Development of an Electroactive and Thermo-Reversible Diels–Alder Epoxy Nanocomposite Doped with Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Manufacturing
2.3. Characterization
3. Results
3.1. Analysis of Nanocomposite Manufacturing and Thermo-Electrical Properties
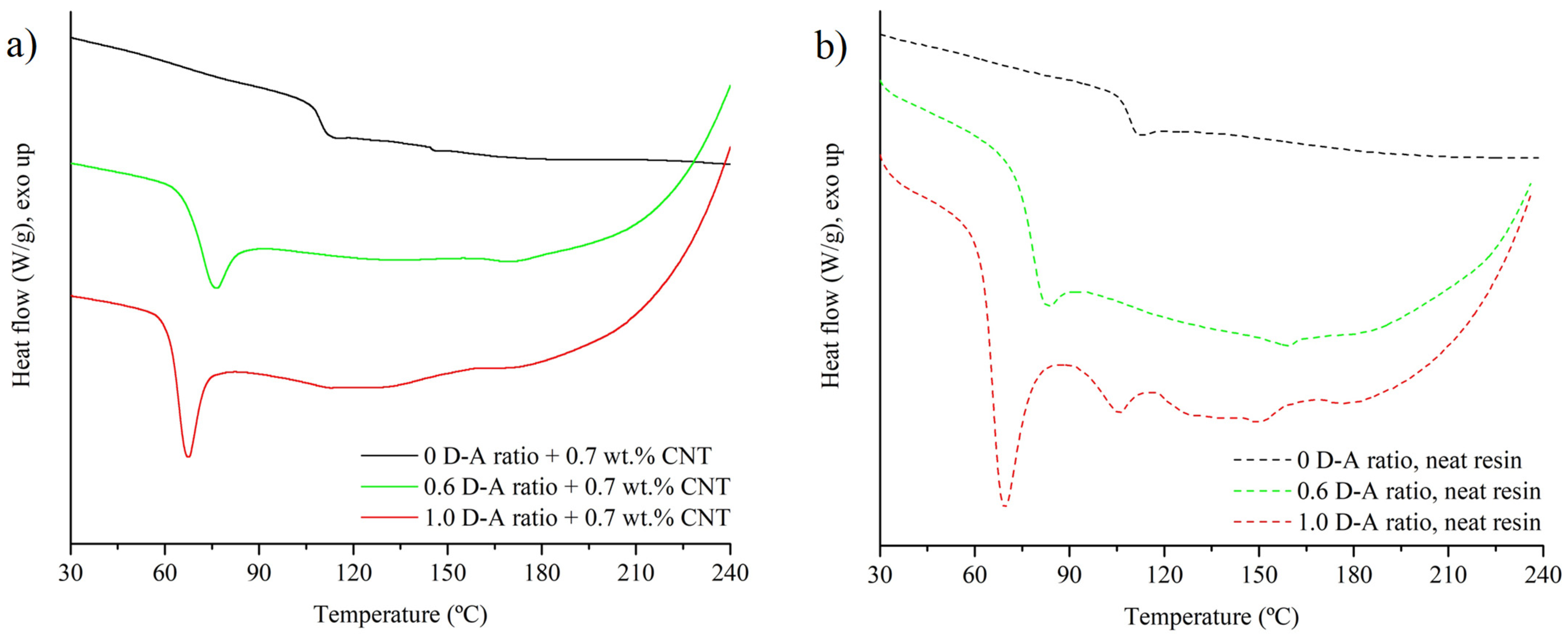
- A notable decrease in Tg induced via the substitution of the DGEBA-MXDA chain with D-A crosslinks, passing from 108 °C in the 0 D-A ratio sample to 64.4 °C in the 1.0 D-A ratio resin.
- An endothermic region, which corresponds to the retro D-A reaction, appears from 90 to 180 °C. At lower temperatures, i.e., closer to 100 °C, the endothermic peaks correspond to -endo D-A isomers’ disengagement; above 130 °C, the endothermic peaks are associated with -exo D-A isomers’ dissociation [12].
- An exothermic region above 190 °C appears due to BMI homopolymerization [36]. The apparition of this reaction confirms that no side irreversible reactions, such as Michael addition, happen to a significant extent during resin manufacturing and curing.
3.2. Mechanical and Thermomechanical Characterization of the 0.6 D-A Resin and Nanocomposite
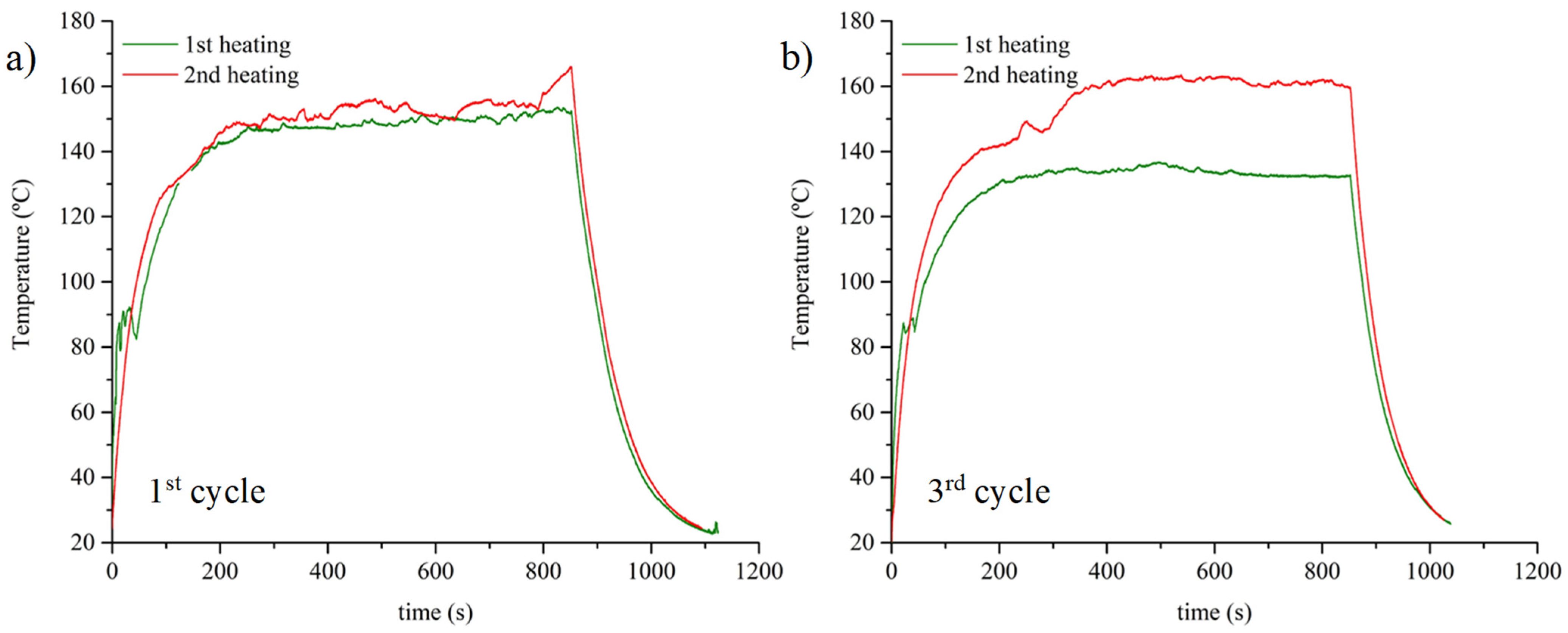
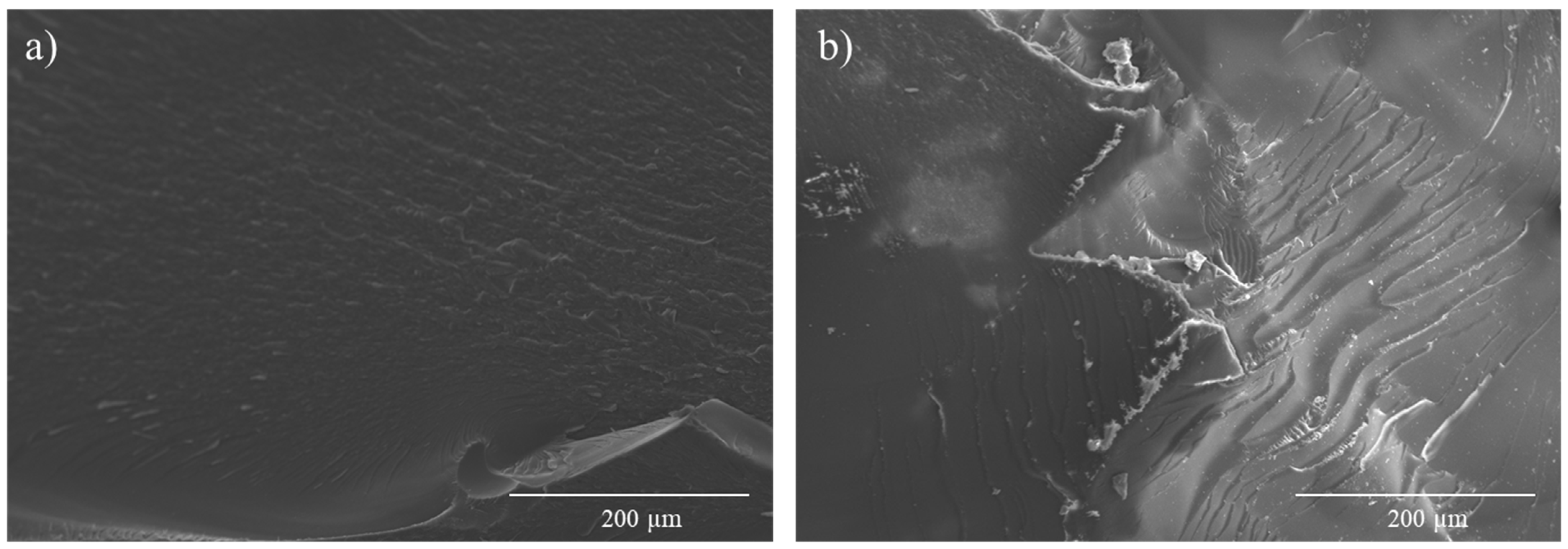
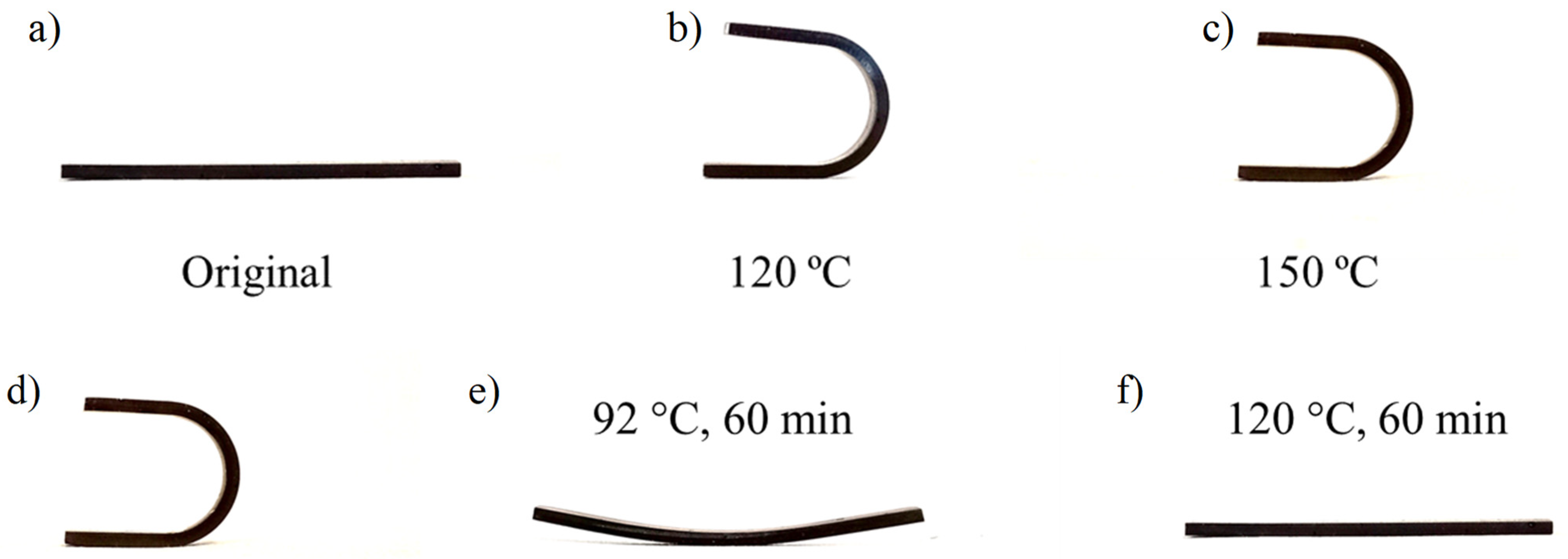
