Recommended Values for the Hydrophobicity and Mechanical Properties of Coating Materials Usable for Preparing Controlled-Release Fertilizers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials, Equipment, and Devices
2.2. Experimental Methods
2.2.1. Preparation of Films
2.2.2. Preparation of CRFs in the Fluidized Bed Spray-Coating Process
2.2.3. Preparation of Modified S Film and Modified SCFs (MSCF)
2.2.4. Determination of the N Release Period of the CRFs
2.2.5. Measurement of the WA of Films
2.2.6. Measurement of the WCA of Films
2.2.7. Measurement of the Mechanical Properties of Films
2.2.8. Statistical Analyses
3. Results
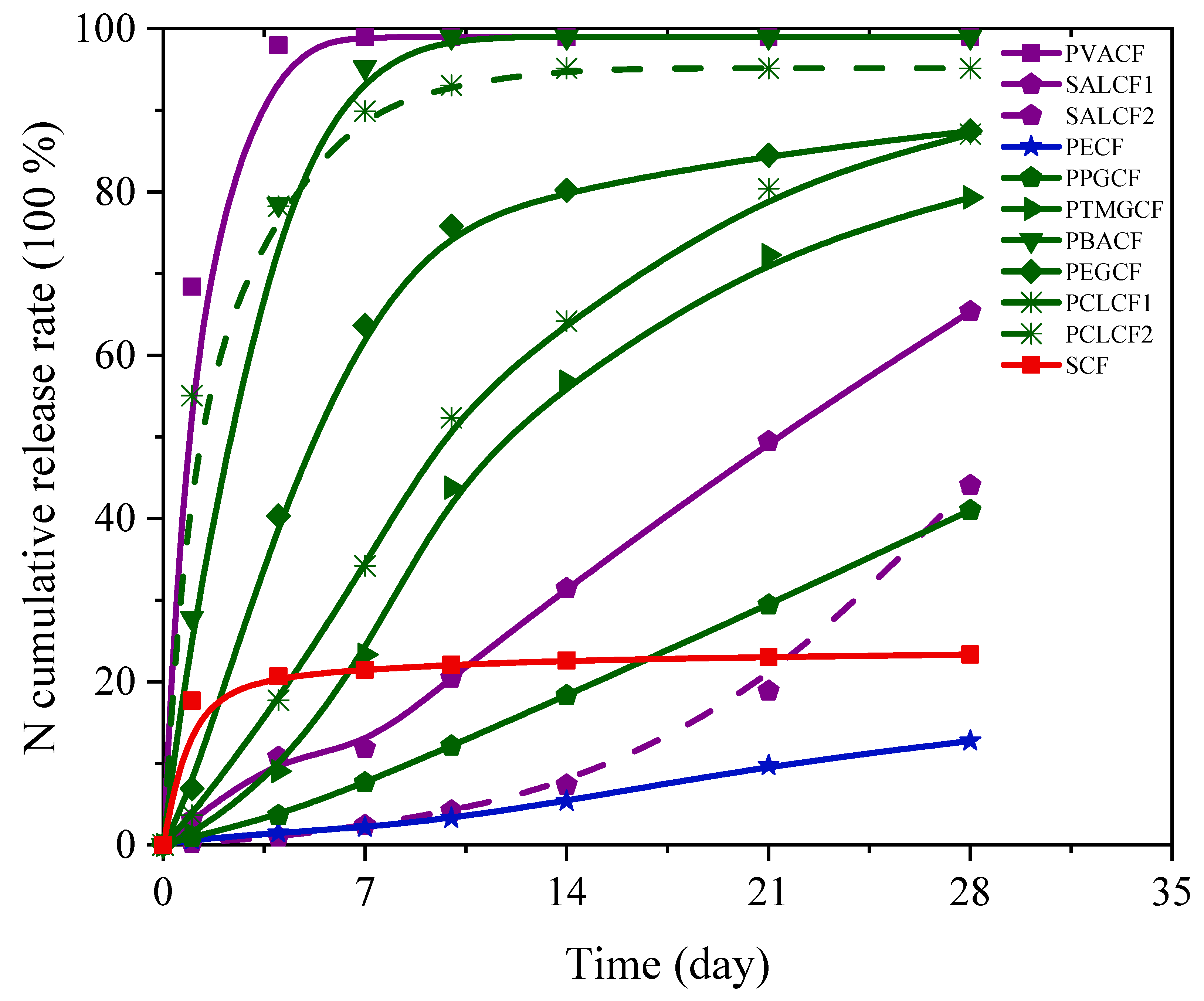
3.1. The N Release Characteristics of CRFs
3.2. The Hydrophobicity of Different Coating Materials
3.3. Mechanical Properties of Different Coating Materials
3.4. Correlations between the Controlled-Release Performance of CRFs and the Physical Properties of the Coating Materials
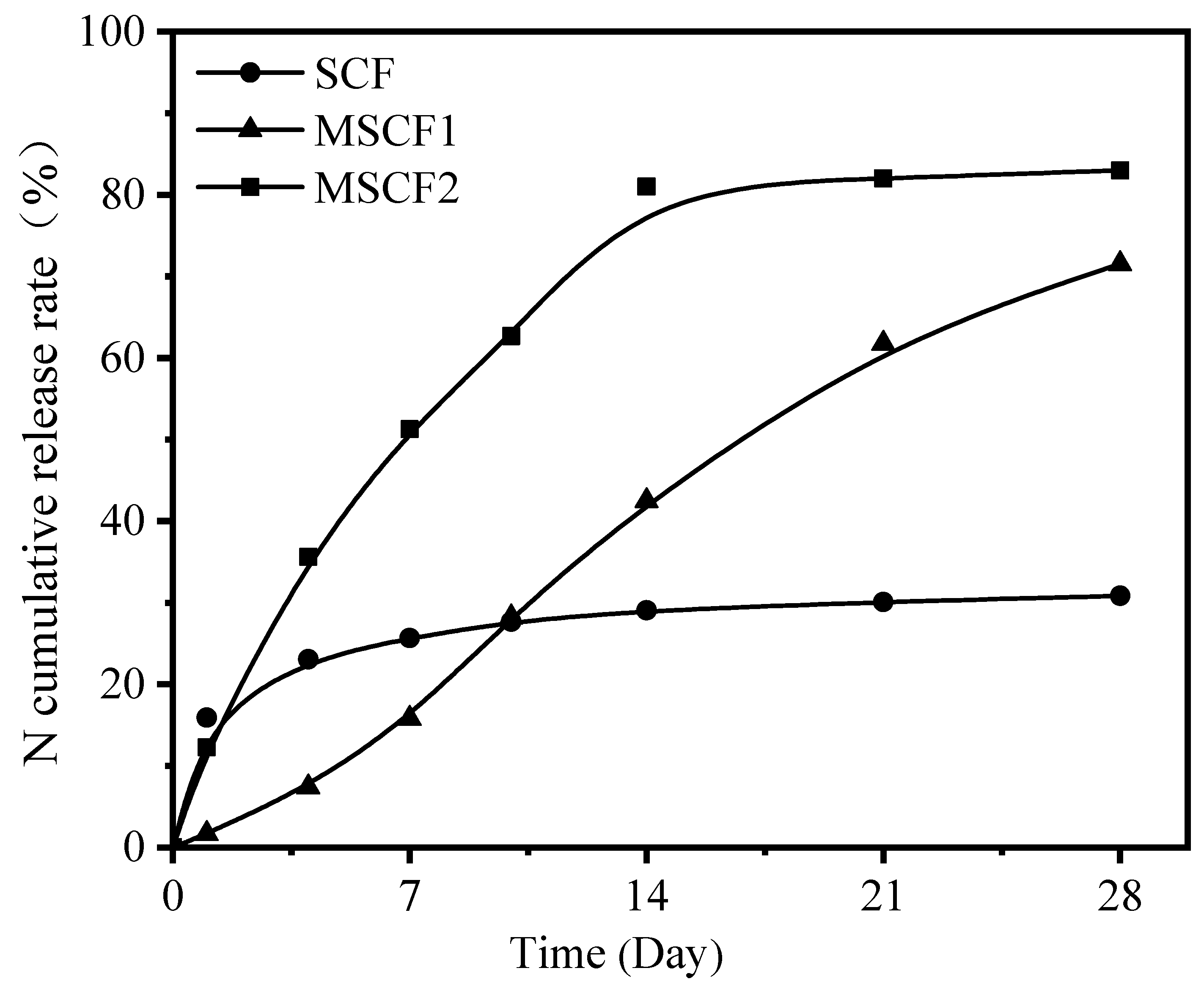
3.5. The Controlled-Release Performance and Physical Properties of MSCFs
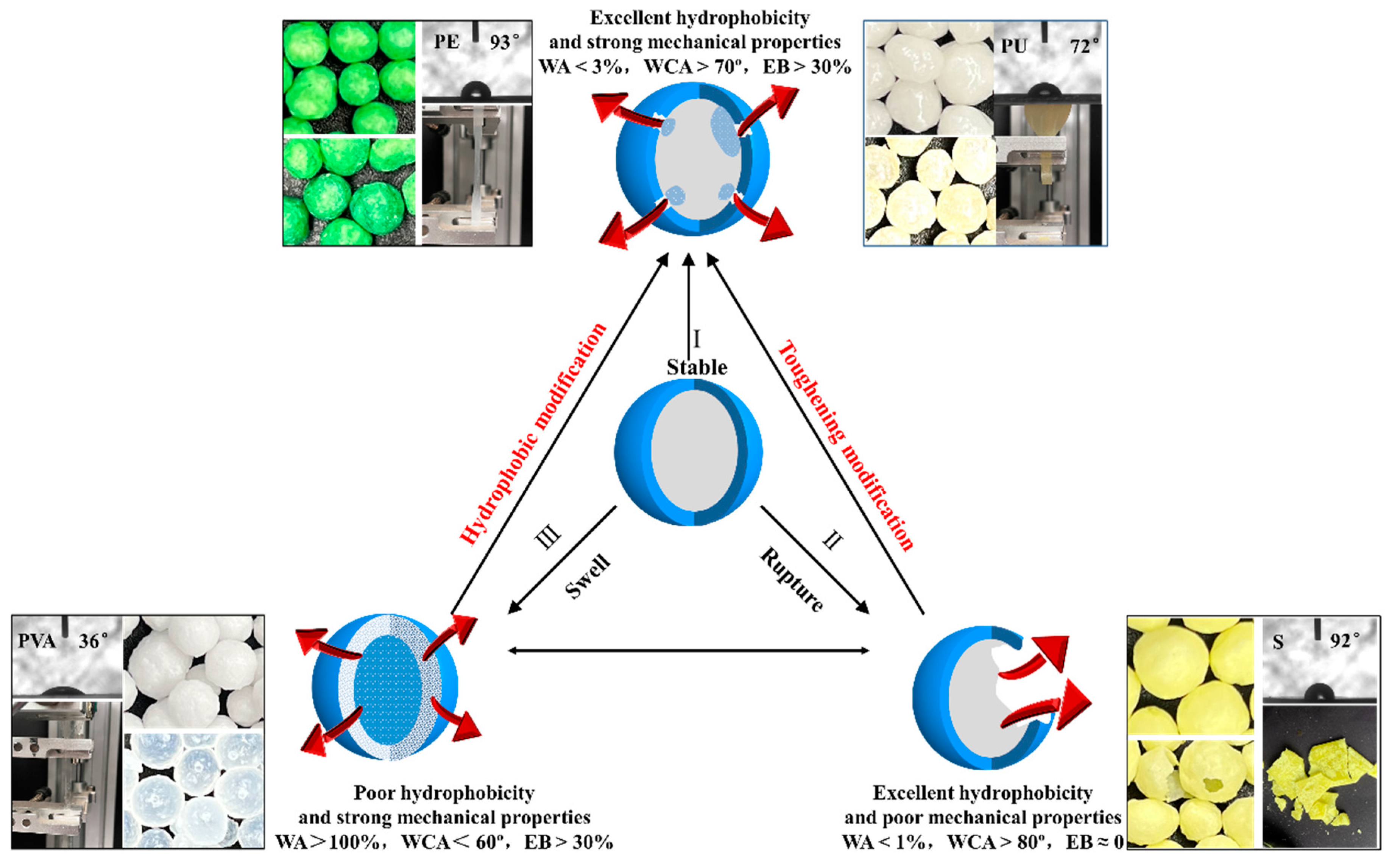
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Sui, B.; Feng, X.F.; Tian, G.L.; Hu, X.Y.; Shen, Q.R.; Guo, S.W. Optimizing nitrogen supply increases rice yield and nitrogen use efficiency by regulating yield formation factors. Field Crops Res. 2013, 150, 99–107. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.F.; Jiang, Y.; Yan, L.; Petropoulos, E.; Wang, J.Y.; Xue, L.H.; Yang, L.Z.; Chen, D.L. Effect of fertilization on nitrogen losses through surface runoffs in Chinese farmlands: A meta-analysis. Sci. Total Environ. 2021, 793, 148554. [Google Scholar] [CrossRef]
- Savci, S. Investigation of effect of chemical fertilizers on environment. APCBEE Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [CrossRef]
