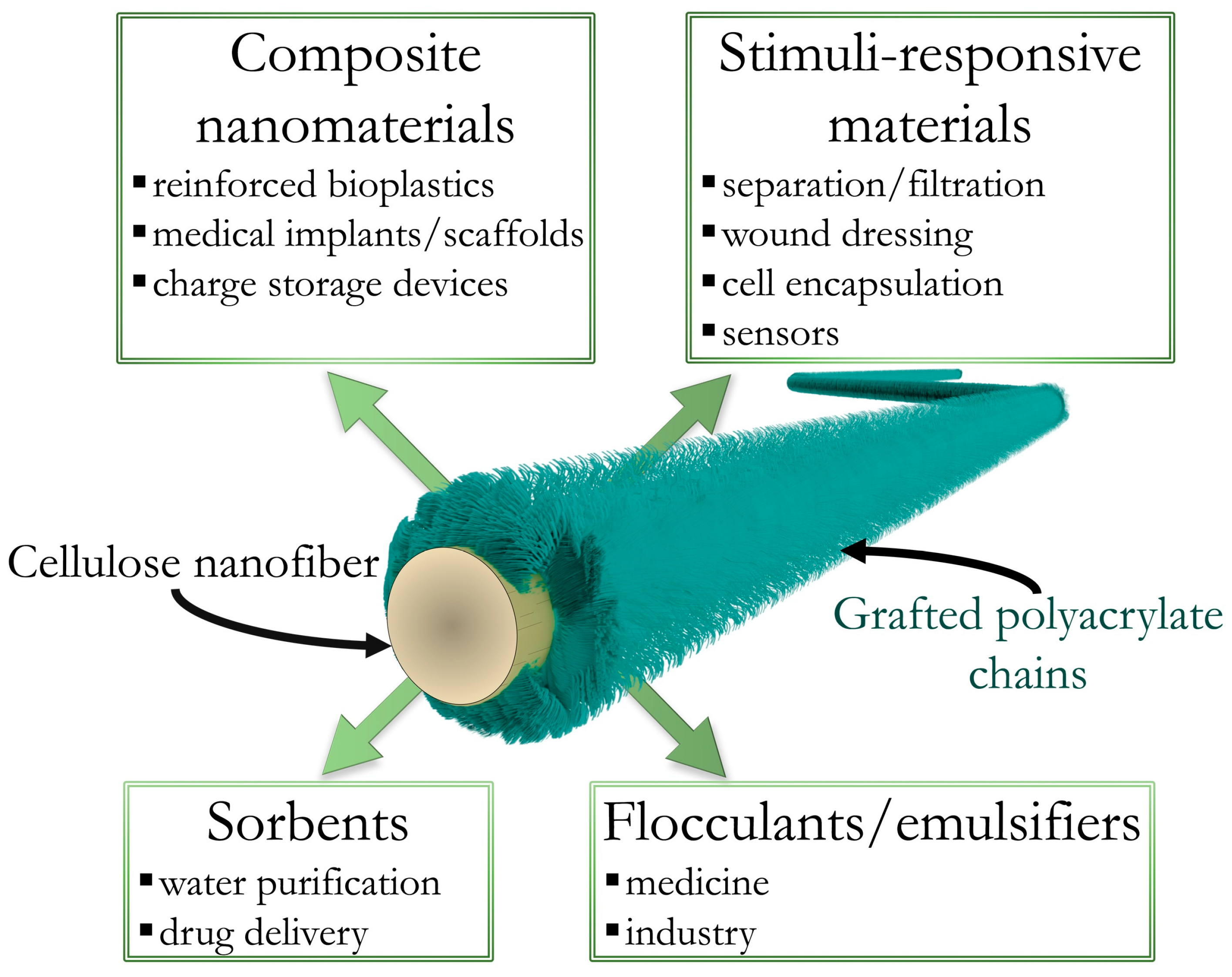
A Polyelectrolyte Colloidal Brush Based on Cellulose: Perspectives for Future Applications
Abstract
:1. Introduction
- (1)
- The sorption properties of CNFs with grafted polyacrylic acid can be utilized for the formation of electroconductive nanofibers via the sorption of a monomer (pyrrole) that can be polymerized, giving rise to a conductive polymer (polypyrrole). Additionally, the selective polymerization inside the polyacrylate shell can be performed via the preliminary sorption of Fe3+ in it. This will govern the polymerization to proceed mainly inside the volume of the shell and result in the formation of fine electroconductive PAA–polypyrrole nanofibers with a hard CNF backbone.
- (2)
- According to the literature discussed above, the enhanced interactions of CNF-PAA colloid brushes with hydrophilic polymers result in the formation of a cooperative network of hydrogen bonds that can act as crosslinks when the CNF-PAA brush is placed inside the solution containing polyacrylamide or another polymer with a polar group. The cooperative and reversible nature of such interactions, along with the high rigidity of the CNF backbone, can lead to the formation of additional sacrificial sub-networks in the hydrogel, which can facilitate an improvement in the mechanical properties of polymer hydrogels, as recently demonstrated for the application of the coordination bonds of a PAA-based polymer network with metal ions [68].
