Fiber-Type Transistor-Based Chemical and Physical Sensors Using Conjugated Polymers
Abstract
:1. Introduction
2. Conjugated Polymer Fibers
2.1. Conjugated Polymers
2.2. Methods for Fabricating Conjugated Polymer Fibers
2.2.1. Electrospinning
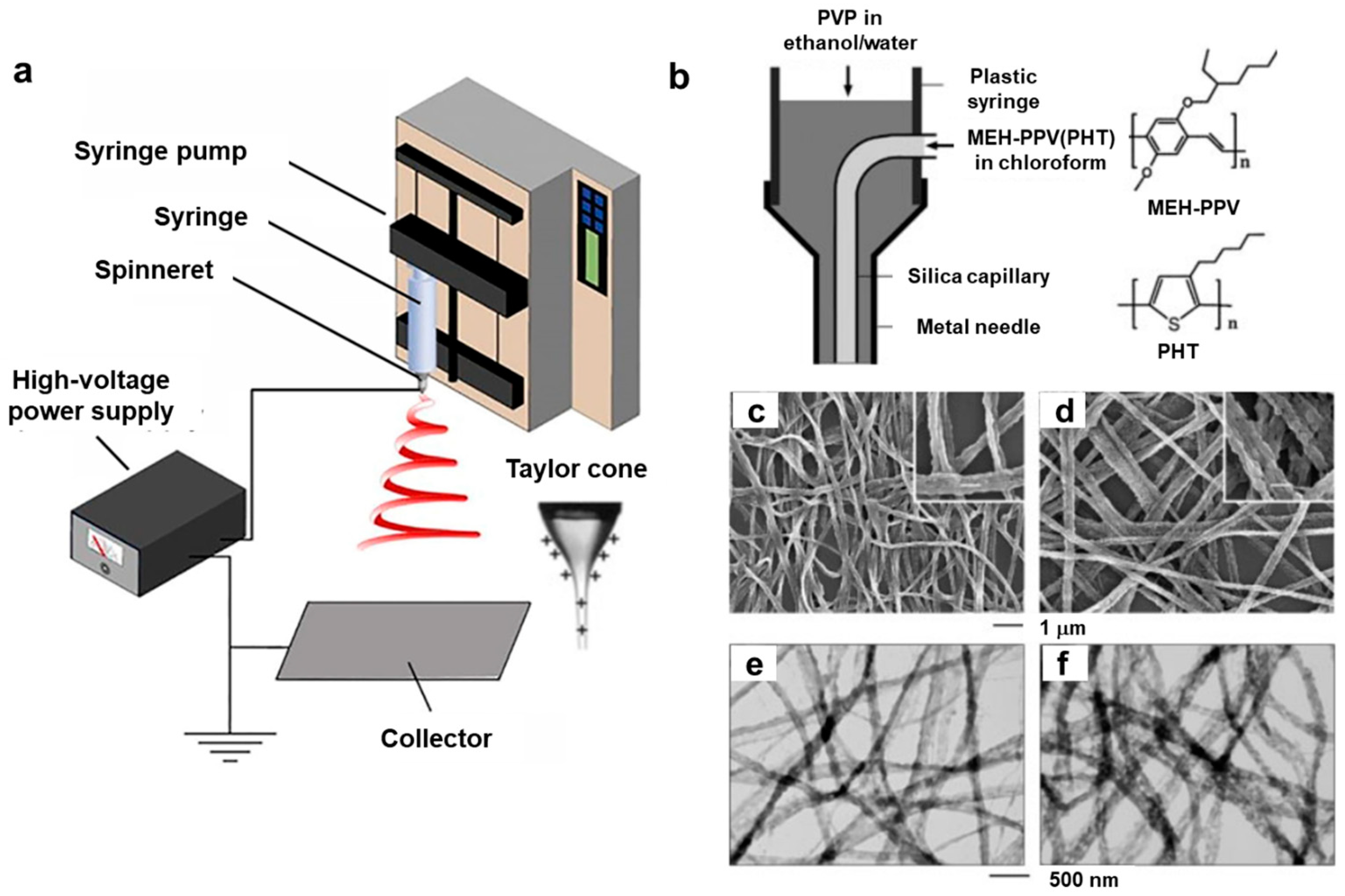
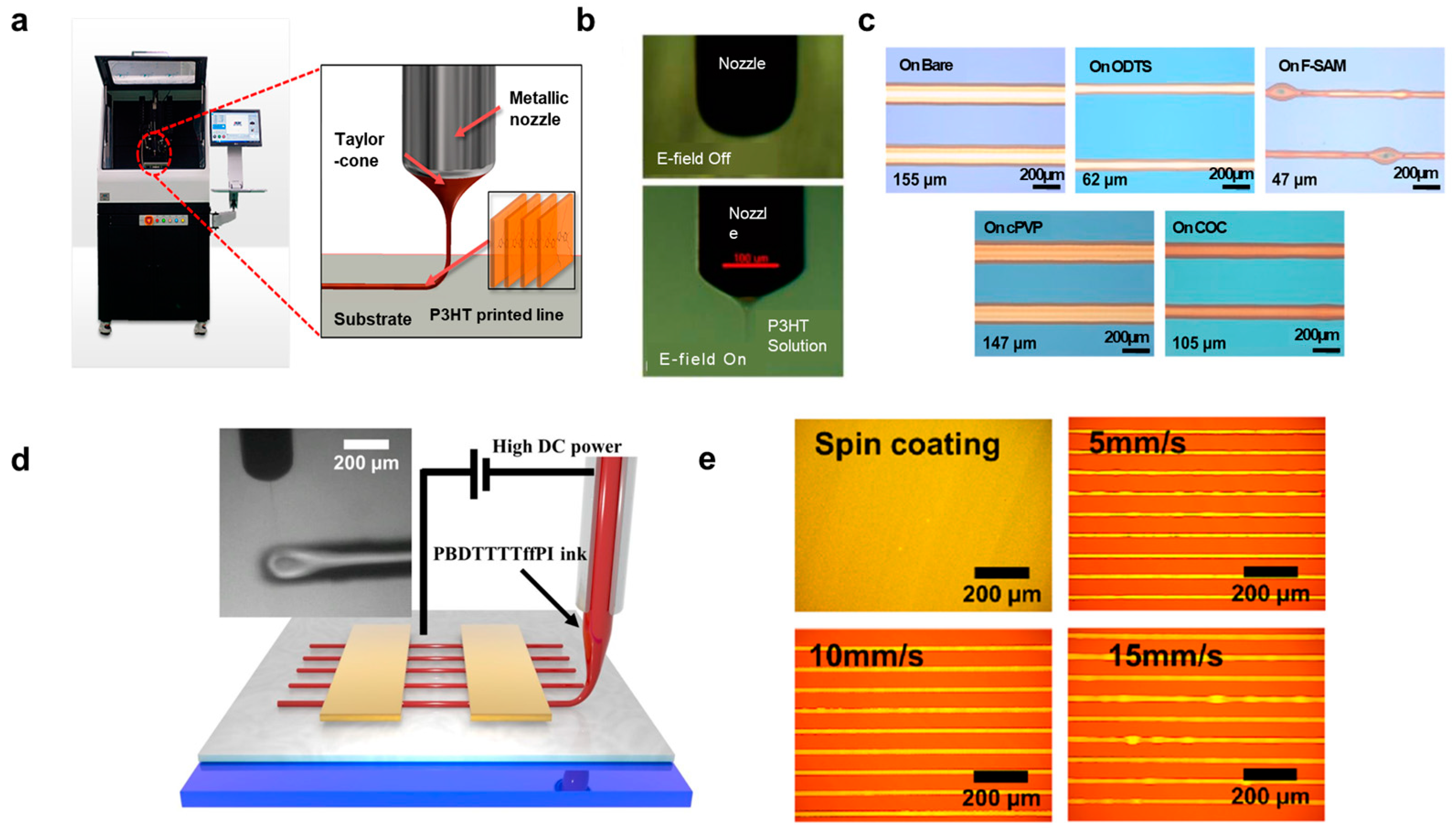
2.2.2. Electrohydrodynamic (EHD) Jet Printing
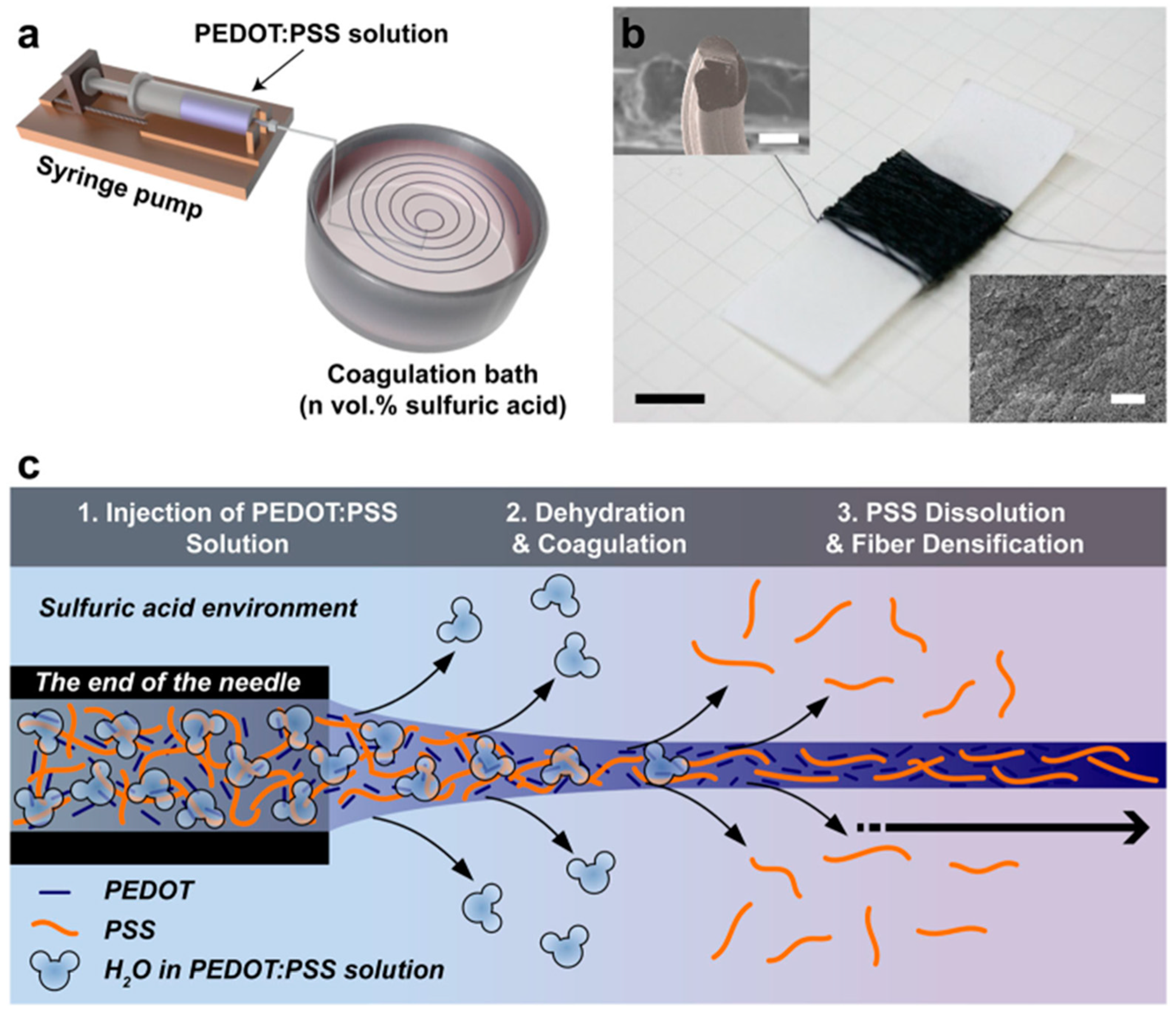
2.2.3. Wet Spinning
2.2.4. Vapor Coating, In Situ Polymerization, and Dip Coating
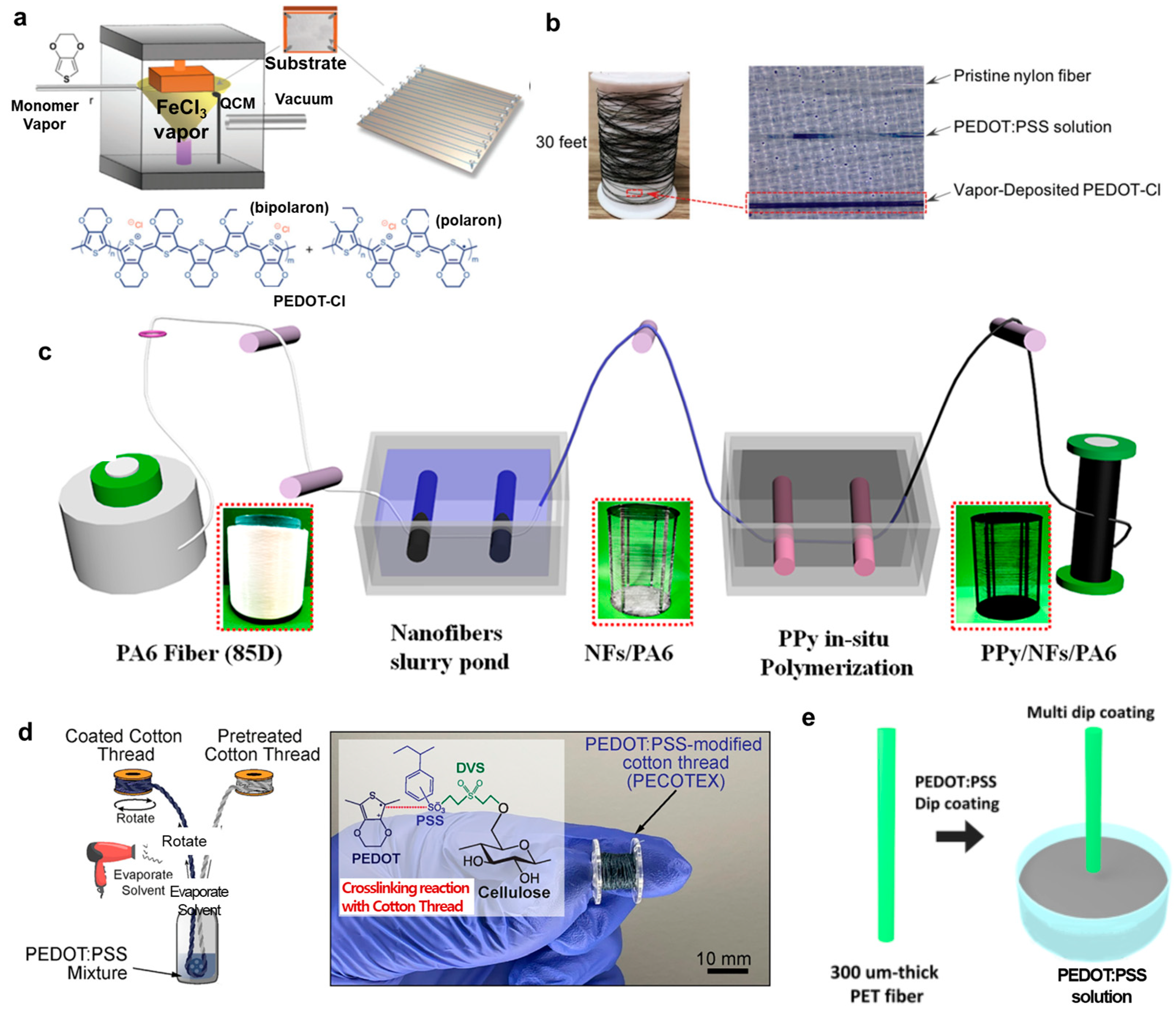
3. Fiber-Type Conjugated Polymer Transistors
3.1. Field-Effect Transistors Using Conjugated Polymer Fibers
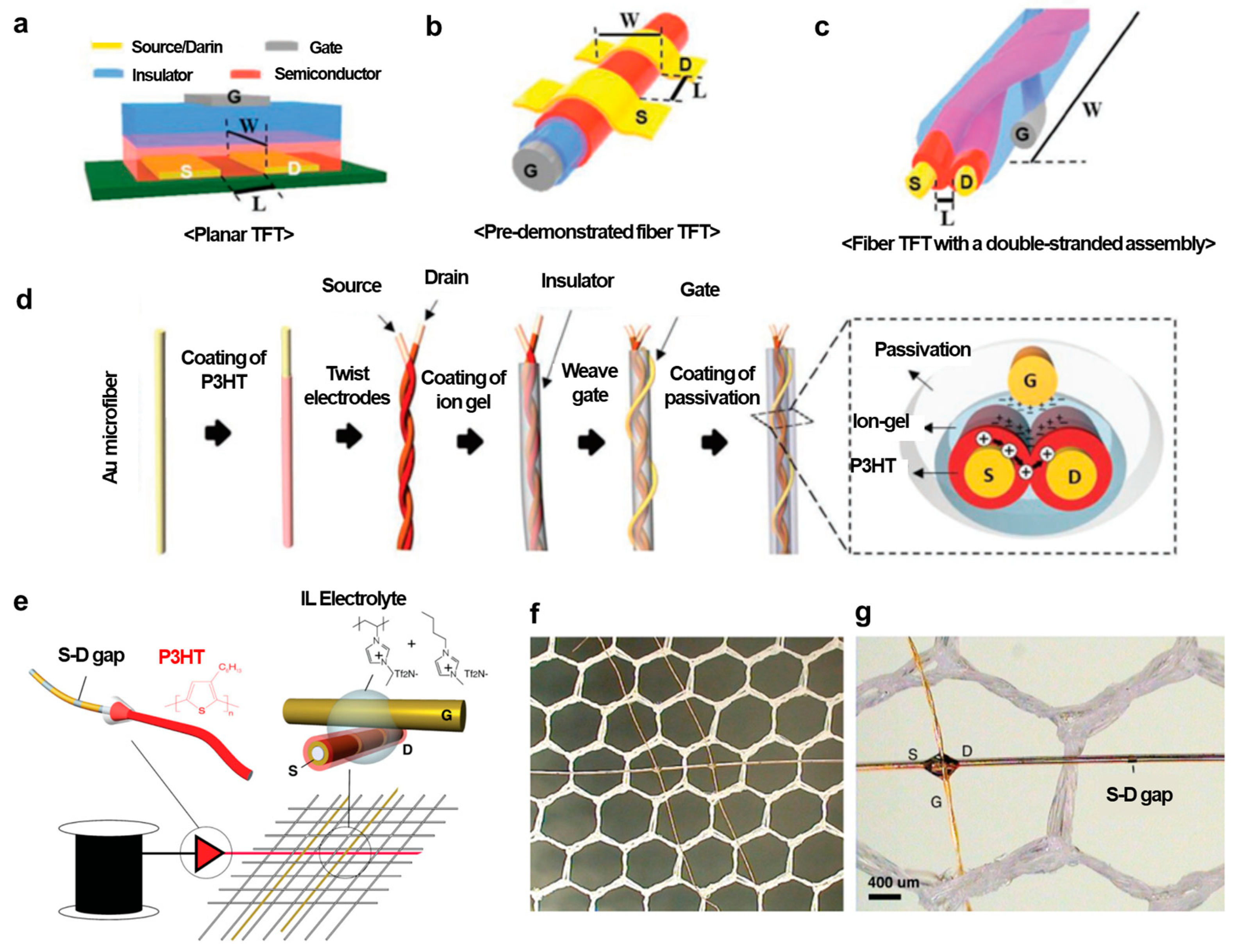
3.2. Fiber-Type Organic Electrochemical Transistors
3.3. Integration of Fiber-Type Transistors into Textiles
4. Application of Fiber-Type Transistors in Sensors
4.1. Chemical Sensors
4.1.1. Glucose Sensors

4.1.2. Ionic Concentration Sensors in Human Sweat

4.1.3. Ascorbic Acid Sensors
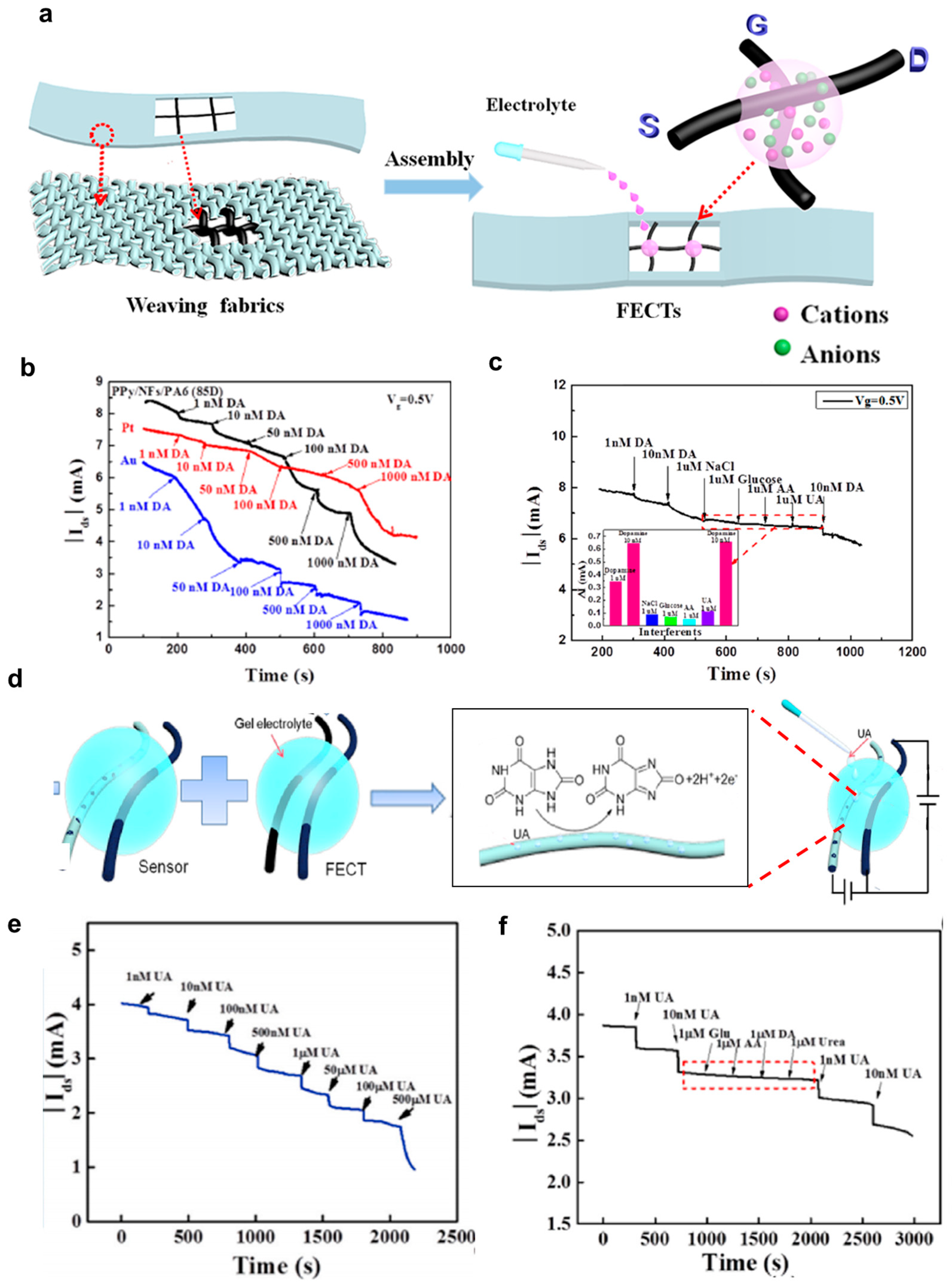
4.1.4. Dopamine Sensors
4.1.5. Uric Acid Sensors
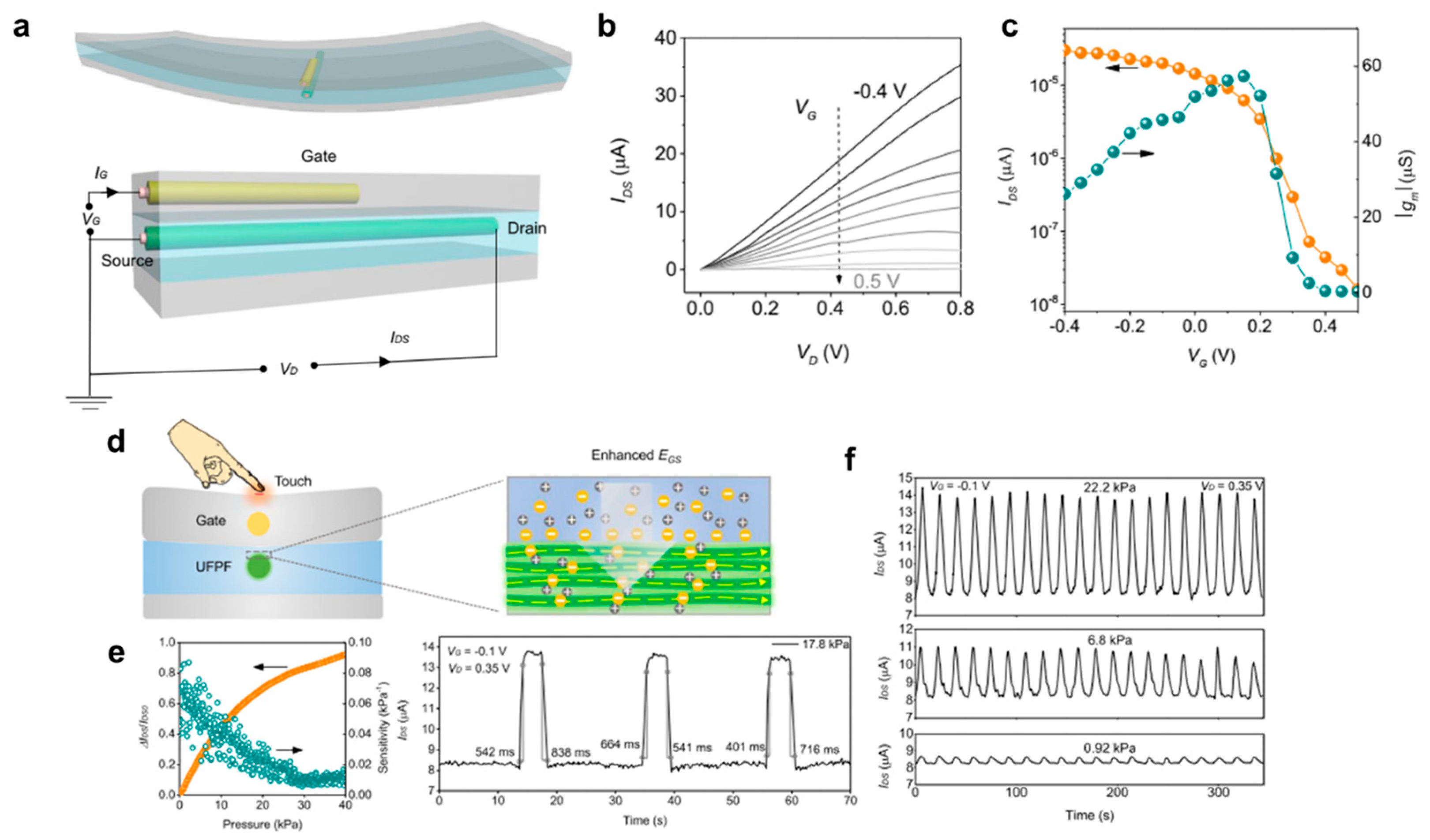
4.2. Physical Sensors
| Transistor Type | Sensing Type | Configuration | Materials | Fiber Preparation Method | References |
|---|---|---|---|---|---|
| OECT | Glucose | Cross geometry | Polypyrrole/rGO/polyamide (PA) filament | In situ polymerization | [52] |
| OECT | Glucose | Cross geometry | PEDOT:PSS/Nylon fibers | Coating | [48] |
| OECT | Ions in human sweat | A single strand fiber | PEDOT:PSS fibers | Wet spinning | [28] |
| OECT | Saline concentration in human sweat | Two parallel fibers | PEDOT:PSS/cotton thread | Soaking | [41] |
| OECT | Ascorbic acid | Two parallel fibers | PEDOT:PSS fibers | Extrusion | [64] |
| OECT | Dopamine | Cross geometry | PPy/NFs/PA6 fiber | In situ polymerization | [34] |
| OECT | Uric acid | Two parallel fibers | PEDOT/rGO/cotton fiber | Reversed microemulsion polymerization | [76] |
| OECT | Tactile | Two parallel fibers | Polyaniline (PANi) fibers | Wet spinning | [81] |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sze, S.M.; Lee, M.-K. Semiconductor Devices: Physics and Technology, 3rd ed.; John Wiley & Sons Pte. Limited: Singapore, 2012. [Google Scholar]
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 432, 488–492. [Google Scholar] [CrossRef]
- Li, F.; Nathan, A.; Wu, Y.; Ong, B.S. Organic Thin Film Transistor Integration: A Hybrid Approach; Wiley: Weiheim, Germany, 2011. [Google Scholar]
- Reese, C.; Roberts, M.; Ling, M.-M.; Bao, Z. Organic thin film transistors. Mater. Today 2004, 7, 20–27. [Google Scholar] [CrossRef]
