Modifying Effect and Mechanism of Polymer Powder on the Properties of Asphalt Binder for Engineering Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Preparation
2.1.1. Asphalt
2.1.2. Polyurea
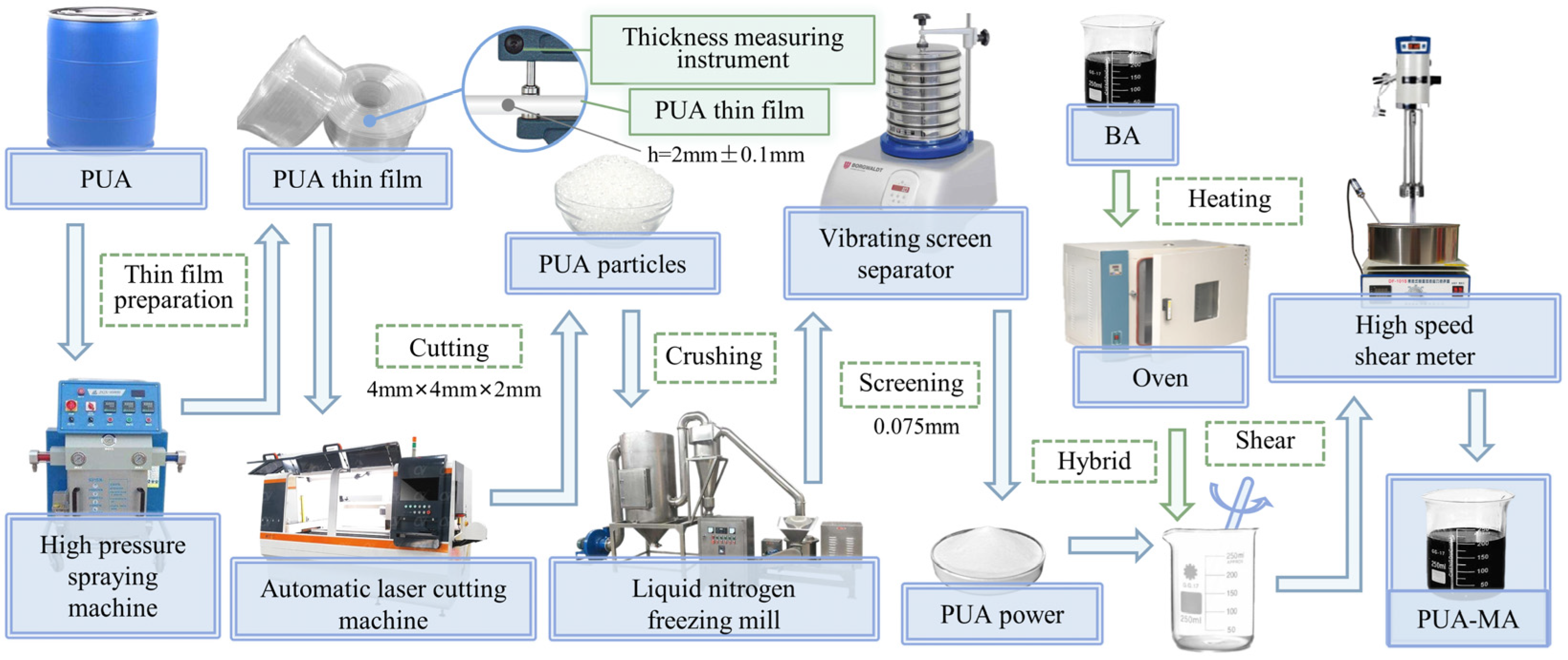
2.1.3. Preparation of PUA and PUA-MA
- I.
- Preparation of PUA modifier
- The PUA raw material of a certain weight was placed in the hopper of a high-pressure spraying machine (H20/35PUA Model, Qindao Sanhesheng Polymer Technology Co., Ltd., Qingdao, China), and the PUA film was produced under high-pressure sealing conditions. PUA film thickness should be controlled within 2 mm (±0.1 mm).
- The 2-mm PUA film was divided into 4–5 mm particles.
- The PUA particles were placed in the storage bin of the liquid nitrogen and then transferred to the mill for fine grinding.
- PUA powder of various particle sizes was screened, and PUA powder of 0.075 mm was chosen as the asphalt modifier.
- II.
- Preparation of PUA-MA
2.2. Experimental Method
2.2.1. Fundamental Performance Test
2.2.2. SEM-EDS Test
2.2.3. TG-FTIR Test
2.2.4. DSC Test
2.2.5. High-Temperature Rheological Properties Test
3. Results and Discussion
3.1. Fundamental Performance of PUA-MA
3.1.1. Physical Property
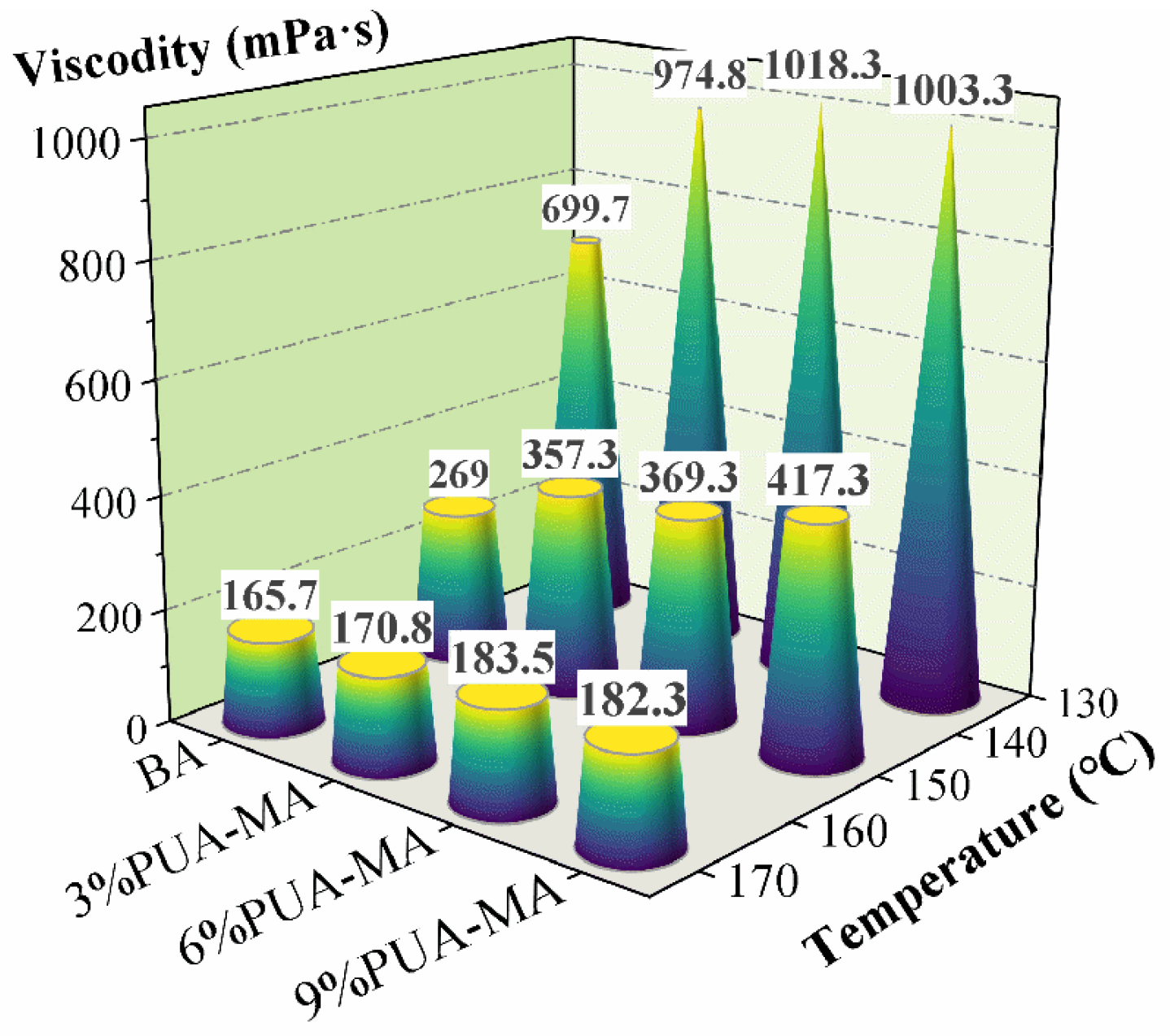
3.1.2. Brookfield Viscosity Test
3.2. Thermal Properties of PUA-MA
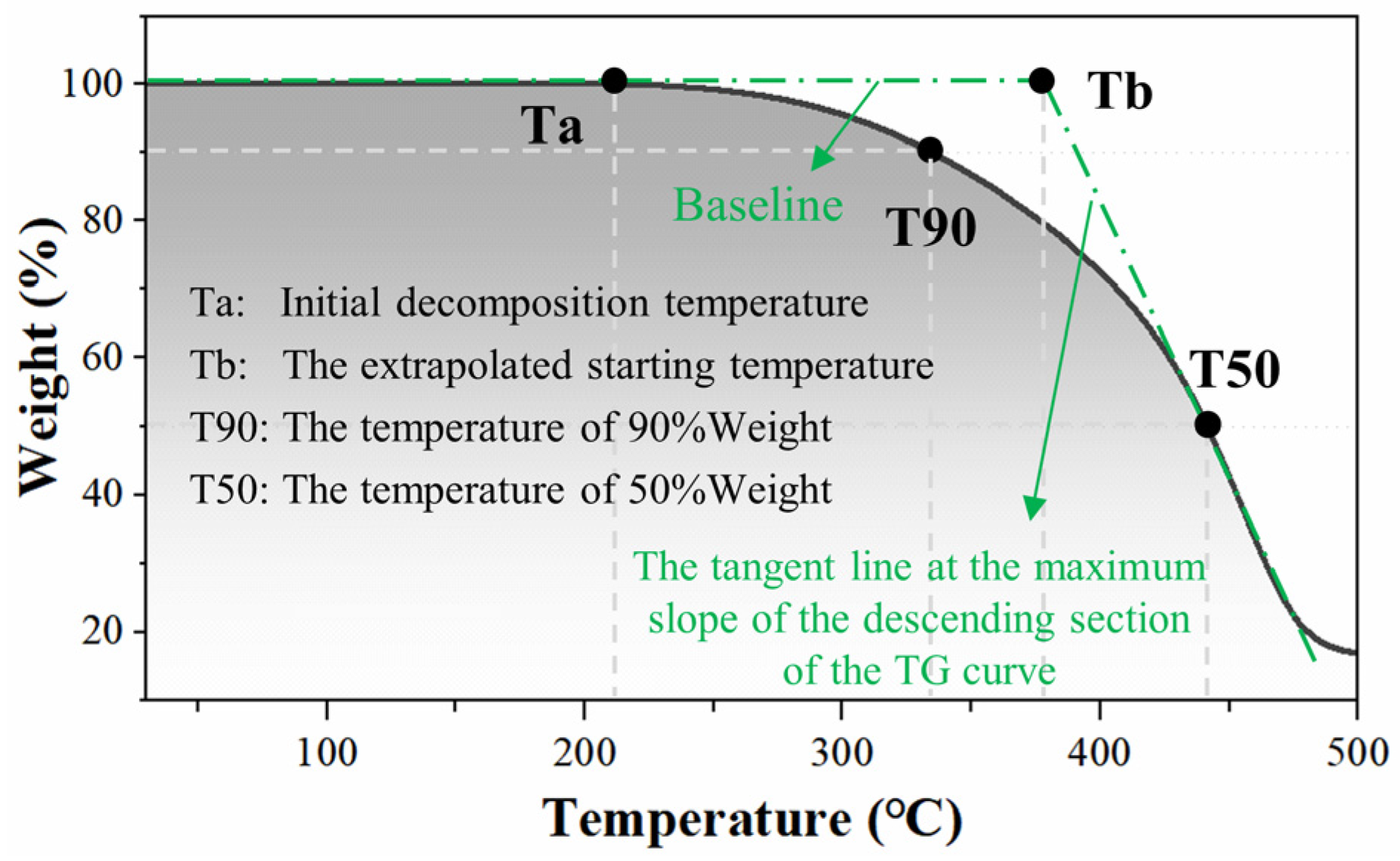
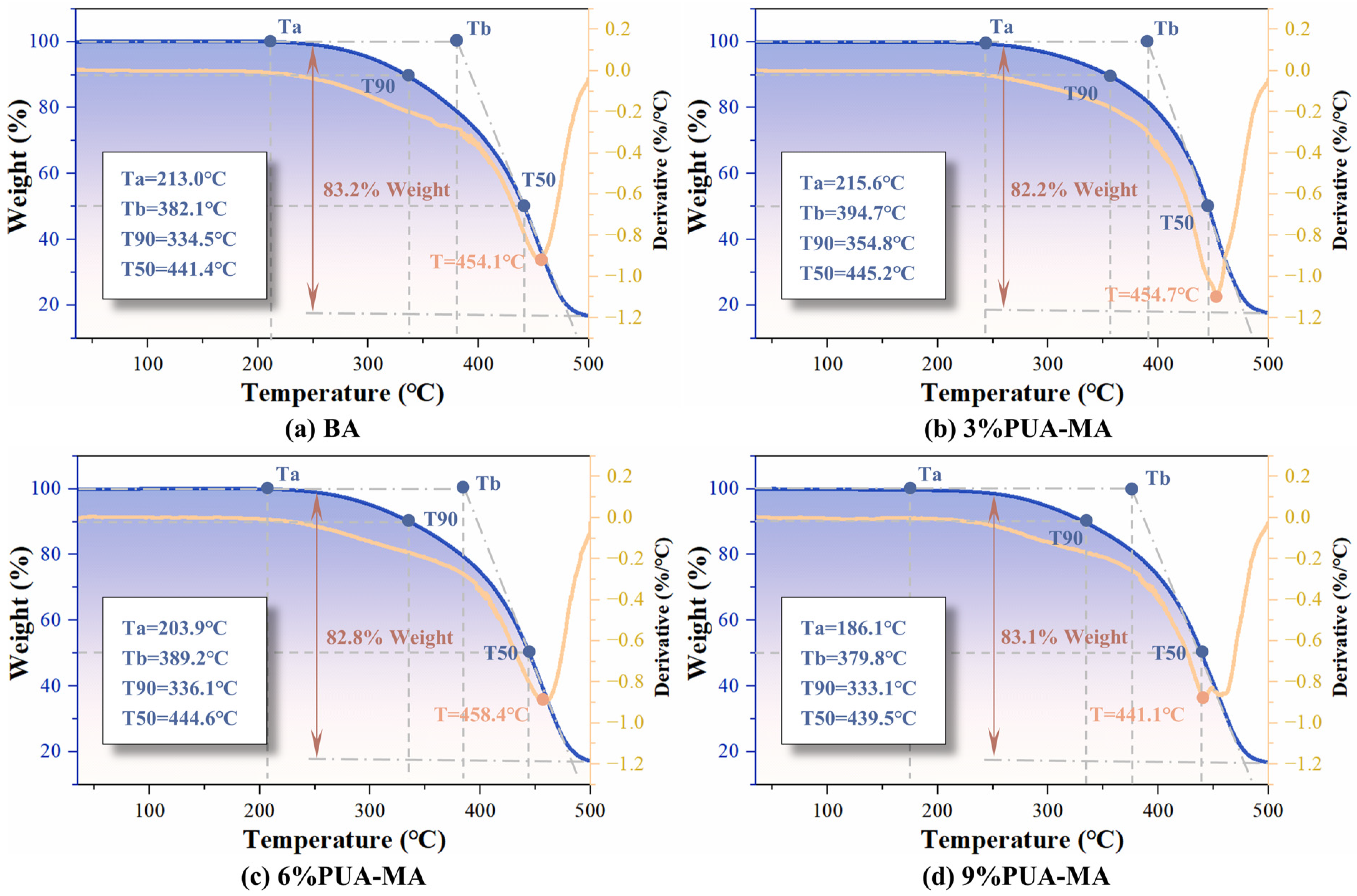
3.2.1. TG Test