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Polyacrylic Acid Grafted Bacterial Cellulose Nanofibers
2.2.2. The Preparation of Electroactive Electrode Material Based on the CNF-PAA Brush (CNF-PAA-PPy)
2.2.3. The Preparation of CNF-PAA-Brush-Reinforced Polyacrylamide Hydrogel (CNF-PAA-PAAm)
2.2.4. Titrimetric Characterization of PAA Content
2.2.5. Fourie Transform Infrared Spectroscopy
2.2.6. Scanning Electron Microscopy
2.2.7. Electrochemical Study of CNF-PAA-PPy
2.2.8. Measurements of Mechanical Properties of Hydrogels
2.2.9. Measurements of CNF-PAA-PAAm Swelling at Various pH
3. Results and Discussion
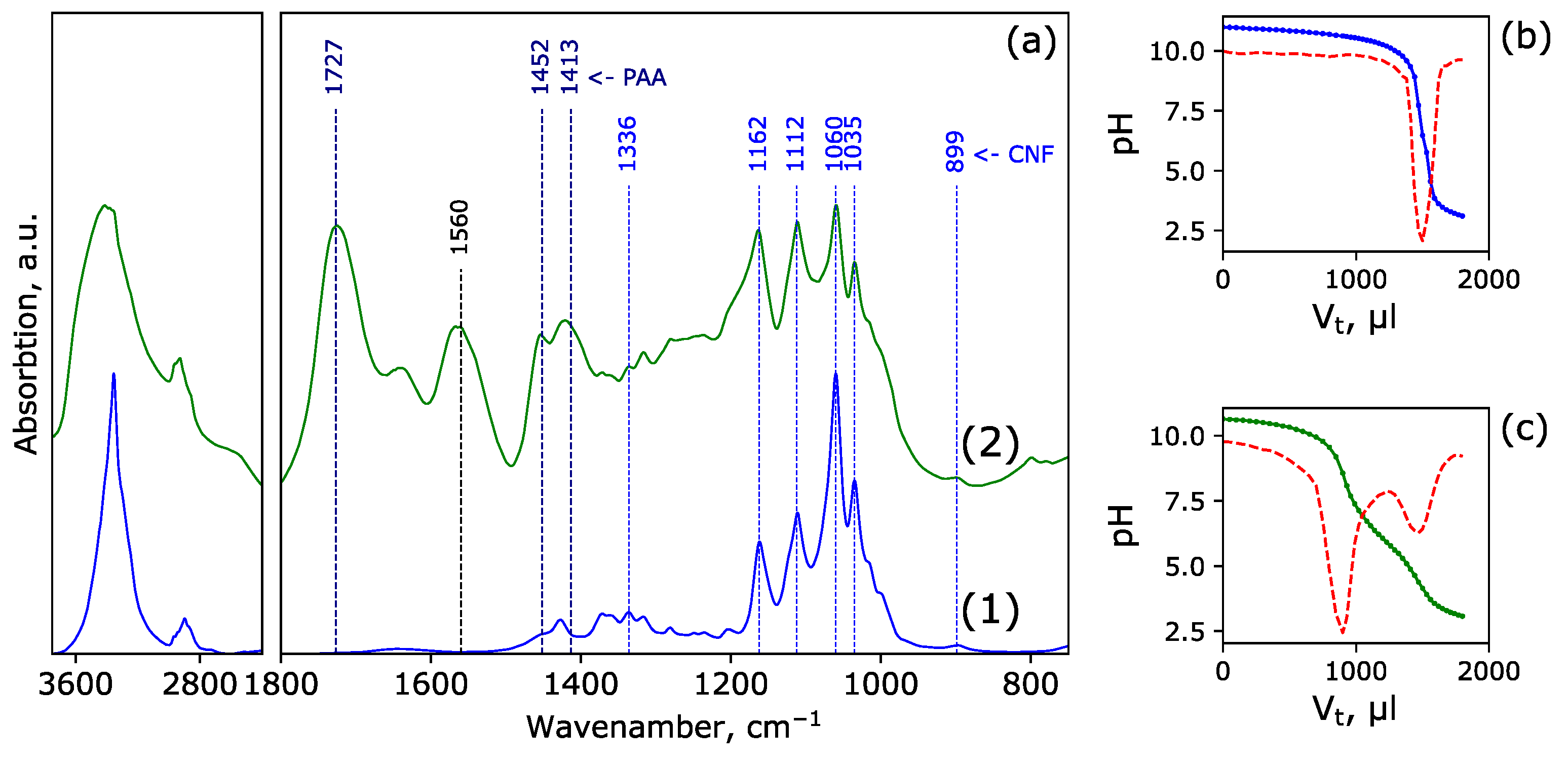
3.1. Characterization of Structure of CNF-PAA
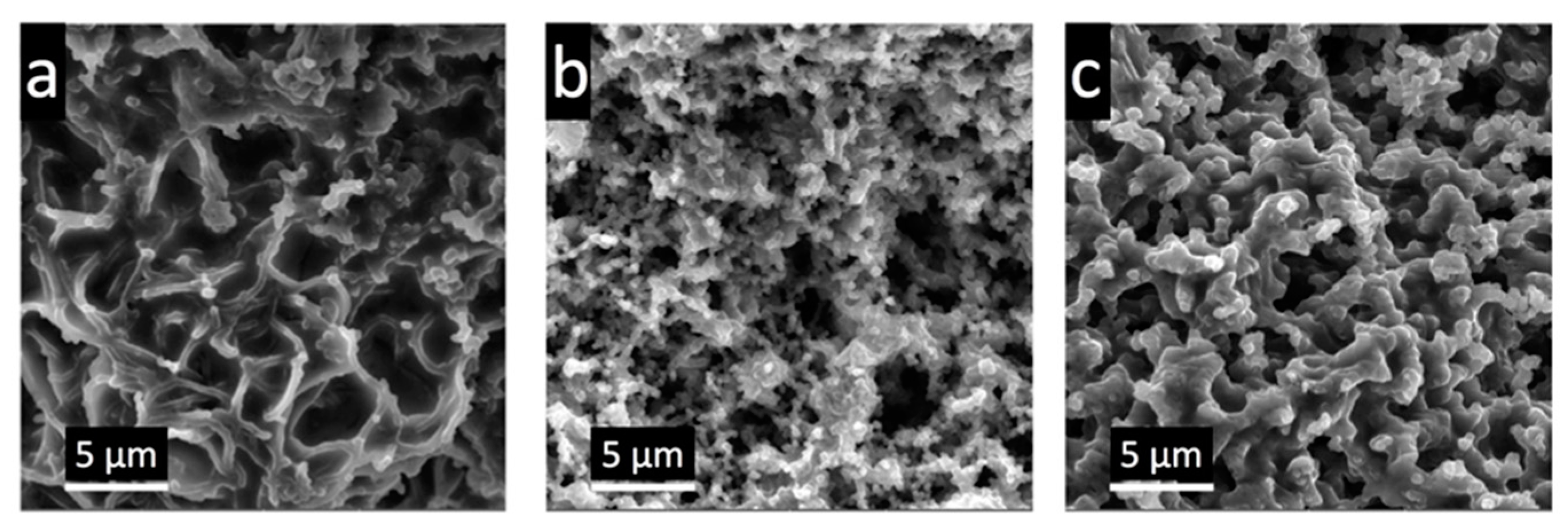
3.2. The Morphology and Electrochemical Performance of the Electroactive Electrode Material CNF-PAA/PPy
3.3. The Mechanical Properties of Reinforced Polyacrylamide Hydrogel PAAm/CNF-PAA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Han, X.; Yang, W.; Hu, J.; Zhang, C.; Liu, K.; Jiang, S. Nanocellulose-based composite aerogels toward the environmental protection: Preparation, modification and applications. Environ. Res. 2023, 236, 116736. [Google Scholar] [CrossRef]
- Ning, L.; You, C.; Jia, Y.; Chen, J.; Zhang, Y.; Li, X.; Rojas, O.J.; Wang, F. Cellulose nanocrystals for crop protection: Leaf adhesion and controlled delivery of bioactive molecules. Green Chem. 2023, 25, 2690–2698. [Google Scholar] [CrossRef]
- Dong, J.; Li, P.; Zeng, J.; Wang, B.; Gao, W.; Xu, J.; Chen, K. Lignin/polypyrrole interpenetrating networks decorated Lignin-containing cellulose nanofibril composite membrane for High-performance supercapacitors. Chem. Eng. J. 2023, 470, 144180. [Google Scholar] [CrossRef]
- Su, S.; Hu, S.; Liu, Q. Application of Polypyrrole Cellulose Nanocrystalline Composite Conductive Material in Garment Design. Adv. Mater. Sci. Eng. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Lustig, M.; Gefen, A. The biomechanical efficacy of a dressing with a soft cellulose fluff core in protecting prone surgical patients from chest injuries on the operating table. Int. Wound J. 2022, 19, 1786–1796. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Li, S.; Luo, J.; Wang, X. Latest Advances on Bacterial Cellulose-Based Antibacterial Materials as Wound Dressings. Front. Bioeng. Biotechnol. 2020, 8, 593768. [Google Scholar] [CrossRef]
- Hakkarainen, T.; Koivuniemi, R.; Kosonen, M.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Vuola, J.; Valtonen, J.; Tammela, P.; Mäkitie, A.; Luukko, K.; et al. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Control. Release 2016, 244, 292–301. [Google Scholar] [CrossRef]
- Blažic, R.; Marušić, K.; Vidović, E. Swelling and Viscoelastic Properties of Cellulose-Based Hydrogels Prepared by Free Radical Polymerization of Dimethylaminoethyl Methacrylate in Cellulose Solution. Gels 2023, 9, 94. [Google Scholar] [CrossRef]
- Enawgaw, H.; Tesfaye, T.; Yilma, K.T.; Limeneh, D.Y. Synthesis of a Cellulose-Co-AMPS Hydrogel for Personal Hygiene Applications Using Cellulose Extracted from Corncobs. Gels 2021, 7, 236. [Google Scholar] [CrossRef]