- Bonfiglio, A.; De Rossi, D.; Kirstein, T.; Locher, I.R.; Mameli, F.; Paradiso, R.; Vozzi, G. Organic field effect transistors for textile applications. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Wang, Z.; He, B.; Chen, M.; Qi, M.; Liu, Y.; Xin, J.; Wei, L. Recent progress of fiber-based transistors: Materials, structures and applications. Front. Optoelectron. 2022, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kim, T.-W. Recent Advances in Fiber-Shaped Electronic Devices for Wearable Applications. Appl. Sci. 2021, 11, 6131. [Google Scholar] [CrossRef]
- Heo, J.S.; Eom, J.; Kim, Y.-H.; Park, S.K. Recent Progress of Textile-Based Wearable Electronics: A Comprehensive Review of Materials, Devices, and Applications. Nano Micro. Small 2018, 14, 1703034. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar]
- Alhashmi Alamer, F.; Althagafy, K.; Alsalmi, O.; Aldeih, A.; Alotaiby, H.; Althebaiti, M.; Alghamdi, H.; Alotibi, N.; Saeedi, A.; Zabarmawi, Y.; et al. Review on PEDOT:PSS-Based Conductive Fabric. ACS Omega 2022, 7, 35371–35386. [Google Scholar] [CrossRef] [PubMed]
- Tseghai, G.B.; Mengistie, D.A.; Malengier, B.; Fante, K.A.; Van Langenhove, L. PEDOT:PSS-Based Conductive Textiles and Their Applications. Sensors 2020, 20, 1881. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Grapenson, S.; Jakob, A. Conductive polypyrrole nanofibers via electrospinning: Electrical and morphological properties. Polymer 2006, 47, 1597–1603. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.-W. Conducting polyaniline nanowire and its applications in chemiresistive sensing. Nanomaterials 2013, 3, 498–523. [Google Scholar] [CrossRef]
- Mirabedini, A.; Foroughi, J.; Wallace, G.G. Developments in conducting polymer fibres: From established spinning methods toward advanced applications. RSC Adv. 2016, 6, 44687–44716. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Bagchi, S.; Achla, R.; Mondal, S.K. Electrospun polypyrrole-polyethylene oxide coated optical fiber sensor probe for detection of volatile compounds. Sens. Actuators B Chem. 2017, 250, 52–60. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Jones, W.E.; Norris, I.D.; Gao, J.; Johnson, A.T.; Pinto, N.J.; Hone, J.; Han, B.; Ko, F.K.; Okuzaki, H.; et al. Electrostatically-generated nanofibers of electronic polymers. Synth. Met. 2001, 119, 27–30. [Google Scholar] [CrossRef]