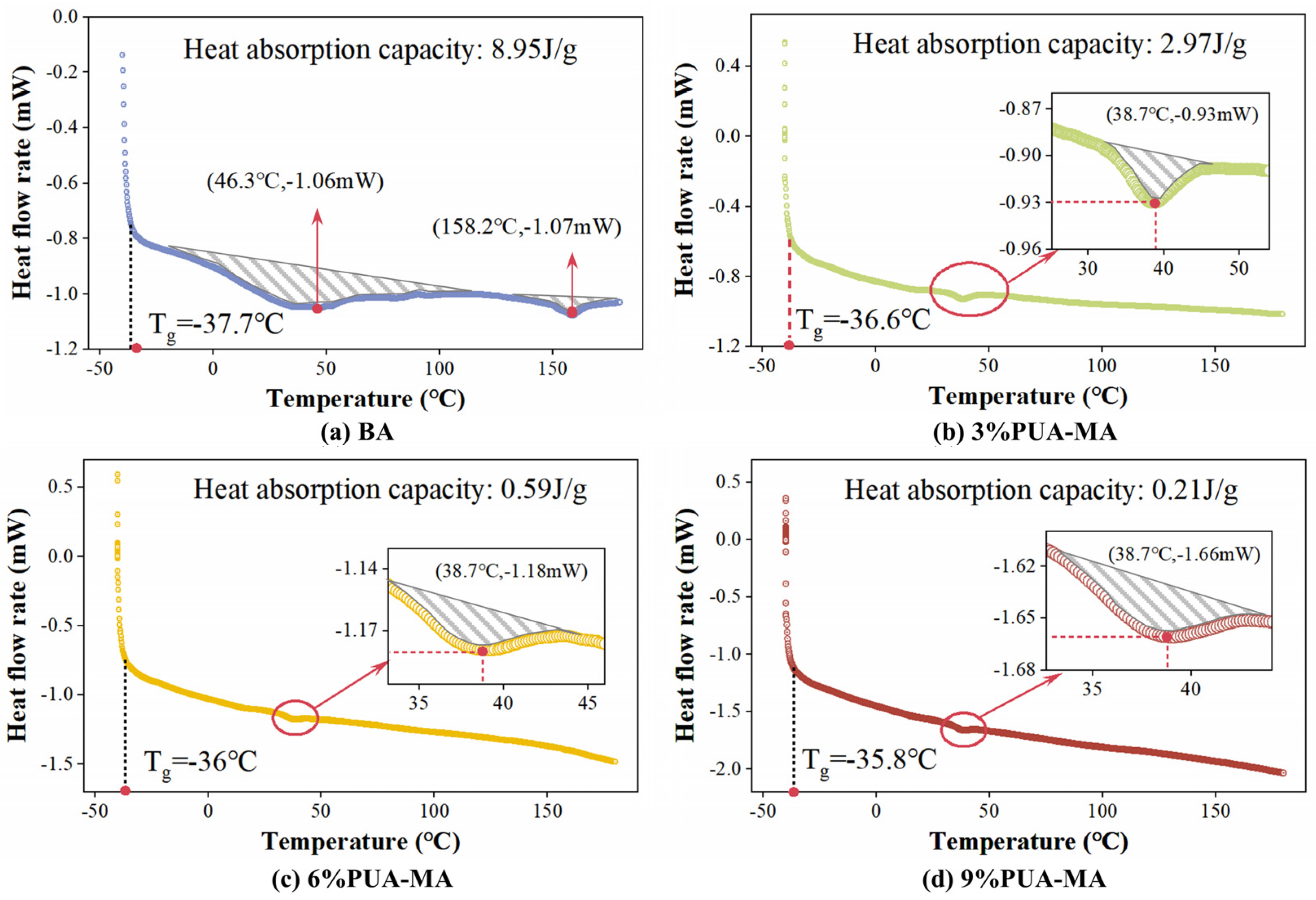
3.2.2. DSC Test
3.3. Microscopic Characteristics of PUA-MA
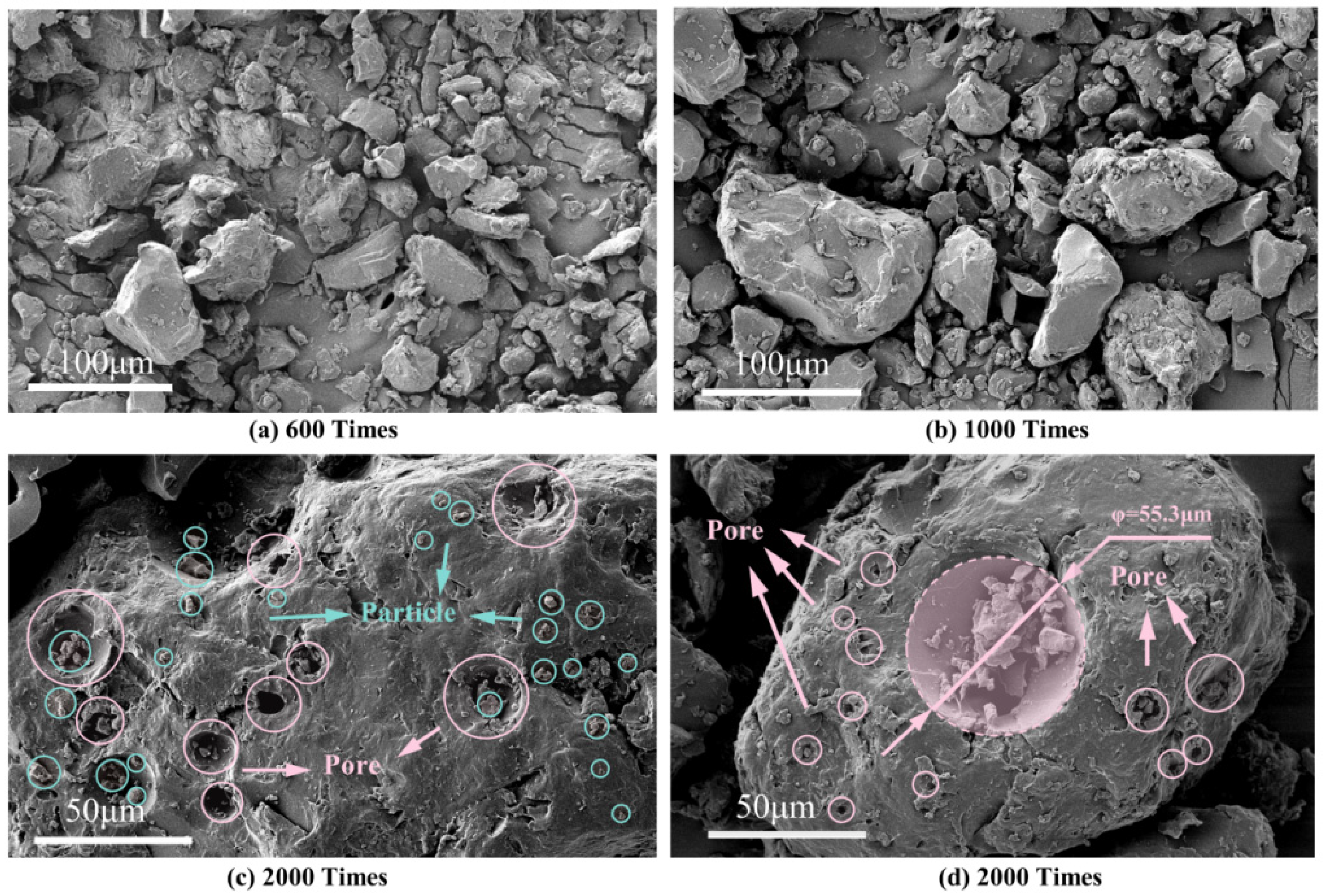
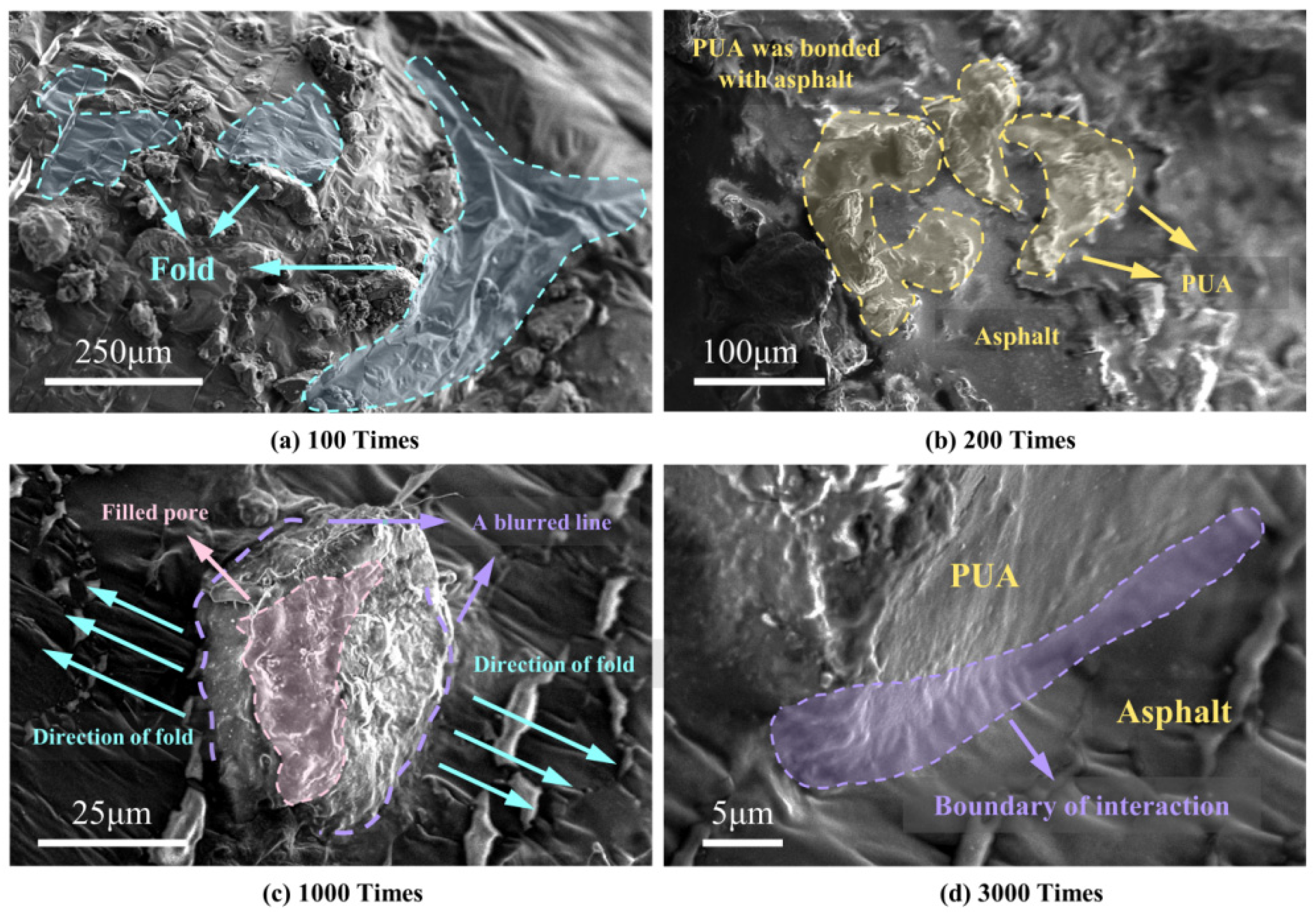
3.3.1. SEM Test
3.3.2. EDS Test
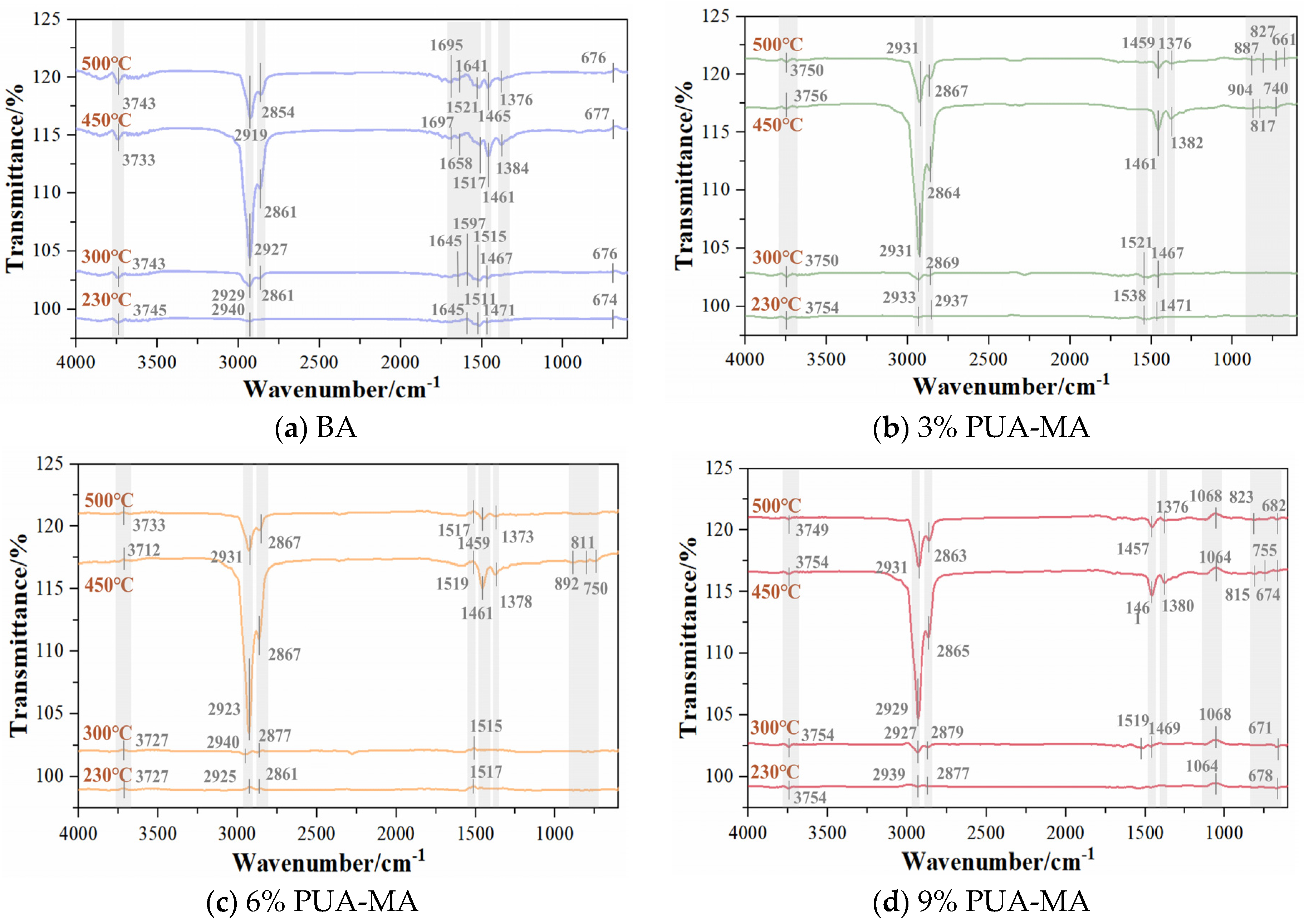
3.3.3. FTIR Test
3.4. High-Temperature Rheological Properties of PUA-MA
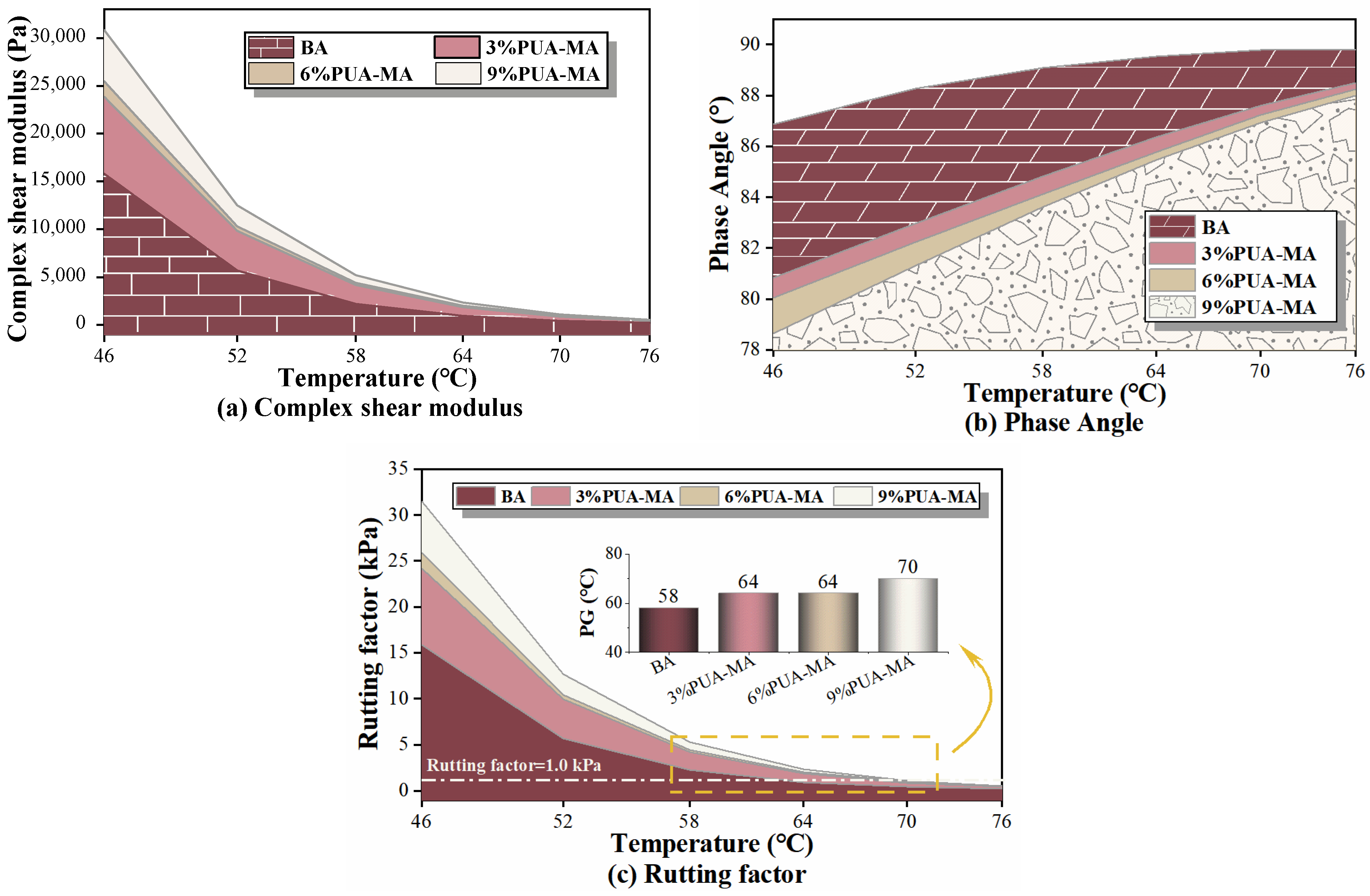
3.4.1. Temperature Sweep Test
3.4.2. Frequency Scanning Test
3.4.3. MSCR Test
4. Conclusions
- The particles of the PUA modifier were mainly massive structures with dense structures and a small number of surface apertures. After being coated by asphalt, the particles formed an infiltration state with excellent physical compatibility.
- PUA substantially improved the thermal stability of asphalt, thereby increasing the thermal decomposition temperature and decreasing the thermal mass loss. However, the glass transition temperature and low-temperature performance were diminished.
- With the addition of PUA and the increase of dosage, softening point and Brookfield viscosity of asphalt were considerably enhanced, as was its performance at high temperatures. However, asphalt’s penetration and elongation decreased, and its low-temperature efficacy was not enhanced.
- PUA modifier significantly improved the rheological properties of asphalt at high temperature. Increasing complex shear modulus, rut factor, PG, and R, and reducing phase Angle and Jnr could enhance asphalt’s rutting resistance at high temperature. The higher the dosage of the modifier, the better the performance.