- Khojastehfar, A.; Mahjoub, S. Application of Nanocellulose Derivatives as Drug Carriers; A Novel Approach in Drug Delivery. Anticancer. Agents Med. Chem. 2021, 21, 692–702. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Ning, L.; Li, L.; He, L.; Lin, H.; You, C.; Wang, F. Efficient in vitro delivery of paclitaxel by a nanocellulose-coated dendritic mesoporous organosilica nanoparticle for enhanced chemodynamic cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 86, 104654. [Google Scholar] [CrossRef]
- Smirnov, M.A.; Fedotova, V.S.; Sokolova, M.P.; Nikolaeva, A.L.; Elokhovsky, V.Y.; Karttunen, M. Polymerizable choline-and imidazolium-based ionic liquids reinforced with bacterial cellulose for 3d-printing. Polymers 2021, 13, 3044. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, F.; Zheng, T.; Li, G.; Wang, W.; Li, Y.; Chen, S.; Li, X.; Lu, Y. Cellulose-based functional hydrogels derived from bamboo for product design. Front. Plant Sci. 2022, 13, 958066. [Google Scholar] [CrossRef] [PubMed]
- Turky, G.; Moussa, M.A.; Hasanin, M.; El-Sayed, N.S.; Kamel, S. Carboxymethyl Cellulose-Based Hydrogel: Dielectric Study, Antimicrobial Activity and Biocompatibility. Arab. J. Sci. Eng. 2021, 46, 17–30. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Moussa, M.A.; Kamel, S.; Turky, G. Development of electrical conducting nanocomposite based on carboxymethyl cellulose hydrogel/silver nanoparticles@polypyrrole. Synth. Met. 2019, 250, 104–114. [Google Scholar] [CrossRef]
- Long, W.; Ouyang, H.; Hu, X.; Liu, M.; Zhang, X.; Feng, Y.; Wei, Y. State-of-art review on preparation, surface functionalization and biomedical applications of cellulose nanocrystals-based materials. Int. J. Biol. Macromol. 2021, 186, 591–615. [Google Scholar] [CrossRef]
- Liyanage, S.; Acharya, S.; Parajuli, P.; Shamshina, J.L.; Abidi, N. Production and Surface Modification of Cellulose Bioproducts. Polymers 2021, 13, 3433. [Google Scholar] [CrossRef]
- Kumar, M.; Gehlot, P.S.; Parihar, D.; Surolia, P.K.; Prasad, G. Promising grafting strategies on cellulosic backbone through radical polymerization processes—A review. Eur. Polym. J. 2021, 152, 110448. [Google Scholar] [CrossRef]
- Garcia-Valdez, O.; Champagne, P.; Cunningham, M.F. Graft modification of natural polysaccharides via reversible deactivation radical polymerization. Prog. Polym. Sci. 2018, 76, 151–173. [Google Scholar] [CrossRef]
- Wan, W.; Ouyang, H.; Long, W.; Yan, W.; He, M.; Huang, H.; Yang, S.; Zhang, X.; Feng, Y.; Wei, Y. Direct Surface Functionalization of Cellulose Nanocrystals with Hyperbranched Polymers through the Anionic Polymerization for pH-Responsive Intracellular Drug Delivery. ACS Sustain. Chem. Eng. 2019, 7, 19202–19212. [Google Scholar] [CrossRef]
- Olsén, P.; Herrera, N.; Berglund, L.A. Polymer Grafting Inside Wood Cellulose Fibers by Improved Hydroxyl Accessibility from Fiber Swelling. Biomacromolecules 2020, 21, 597–603. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Grafting of cellulose with N-isopropylacrylamide and glycidyl methacrylate for efficient removal of Ni(II), Cu(II) and Pd(II) ions from aqueous solution. Sep. Purif. Technol. 2019, 219, 249–259. [Google Scholar] [CrossRef]