- Wu, S.; Liu, P.; Zhang, Y.; Zhang, H.; Qin, X. Flexible and conductive nanofiber-structured single yarn sensor for smart wearable devices. Sens. Actuators B Chem. 2017, 252, 697–705. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Lo, T.-Y.; Chen, C.-H.; Wu, K.-H.; Lin, C.-M.; Whang, W.-T. Electrospinning of magnesium-ion linked binder-less PEDOT:PSS nanofibers for sensing organic gases. Sens. Actuators B Chem. 2015, 216, 603–607. [Google Scholar] [CrossRef]
- Li, D.; Babel, A.; Jenekhe, S.A.; Xia, Y. Nanofibers of Conjugated Polymers Prepared by Electrospinning with a Two-Capillary Spinneret. Adv. Mater. 2004, 16, 2062–2066. [Google Scholar] [CrossRef]
- Babel, A.; Li, D.; Xia, Y.; Jenekhe, S.A. Electrospun Nanofibers of Blends of Conjugated Polymers: Morphology, Optical Properties, and Field-Effect Transistors. Macromolecules 2005, 38, 4705–4711. [Google Scholar] [CrossRef]
- Mkhize, N.; Bhaskaran, H. Electrohydrodynamic Jet Printing: Introductory Concepts and Considerations. Small Sci. 2022, 2, 2100073. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Lee, H.; Lee, B.S.; Park, S.; Yudistira, H.T.; Choong, C.L.; Park, J.J.; Park, C.E.; Byun, D. Directly drawn poly(3-hexylthiophene) field-effect transistors by electrohydrodynamic jet printing: Improving performance with surface modification. ACS Appl. Mater. Interfaces 2014, 6, 10736–10743. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jeong, Y.J.; Hong, J.; Kwon, H.-j.; Ye, H.; Wang, R.; Choi, H.H.; Kong, H.; Hwang, H.; Kim, S.H.; et al. Electrohydrodynamic-Jet-Printed Phthalimide-Derived Conjugated Polymers for Organic Field-Effect Transistors and Logic Gates. ACS Appl. Mater. Interfaces 2022, 14, 7073–7081. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; He, J.; Lei, Q.; Li, D. Electrohydrodynamic Printing of Microscale PEDOT:PSS-PEO Features with Tunable Conductive/Thermal Properties. ACS Appl. Mater. Interfaces 2018, 10, 19116–19122. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Kwon, H.-j.; Ye, H.; Kim, J.Y.; Lee, J.; Jeong, Y.J.; Kim, S.H. Enhanced solvent resistance and electrical performance of electrohydrodynamic jet printed PEDOT:PSS composite patterns: Effects of hardeners on the performance of organic thin-film transistors. Phys. Chem. Chem. Phys. 2019, 21, 25690–25699. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lim, T.; Kim, C.-H.; Yeo, C.S.; Seo, K.; Kim, S.-M.; Kim, J.; Park, S.Y.; Ju, S.; Yoon, M.-H. Organic electrochemical transistor-based channel dimension-independent single-strand wearable sweat sensors. NPG Asia Mater. 2018, 10, 1086–1095. [Google Scholar] [CrossRef]