3.3. Smart Abilities: Thermally and Thermoelectrically Stimulated Crack-Healing and the Shape Memory of the 0.6 D-A Resin Nanocomposite Doped with 0.7 wt.% of CNT
4. Conclusions
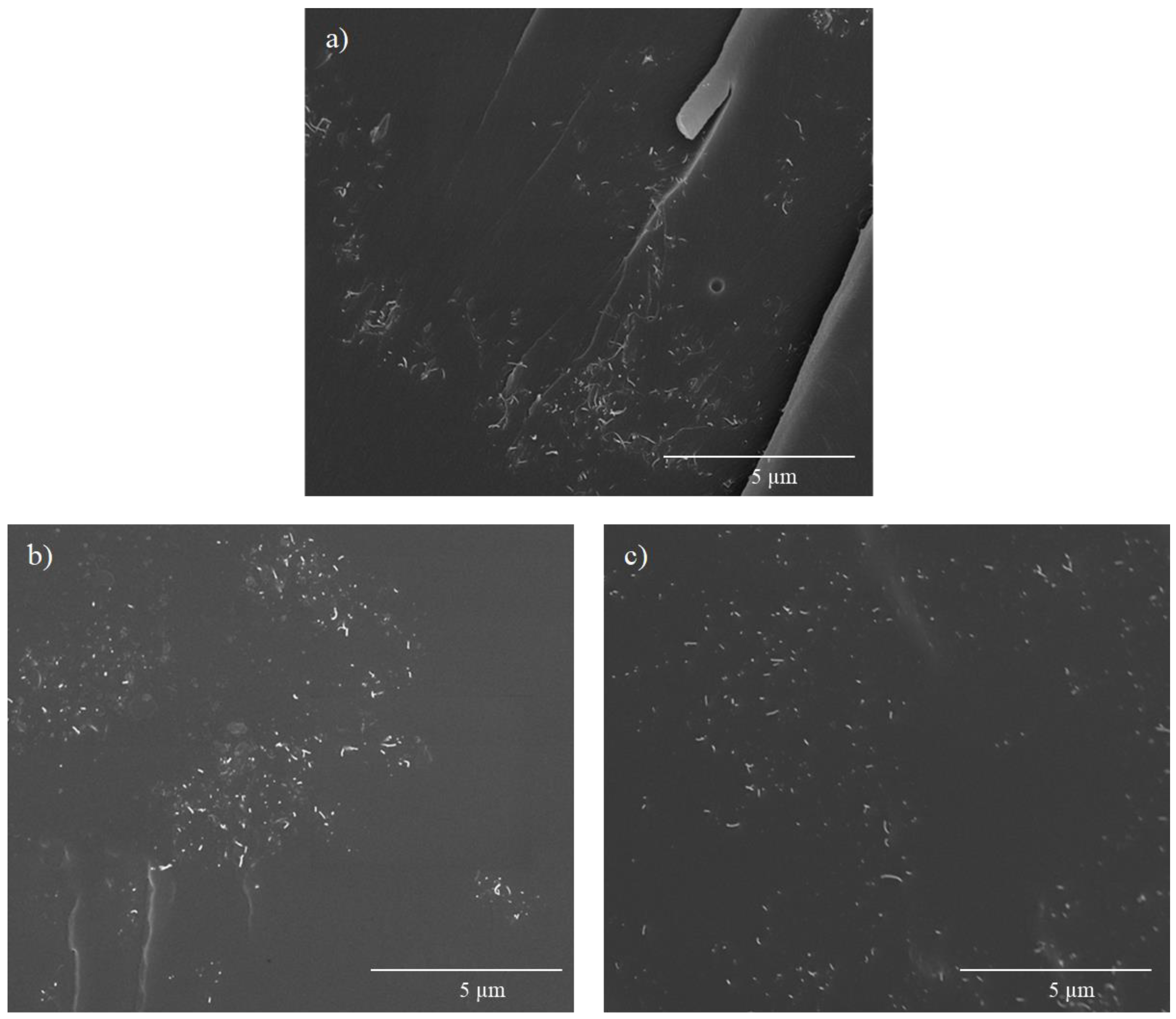
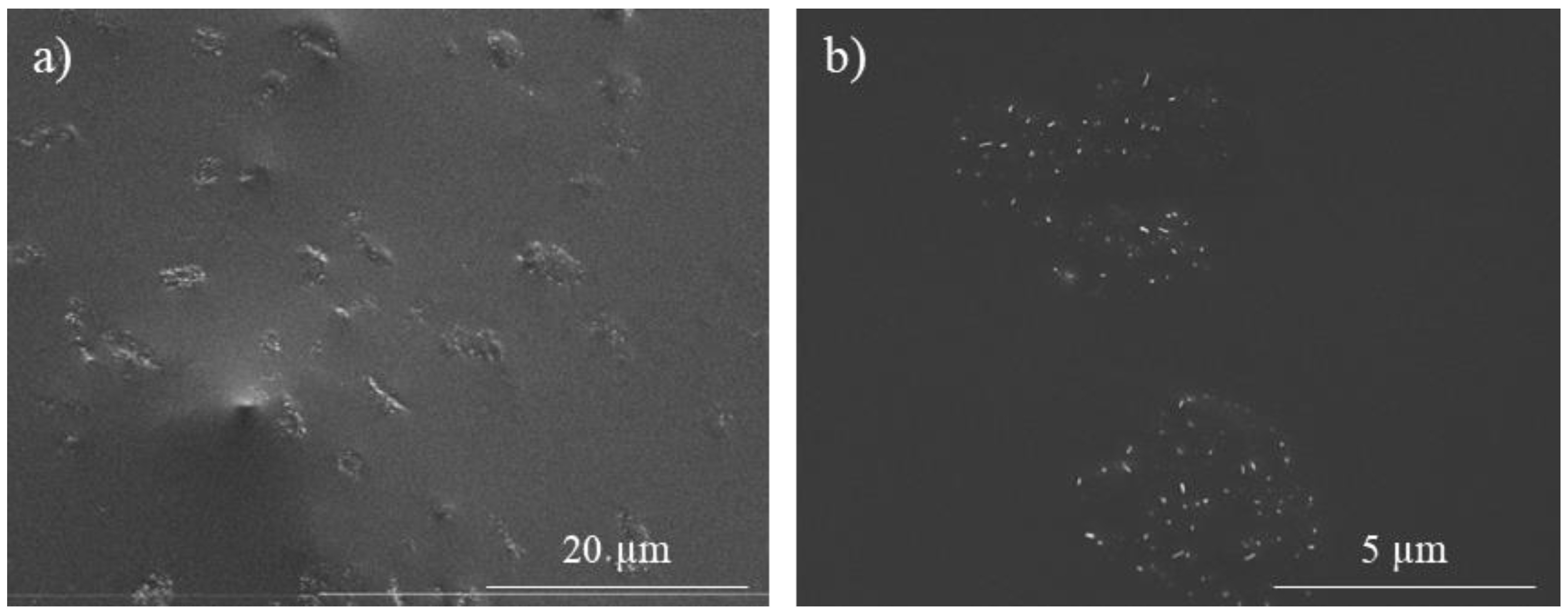
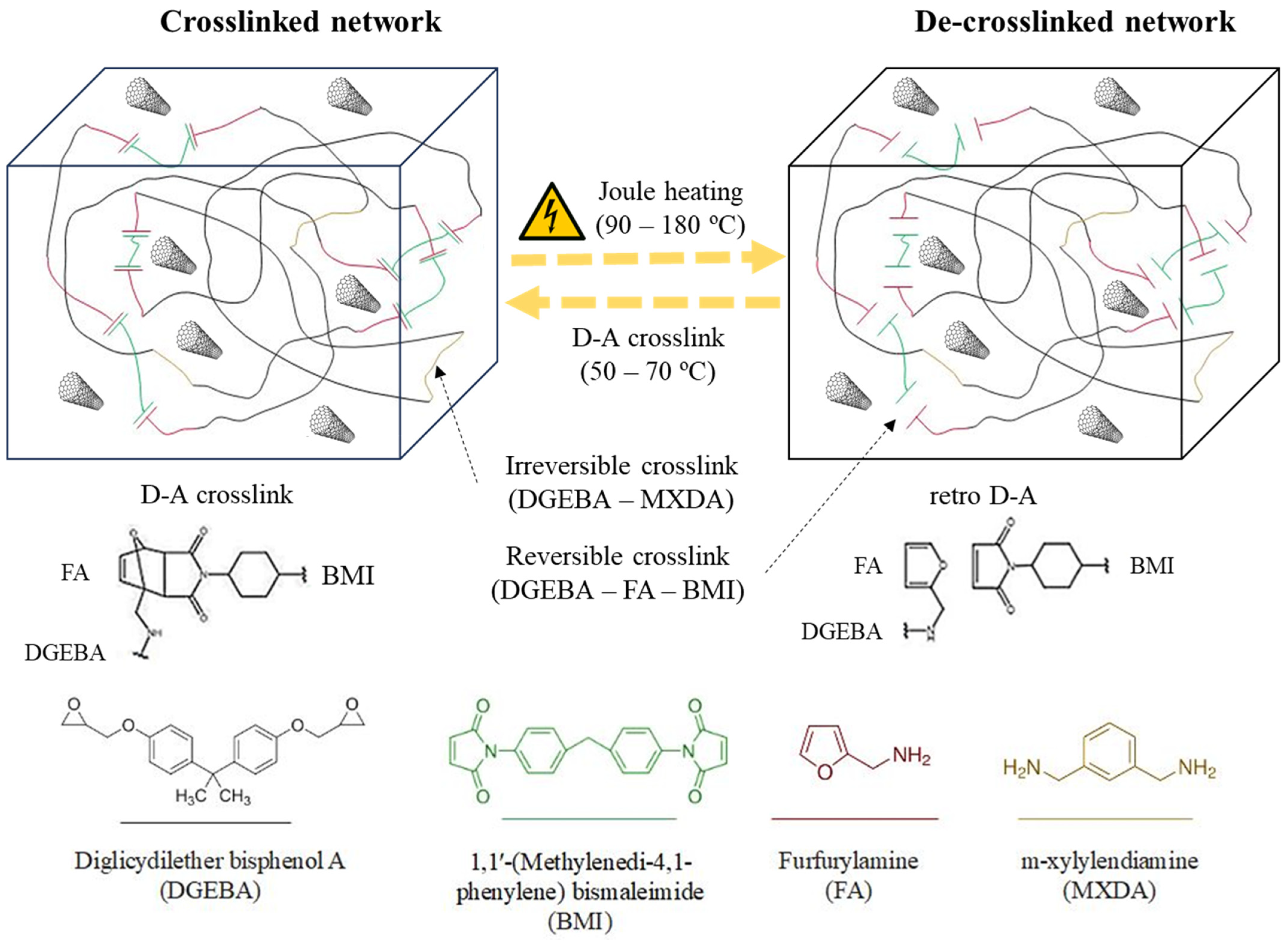
- The addition of BMI and its formation of aggregates hinder the effectiveness of CNT dispersion and make difficult direct contact between them. Thus, D-A nanocomposites show lower electrical conductivities, but they have ohmic behaviors and predictable heating through the Joule effect.
- The addition of CNT up to 0.7 wt.% allows the optimum thermoelectric responses of D-A nanocomposites, which are able to reach a range of temperatures between 150 and 170 °C and trigger reversible network disengagement through retro D-A reaction.
- The 1.0 D-A ratio nanocomposites have a high brittle behavior with smooth fracture surfaces that reduce their usability. Meanwhile, the 0.6 D-A ratio nanocomposites show a more balanced compendium of properties despite their lower electrical conductivity.
- CNT addition reduces chain mobility at high temperatures. Nonetheless, a 0.6 D-A ratio nanocomposite can heal cracks via Joule heating and reduce its original volume by more than 50%.