- Chen, Z.M.; Wang, Q.; Ma, J.W.; Zou, P.; Jiang, L.N. Impact of controlled-release urea on rice yield, nitrogen use efficiency and soil fertility in a single rice cropping system. Sci. Rep. 2020, 10, 10432. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tang, L.; Han, Y.L.; Yang, L.; Xie, G.X.; Peng, J.W.; Tian, C.; Zhou, X.; Liu, Q.; Rong, X.M.; et al. Reduction in nitrogen fertilizer applications by the use of polymer-coated urea: Effect on maize yields and environmental impacts of nitrogen losses. J. Sci. Food Agric. 2019, 99, 2259–2266. [Google Scholar] [CrossRef]
- Qiao, D.L.; Liu, H.S.; Yu, L.; Bao, X.Y.; Simonb, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef]
- Boller, R.A.; Graver, R.B. Two-Package Coating System Comprising a Polyester Having an Acid Number of at Least 50 in One of the Packages Thereof. U.S. Patent 3,218,274, 16 November 1961. [Google Scholar]
- Stansbury, R.L.; Lynch, C.S.; Kamil, S.; Linden, N.J. Slow-Release Fertilizer Composition Consisting of Asphalt Wax Binder and Inert Filler. U.S. Patent 3,276,857, 5 October 1964. [Google Scholar]
- Fujita, T.; Takahashi, C.; Ohshima, M.; Ushioda, T.; Shimizu, H. Method for Producing Granular Coated Fertilizer. U.S. Patent 4,019,890, 3 December 1974. [Google Scholar]
- Geiger, A.J.; Stelmack, E.G.; Babiak, N.M. Controlled Release Fertilizer and Method for Production Thereof. U.S. Patent 6,663,686 B1, 16 December 2000. [Google Scholar]
- Liu, Y.H.; Wang, T.J.; Kan, C.Y.; Wang, M.H.; Jin, Y. Development in polymer latex coated fertilizer for controlled release. Chem. Indus. Engin. Prog. 2009, 28, 1589–1595. (In Chinese) [Google Scholar]
- Lawrencia, D.; Wong, S.K.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled release fertilizers: A review on coating materials and mechanism of release. Plants 2021, 10, 238. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, J.; Yang, X.D. The diffusion model of nutrient release from membrane pore of controlled release fertilizer. Environ. Technol. Innov. 2022, 25, 102256. [Google Scholar] [CrossRef]
- Yang, X.D.; Jiang, R.F.; Lin, Y.Z.; Li, Y.T.; Li, J.; Zhao, B.Q. Nitrogen release characteristics of polyethylene-coated controlled-release fertilizers and their dependence on membrane pore structure. Particuology 2018, 36, 158–164. [Google Scholar] [CrossRef]