- Feng, D.; Wang, P.; Wang, M.; Zhu, C.; Gao, Q.; Shen, M. A Facile Route Toward Continuous Wet-spinning of PEDOT: PSS Fibers with Enhanced Strength and Electroconductivity. Fibers Polym. 2021, 22, 1491–1495. [Google Scholar] [CrossRef]
- Foroughi, J.; Spinks, G.M.; Wallace, G.G. A reactive wet spinning approach to polypyrrole fibres. J. Mater. Chem. 2011, 21, 6421–6426. [Google Scholar] [CrossRef]
- Zhang, L.; Andrew, T. Vapor-Coated Monofilament Fibers for Embroidered Electrochemical Transistor Arrays on Fabrics. Adv. Electron. Mater. 2018, 4, 1800271. [Google Scholar] [CrossRef]
- Zhang, L.; Fairbanks, M.; Andrew, T.L. Rugged Textile Electrodes for Wearable Devices Obtained by Vapor Coating Off-the-Shelf, Plain-Woven Fabrics. Adv. Funct. Mater. 2017, 27, 1700415. [Google Scholar] [CrossRef]
- Cetin, M.Z.; Camurlu, P. An amperometric glucose biosensor based on PEDOT nanofibers. RSC Adv. 2018, 8, 19724–19731. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; Wang, Y.; Zhang, Y.; Ding, X.; Zhong, W.; Wang, D.; Wang, W.; Liu, Q.; Liu, K.; Li, M.; et al. Wearable Fiber-Based Organic Electrochemical Transistors as a Platform for Highly Sensitive Dopamine Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 13105–13113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Z.; Qing, X.; Zhong, W.; Liu, Q.; Wang, W.; Li, M.; Liu, K.; Wang, D. Ion sensors based on novel fiber organic electrochemical transistors for lead ion detection. Anal. Bioanal. Chem. 2016, 408, 5779–5787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Qing, X.; Wang, Y.; Zhong, W.; Wang, W.; Chen, Y.; Liu, Q.; Li, M.; Wang, D. Fiber organic electrochemical transistors based on multi-walled carbon nanotube and polypyrrole composites for noninvasive lactate sensing. Anal. Bioanal. Chem. 2020, 412, 7515–7524. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kim, H.; Choi, S.; Jeong, E.G.; Kim, D.; Lee, S.; Lee, H.S.; Seo, Y.C.; Choi, K.C. Weavable and Highly Efficient Organic Light-Emitting Fibers for Wearable Electronics: A Scalable, Low-Temperature Process. Nano Lett. 2018, 18, 347–356. [Google Scholar] [CrossRef]
- Alshabouna, F.; Lee, H.S.; Barandun, G.; Tan, E.; Cotur, Y.; Asfour, T.; Gonzalez-Macia, L.; Coatsworth, P.; Núnez-Bajo, E.; Kim, J.-S.; et al. PEDOT:PSS-modified cotton conductive thread for mass manufacturing of textile-based electrical wearable sensors by computerized embroidery. Mater. Today 2022, 59, 56–67. [Google Scholar] [CrossRef]
- Hamedi, M.; Forchheimer, R.; Inganas, O. Towards woven logic from organic electronic fibres. Nat. Mater. 2007, 6, 357–362. [Google Scholar] [CrossRef]