- Khalilzadeh, M.A.; Hosseini, S.; Rad, A.S.; Venditti, R.A. Synthesis of Grafted Nanofibrillated Cellulose-Based Hydrogel and Study of Its Thermodynamic, Kinetic, and Electronic Properties. J. Agric. Food Chem. 2020, 68, 8710–8719. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Rapid synthesis of graft copolymers from natural cellulose fibers. Carbohydr. Polym. 2013, 98, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wen, Y.; Qu, J.; Geng, X.; Chen, B.; Wei, B.; Wu, B.; Yang, S.; Zhang, H.; Ni, Y. Improving salt tolerance and thermal stability of cellulose nanofibrils by grafting modification. Carbohydr. Polym. 2019, 211, 257–265. [Google Scholar] [CrossRef]
- Mzinyane, N.N.; Chiririwa, H.; Ofomaja, A.E.; Naidoo, E.B. Effect of ammonium ceric nitrate as initiator in grafting of acrylic acid onto pine cone powder. Cellul. Chem. Technol. 2019, 53, 971–979. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Grafted cellulose: A bio-based polymer for durable applications. Polym. Bull. 2018, 75, 2213–2242. [Google Scholar] [CrossRef]
- Grishkewich, N.; Akhlaghi, S.P.; Zhaoling, Y.; Berry, R.; Tam, K.C. Cellulose nanocrystal-poly(oligo(ethylene glycol) methacrylate) brushes with tunable LCSTs. Carbohydr. Polym. 2016, 144, 215–222. [Google Scholar] [CrossRef]
- Hatton, F.L.; Kedzior, S.A.; Cranston, E.D.; Carlmark, A. Grafting-from cellulose nanocrystals via photoinduced Cu-mediated reversible-deactivation radical polymerization. Carbohydr. Polym. 2017, 157, 1033–1040. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, C.; Lei, S.; Chen, J.; Lei, S.; Li, Z. Ultraviolet light-, temperature- and pH-responsive fluorescent sensors based on cellulose nanocrystals. Polym. Chem. 2018, 9, 3098–3107. [Google Scholar] [CrossRef]
- Boujemaoui, A.; Cobo Sanchez, C.; Engström, J.; Bruce, C.; Fogelström, L.; Carlmark, A.; Malmström, E. Polycaprolactone Nanocomposites Reinforced with Cellulose Nanocrystals Surface-Modified via Covalent Grafting or Physisorption: A Comparative Study. ACS Appl. Mater. Interfaces 2017, 9, 35305–35318. [Google Scholar] [CrossRef] [PubMed]
- Hansson, S.; Östmark, E.; Carlmark, A.; Malmström, E. ARGET ATRP for Versatile Grafting of Cellulose Using Various Monomers. ACS Appl. Mater. Interfaces 2009, 1, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- Carlmark, A.; Malmström, E.E. ATRP Grafting from Cellulose Fibers to Create Block-Copolymer Grafts. Biomacromolecules 2003, 4, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Nyström, D.; Lindqvist, J.; Östmark, E.; Hult, A.; Malmström, E. Superhydrophobic bio-fibre surfaces via tailored grafting architecture. Chem. Commun. 2006, 34, 3594–3596. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.J.; Karnati, R.; Wilson, S.M.; Cheng, W.; Liu, M.; Zhang, Z. Use of Nanocrystaline Cellulose and Polymer Grafted Nanocrystaline Cellulose for Increasing Retention, Wet Strength, and Dry Strength in Papermaking. Process. Patent USOO9034145B2, 1 October 2013. Available online: https://patents.google.com/patent/US9034145B2 (accessed on 19 May 2015).