- Lu, P.F.; Zhang, M.; Li, Q.; Xu, Y. Structure and Properties of Controlled Release Fertilizers Coated with Thermosetting Resin. Polym.-Plast. Technol. Eng. 2013, 52, 381–386. [Google Scholar] [CrossRef]
- Shaviv, A.; Raban, S.; Zaidel, E. Modeling controlled nutrient release from a population of polymer Coated fertilizers: Statistically based model for diffusion release. Environ. Sci. Technol. 2003, 37, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.H.; KuShaari, K.; Basit, A. Modeling the release of nitrogen from controlled release fertilizer with imperfect coating in soils and water. Ind. Eng. Chem. Res. 2015, 54, 6724–6733. [Google Scholar] [CrossRef]
- Cruz, D.; Bortoletto-Santos, R.; Guimarães, G.; Polito, W.L.; Ribeiro, C. Role of polymeric coating on the phosphate availability as a fertilizer: Insight from phosphate release by castor polyurethane coatings. J. Agric. Food Chem. 2017, 65, 5890–5895. [Google Scholar] [CrossRef]
- Du, C.W.; Zhou, J.; Shaviv, A.; Wang, H. Mathematical model for potassium release from polymer-coated fertilizer. Biosyst. Eng. 2004, 88, 395–400. [Google Scholar] [CrossRef]
- Wang, G.D.; Yang, L.; Lan, R.; Wang, T.J.; Jin, Y. Granulation by spray coating aqueous solution of ammonium sulfate to produce large spherical granules in a fluidized bed. Particuology 2013, 11, 483–489. [Google Scholar] [CrossRef]
- Lan, R.; Wang, G.D.; Yang, L.; Wang, T.J.; Kan, C.Y.; Jin, Y. Prediction of release characteristics of film-coated urea from structure characterization data of the film. Chem. Eng. Technol. 2013, 36, 347–354. [Google Scholar] [CrossRef]
- Yang, L.; An, D.; Wang, T.J.; Kan, C.Y.; Jin, Y. A model for the swelling of and diffusion from a hydrophilic film for controlled-release urea particles. Particuology 2017, 30, 73–82. [Google Scholar] [CrossRef]
- Ariyanti, S.; Man, Z.; Bustam, M.A. Improvement of hydrophobicity of urea modified tapioca starch film with lignin for slow release fertilizer. Adv. Mater. Res. 2020, 626, 350–354. [Google Scholar] [CrossRef]