- The shape fixing and shape recovery efficiency of a 0.6 D-A ratio nanocomposite is close to 100 °C when the thermoconforming and shape recovery temperatures are higher than the Tg, i.e., approximately Tg + 20 °C.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| 0 D-A Ratio | 0.6 D-A Ratio | 1.0 D-A Ratio | |
|---|---|---|---|
| E’30 °C (MPa) | 2656 ± 146 | 3129 ± 233 | 2045 ± 487 |
| E’min (MPa) | 21.5 ± 1.6 | 3.3 ± 1.3 | - |
| Tg (°C) | 126.5 ± 0.3 | 91.1 ± 1.5 | 80.3 ± 1.9 |
| σ flex (MPa) | 152.2 ± 13.9 | 75.0 ± 11.8 | 7.2 ± 0.6 |
| E flex (MPa) | 3097.3 ± 224.0 | 4503.9 ± 246.5 | 2364.4 ± 245.0 |
| ε flex (%) | 5.9 ± 1.0 | 2.5 ± 0.5 | 0.6 ± 0.1 |
References
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; Ji, Y.; Wei, Y. Functional epoxy vitrimers and composites. Prog. Mater. Sci. 2021, 120, 100710. [Google Scholar] [CrossRef]
- Briou, B.; Améduri, B.; Boutevin, B. Trends in the Diels–Alder reaction in polymer chemistry. Chem. Soc. Rev. 2021, 50, 11055–11097. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, X.; Pei, Z.; Yang, Y.; Wei, Y.; Ji, Y. Multi-stimuli responsive and multi-functional oligoaniline-modified vitrimers. Chem. Sci. 2016, 8, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Capelot, M.; Unterlass, M.M.; Tournilhac, F.; Leibler, L. Catalytic Control of the Vitrimer Glass Transition. ACS Macro Lett. 2012, 1, 789–792. [Google Scholar] [CrossRef]
- de Luzuriaga, A.R.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Mater. Horizons 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Memon, H.; Liu, H.; Rashid, M.A.; Chen, L.; Jiang, Q.; Zhang, L.; Wei, Y.; Liu, W.; Qiu, Y. Vanillin-Based Epoxy Vitrimer with High Performance and Closed-Loop Recyclability. Macromolecules 2020, 53, 621–630. [Google Scholar] [CrossRef]
- Lorero, I.; Rodríguez, A.; Campo, M.; Prolongo, S. Thermally remendable, weldable, and recyclable epoxy network crosslinked with reversible Diels-alder bonds. Polymer 2022, 259, 125334. [Google Scholar] [CrossRef]
- Zolghadr, M.; Shakeri, A.; Zohuriaan-Mehr, M.J.; Salimi, A. Self-healing semi-IPN materials from epoxy resin by solvent-free furan–maleimide Diels–Alder polymerization. J. Appl. Polym. Sci. 2019, 136, 48015. [Google Scholar] [CrossRef]
- Bai, N.; Saito, K.; Simon, G.P. Synthesis of a diamine cross-linker containing Diels–Alder adducts to produce self-healing thermosetting epoxy polymer from a widely used epoxy monomer. Polym. Chem. 2012, 4, 724–730. [Google Scholar] [CrossRef]
- Cuvellier, A.; Verhelle, R.; Brancart, J.; Vanderborght, B.; Van Assche, G.; Rahier, H. The influence of stereochemistry on the reactivity of the Diels–Alder cycloaddition and the implications for reversible network polymerization. Polym. Chem. 2018, 10, 473–485. [Google Scholar] [CrossRef]
- Liu, X.; Du, P.; Liu, L.; Zheng, Z.; Wang, X.; Joncheray, T.; Zhang, Y. Kinetic study of Diels–Alder reaction involving in maleimide–furan compounds and linear polyurethane. Polym. Bull. 2013, 70, 2319–2335. [Google Scholar] [CrossRef]
- Strachota, B.; Hodan, J.; Dybal, J.; Matějka, L. Self-Healing Epoxy and Reversible Diels-Alder Based Interpenetrating Networks. Macromol. Mater. Eng. 2021, 306, 2000474. [Google Scholar] [CrossRef]
- Singh, N.P.; Gupta, V.; Singh, A.P. Graphene and carbon nanotube reinforced epoxy nanocomposites: A review. Polymer 2019, 180, 121724. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, H.; Tzounis, L.; Santagiuliana, G.; Bilotti, E.; Papageorgiou, D.G. Multifunctional epoxy nanocomposites reinforced by two-dimensional materials: A review. Carbon 2021, 185, 57–81. [Google Scholar] [CrossRef]