- Zhang, W.; Zhao, J.Q.; Lu, C.H. Cellulose Nano-Fibrous Hyperbranched Method of. Modifying. Patent CN104761749B, 3 April 2015. Available online: https://patents.google.com/patent/CN104761749B (accessed on 21 November 2017).
- Moghaddam, P.N.; Avval, M.E.; Fareghi, A.R. Modification of cellulose by graft polymerization for use in drug delivery systems. Colloid Polym. Sci. 2014, 292, 77–84. [Google Scholar] [CrossRef]
- Cheng, D.; Wen, Y.; An, X.; Zhu, X.; Cheng, X.; Zheng, L.; E Nasrallah, J. Improving the colloidal stability of Cellulose nano-crystals by surface chemical grafting with polyacrylic acid. J. Bioresour. Bioprod. 1996, 1, 114–119. [Google Scholar] [CrossRef]
- Habibi, Y.; Chanzy, H.; Vignon, M.R. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef]
- Li, S.; Xiao, M.; Zheng, A.; Xiao, H. Cellulose Microfibrils Grafted with PBA via Surface-Initiated Atom Transfer Radical Polymerization for Biocomposite Reinforcement. Biomacromolecules 2011, 12, 3305–3312. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.-C.; Zhang, X.; Ren, S.; Dong, L.; Lee, S.; Cheng, H.N.; Lei, T.; Wu, Q. Surface modified cellulose nanocrystals for tailoring interfacial miscibility and microphase separation of polymer nanocomposites. Cellulose 2019, 26, 4301–4312. [Google Scholar] [CrossRef]
- Dalloul, F.; Mietner, J.B.; Navarro, J.R.G. Production and 3D Printing of a Nanocellulose-Based Composite Filament Composed of Polymer-Modified Cellulose Nanofibrils and High-Density Polyethylene (HDPE) for the Fabrication of 3D Complex Shapes. Fibers 2022, 10, 91. [Google Scholar] [CrossRef]
- Vakili, M.R.; Mohammed-Saeid, W.; Aljasser, A.; Hopwood-Raja, J.; Ahvazi, B.; Hrynets, Y.; Betti, M.; Lavasanifar, A. Development of mucoadhesive hydrogels based on polyacrylic acid grafted cellulose nanocrystals for local cisplatin delivery. Carbohydr. Polym. 2021, 255, 117332. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, I.; Islam, M.; Saeed, H.; Haider, A.; Shahzadi, A.; Haider, J.; Ahmed, N.; Ul-Hamid, A.; Nabgan, W.; Ikram, M.; et al. Formation of biocompatible MgO/cellulose grafted hydrogel for efficient bactericidal and controlled release of doxorubicin. Int. J. Biol. Macromol. 2022, 220, 1277–1286. [Google Scholar] [CrossRef]
- Krasnopeeva, E.L.; Melenevskaya, E.Y.; Klapshina, L.G.; Shilyagina, N.Y.; Balalaeva, I.V.; Smirnov, N.N.; Smirnov, M.A.; Yakimansky, A.V. Poly(methacrylic Acid)-Cellulose Brushes as Anticancer Porphyrazine Carrier. Nanomaterials 2021, 11, 1997. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.; Gomathi, T.; Vijayalakshmi, K.; Saranya, M.; Sudha, P.N. Banana fiber Cellulose Nano Crystals grafted with butyl acrylate for heavy metal lead (II) removal. Int. J. Biol. Macromol. 2019, 131, 461–472. [Google Scholar] [CrossRef]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [CrossRef]
- Jumadilov, T.; Malimbayeva, Z.; Yskak, L.; Suberlyak, O.; Kondaurov, R.; Imangazy, A.; Agibayeva, L.; Akimov, A.; Khimersen, K.; Zhuzbayeva, A. Comparative Characteristics of Polymethacrylic Acid Hydrogel Sorption Activity in Relation to Lanthanum Ions in Different Intergel Systems. Chem. Chem. Technol. 2022, 16, 418–431. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Yuan, B.; Fu, M.-L. A facile foaming-polymerization strategy to prepare 3D MnO2 modified biochar-based porous hydrogels for efficient removal of Cd(II) and Pb(II). Chemosphere 2020, 239, 124745. [Google Scholar] [CrossRef]