- Shen, Y.Z.; Zhou, J.M.; Du, C.W.; Zhou, Z.J. Hydrophobic modification of waterborne polymer slows urea release and improves nitrogen use efficiency in rice. Sci. Total Environ. 2021, 794, 148612. [Google Scholar] [CrossRef] [PubMed]
- Treinyte, J.; Grazuleviciene, V.; Paleckiene, R.; Ostrauskaite, J.; Cesoniene, L. Biodegradable polymer composites as coating materials for granular fertilizers. J. Polym. Environ. 2018, 26, 543–554. [Google Scholar] [CrossRef]
- Han, X.Z.; Chen, S.S.; Hu, X.G. Controlled-release fertilizer encapsulated by starch/polyvinyl alcohol coating. Desalination 2009, 240, 21–26. [Google Scholar] [CrossRef]
- Chen, S.L.; Jiang, Y.F.; Chang, B.; Yang, M.; Zou, H.T.; Zhang, Y.L. Preparation and characteristics of urea coated with water-based copolymer-biochar composite film material. J. Plant Nutr. Fert. Sci. 2018, 24, 1245–1254. (In Chinese) [Google Scholar] [CrossRef]
- Sarkar, A.; Biswas, D.R.; Datta, S.C.; Dwivedi, B.S.; Bhattacharyya, R.; Kumar, R.; Bandyopadhyay, K.K.; Saha, M.; Chawla, G.; Saha, J.K.; et al. Preparation of novel biodegradable starch/poly (vinyl alcohol)/bentonite grafted polymeric films for fertilizer encapsulation. Carbohydr. Polym. 2021, 259, 117679. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, S.L.; Fan, L.J.; Yang, M.; Zou, H.T.; Zhang, Y.L. Preparation and properties of nano-SiO2–polyvinyl alcohol–γ-polyglutamic acid composite film materials. J. Plant Nutr. Fert. Sci. 2019, 25, 2044–2052. (In Chinese) [Google Scholar] [CrossRef]
- Yang, Y.; Zou, H.T.; Wang, J.; Xu, M.; Liu, Y.; Zhang, Y.L. Preparation and properties of modified polyvinyl alcohol film for encapsulation of fertilizer. J. Plant Nutr. Fert. Sci. 2012, 18, 1286–1292. (In Chinese) [Google Scholar]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in superhydrophobic surface development. Soft Matter. 2008, 4, 24–40. [Google Scholar] [CrossRef]
