Development of Inkjet Printable Formulations Based on Polyorganosilazane and Divinylbenzene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of UV-Curable Inks and SiCN Ceramics
2.3. Characterization
3. Results and Discussion
3.1. Rheology and Wetting Behavior of the Ink
3.2. Crosslinking Behavior and FTIR Analysis
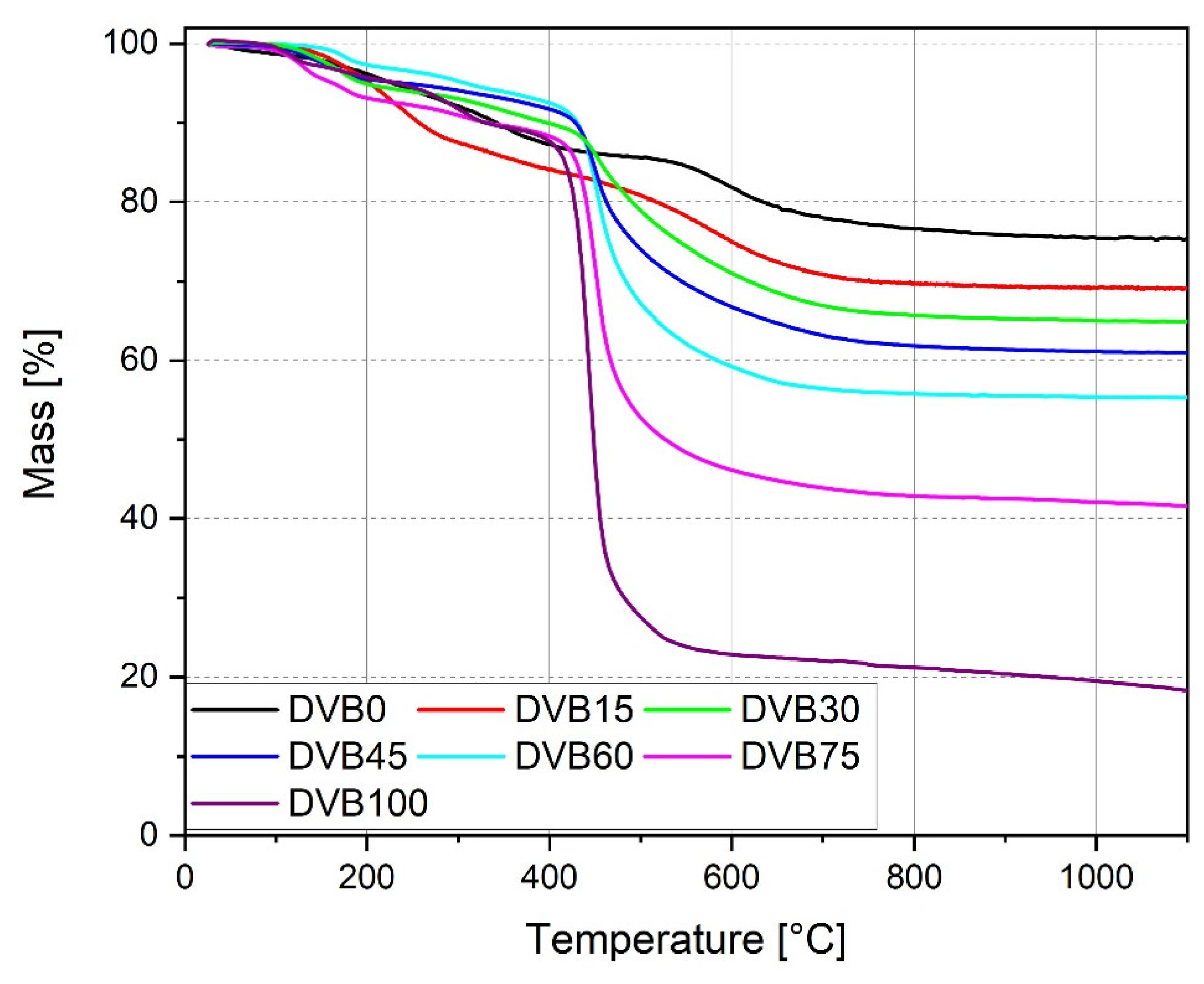
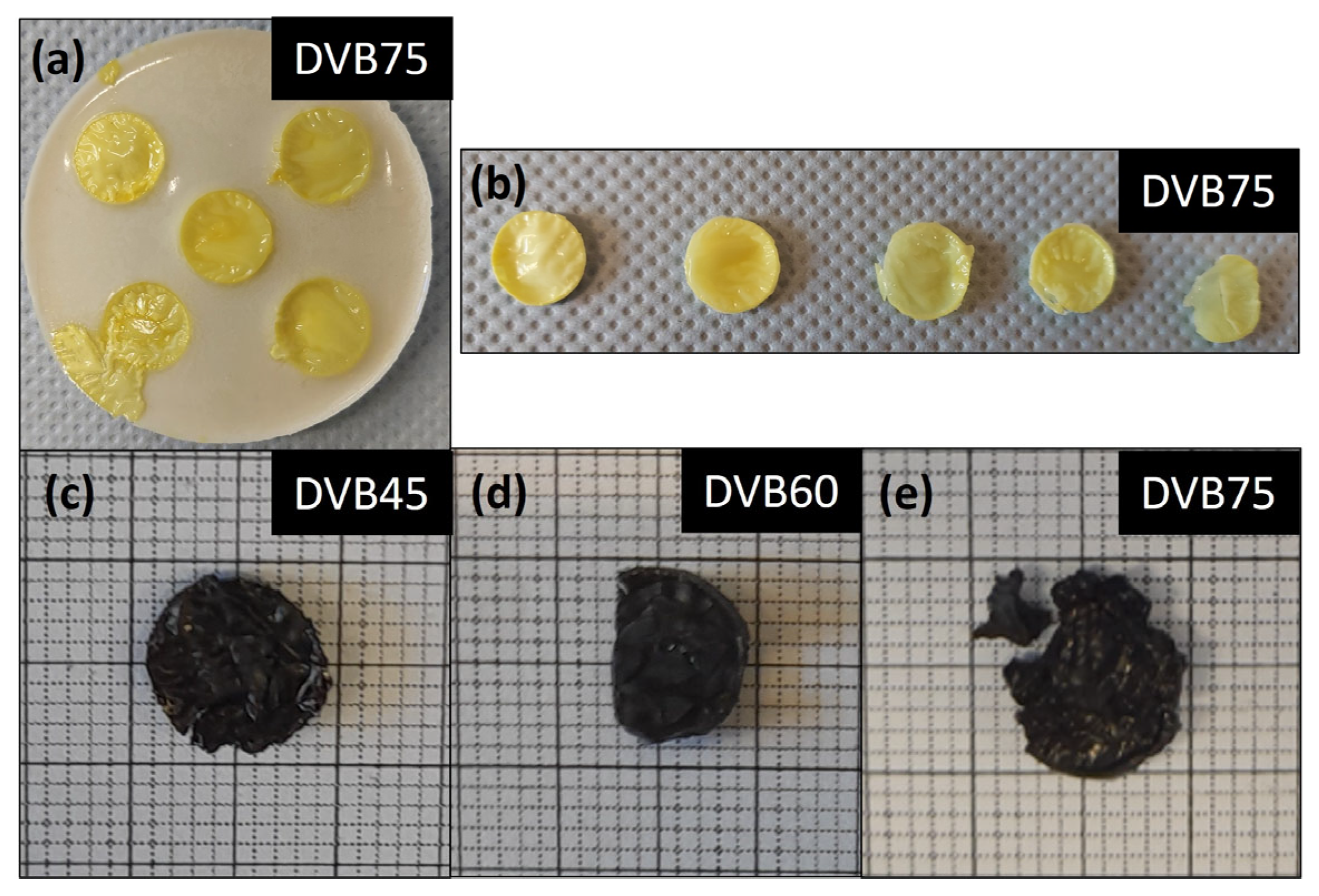
3.3. Thermal Analysis and Ceramization Process
3.4. Electrical Conductivity of the Bulk Material
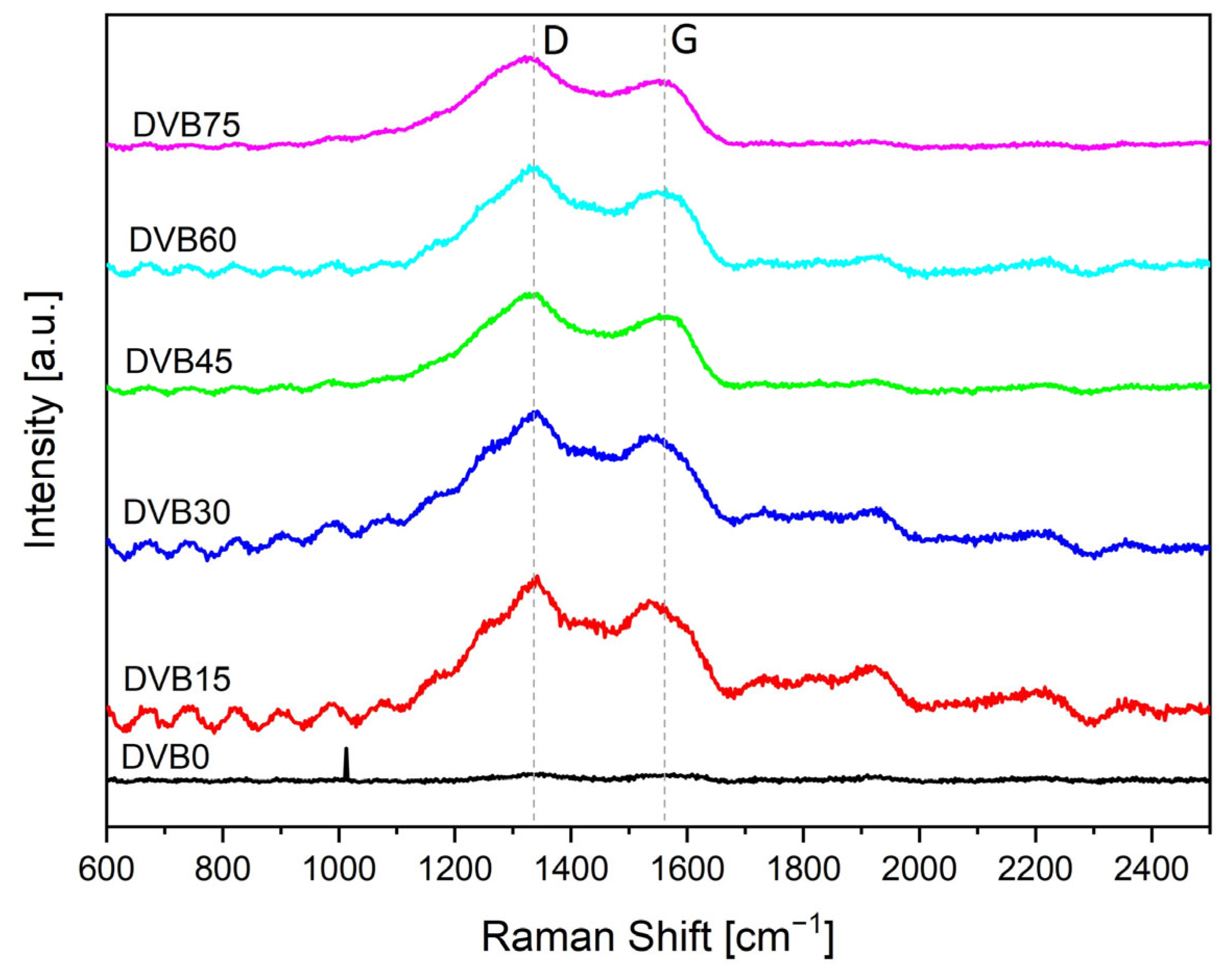
3.5. Raman Spectroscopy
3.6. Inkjet Printing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-Derived Ceramics: 40 Years of Research and Innovation in Advanced Ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Fonblanc, D.; Lopez-Ferber, D.; Wynn, M.; Lale, A.; Soleilhavoup, A.; Leriche, A.; Iwamoto, Y.; Rossignol, F.; Gervais, C.; Bernard, S. Crosslinking Chemistry of Poly(Vinylmethyl-Co-Methyl)Silazanes toward Low-Temperature Formable Preceramic Polymers as Precursors of Functional Aluminium-Modified Si–C–N Ceramics. Dalton Trans. 