- Muller, C.; Hamedi, M.; Karlsson, R.; Jansson, R.; Marcilla, R.; Hedhammar, M.; Inganas, O. Woven electrochemical transistors on silk fibers. Adv. Mater. 2011, 23, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Tarabella, G.; Villani, M.; Calestani, D.; Mosca, R.; Iannotta, S.; Zappettini, A.; Coppedè, N. A single cotton fiber organic electrochemical transistor for liquid electrolyte saline sensing. J. Mater. Chem. 2012, 22, 23830–23834. [Google Scholar] [CrossRef]
- Battista, E.; Lettera, V.; Villani, M.; Calestani, D.; Gentile, F.; Netti, P.A.; Iannotta, S.; Zappettini, A.; Coppedè, N. Enzymatic sensing with laccase-functionalized textile organic biosensors. Org. Electron. 2017, 40, 51–57. [Google Scholar] [CrossRef]
- Tadesse, M.G.; Mengistie, D.A.; Chen, Y.; Wang, L.; Loghin, C.; Nierstrasz, V. Electrically conductive highly elastic polyamide/lycra fabric treated with PEDOT:PSS and polyurethane. J. Mater. Sci. 2019, 54, 9591–9602. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.; Ahn, J.; Hwang, D.K.; Ju, H.; Park, M.-C.; Yang, H.; Kim, S.H.; Jang, H.W.; Lim, J.A. A New Architecture for Fibrous Organic Transistors Based on a Double-Stranded Assembly of Electrode Microfibers for Electronic Textile Applications. Adv. Mater. 2019, 31, 1900564. [Google Scholar] [CrossRef]
- Owyeung, R.E.; Terse-Thakoor, T.; Rezaei Nejad, H.; Panzer, M.J.; Sonkusale, S.R. Highly Flexible Transistor Threads for All-Thread Based Integrated Circuits and Multiplexed Diagnostics. ACS Appl. Mater. Interfaces 2019, 11, 31096–31104. [Google Scholar] [CrossRef]
- Hamedi, M.; Herlogsson, L.; Crispin, X.; Marcilla, R.; Berggren, M.; Inganas, O. Fiber-Embedded Electrolyte-Gated Field-Effect Transistors for e-Textiles. Adv. Mater. 2009, 21, 573–577. [Google Scholar] [CrossRef]
- Lund, A.; van der Velden, N.M.; Persson, N.-K.; Hamedi, M.M.; Müller, C. Electrically conducting fibres for e-textiles: An open playground for conjugated polymers and carbon nanomaterials. Mater. Sci. Eng. R Rep. 2018, 126, 1–29. [Google Scholar] [CrossRef]
- Yang, A.; Li, Y.; Yang, C.; Fu, Y.; Wang, N.; Li, L.; Yan, F. Fabric Organic Electrochemical Transistors for Biosensors. Adv. Mater. 2018, 30, e1800051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liao, C.; Mak, C.H.; You, P.; Mak, C.L.; Yan, F. Highly sensitive glucose sensors based on enzyme-modified whole-graphene solution-gated transistors. Sci. Rep. 2015, 5, 8311. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Wang, Y.; Qing, X.; Zhou, Q.; Zhang, Y.; Liu, Q.; Liu, K.; Wang, W.; Li, M.; Lu, Z.; Chen, Y.; et al. The woven fiber organic electrochemical transistors based on polypyrrole nanowires/reduced graphene oxide composites for glucose sensing. Biosens. Bioelectron. 