- Grenda, K.; Arnold, J.; Gamelas, J.A.F.; Cayre, O.J.; Rasteiro, M.G. Flocculation of silica nanoparticles by natural, wood-based polyelectrolytes. Sep. Purif. Technol. 2020, 231, 115888. [Google Scholar] [CrossRef]
- Aloulou, F.; Boufi, S.; Labidi, J. Modified cellulose fibres for adsorption of organic compound in aqueous solution. Sep. Purif. Technol. 2006, 52, 332–342. [Google Scholar] [CrossRef]
- Kono, H. Cationic flocculants derived from native cellulose: Preparation, biodegradability, and removal of dyes in aqueous solution. Resour. Technol. 2017, 3, 55–63. [Google Scholar] [CrossRef]
- Quinlan, P.J.; Tanvir, A.; Tam, K.C. Application of the central composite design to study the flocculation of an anionic azo dye using quaternized cellulose nanofibrils. Carbohydr. Polym. 2015, 133, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Nourani, M.; Baghdadi, M.; Javan, M.; Bidhendi, G.N. Production of a biodegradable flocculant from cotton and evaluation of its performance in coagulation-flocculation of kaolin clay suspension: Optimization through response surface methodology (RSM). J. Environ. Chem. Eng. 2016, 4, 1996–2003. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, H.; Wang, B.; Xiong, L.; Shi, S.; Chen, X. Homogeneous synthesis and characterization of polyacrylamide-grafted cationic cellulose flocculants. J. Appl. Polym. Sci. 2016, 133, 43106. [Google Scholar] [CrossRef]
- Parviainen, H.; Hiltunen, M.; Maunu, S.L. Preparation and flocculation behavior of cellulose-g-PMOTAC copolymer. J. Appl. Polym. Sci. 2014, 131, 40448. [Google Scholar] [CrossRef]
- Mir, A.; Kumar, A.; Riaz, U. A short review on the synthesis and advance applications of polyaniline hydrogels. RSC Adv. 2022, 12, 19122–19132. [Google Scholar] [CrossRef]
- Du, J.; Zhu, W.; Yang, Q.; She, X.; Wu, H.; Tsou, C.; Manuel, D.G.; Huang, H. Strong conductive hybrid hydrogel electrode based on inorganic hybrid crosslinking. Colloid Polym. Sci. 2022, 300, 111–124. [Google Scholar] [CrossRef]
- He, X.; Zhuang, T.; Ruan, S.; Xia, X.; Xia, Y.; Zhang, J.; Huang, H.; Gan, Y.; Zhang, W. An innovative poly(ionic liquid) hydrogel-based anti-freezing electrolyte with high conductivity for supercapacitor. Chem. Eng. J. 2023, 466, 143209. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Z.; Zhong, W.; Yang, W. Hydrothermal direct synthesis of polyaniline, graphene/polyaniline and N-doped graphene/polyaniline hydrogels for high performance flexible supercapacitors. J. Mater. Chem. A 2018, 6, 9245–9256. [Google Scholar] [CrossRef]
- Ye, J.; Shi, D.; Yang, Z.; Chen, M. Interpenetrating Network Hydrogels based on Nanostructured Conductive Polymers for Flexible Supercapacitor. Polym. Sci. Ser. A 2018, 60, 647–654. [Google Scholar] [CrossRef]
- Smirnov, M.A.; Sokolova, M.P.; Geydt, P.; Smirnov, N.N.; Bobrova, N.V.; Toikka, A.M.; Lahderanta, E. Dual doped electroactive hydrogelic fibrous mat with high areal capacitance. Mater. Lett. 2017, 199, 192–195. [Google Scholar] [CrossRef]