- Sofla, R.L.M.; Rezaei, M.; Babaie, A.; Nasiri, M. Preparation of electroactive shape memory polyurethane/graphene nanocomposites and investigation of relationship between rheology, morphology and electrical properties. Compos. Part B Eng. 2019, 175, 107090. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Giannetti, A.; Lima, G.M.R.; Orozco, F.; Picchioni, F.; Mattoli, V.; Bose, R.K.; Pucci, A. Thermally Switchable Electrically Conductive Thermoset rGO/PK Self-Healing Composites. Polymers 2021, 13, 339. [Google Scholar] [CrossRef]
- Cortés, A.; Pérez-Chao, N.; Jiménez-Suárez, A.; Campo, M.; Prolongo, S. Sequential and selective shape memory by remote electrical control. Eur. Polym. J. 2022, 164, 110888. [Google Scholar] [CrossRef]
- Gong, C.; Liang, J.; Hu, W.; Niu, X.; Ma, S.; Hahn, H.T.; Pei, Q. A Healable, Semitransparent Silver Nanowire-Polymer Composite Conductor. Adv. Mater. 2013, 25, 4186–4191. [Google Scholar] [CrossRef]
- Li, J.; Liang, J.; Li, L.; Ren, F.; Hu, W.; Li, J.; Qi, S.; Pei, Q. Healable Capacitive Touch Screen Sensors Based on Transparent Composite Electrodes Comprising Silver Nanowires and a Furan/Maleimide Diels–Alder Cycloaddition Polymer. ACS Nano 2014, 8, 12874–12882. [Google Scholar] [CrossRef]
- Kim, T.G.; Kim, J.M.; Jang, K.-S.; Lee, S.J. Dispersibility-tailored conductive epoxy nanocomposites with silica nanoparticle-embedded silver nanowires. Polym. Test. 2021, 96, 107111. [Google Scholar] [CrossRef]
- Sverdrup, H.; Koca, D.; Ragnarsdottir, K.V. Investigating the sustainability of the global silver supply, reserves, stocks in society and market price using different approaches. Resour. Conserv. Recycl. 2014, 83, 121–140. [Google Scholar] [CrossRef]
- Li, J.; Ma, P.C.; Chow, W.S.; To, C.K.; Tang, B.Z.; Kim, J. Correlations between Percolation Threshold, Dispersion State, and Aspect Ratio of Carbon Nanotubes. Adv. Funct. Mater. 2007, 17, 3207–3215. [Google Scholar] [CrossRef]
- Sánchez-Romate, X.F.; Artigas, J.; Jiménez-Suárez, A.; Sánchez, M.; Güemes, A.; Ureña, A. Critical parameters of carbon nanotube reinforced composites for structural health monitoring applications: Empirical results versus theoretical predictions. Compos. Sci. Technol. 2019, 171, 44–53. [Google Scholar] [CrossRef]
- Willocq, B.; Bose, R.K.; Khelifa, F.; Garcia, S.J.; Dubois, P.; Raquez, J.-M. Healing by the Joule effect of electrically conductive poly(ester-urethane)/carbon nanotube nanocomposites. J. Mater. Chem. A 2016, 4, 4089–4097. [Google Scholar] [CrossRef]
- Wang, T.; Yu, W.-C.; Zhou, C.-G.; Sun, W.-J.; Zhang, Y.-P.; Jia, L.-C.; Gao, J.-F.; Dai, K.; Yan, D.-X.; Li, Z.-M. Self-healing and flexible carbon nanotube/polyurethane composite for efficient electromagnetic interference shielding. Compos. Part B Eng. 2020, 193, 108015. [Google Scholar] [CrossRef]
- Handique, J.; Dolui, S.K. A thermally remendable multiwalled carbon nanotube/epoxy composites via Diels-Alder bonding. J. Polym. Res. 2019, 26, 163. [Google Scholar] [CrossRef]
- Macedo, R.; Lima, G.; Orozco, F.; Picchioni, F.; Moreno-Villoslada, I.; Pucci, A.; Bose, R.K.; Araya-Hermosilla, R. Electrically Self-Healing Thermoset MWCNTs Composites Based on Diels-Alder and Hydrogen Bonds. Polymers 2019, 11, 1885. [Google Scholar] [CrossRef]
- Jiménez-Suárez, A.; Campo, M.; Gaztelumendi, I.; Markaide, N.; Sánchez, M.; Ureña, A. The influence of mechanical dispersion of MWCNT in epoxy matrix by calendering method: Batch method versus time controlled. Compos. Part B Eng. 2013, 48, 88–94. [Google Scholar] [CrossRef]