- Although the high-temperature performance and thermal stability of asphalt were the best when the content of PUA was 6–9%, the low-temperature performance of asphalt was not improved effectively at high content. Therefore, the recommended content of the PUA modifier was 6%.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Behnood, A.; Gharehveran, M.M. Morphology, rheology, and physical properties of polymer-modified asphalt binders. Eur. Polym. J. 2019, 112, 766–791. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, Y.; Rahman, A.; Ding, H. Application of steel slag powder to enhance the low-temperature fracture properties of asphalt mastic and its corresponding mechanism. J. Clean Prod. 2018, 184, 21–31. [Google Scholar] [CrossRef]
- Porto, M.; Caputo, P.; Loise, V.; Eskandarsefat, S.; Teltayev, B.; Rossi, C.O. Asphalt and Asphalt Modification: A Review on Latest Advances. Appl. Sci. 2019, 9, 174. [Google Scholar] [CrossRef]
- Afzal, A.; Kausar, A.; Siddiq, M. Role of polymeric composite in civil engineering applications: A review. Polym.-Plast. Tech. Mater. 2020, 59, 1023–1040. [Google Scholar] [CrossRef]
- Khatoon, H.; Ahmad, S. A review on conducting polymer reinforced polyurethane composites. J. Ind. Eng. Chem. 2017, 53, 1–22. [Google Scholar] [CrossRef]
