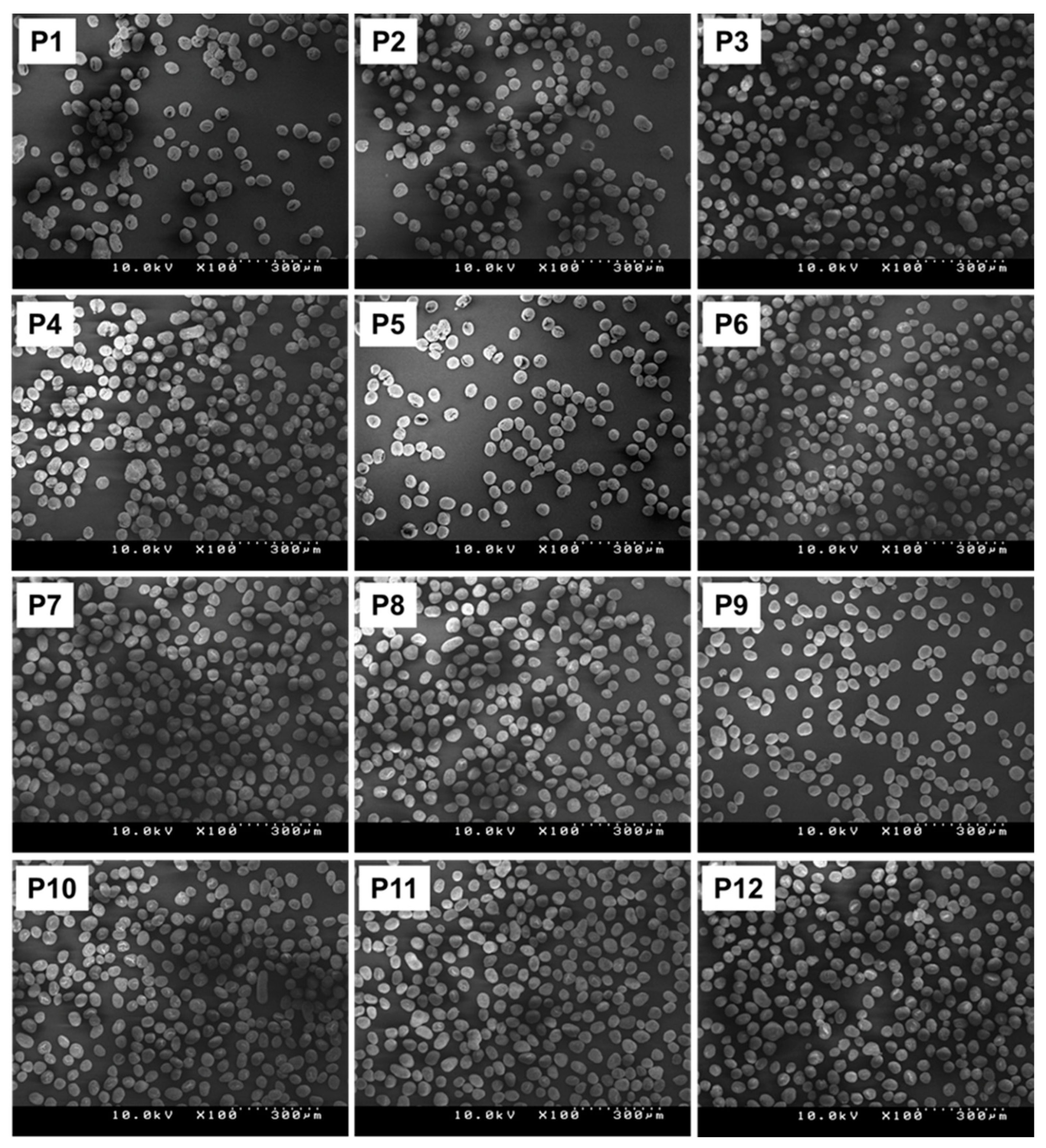
| Run No. | Particle Characteristic a | Chemical Composition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D10 (µm) | D50 (µm) | D90 (µm) | RSF | Circularity | Ti b (mmol g−1) | DBP c (mmol g−1) | DEP c (mmol g−1) | Total ID c (mmol g−1) | OEt c (mmol g−1) | |
| P1 | 38.4 | 44.0 | 50.9 | 0.28 | 0.76 | 0.48 | 0.40 | 0.10 | 0.51 | 0.20 |
| P2 | 38.1 | 43.4 | 49.2 | 0.26 | 0.71 | 0.48 | 0.43 | 0.09 | 0.52 | 0.22 |
| P3 | 39.8 | 44.8 | 50.3 | 0.23 | 0.7 | 0.44 | 0.41 | 0.11 | 0.52 | 0.18 |
| P4 | 38.5 | 43.6 | 48.4 | 0.23 | 0.76 | 0.46 | 0.46 | 0.09 | 0.55 | 0.22 |
| P5 | 37.8 | 42.7 | 48.2 | 0.24 | 0.71 | 0.42 | 0.45 | 0.09 | 0.54 | 0.20 |
| P6 | 38.6 | 44.5 | 51.0 | 0.28 | 0.74 | 0.44 | 0.43 | 0.09 | 0.51 | 0.24 |