2017, 95, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Shim, N.Y.; Bernards, D.A.; Macaya, D.J.; DeFranco, J.A.; Nikolou, M.; Owens, R.M.; Malliaras, G.G. All-plastic electrochemical transistor for glucose sensing using a ferrocene mediator. Sensors 2009, 9, 9896–9902. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Zhang, M.; Niu, L.; Zheng, Z.; Yan, F. Highly selective and sensitive glucose sensors based on organic electrochemical transistors with graphene-modified gate electrodes. J. Mater. Chem. B 2013, 1, 3820–3829. [Google Scholar] [CrossRef] [PubMed]
- Bernards, D.A.; Macaya, D.J.; Nikolou, M.; DeFranco, J.A.; Takamatsu, S.; Malliaras, G.G. Enzymatic sensing with organic electrochemical transistors. J. Mater. Chem. 2008, 18, 116–120. [Google Scholar] [CrossRef]
- Tang, H.; Lin, P.; Chan, H.L.; Yan, F. Highly sensitive dopamine biosensors based on organic electrochemical transistors. Biosens. Bioelectron. 2011, 26, 4559–4563. [Google Scholar] [CrossRef]
- Lin, P.; Luo, X.; Hsing, I.M.; Yan, F. Organic electrochemical transistors integrated in flexible microfluidic systems and used for label-free DNA sensing. Adv. Mater. 2011, 23, 4035–4040. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Stavrinidou, E.; Leleux, P.; Rajaona, H.; Khodagholy, D.; Rivnay, J.; Lindau, M.; Sanaur, S.; Malliaras, G.G. Direct measurement of ion mobility in a conducting polymer. Adv. Mater. 2013, 25, 4488–4493. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Collins, B.A.; Sessolo, M.; Stavrinidou, E.; Strakosas, X.; Tassone, C.; Delongchamp, D.M.; Malliaras, G.G. Structural control of mixed ionic and electronic transport in conducting polymers. Nat. Commun. 2016, 7, 11287. [Google Scholar] [CrossRef]
- Khodagholy, D.; Doublet, T.; Quilichini, P.; Gurfinkel, M.; Leleux, P.; Ghestem, A.; Ismailova, E.; Herve, T.; Sanaur, S.; Bernard, C.; et al. In vivo recordings of brain activity using organic transistors. Nat. Commun. 2013, 4, 1575. [Google Scholar] [CrossRef]
- Coppedè, N.; Tarabella, G.; Villani, M.; Calestani, D.; Iannotta, S.; Zappettini, A. Human stress monitoring through an organic cotton-fiber biosensor. J. Mater. Chem. B 2014, 2, 5620–5626. [Google Scholar] [CrossRef]
- Coppedè, N.; Giannetto, M.; Villani, M.; Lucchini, V.; Battista, E.; Careri, M.; Zappettini, A. Ion selective textile organic electrochemical transistor for wearable sweat monitoring. Org. Electron. 2020, 78, 105579. [Google Scholar] [CrossRef]