- ASTM Subcommittee D20; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. D790. American Society for Testing Materials: Conshohocken, PA, USA, 2002.
- Hu, N.; Masuda, Z.; Yamamoto, G.; Fukunaga, H.; Hashida, T.; Qiu, J. Effect of fabrication process on electrical properties of polymer/multi-wall carbon nanotube nanocomposites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 893–903. [Google Scholar] [CrossRef]
- Bao, W.; Meguid, S.; Zhu, Z.; Pan, Y.; Weng, G. A novel approach to predict the electrical conductivity of multifunctional nanocomposites. Mech. Mater. 2012, 46, 129–138. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Radue, M.S.; Gaikwad, P.; Bamane, S.; Patil, S.U.; Pisani, W.A.; Odegard, G.M. Prediction of the Interfacial Properties of High-Performance Polymers and Flattened CNT-Reinforced Composites Using Molecular Dynamics. Langmuir 2021, 37, 11526–11534. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Medvedev, G.A.; Lee, E.-W.; Kim, J.; Caruthers, J.M.; Strachan, A. Molecular dynamics simulations and experimental studies of the thermomechanical response of an epoxy thermoset polymer. Polymer 2012, 53, 4222–4230. [Google Scholar] [CrossRef]
- Grenier-Loustalot, M.-F.; Gouarderes, F.; Joubert, F.; Grenier, P. Synthesis, mechanism and kinetics of radical polymerization of bismaleimide-type telechelic oligomers in solvent and in the solid state. Polymer 1993, 34, 3848–3859. [Google Scholar] [CrossRef]
- Odegard, G.M.; Bandyopadhyay, A. Physical aging of epoxy polymers and their composites. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1695–1716. [Google Scholar] [CrossRef]
- Flory, A.L.; Ramanathan, T.; Brinson, L.C. Physical Aging of Single Wall Carbon Nanotube Polymer Nanocomposites: Effect of Functionalization of the Nanotube on the Enthalpy Relaxation. Macromolecules 2010, 43, 4247–4252. [Google Scholar] [CrossRef]
- Martone, A.; Grassia, L.; Zarrelli, M.; Giordano, M.; D’amore, A. Enthalpy relaxation of an epoxy matrix/carbon nanotubes. AIP Conf. Proc. 2012, 1459, 347–349. [Google Scholar] [CrossRef]
- Chang, C.-M.; Liu, Y.-L. Functionalization of multi-walled carbon nanotubes with furan and maleimide compounds through Diels–Alder cycloaddition. Carbon 2009, 47, 3041–3049. [Google Scholar] [CrossRef]
- Liu, Y.-L. Effective approaches for the preparation of organo-modified multi-walled carbon nanotubes and the corresponding MWCNT/polymer nanocomposites. Polym. J. 2016, 48, 351–358. [Google Scholar] [CrossRef]
- Polgar, L.M.; Criscitiello, F.; Van Essen, M.; Araya-Hermosilla, R.; Migliore, N.; Lenti, M.; Raffa, P.; Picchioni, F.; Pucci, A. Thermoreversibly Cross-Linked EPM Rubber Nanocomposites with Carbon Nanotubes. Nanomaterials 2018, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.-R.; Lee, D.-I.; Park, J.-H.; Lee, D.-S. Thermally Healable and Recyclable Graphene-Nanoplate/Epoxy Composites Via an In-Situ Diels-Alder Reaction on the Graphene-Nanoplate Surface. Polymers 2019, 11, 1057. [Google Scholar] [CrossRef] [PubMed]
- Ciecierska, E.; Boczkowska, A.; Kurzydlowski, K.J.; Rosca, I.D.; Van Hoa, S. The effect of carbon nanotubes on epoxy matrix nanocomposites. J. Therm. Anal. Calorim. 2013, 111, 1019–1024. [Google Scholar] [CrossRef]