- Xie, J.Z.; Yang, Y.C.; Gao, B.; Wan, Y.S.; Li, Y.C.C.; Xu, J.; Zhao, Q.H. Biomimetic superhydrophobic biobased polyurethane-coated fertilizer with atmosphere “outerwear”. ACS Appl. Mater. Interfaces 2017, 9, 15868–15879. [Google Scholar] [CrossRef]
- Zhang, S.G.; Yang, Y.C.; Gao, B.; Li, Y.C.C.; Liu, Z.G. Superhydrophobic controlled-release fertilizers coated with bio-based polymers with organosilicon and nano-silica modifications. J. Mater. Chem. A 2017, 5, 19943–19953. [Google Scholar] [CrossRef]
- Yang, X.D.; Zhao, B.Q.; Li, Y.T.; Li, J.; Lin, Z.A.; Yuan, L. Method for Producing Controlled-Release Fertilizer Coated with Polyurethane. U.S. Patent 9,416,064 B2, 16 August 2014. [Google Scholar]
- Li, L.X.; Sun, Y.M.; Cao, B.; Song, H.H.; Xiao, Q.; Yi, W.P. Preparation and performance of polyurethane/mesoporous silica composites for coated urea. Mater. Des. 2016, 99, 21–25. [Google Scholar] [CrossRef]
- Dai, C.; Yang, L.; Xie, J.R.; Wang, T.J. Nutrient diffusion control of fertilizer granules coated with a gradient hydrophobic film. Colloids Surf. A 2020, 588, 124361. [Google Scholar] [CrossRef]
- Kassem, I.; Ablouh, E.H.; Bouchtaoui, F.Z.E.; Kassab, Z.; Khouloud, M.; Sehaqui, H.; Ghalfi, H.; Alami, J.; Achaby, M.E. Cellulose nanocrystals-filled poly (vinyl alcohol) nanocomposites as waterborne coating materials of NPK fertilizer with slow release and water retention properties. Int. J. Biol. Macromol. 2021, 189, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Wang, T.J.; Qin, L.; Jin, Y. Urea particle coating for controlled release by using DCPD modified sulfur. Powder Technol. 2008, 183, 88–93. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.D.; Wang, N.; Zhang, X.; Zhang, J.; Jiang, Z.G. Prepartion and characterization of modified SiO2/PU composite coating for controlled- release fertilizer. New Chem. Mater. 2020, 48, 146–150. (In Chinese) [Google Scholar] [CrossRef]
- Wang, S.P.; Li, X.; Ren, K.; Huang, R.; Lei, G.C.; Shen, L.J. Surface modification of pyrophyllite for optimizing properties of castor oil-based polyurethane composite and its application in controlled-release fertilizer. Arab. J. Chem. 2023, 16, 104400. [Google Scholar] [CrossRef]