| P7 | 38.6 | 45.0 | 50.4 | 0.26 | 0.72 | 0.44 | 0.45 | 0.09 | 0.55 | 0.22 |
| P8 | 37.9 | 42.9 | 48.7 | 0.25 | 0.68 | 0.46 | 0.42 | 0.11 | 0.53 | 0.18 |
| P9 | 37.8 | 43.2 | 49.0 | 0.26 | 0.83 | 0.48 | 0.41 | 0.11 | 0.52 | 0.22 |
| P10 | 36.9 | 42.6 | 49.1 | 0.29 | 0.76 | 0.46 | 0.40 | 0.10 | 0.50 | 0.20 |
| P11 | 36.3 | 42.6 | 48.4 | 0.28 | 0.74 | 0.44 | 0.38 | 0.09 | 0.47 | 0.20 |
| P12 | 38.4 | 44.0 | 50.9 | 0.28 | 0.76 | 0.48 | 0.41 | 0.09 | 0.50 | 0.16 |
| AVG d | 38.1 | 43.6 | 49.5 | 0.26 | 0.74 | 0.46 | 0.42 | 0.10 | 0.52 | 0.20 |
| STD d | 0.89 | 0.85 | 1.08 | 0.02 | 0.04 | 0.020 | 0.023 | 0.008 | 0.021 | 0.025 |
| STE d | 0.26 | 0.25 | 0.31 | 0.01 | 0.01 | 0.006 | 0.007 | 0.002 | 0.006 | 0.007 |
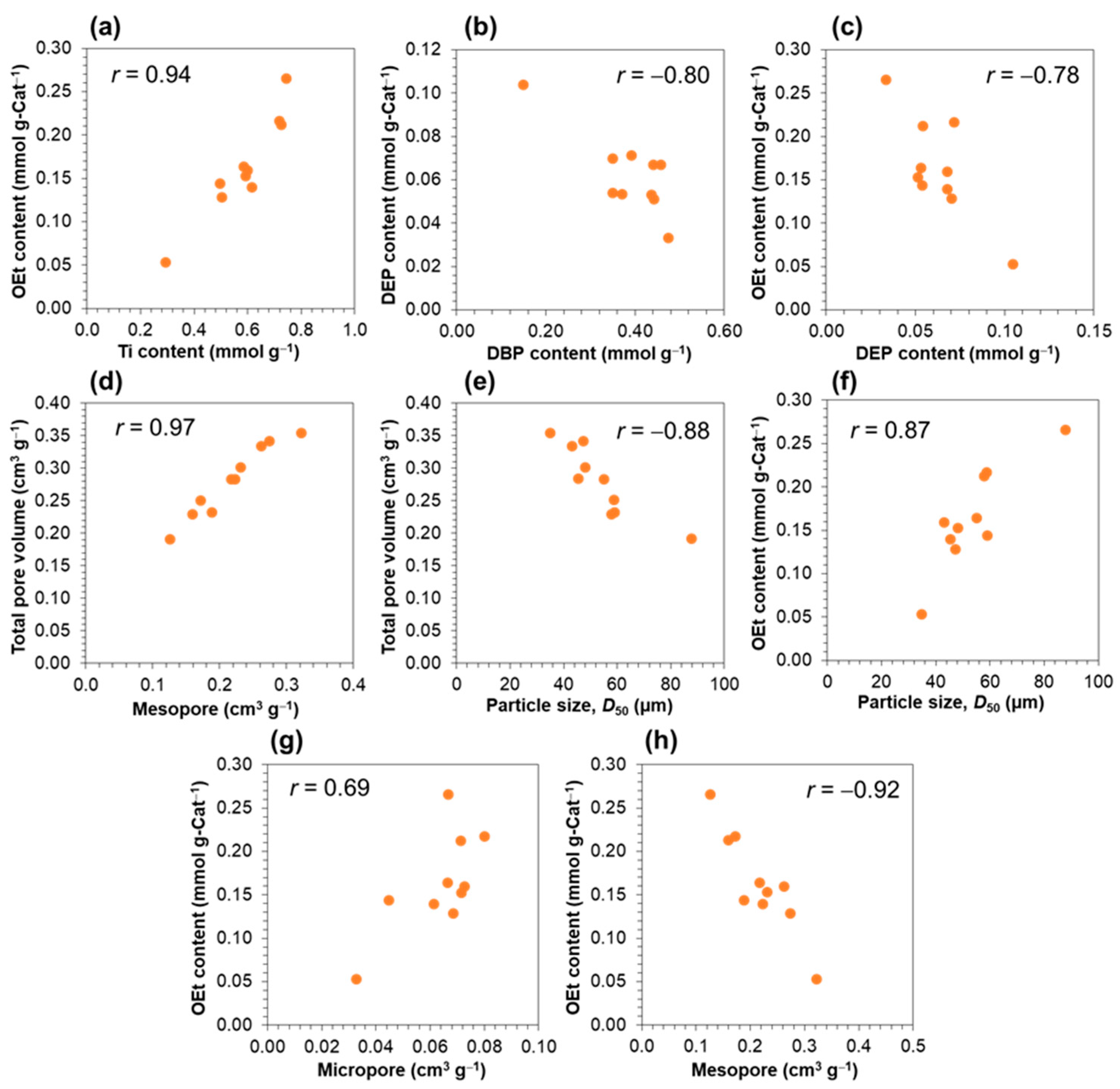