- Somarathna, H.M.C.C.; Raman, S.N.; Mohotti, D.; Mutalib, A.A.; Badri, K.H. The use of polyurethane for structural and infrastructural engineering applications: A state-of-the-art review. Constr. Build. Mater. 2018, 190, 995–1014. [Google Scholar] [CrossRef]
- Visakh, P.M.; Semkin, A.O.; Rezaev, I.A.; Fateev, A.V. Review on soft polyurethane flame retardant. Constr. Build. Mater. 2019, 227, 11673. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wang, J.; Yuan, J.; Jiang, F.; Yu, X.; Xiao, F. Recent applications and developments of Polyurethane materials in pavement engineering. Constr. Build. Mater. 2021, 304, 124639. [Google Scholar] [CrossRef]
- Polacco, G.; Filippi, S.; Merusi, F.; Stastna, G. A review of the fundamentals of polymer-modified asphalts: Asphalt/polymer interactions and principles of compatibility. Adv. Colloid Interface Sci. 2015, 224, 72–112. [Google Scholar] [CrossRef]
- Shatanawi, K.M.; Biro, S.; Geiger, A.; Amirkhanian, S.N. Effects of furfural activated crumb rubber on the properties of rubberized asphalt. Constr. Build. Mater. 2012, 28, 96–103. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Q.; Yang, Q.; Tovar, C.; Tan, Y.; Oeser, M. Thermaloxidative and ultraviolet ageing behavior of nano-montmorillonite modified asphalt. Road Mater. Pavement 2021, 22, 121–139. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. Rsc Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Cong, L.; Yang, F.; Guo, G.; Ren, M.; Shi, J.; Tan, L. The use of polyurethane for asphalt pavement engineering applications: A state-of-the-art review. Constr. Build. Mater. 2019, 225, 1012–1025. [Google Scholar] [CrossRef]