- Feng, J.; Fang, Y.; Wang, C.; Chen, C.; Tang, C.; Guo, Y.; Wang, L.; Yang, Y.; Zhang, K.; Wang, J.; et al. All-Polymer Fiber Organic Electrochemical Transistor for Chronic Chemical Detection in the Brain. Adv. Funct. Mater. 2023, 33, 2214945. [Google Scholar] [CrossRef]
- Yang, Y.; Jo, A.; Lee, Y.; Lee, C. Electrodeposited nanoporous ruthenium oxide for simultaneous quantification of ascorbic acid and uric acid using chronoamperometry at two different potentials. Sens. Actuators B Chem. 2018, 255, 316–324. [Google Scholar] [CrossRef]
- Cheng, H.; Li, L.; Zhang, M.; Jiang, Y.; Yu, P.; Ma, F.; Mao, L. Recent advances on in vivo analysis of ascorbic acid in brain functions. TrAC Trends Anal. Chem. 2018, 109, 247–259. [Google Scholar] [CrossRef]
- Fang, Y.; Feng, J.; Shi, X.; Yang, Y.; Wang, J.; Sun, X.; Li, W.; Sun, X.; Peng, H. Coaxial fiber organic electrochemical transistor with high transconductance. Nano Res. 2023, 16, 11885–11892. [Google Scholar] [CrossRef]
- Yang, W.; Yu, Y.; Tang, Y.; Li, K.; Zhao, Z.; Li, M.; Yin, G.; Li, H.; Sun, S. Enhancing electrochemical detection of dopamine via dumbbell-like FePt–Fe3O4 nanoparticles. Nanoscale 2017, 9, 1022–1027. [Google Scholar] [CrossRef]
- Li, B.-R.; Hsieh, Y.-J.; Chen, Y.-X.; Chung, Y.-T.; Pan, C.-Y.; Chen, Y.-T. An Ultrasensitive Nanowire-Transistor Biosensor for Detecting Dopamine Release from Living PC12 Cells under Hypoxic Stimulation. J. Am. Chem. Soc. 2013, 135, 16034–16037. [Google Scholar] [CrossRef]
- Sun, C.; Wang, X.; Auwalu, M.A.; Cheng, S.; Hu, W. Organic thin film transistors-based biosensors. EcoMat 2021, 3, e12094. [Google Scholar] [CrossRef]
- Wang, N.; Yang, A.; Fu, Y.; Li, Y.; Yan, F. Functionalized Organic Thin Film Transistors for Biosensing. Acc. Chem. Res. 2019, 52, 277–287. [Google Scholar] [CrossRef]
- Song, J.; Zheng, J.; Yang, A.; Liu, H.; Zhao, Z.; Wang, N.; Yan, F. Metal–organic framework transistors for dopamine sensing. Mater. Chem. Front. 2021, 5, 3422–3427. [Google Scholar] [CrossRef]
- Casalini, S.; Leonardi, F.; Cramer, T.; Biscarini, F. Organic field-effect transistor for label-free dopamine sensing. Org. Electron. 2013, 14, 156–163. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Fu, Y.; Yan, F. AC Measurements Using Organic Electrochemical Transistors for Accurate Sensing. ACS Appl. Mater. Interfaces 2018, 10, 25834–25840. [Google Scholar] [CrossRef]