- Smirnov, M.A.; Tarasova, E.V.; Vorobiov, V.K.; Kasatkin, I.A.; Mikli, V.; Sokolova, M.P.; Bobrova, N.V.; Vassiljeva, V.; Krumme, A.; Yakimanskiy, A.V. Electroconductive fibrous mat prepared by electrospinning of polyacrylamide-g-polyaniline copolymers as electrode material for supercapacitors. J. Mater. Sci. 2019, 54, 4859–4873. [Google Scholar] [CrossRef]
- Smirnov, M.A.; Bobrova, N.V.; Dmitriev, I.Y.; Bukolšek, V.; Elyashevich, G.K. Electroactive hydrogels based on poly(acrylic acid) and polypyrrole. Polym. Sci. Ser. A 2011, 53, 67–74. [Google Scholar] [CrossRef]
- Tu, C.-W.; Tsai, F.-C.; Chen, J.-K.; Wang, H.-P.; Lee, R.-H.; Zhang, J.; Chen, T.; Wang, C.-C.; Huang, C.-F. Preparations of Tough and Conductive PAMPS/PAA Double Network Hydrogels Containing Cellulose Nanofibers and Polypyrroles. Polymers 2020, 12, 2835. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Fan, L.; Meng, Z.; Zhou, J.; Ye, D.; Xu, W.; Xu, J. Flexible quasi-solid-state supercapacitors for anti-freezing power sources based on polypyrrole@cation-grafted bacterial cellulose. Carbohydr. Polym. 2024, 324, 121502. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, W.; Li, K.; Long, S.; Li, X.; Huang, Y. Toughening Weak Polyampholyte Hydrogels with Weak Chain Entanglements via a Secondary Equilibrium Approach. Polymers 2023, 15, 2644. [Google Scholar] [CrossRef]
- Valtasaari, L. The configuration of cellulose dissolved in iron-sodium tartrate. Die Makronwlekulare Chem. 1971, 150, 117–126. [Google Scholar] [CrossRef]
- Ardizzone, S.; Fregonara, G.; Trasatti, S. “Inner” and “outer” active surface of RuO2 electrodes. Electrochim. Acta 1990, 35, 263–267. [Google Scholar] [CrossRef]
- Liu, P.; Borrell, P.F.; Božič, M.; Kokol, V.; Oksman, K.; Mathew, A.P. Nanocelluloses and their phosphorylated derivatives for selective adsorption of Ag+, Cu2+ and Fe3+ from industrial effluents. J. Hazard. Mater. 2015, 294, 177–185. [Google Scholar] [CrossRef]
- Sriplai, N.; Pinitsoontorn, S. Bacterial cellulose-based magnetic nanocomposites: A review. Carbohydr. Polym. 2021, 254, 117228. [Google Scholar] [CrossRef]
- Cheng, Y.; Ren, X.; Duan, L.; Gao, G. A transparent and adhesive carboxymethyl cellulose/polypyrrole hydrogel electrode for flexible supercapacitors. J. Mater. Chem. C 2020, 8, 8234–8242. [Google Scholar] [CrossRef]
- Sun, Z.; Thielemans, W. Interconnected and high cycling stability polypyrrole supercapacitors using cellulose nanocrystals and commonly used inorganic salts as dopants. J. Energy Chem. 2023, 76, 165–174. [Google Scholar] [CrossRef]
- Xu, H.; Cui, L.; Pan, X.; An, Y.; Jin, X. Carboxymethylcellulose-polyaniline/carbon nanotube (CMC-PANI/CNT) film as flexible and highly electrochemical active electrode for supercapacitors. Int. J. Biol. Macromol. 2022, 219, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Li, L.; Ai, Z.; Gao, X.; Qian, J.; Xu, H.; Su, X.; Wu, J.; Gao, Y. One-Step Fabrication of Ice-Templated Pure Polypyrrole Nanoparticle Hydrogels for High-Rate Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 11940–11947. [Google Scholar] [CrossRef]