- Izquierdo, M.A.; Garcia-Morales, M.; Martinez-Boza, F.J.; Navarro, F.J. Thermo-mechanical properties and microstructural considerations of MDI isocyanate-based bituminous foams. Mater. Chem. Phys. 2014, 146, 261–268. [Google Scholar] [CrossRef]
- Li, T.; Guo, Z.; Liang, D.; Luo, S.; Zhang, Y.; Hong, B.; Lu, G.; Wang, D.; Oeser, M. Chemical and physical effects of polyurethane-precursor-based reactive modifier on the low-temperature performance of asphalt. Constr. Build. Mater. 2022, 328, 127055. [Google Scholar] [CrossRef]
- Li, T.; Guo, Z.; Lu, G.; Liang, D.; Luo, S.; Hong, B.; Wang, D.; Oeser, M. Experimental investigations and quantum chemical calculations of methylene diphenyl diisocyanate (MDI)-based chemically modified asphalt and its crosslinking behaviours. Fuel 2022, 321, 124084. [Google Scholar] [CrossRef]
- Sardon, H.; Pascual, A.; Mecerreyes, D.; Taton, D.; Cramail, H.; Hedrick, J.L. Synthesis of Polyurethanes Using Organocatalysis: A Perspective. Macromolecules 2015, 48, 3153–3165. [Google Scholar] [CrossRef]
- Hermida-Merino, D.; O’Driscoll, B.; Hart, L.R.; Harris, P.J.; Colquhoun, H.M.; Slark, A.T.; Prisacariu, C.; Hamley, I.W.; Hayes, W. Enhancement of microphase ordering and mechanical properties of supramolecular hydrogen-bonded polyurethane networks. Polym. Chem. 2018, 9, 3406–3414. [Google Scholar] [CrossRef]