- Cao, Y.P.; Yang, X.D.; Jiang, R.F.; Zhang, F.S.; Hu, S.W. A Polymer Coated Controlled Release Fertilizer and Its Production Method and Special Coating Material. ZL 200710099144.7, 14 May 2007. (In Chinese). [Google Scholar]
- ISO 18644:2016; Fertilizers and Soil Conditioners—Controlled-release Fertilizer: General Requirements. ISO: Geneva, Switzerland, 2016.
- Lu, H.; Dun, C.P.; Jariwala, H.; Wang, R.; Cui, P.Y.; Zhang, H.P.; Dai, Q.G.; Yang, S.; Zhang, H.C. Improvement of bio-based polyurethane and its optimal application in controlled release fertilizer. J. Control. Release 2022, 350, 748–760. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Madera-Santana, T.J.; Rodríguez-Félix, F.; Barreras-Urbina, C.G. Controlled and prolonged release systems of urea from micro and nanomaterials as an alternative for developing a sustainable agriculture: A review. J. Nanomater. 2022, 2022, 5697803. [Google Scholar] [CrossRef]
- Duan, L.L.; Zhang, M.; Liu, G.; Yang, Y.C.; Yang, Y. Membrane microstructures and nutrient release mechanism of thermoplastic coated urea. J. Plant Nutr. Fert. Sci. 2019, 15, 1170–1178. (In Chinese) [Google Scholar]
- Ibrahim, K.R.M.; Babadi, F.E.; Yunus, R. Comparative performance of different urea coating materials for slow release. Particuology 2014, 17, 165–172. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Kaewpirom, S. Potassium release kinetics and water retention of controlled-release fertilizers based on chitosan hydrogels. J. Polym. Environ. 2010, 18, 413–421. [Google Scholar] [CrossRef]
- Mulder, W.J.; Gosselink, R.J.A.; Vingerhoeds, M.H.; Harmsen, P.F.H.; Eastham, D. Lignin based controlled release coatings. Ind. Crops Prod. 2011, 34, 915–920. [Google Scholar] [CrossRef]
- Ferna, M.; Garrido-Herrera, F.J.; Gonza, E.; Villafranca-Sa, M.; Flores-Ce, F. Lignin and ethylcellulose as polymers in controlled release formulations of urea. J. Appl. Polym. Sci. 2008, 108, 3796–3803. [Google Scholar] [CrossRef]