- Gualandi, I.; Tonelli, D.; Mariani, F.; Scavetta, E.; Marzocchi, M.; Fraboni, B. Selective detection of dopamine with an all PEDOT:PSS Organic Electrochemical Transistor. Sci. Rep. 2016, 6, 35419. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, Y.; Zhu, R.; Chen, Y.; Liu, X.; Li, M.; Yang, L.; Wang, Y.; Wang, D. Fiber based organic electrochemical transistor integrated with molecularly imprinted membrane for uric acid detection. Talanta 2022, 238, 123055. [Google Scholar] [CrossRef] [PubMed]
- El Ridi, R.; Tallima, H. Physiological functions and pathogenic potential of uric acid: A review. J. Adv. Res. 2017, 8, 487–493. [Google Scholar] [CrossRef]
- Lin, P.; Yan, F. Organic Thin-Film Transistors for Chemical and Biological Sensing. Adv. Mater. 2012, 24, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Mak, C.; Zhang, M.; Chan, H.L.; Yan, F. Flexible organic electrochemical transistors for highly selective enzyme biosensors and used for saliva testing. Adv. Mater. 2015, 27, 676–681. [Google Scholar] [CrossRef]
- Hao, P.; Zhu, R.; Tao, Y.; Jiang, W.; Liu, X.; Tan, Y.; Wang, Y.; Wang, D. Dual-Analyte Sensing with a Molecularly Imprinted Polymer Based on Enhancement-Mode Organic Electrochemical Transistors. ACS Appl. Mater. Interfaces 2023, 15, 30567–30579. [Google Scholar] [CrossRef]
- Fang, B.; Yan, J.; Chang, D.; Piao, J.; Ma, K.M.; Gu, Q.; Gao, P.; Chai, Y.; Tao, X. Scalable production of ultrafine polyaniline fibres for tactile organic electrochemical transistors. Nat. Commun. 2022, 13, 2101. [Google Scholar] [CrossRef]
- Lee, S.; Franklin, S.; Hassani, F.A.; Yokota, T.; Nayeem, M.O.G.; Wang, Y.; Leib, R.; Cheng, G.; Franklin, D.W.; Someya, T. Nanomesh pressure sensor for monitoring finger manipulation without sensory interference. Science 2020, 370, 966–970. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, K.V.; Lee, D.; Kim, Y.; Lee, W.H. Fiber-Type Transistor-Based Chemical and Physical Sensors Using Conjugated Polymers. Polymers 2023, 15, 4062. https://doi.org/10.3390/polym15204062
Nguyen KV, Lee D, Kim Y, Lee WH. Fiber-Type Transistor-Based Chemical and Physical Sensors Using Conjugated Polymers. Polymers. 2023; 15(20):4062. https://doi.org/10.3390/polym15204062
Chicago/Turabian StyleNguyen, Ky Van, Donggeun Lee, Youngnan Kim, and Wi Hyoung Lee. 2023. "Fiber-Type Transistor-Based Chemical and Physical Sensors Using Conjugated Polymers" Polymers 15, no. 20: 4062. https://doi.org/10.3390/polym15204062
APA StyleNguyen, K. V., Lee, D., Kim, Y., & Lee, W. H. (2023). Fiber-Type Transistor-Based Chemical and Physical Sensors Using Conjugated Polymers. Polymers, 15(20), 4062. https://doi.org/10.3390/polym15204062