- Gao, W.; Bie, M.; Quan, Y.; Zhu, J.; Zhang, W. Self-healing, reprocessing and sealing abilities of polysulfide-based polyurethane. Polymer 2018, 151, 27–33. [Google Scholar] [CrossRef]
- Jomaa, M.H.; Roiban, L.; Dhungana, D.S.; Xiao, J.; Cavaille, J.Y.; Seveyrat, L.; Lebrun, L.; Diguet, G.; Masenelli-Varlot, K. Quantitative analysis of grafted CNT dispersion and of their stiffening of polyurethane (PU). Compos. Sci. Technol. 2019, 171, 103–110. [Google Scholar] [CrossRef]
- Bazmara, B.; Tahersima, M.; Behravan, A. Influence of thermoplastic polyurethane and synthesized polyurethane additive in performance of asphalt pavements (Retracted article. See vol. 380, 2023). Constr. Build. Mater. 2018, 166, 1–11. [Google Scholar] [CrossRef]
- Sun, M.; Zheng, M.; Qu, G.; Yuan, K.; Bi, Y.; Wang, J. Performance of polyurethane modified asphalt and its mixtures. Constr. Build. Mater. 2018, 191, 386–397. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, J.; Jia, M.; Ban, X.; Wang, L.; Chen, L.; Huang, T.; Liu, H. Effects of Polyurethane Thermoplastic Elastomer on Properties of Asphalt Binder and Asphalt Mixture. J. Mater. Civ. Eng. 2021, 33, 04020477. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, J.; Jia, M.; Qi, B.; Zhang, H.; Lv, W.; Mao, Z.; Chang, P.; Peng, J.; Liu, Y. Study on a thermosetting polyurethane modified asphalt suitable for bridge deck pavements: Formula and properties. Constr. Build. Mater. 2020, 241, 118122. [Google Scholar] [CrossRef]