- Buyanov, A.L.; Gofman, I.V.; Bozhkova, S.A.; Saprykina, N.N.; Kochish, A.Y.; Netyl’ko, G.I.; Khripunov, A.K.; Smyslov, R.Y.; Afanas’ev, A.V.; Panarin, E.F. Composite hydrogels based on polyacrylamide and cellulose: Synthesis and functional properties. Russ. J. Appl. Chem. 2016, 89, 772–779. [Google Scholar] [CrossRef]
- Dmitriev, I.; Kuryndin, I.; Bobrova, N.; Smirnov, M. Swelling behavior and network characterization of hydrogels from linear polyacrylamide crosslinked with glutaraldehyde. Mater. Today Commun. 2015, 4, 93–100. [Google Scholar] [CrossRef]
- Ballauff, M.; Borisov, O. Polyelectrolyte brushes. Curr. Opin. Colloid Interface Sci. 2006, 11, 316–323. [Google Scholar] [CrossRef]
- Borisov, O.V.; Birshtein, T.M.; Zhulina, E.B. Collapse of grafted polyelectrolyte layer. J. Phys. II 1991, 1, 521–526. [Google Scholar] [CrossRef]
- Ross, R.S.; Pincus, P. The polyelectrolyte brush: Poor solvent. Macromolecules 1992, 25, 2177–2183. [Google Scholar] [CrossRef]
- Zhulina, E.B.; Borisov, O.V. Polyelectrolytes Grafted to Curved Surfaces. Macromolecules 1996, 29, 2618–2626. [Google Scholar] [CrossRef]
- Borisov, O.V.; Zhulina, E.B. Conformations of polyelectrolyte molecular brushes: A mean-field theory. J. Chem. Phys. 2018, 149, 184904. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.S. Limiting Laws and Counterion Condensation in Polyelectrolyte Solutions I. Colligative Properties. J. Chem. Phys. 1969, 51, 924–933. [Google Scholar] [CrossRef]
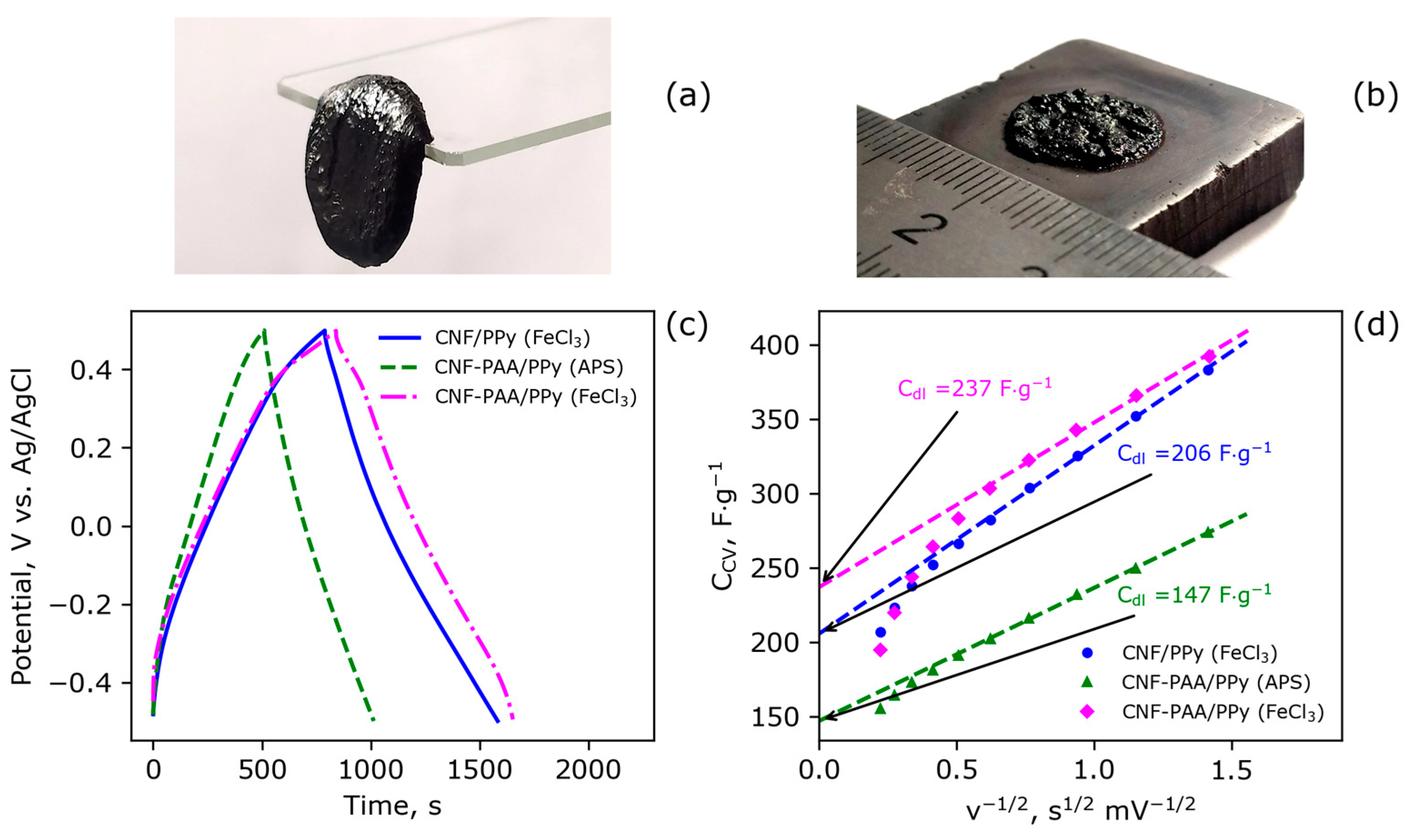
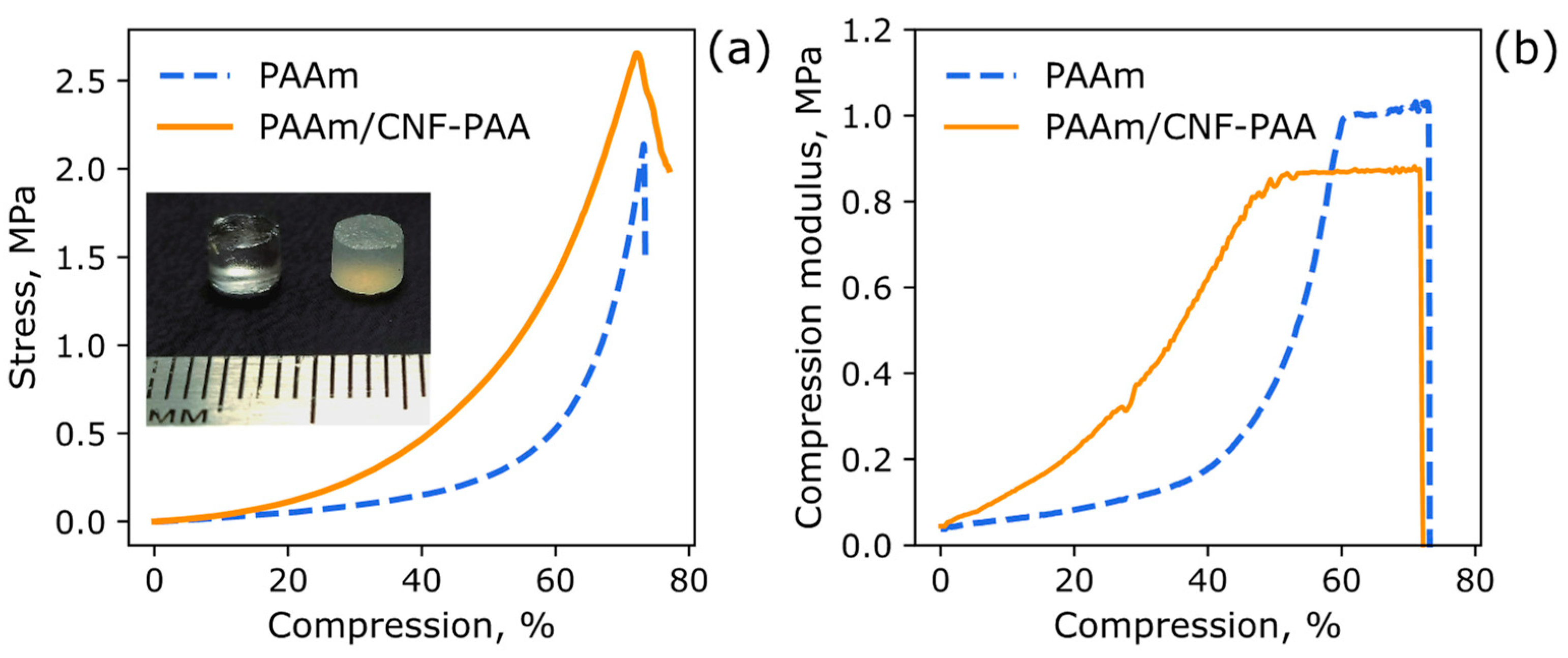
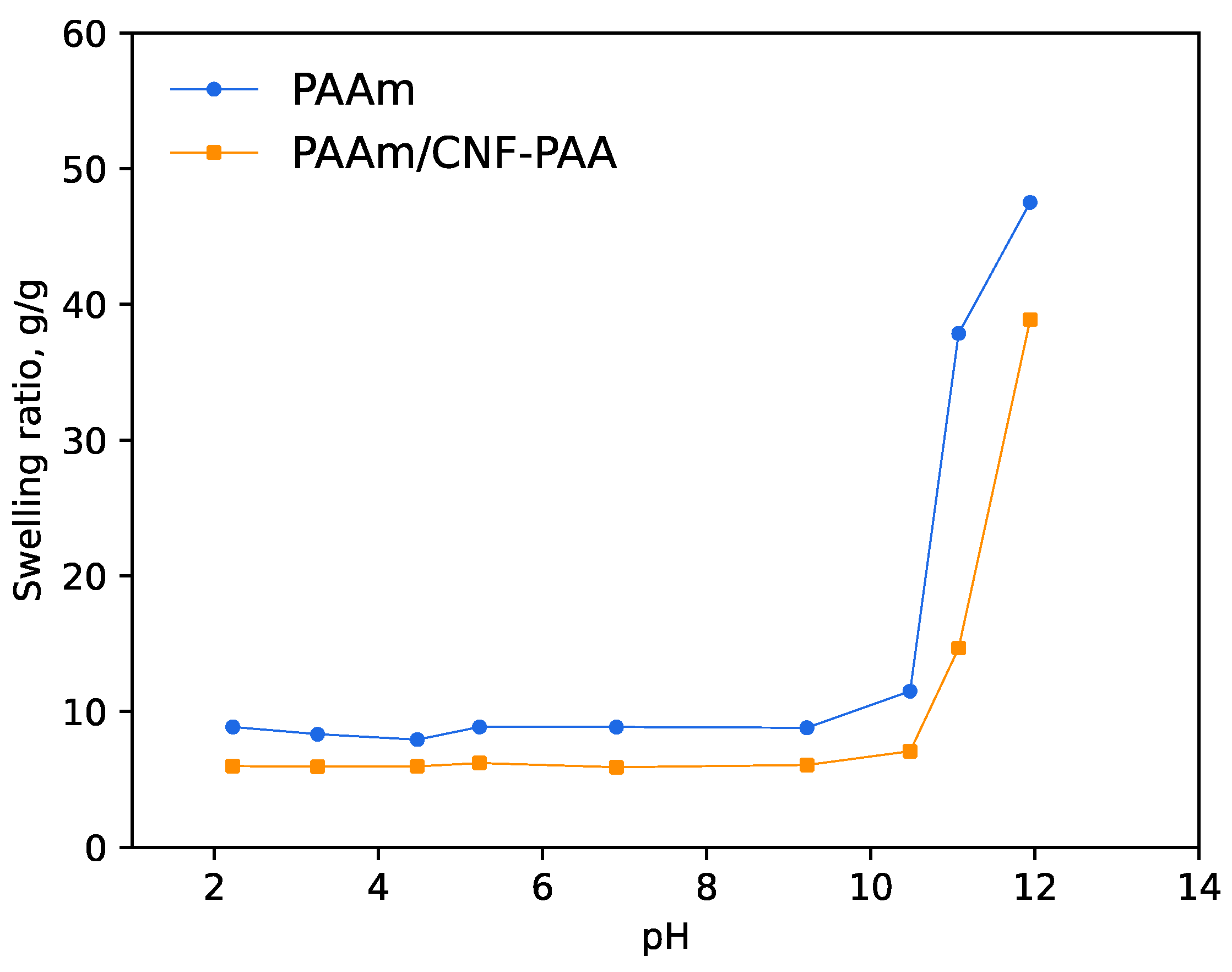
| Sample | Initiator | Csp 1, F/g | Cdl, F/g | Q, g/g |
|---|---|---|---|---|
| CNF/PPy | FeCl3 | 401 | 206 | 6.7 |
| CNF-PAA/PPy | FeCl3 | 414 | 237 | 6.2 |
| CNF-PAA/PPy | APS | 254 | 147 | 6.5 |
| Sample | Young’s Modulus, kPa (ε 1 = 0–10%) | Compression Strength, MPa | Compression at Break, % |
|---|---|---|---|
| PAAm | 150 ± 40 | 1.9 ± 0.2 | 76 ± 2 |
| PAAm/CNF-PAA | 400 ± 60 | 2.4 ± 0.2 | 73 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirnov, M.A.; Vorobiov, V.K.; Fedotova, V.S.; Sokolova, M.P.; Bobrova, N.V.; Smirnov, N.N.; Borisov, O.V. A Polyelectrolyte Colloidal Brush Based on Cellulose: Perspectives for Future Applications. Polymers 2023, 15, 4526. https://doi.org/10.3390/polym15234526
Smirnov MA, Vorobiov VK, Fedotova VS, Sokolova MP, Bobrova NV, Smirnov NN, Borisov OV. A Polyelectrolyte Colloidal Brush Based on Cellulose: Perspectives for Future Applications. Polymers. 2023; 15(23):4526. https://doi.org/10.3390/polym15234526
Chicago/Turabian StyleSmirnov, Michael A., Vitaly K. Vorobiov, Veronika S. Fedotova, Maria P. Sokolova, Natalya V. Bobrova, Nikolay N. Smirnov, and Oleg V. Borisov. 2023. "A Polyelectrolyte Colloidal Brush Based on Cellulose: Perspectives for Future Applications" Polymers 15, no. 23: 4526. https://doi.org/10.3390/polym15234526
APA StyleSmirnov, M. A., Vorobiov, V. K., Fedotova, V. S., Sokolova, M. P., Bobrova, N. V., Smirnov, N. N., & Borisov, O. V. (2023). A Polyelectrolyte Colloidal Brush Based on Cellulose: Perspectives for Future Applications. Polymers, 15(23), 4526. https://doi.org/10.3390/polym15234526