- Che, B.D.; Nguyen, B.Q.; Nguyen, L.-T.T.; Nguyen, H.T.; Nguyen, V.Q.; Van Le, T.; Nguyen, N.H. The impact of different multi-walled carbon nanotubes on the X-band microwave absorption of their epoxy nanocomposites. Chem. Central J. 2015, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Pras, M.; Gérard, J.-F.; Golanski, L.; Quintard, G.; Duchet-Rumeau, J. Key Role of the Dispersion of Carbon Nanotubes (CNTs) within Epoxy Networks on their Ability to Release. Polymers 2020, 12, 2530. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.I.; Halder, S.; Wang, J. Diels-Alder based epoxy matrix and interfacial healing of bismaleimide grafted GNP infused hybrid nanocomposites. Polym. Test. 2019, 74, 138–151. [Google Scholar] [CrossRef]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Donchak, V.; Stetsyshyn, Y.; Bratychak, M.; Broza, G.; Harhay, K.; Stepina, N.; Kostenko, M.; Voronov, S. Nanoarchitectonics at surfaces using multifunctional initiators of surface-initiated radical polymerization for fabrication of the nanocomposites. Appl. Surf. Sci. Adv. 2021, 5, 100104. [Google Scholar] [CrossRef]
- Fang, L.; Chen, J.; Zou, Y.; Xu, Z.; Lu, C. Thermally-Induced Self-Healing Behaviors and Properties of Four Epoxy Coatings with Different Network Architectures. Polymers 2017, 9, 333. [Google Scholar] [CrossRef]
- Terryn, S.; Roels, E.; Brancart, J.; Van Assche, G.; Vanderborght, B. Self-Healing and High Interfacial Strength in Multi-Material Soft Pneumatic Robots via Reversible Diels–Alder Bonds. Actuators 2020, 9, 34. [Google Scholar] [CrossRef]
- Shymborska, Y.; Budkowski, A.; Raczkowska, J.; Donchak, V.; Melnyk, Y.; Vasiichuk, V.; Stetsyshyn, Y. Switching it Up: The Promise of Stimuli-Responsive Polymer Systems in Biomedical Science. Chem. Rec. 2023, e202300217. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Kim, M.S.; Huber, G.W.; Dumesic, J.A. Design of closed-loop recycling production of a Diels–Alder polymer from a biomass-derived difuran as a functional additive for polyurethanes. Green Chem. 2021, 23, 9479–9488. [Google Scholar] [CrossRef] [PubMed]

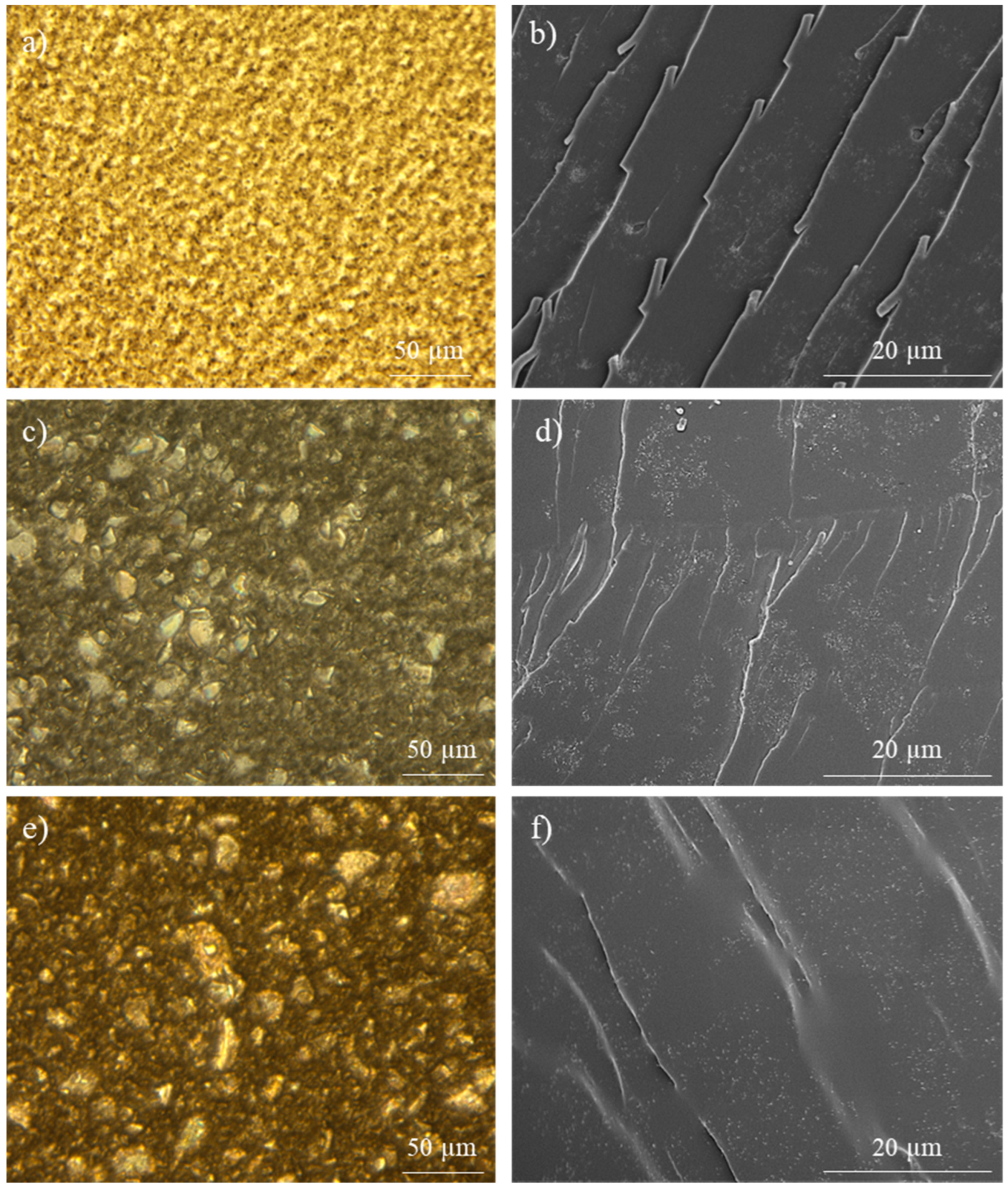
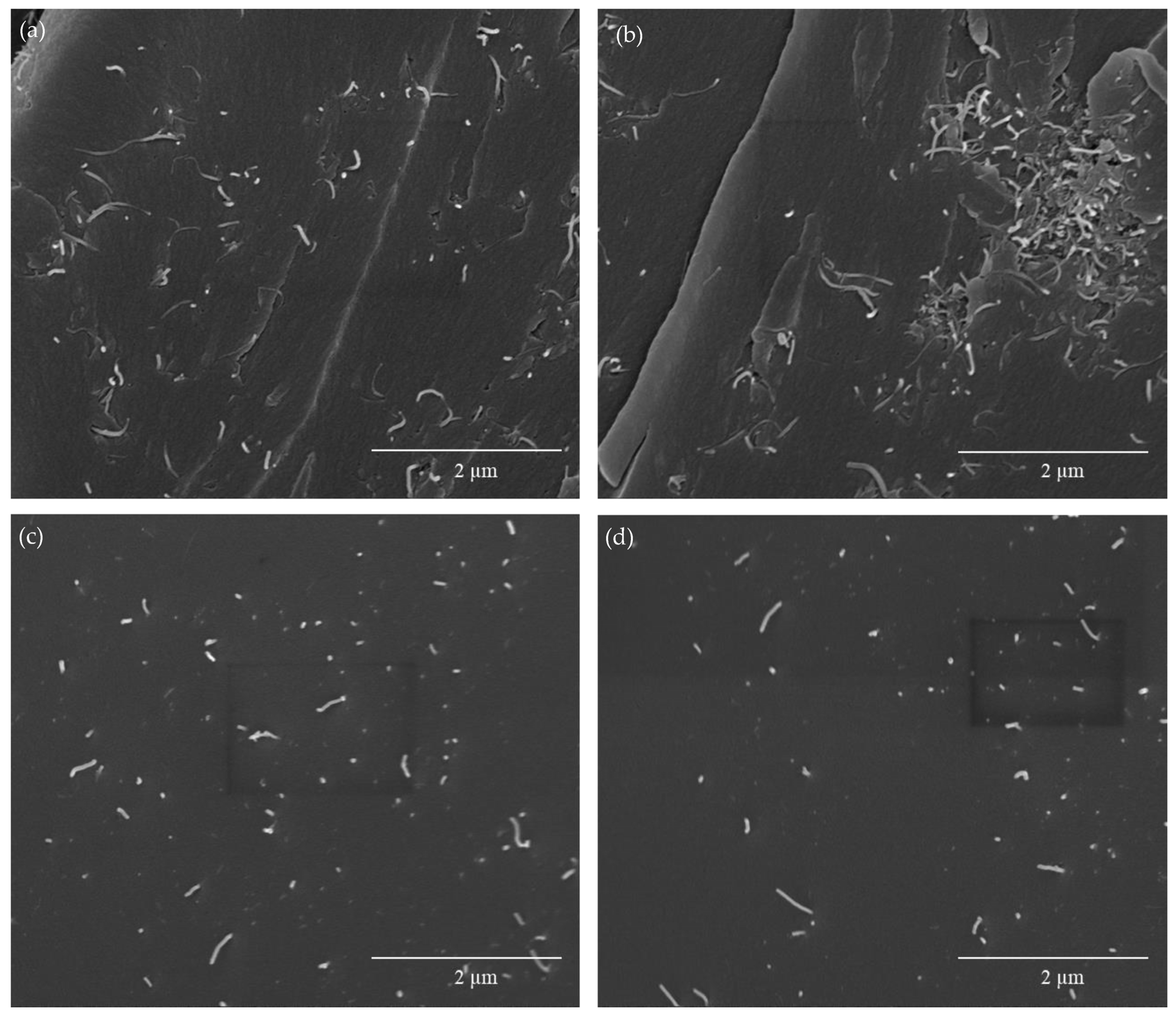




| Tg (°C) | ΔHrD-A (J/g) | |
|---|---|---|
| 0 D-A ratio + 0.7 wt.% CNT | 107.7 | - |
| 0.6 D-A ratio + 0.7 wt.% CNT | 69.8 | 6.5 |
| 1.0 D-A ratio + 0.7 wt.% CNT | 66.7 | 12.2 |
| 0 D-A ratio, neat resin | 108 | - |
| 0.6 D-A ratio, neat resin | 67.6 | 4.7 |
| 1.0 D-A ratio, neat resin | 64.4 | 8.2 |
| 0.6 D-A Ratio Neat Resin | 0.6 D-A Ratio + 0.7 CNT wt.% | |
|---|---|---|
| E’G, 30 °C (MPa) | 3129 ± 233 | 3336 ± 119 |
| E’R, min (MPa) | 3.3 ± 1.3 | 4.9 ± 1.0 |
| Tg (°C) | 91.1 ± 1.5 | 91.0 ± 1.0 |
| E’30 °C/E’Tg | 133–168 | 112–136 |
| E’G, 30 °C/E’R, min | 1070–1470 | 570–820 |
| σ flex (MPa) | 75.0 ± 11.8 | 33.9 ± 5.4 |
| ε u, flex (%) | 2.5 ± 0.5 | 1.4 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorero, I.; Rodríguez, Á.; Campo, M.; Prolongo, S.G. Development of an Electroactive and Thermo-Reversible Diels–Alder Epoxy Nanocomposite Doped with Carbon Nanotubes. Polymers 2023, 15, 4715. https://doi.org/10.3390/polym15244715
Lorero I, Rodríguez Á, Campo M, Prolongo SG. Development of an Electroactive and Thermo-Reversible Diels–Alder Epoxy Nanocomposite Doped with Carbon Nanotubes. Polymers. 2023; 15(24):4715. https://doi.org/10.3390/polym15244715
Chicago/Turabian StyleLorero, Isaac, Álvaro Rodríguez, Mónica Campo, and Silvia G. Prolongo. 2023. "Development of an Electroactive and Thermo-Reversible Diels–Alder Epoxy Nanocomposite Doped with Carbon Nanotubes" Polymers 15, no. 24: 4715. https://doi.org/10.3390/polym15244715
APA StyleLorero, I., Rodríguez, Á., Campo, M., & Prolongo, S. G. (2023). Development of an Electroactive and Thermo-Reversible Diels–Alder Epoxy Nanocomposite Doped with Carbon Nanotubes. Polymers, 15(24), 4715. https://doi.org/10.3390/polym15244715