- Yu, R.; Zhu, X.; Zhang, M.; Fang, C. Investigation on the Short-Term Aging-Resistance of Thermoplastic Polyurethane-Modified Asphalt Binders. Polymers 2018, 10, 1189. [Google Scholar] [CrossRef]
- Fang, J.; Tu, J. Effect of ultraviolet (UV) aging on rheology properties and microstructure of polyurethane (PU) modified asphalt. Mater. Res. Express. 2019, 6, 125318. [Google Scholar] [CrossRef]
- Kazemi, M.; Goli, A.; Nasimifar, M. Evaluation of the self-healing performance of polyurethane-modified asphalt using asphalt bond strength (BBS) test and CT scan. Int. J. Pavement Res. Technol. 2021, 14, 168–173. [Google Scholar] [CrossRef]
- Carrera, V.; Partal, P.; Garcia-Morales, M.; Gallegos, C.; Perez-Lepe, A. Effect of processing on the rheological properties of poly-urethane/urea bituminous products. Fuel Process. Technol. 2010, 91, 1139–1145. [Google Scholar] [CrossRef]
- Izquierdo, M.A.; Navarro, F.J.; Martinez-Boza, F.J.; Gallegos, C. Structure-property relationships in the development of bituminous foams from MDI based prepolymers. Rheol. Acta. 2014, 53, 123–131. [Google Scholar] [CrossRef]
- Ministry of Transport. The Standard Test Methods of Asphalt and Bituminous Mixtures for Highway Engineering: JTG E20-2011; China Communications Press: Beijing, China, 2021.