| Sample | MGE Source | Particle Characteristic | Catalyst Chemical Composition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D10 (µm) | D50 (µm) | D90 (µm) | RSF | Circularity | Ti (mmol g−1) | DBP (mmol g−1) | DEP (mmol g−1) | Total ID (mmol g−1) | OEt (mmol g−1) | ||
| CAT1 | MGE-STD | 39.2 | 45.3 | 54.6 | 0.34 | 0.84 | 0.62 | 0.46 | 0.07 | 0.52 | 0.14 |
| CAT2 | MGE-MND | 40.7 | 48.0 | 59.9 | 0.40 | 0.81 | 0.58 | 0.44 | 0.05 | 0.49 | 0.15 |
| CAT3 | MGE-MGI | 36.8 | 47.2 | 59.4 | 0.48 | 0.76 | 0.49 | 0.35 | 0.07 | 0.42 | 0.13 |
| CAT4 | MGE-DES | 29.7 | 34.8 | 44.5 | 0.43 | 0.82 | 0.29 | 0.15 | 0.10 | 0.25 | 0.05 |
| CAT5 | MGE-MLA | 37.0 | 43.0 | 52.9 | 0.37 | 0.81 | 0.59 | 0.44 | 0.07 | 0.51 | 0.16 |
| CAT6 | MGE-DCA | 46.7 | 54.9 | 62.7 | 0.29 | 0.84 | 0.61 | 0.44 | 0.05 | 0.49 | 0.16 |