- Wang, F.Y.; Liu, M.Z.; Ni, B.O.; Xie, L.H. κ-carrageenan–sodium alginate beads and superabsorbent coated nitrogen fertilizer with slow-release, water-retention, and anticompaction properties. Ind. Eng. Chem. Res. 2012, 51, 1413–1422. [Google Scholar] [CrossRef]
- Li, L.X.; Wang, M.; Wu, X.D.; Yi, W.P.; Xiao, Q. Bio-based polyurethane nanocomposite thin coatings from two comparable POSS with eight same vertex groups for controlled release urea. Sci. Rep. 2021, 11, 9917. [Google Scholar] [CrossRef]
- Zhao, M.H.; Wang, Y.Q.; Liu, L.X.; Liu, L.X.; Chen, M.; Zhang, C.Q.; Lu, Q.M. Green coatings from renewable modified bentonite and vegetable oil based polyurethane for slow release fertilizers. Polym. Compos. 2017, 39, 4355–4363. [Google Scholar] [CrossRef]
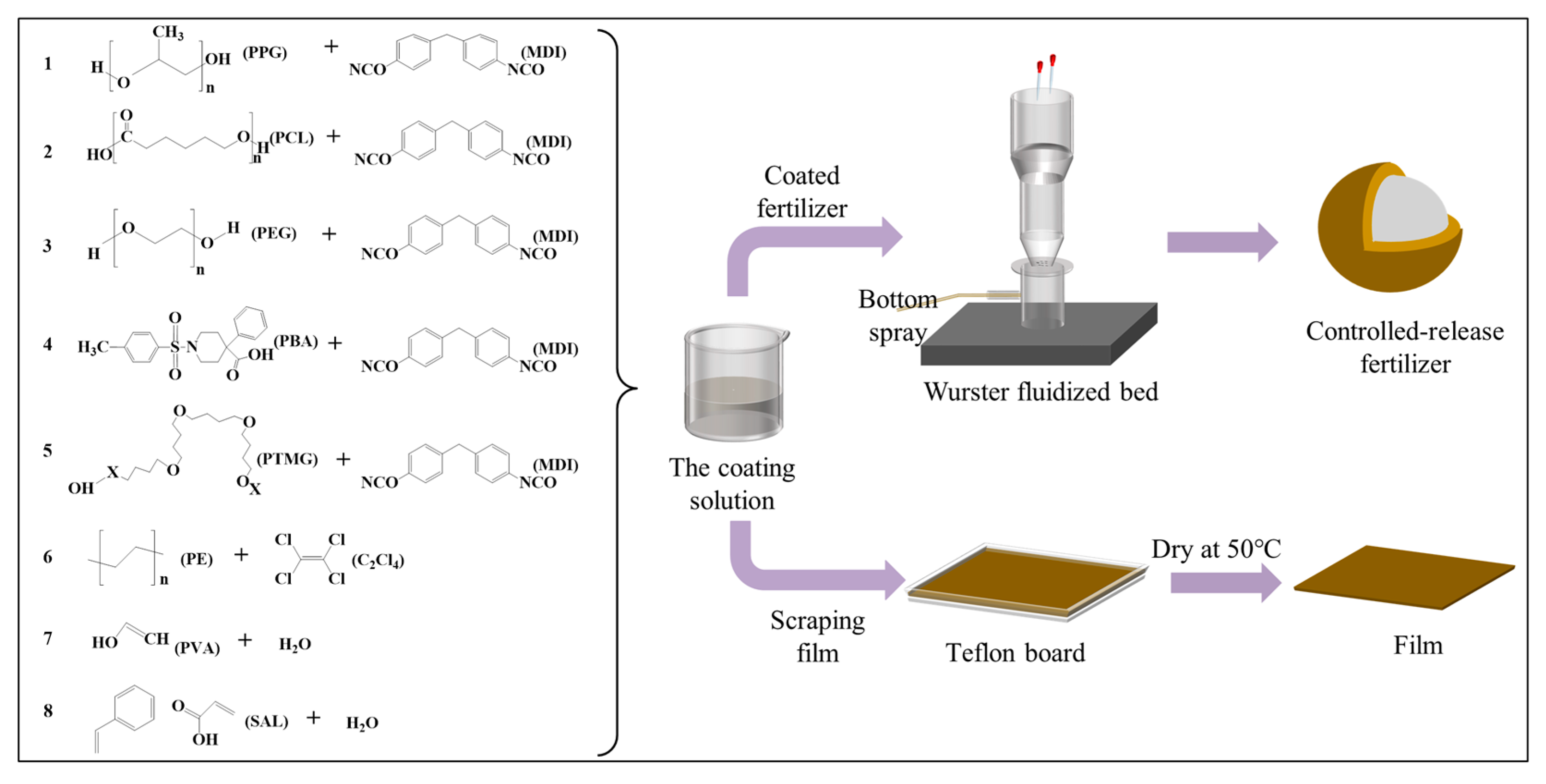


| NO. | Treatment | Quantity of Coating Materials | Urea (kg) | Coating Rate (%) | Temperature (°C) |
|---|---|---|---|---|---|
| 1 | PVACF | 1000 g PVA solution (5% w/w) | 1 | 5 | 80 |
| 2 | SALCF1 | 267 g SAL1 solution (30% w/w) | 1 | 8 | 45 |
| 3 | SALCF2 | 267 g SAL2 solution (30% w/w) | 1 | 8 | 45 |
| 4 | PECF | 625 g PE solution (8% w/w) | 1 | 5 | 85 |
| 5 | PPGCF | 19.96 g PPG, 20.04 g MDI | 1 | 4 | 70 |
| 6 | PTMGCF | 19.88 g PTMG250, 20.21 MDI | 1 | 4 | 70 |
| 7 | PBACF | 30.8 g PBA 1000, 9.2 g MDI | 1 | 4 | 70 |
| 8 | PEGCF | 18 g PEG200, 23.8 g MDI | 1 | 4 | 70 |
| 9 | PCLCF1 | 25.76 g PCL3050, 16.6 g MDI | 1 | 4 | 70 |
| 10 | PCLCF2 | 32 g PCL2105, 8 g MDI | 1 | 4 | 70 |
| Coating Materials | Treatment | Time | η△t | T | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | 4 d | 7 d | 10 d | 14 d | 21 d | 28 d | ||||
| PVA | PVACF | 68.4 | 98.1 | 99.0 | 99.0 | 99.0 | 99.0 | 99.0 | -- | 1.5 |
| SAL | SALCF1 | 0.2 | 1.0 | 2.4 | 4.2 | 7.4 | 18.9 | 44.1 | 1.6 | 50 |
| SALCF2 | 3.1 | 10.8 | 11.9 | 20.5 | 31.4 | 49.5 | 65.4 | 2.3 | 34 | |
| PE | PECF | 0.6 | 1.5 | 2.2 | 3.3 | 5.4 | 9.7 | 12.8 | 0.4 | 178 |
| PU | PPGCF | 1.0 | 3.6 | 7.6 | 12.1 | 18.3 | 29.4 | 41.0 | 1.5 | 54 |
| PTMGCF | 1.2 | 9.0 | 23.3 | 43.8 | 56.8 | 72.3 | 79.3 | 2.9 | 28 | |