- Yan, T.; Ran, X.; Chen, Z. Preparation and Research on Performance of Asphalt Modified by Polyurethane and Waste Tire Rubber. Highway 2019, 64, 214–219. [Google Scholar]
- Wang, L.; Zhang, Z.; Zhu, Y.; Liu, H.; Chen, L.; Ban, X. Properties and Microscopic Mechanism of PAPI Type Polyurethane Modified Asphalt. Bull. Chin. Ceram. Soc. 2021, 40, 4158–4166. [Google Scholar]
- Gunay, T.; Tomkovic, T.; Hatzikiriakos, S.G. Thermorheological properties of asphalt binders. Can. J. Chem. Eng. 2020, 98, 1803–1814. [Google Scholar] [CrossRef]
- Jin, X.; Guo, N.; You, Z.; Wang, L.; Wen, Y.; Tan, Y. Rheological properties and micro-characteristics of polyurethane composite modified asphalt. Constr. Build. Mater. 2020, 234, 117395. [Google Scholar] [CrossRef]
- Jin, X.; Guo, N.; Yan, Z.; Tan, Y. Preparation and Performance Evaluation on Polyurethane Composite Modified Asphalt, China. J. Highw. Transp. 2021, 34, 80–94. [Google Scholar]
- Zhang, H.; Zhang, G.; Han, F.; Zhang, Z.; Lv, W. A lab study to develop a bridge deck pavement using bisphenol A unsaturated polyester resin modified asphalt mixture. Constr. Build. Mater. 2018, 159, 83–98. [Google Scholar] [CrossRef]
- Yu, R.; Zhu, X.; Zhou, X.; Kou, Y.; Zhang, M.; Fang, C. Rheological properties and storage stability of asphalt modified with nanoscale polyurethane emulsion. Pet. Sci. Technol. 2018, 36, 85–90. [Google Scholar] [CrossRef]
- Jiang, X.; Gabrielson, J.; Huang, B.; Bai, Y.; Polaczyk, P.; Zhang, M.; Hu, W.; Xiao, R. Evaluation of inverted pavement by structural condition indicators from falling weight deflectometer. Constr. Build. Mater. 2022, 319, 125991. [Google Scholar] [CrossRef]
- Sun, X.; Ou, Z.; Zhao, T.; Qin, X.; Jin, J.; Yu, H.; Li, L. Interaction state and element leaching of waste incineration fly ash-asphalt mortar based on fillerization. Constr. Build. Mater. 2023, 398, 132463. [Google Scholar] [CrossRef]
- Lu, G.; Liu, P.; Toerzs, T.; Wang, D.; Oeser, M.; Grabe, J. Numerical analysis for the influence of saturation on the base course of permeable pavement with a novel polyurethane binder. Constr. Build. Mater. 2020, 240, 117930. [Google Scholar] [CrossRef]
- Jiang, X.; Titi, H.; Ma, Y.; Polaczyk, P.; Zhang, M.; Gabrielson, J.; Bai, Y.; Huang, B. Evaluating the performance of inverted pavement structure using the accelerated pavement test (APT). Constr. Build. Mater. 2022, 346, 128489. [Google Scholar] [CrossRef]
- Kavinmathi, K.; Narayan, S.P.A.; Subramanian, S.C. Impact of lateral load transfer in heavy road vehicles at horizontal curves on the distress of asphalt pavements. Road Mater. Pavement Des. 2022, 23, 2486–2506. [Google Scholar] [CrossRef]
- Carrera, V.; Partal, P.; Garcia-Morales, M.; Gallegos, C.; Paez, A. Influence of Asphalt Colloidal Nature on the Design of Isocyanate-Based Bituminous Products with Enhanced Rheological Properties. Ind. Eng. Chem. Res. 2009, 48, 8464–8470. [Google Scholar] [CrossRef]
- Nian, T.; Li, P.; Wei, X.; Wang, P.; Li, H.; Guo, R. The effect of freeze-thaw cycles on durability properties of SBS-modified asphalt. Constr. Build. Mater. 2018, 187, 77–88. [Google Scholar] [CrossRef]
- Mirsepahi, M.; Tanzadeh, J.; Ghanoon, S.A. Laboratory evaluation of dynamic performance and viscosity improvement in modified asphalt by combining nanomaterials and polymer. Constr. Build. Mater. 2020, 233, 117183. [Google Scholar] [CrossRef]
- Liu, S.; Jin, J.; Yu, H.; Gao, Y.; Du, Y.; Sun, X.; Qian, G. Performance enhancement of modified asphalt via coal gangue with microstructure control. Constr. Build. Mater. 2023, 367, 130287. [Google Scholar] [CrossRef]







| Technical Indices | Measured Value | JTG E20-2011 [30] | |
|---|---|---|---|
| Softening point/°C | 49 | 44–54 | |
| Ductility (10 °C, 5 cm/min)/cm | >100 | ≥100 | |
| Penetration (25 °C, 100 g, 5 s)/0.1 mm | 67.8 | 60–80 | |
| Brookfield viscosity (135 °C)/mPa·s | 699.7 | — | |
| PG grading | 58 | — | |
| TFOT Residue | Mass change/% | 0.2 | ≤0.8 |
| Residual penetration ratio/% | 70 | ≥61 | |
| Residual ductility (10 °C)/cm | 18 | ≥15 | |
| Type | Solid Content/% | Viscosity/cps | Tensile Strength/MPa | Tensile Strength/% |
|---|---|---|---|---|
| PUA-100 | 81–85 | ≤800 | 28 | 375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Sun, X.; Ou, Z.; Li, Z.; Liu, Z.; Qin, X. Modifying Effect and Mechanism of Polymer Powder on the Properties of Asphalt Binder for Engineering Application. Polymers 2023, 15, 4659. https://doi.org/10.3390/polym15244659
Zhao W, Sun X, Ou Z, Li Z, Liu Z, Qin X. Modifying Effect and Mechanism of Polymer Powder on the Properties of Asphalt Binder for Engineering Application. Polymers. 2023; 15(24):4659. https://doi.org/10.3390/polym15244659
Chicago/Turabian StyleZhao, Wensheng, Xiaolong Sun, Zhixin Ou, Zhijian Li, Zhisheng Liu, and Xiao Qin. 2023. "Modifying Effect and Mechanism of Polymer Powder on the Properties of Asphalt Binder for Engineering Application" Polymers 15, no. 24: 4659. https://doi.org/10.3390/polym15244659
APA StyleZhao, W., Sun, X., Ou, Z., Li, Z., Liu, Z., & Qin, X. (2023). Modifying Effect and Mechanism of Polymer Powder on the Properties of Asphalt Binder for Engineering Application. Polymers, 15(24), 4659. https://doi.org/10.3390/polym15244659