| CAT7 | MGE-PPA | 47.2 | 58.6 | 67.3 | 0.34 | 0.84 | 0.72 | 0.39 | 0.07 | 0.46 | 0.22 |
| CAT8 | MGE-HXA | 48.2 | 57.7 | 67.4 | 0.33 | 0.81 | 0.73 | 0.35 | 0.05 | 0.40 | 0.21 |
| CAT9 | MGE-ACA | 72.5 | 87.7 | 102.4 | 0.34 | 0.77 | 0.74 | 0.47 | 0.03 | 0.51 | 0.27 |
| CAT10 | MGE-OLA | 51.6 | 58.8 | 66.0 | 0.24 | 0.75 | 0.49 | 0.37 | 0.05 | 0.42 | 0.14 |
| CAT8 * | MGE-HXA | 51.3 | 59.3 | 68.7 | 0.29 | 0.74 | 0.73 | 0.38 | 0.05 | 0.43 | 0.24 |
| CAT9 * | MGE-ACA | 72.7 | 88.1 | 102.6 | 0.34 | 0.77 | 0.73 | 0.50 | 0.04 | 0.54 | 0.29 |
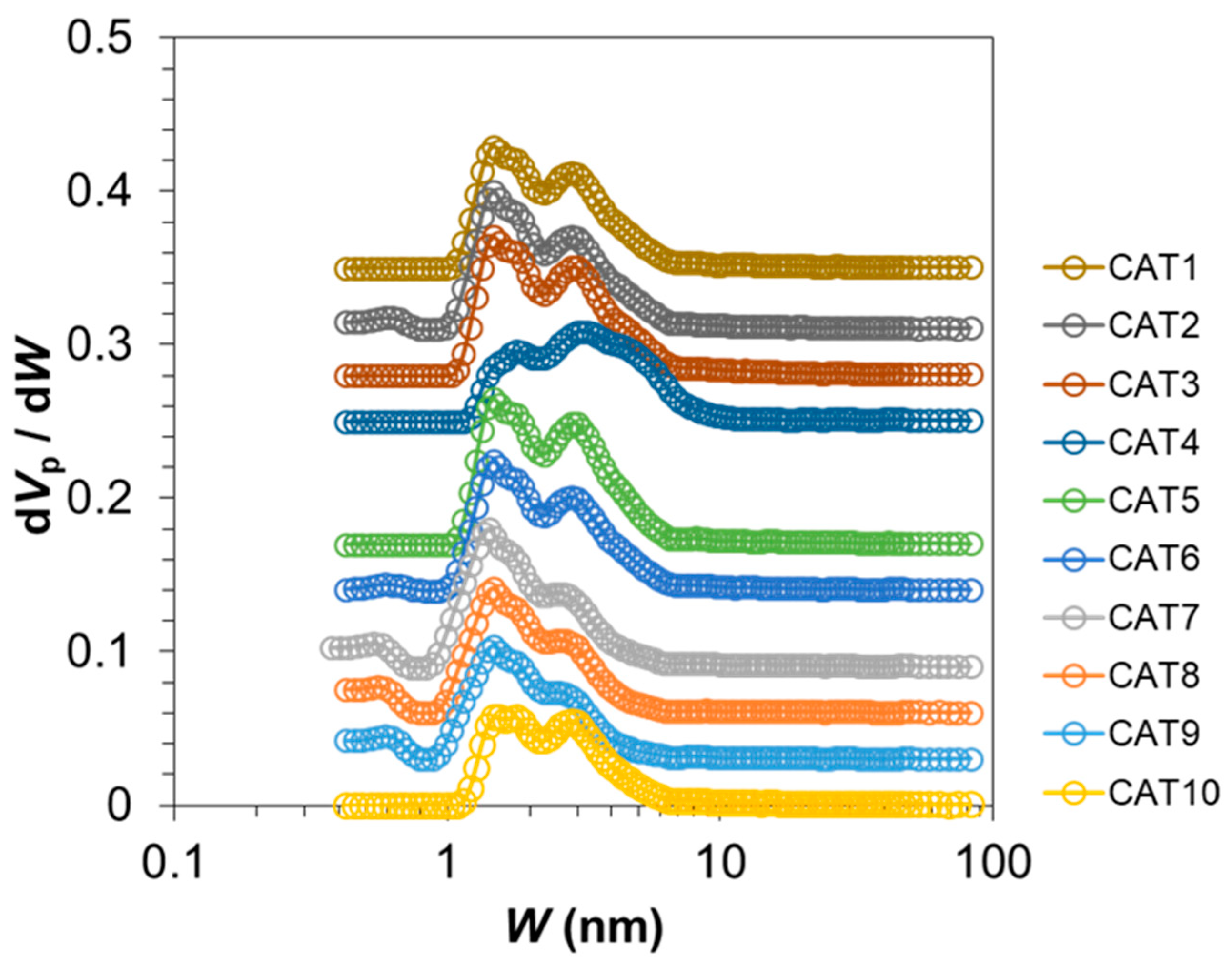
| Catalyst | Total Pore Volume a (cm3 g−1) | Micropore b (cm3 g−1) | Mesopore c (cm3 g−1) |
|---|---|---|---|
| CAT1 | 0.284 | 0.061 | 0.223 |
| CAT2 | 0.302 | 0.071 | 0.230 |
| CAT3 | 0.342 | 0.068 | 0.273 |
| CAT4 | 0.354 | 0.033 | 0.322 |