| PBACF | 27.8 | 78.5 | 95.3 | 99.0 | 99.0 | 99.0 | 99.0 | 16.9 | 4 | |
| PEGCF | 6.9 | 40.3 | 63.7 | 75.8 | 80.2 | 84.5 | 87.5 | 3.0 | 25 | |
| PCLCF1 | 3.7 | 17.8 | 34.2 | 52.3 | 64.2 | 80.4 | 87.1 | 3.1 | 26 | |
| PCLCF2 | 55.1 | 78.2 | 89.9 | 93.1 | 95.2 | 95.2 | 95.2 | 7.7 | 4 | |
| S | SCF | 17.7 | 20.7 | 21.5 | 22.1 | 22.6 | 23.0 | 23.4 | -- | -- |
| Kinetic Equation | r | Release Period (Day) | ||||
|---|---|---|---|---|---|---|
| 30 | 90 | |||||
| y1 | y2 | y1 | y2 | |||
| WA (%) | y = 166.06x−1.24 | 0.986 | 2.4 | 3.0 | 0.6 | 1.0 |
| WCA (°) | y = 37.28x0.18 | 0.701 | 68.8 | 60.0 | 83.8 | 80.0 |
| EB (%) | y = −19.42 + 2.57x | 0.737 | 57.7 | 30.0 | 211.9 | 100.0 |
| Treatment | WA (%) | WCA (°) | EB (%) | TS (MPa) | Release Period (Day) |
|---|---|---|---|---|---|
| SCF | 0.1 | 92.1 | -- | -- | -- |
| MSCF1 | 2.4 | 63.3 | 48.3 | 2.8 | 40 |
| MSCF2 | 2.2 | 75.9 | 10.4 | 1.2 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, J.; Lin, R.; Gu, D.; Zhou, Y.; Li, H.; Yang, X. Recommended Values for the Hydrophobicity and Mechanical Properties of Coating Materials Usable for Preparing Controlled-Release Fertilizers. Polymers 2023, 15, 4687. https://doi.org/10.3390/polym15244687
Wang Y, Li J, Lin R, Gu D, Zhou Y, Li H, Yang X. Recommended Values for the Hydrophobicity and Mechanical Properties of Coating Materials Usable for Preparing Controlled-Release Fertilizers. Polymers. 2023; 15(24):4687. https://doi.org/10.3390/polym15244687
Chicago/Turabian StyleWang, Yajing, Juan Li, Ru Lin, Dianrun Gu, Yuanfang Zhou, Han Li, and Xiangdong Yang. 2023. "Recommended Values for the Hydrophobicity and Mechanical Properties of Coating Materials Usable for Preparing Controlled-Release Fertilizers" Polymers 15, no. 24: 4687. https://doi.org/10.3390/polym15244687
APA StyleWang, Y., Li, J., Lin, R., Gu, D., Zhou, Y., Li, H., & Yang, X. (2023). Recommended Values for the Hydrophobicity and Mechanical Properties of Coating Materials Usable for Preparing Controlled-Release Fertilizers. Polymers, 15(24), 4687. https://doi.org/10.3390/polym15244687