| CAT5 | 0.335 | 0.072 | 0.262 |
| CAT6 | 0.283 | 0.066 | 0.217 |
| CAT7 | 0.251 | 0.080 | 0.171 |
| CAT8 | 0.230 | 0.071 | 0.159 |
| CAT9 | 0.192 | 0.067 | 0.125 |
| CAT10 | 0.233 | 0.045 | 0.188 |
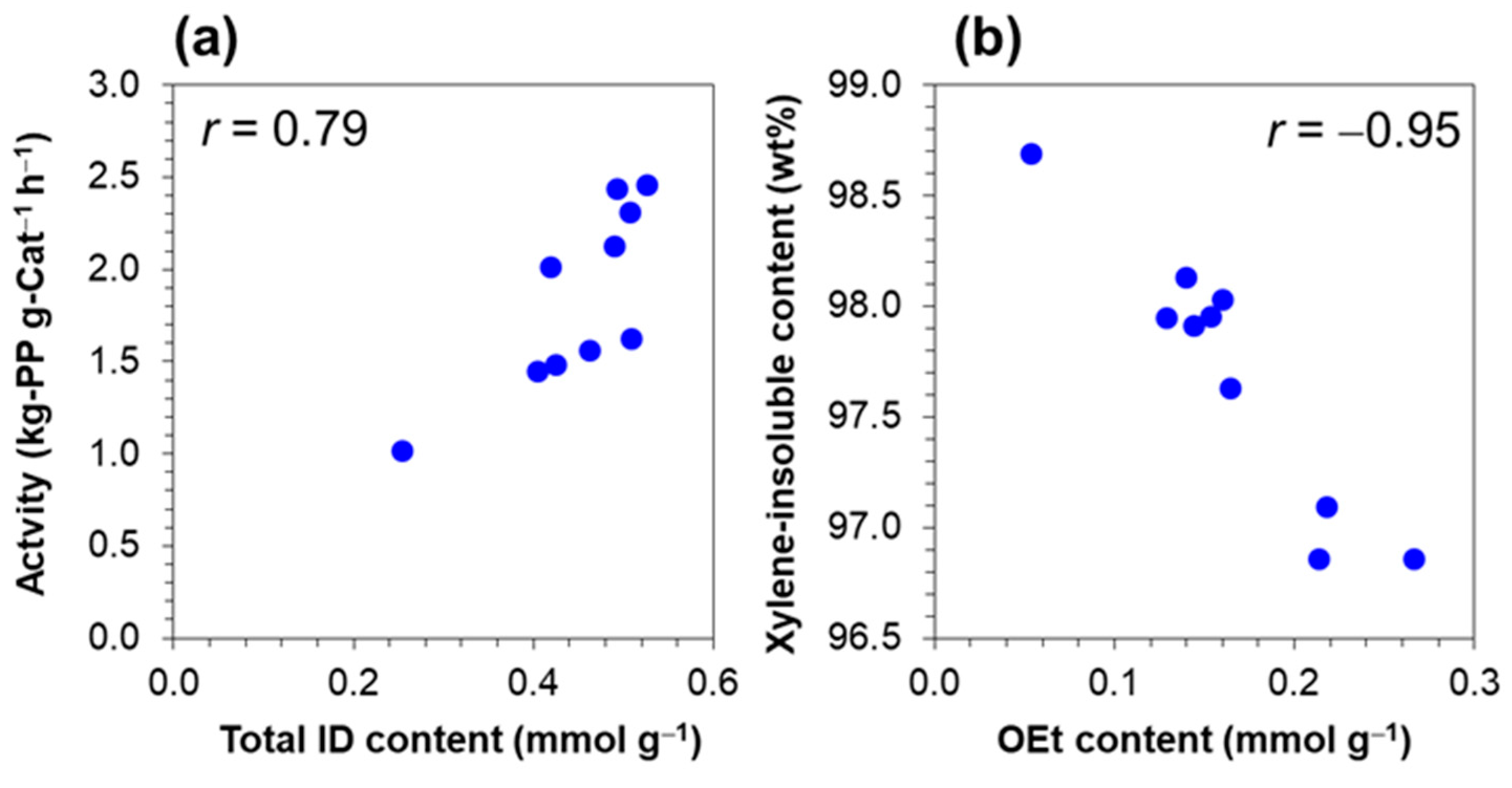
| Catalyst Sample | Activity a (kg-PP g-Cat−1 h−1) | XI (wt%) |
|---|---|---|
| CAT1 | 2.46 | 98.13 |
| CAT2 | 2.44 | 97.96 |
| CAT3 | 2.01 | 97.95 |
| CAT4 | 0.98 | 98.70 |
| CAT5 | 2.31 | 98.03 |
| CAT6 | 2.13 | 97.63 |
| CAT7 | 1.56 | 97.10 |
| CAT8 | 1.45 | 96.86 |
| CAT9 | 1.63 | 96.86 |
| CAT10 | 1.49 | 97.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chammingkwan, P.; Khoshsefat, M.; Terano, M.; Taniike, T. Parallel Catalyst Synthesis Protocol for Accelerating Heterogeneous Olefin Polymerization Research. Polymers 2023, 15, 4729. https://doi.org/10.3390/polym15244729
Chammingkwan P, Khoshsefat M, Terano M, Taniike T. Parallel Catalyst Synthesis Protocol for Accelerating Heterogeneous Olefin Polymerization Research. Polymers. 2023; 15(24):4729. https://doi.org/10.3390/polym15244729
Chicago/Turabian StyleChammingkwan, Patchanee, Mostafa Khoshsefat, Minoru Terano, and Toshiaki Taniike. 2023. "Parallel Catalyst Synthesis Protocol for Accelerating Heterogeneous Olefin Polymerization Research" Polymers 15, no. 24: 4729. https://doi.org/10.3390/polym15244729
APA StyleChammingkwan, P., Khoshsefat, M., Terano, M., & Taniike, T. (2023). Parallel Catalyst Synthesis Protocol for Accelerating Heterogeneous Olefin Polymerization Research. Polymers, 15(24), 4729. https://doi.org/10.3390/polym15244729