2018, 47, 14580–14593. [Google Scholar] [CrossRef] [PubMed]
- Parchovianský, M.; Parchovianská, I.; Švančárek, P.; Motz, G.; Galusek, D. PDC Glass/Ceramic Coatings Applied to Differently Pretreated AISI441 Stainless Steel Substrates. Materials 2020, 13, 629. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Zahedtalaban, M.; Denk, J.; Horcher, A.; Ruckdäschel, H.; Schafföner, S.; Motz, G. Photoinduced Thiol-Ene “Click” Chemistry for Resource-Efficient Curing of Polysilazane-Based Coatings and Its Effects on Coating Property Profile. Open Ceram. 2023, 15, 100384. [Google Scholar] [CrossRef]
- Barroso, G.; Li, Q.; Bordia, R.K.; Motz, G. Polymeric and Ceramic Silicon-Based Coatings—A Review. J. Mater. Chem. A Mater. 2019, 7, 1936–1963. [Google Scholar] [CrossRef]
- Liu, G.; Kaspar, J.; Reinold, L.M.; Graczyk-Zajac, M.; Riedel, R. Electrochemical Performance of DVB-Modified SiOC and SiCN Polymer-Derived Negative Electrodes for Lithium-Ion Batteries. Electrochim. Acta 2013, 106, 101–108. [Google Scholar] [CrossRef]
- Ahilan, V.; Wilhelm, M.; Rezwan, K. Porous Polymer Derived Ceramic (PDC)-Montmorillonite-H3PMo12O40/SiO2 Composite Membranes for Microbial Fuel Cell (MFC) Application. Ceram. Int. 2018, 44, 19191–19199. [Google Scholar] [CrossRef]
- Kumar, B.; Prasad, R.M. Polymer-Derived Microporous SiOC Ceramic Coated Gallium Nitride Sensor for Selective H2/CO Detection. Sens. Actuators B Chem. 2023, 379, 133226. [Google Scholar] [CrossRef]
- Silva, T.C.D.A.; Mooste, M.; Kibena-Põldsepp, E.; Matisen, L.; Merisalu, M.; Kook, M.; Sammelselg, V.; Tammeveski, K.; Wilhelm, M.; Rezwan, K. Polymer-Derived Co/Ni-SiOC(N) Ceramic Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells. Catal. Sci. Technol. 2019, 9, 854–866. [Google Scholar] [CrossRef]
- Stiharu, I.; Andronenko, S.; Zinnatullin, A.; Vagizov, F. SiCNFe Ceramics as Soft Magnetic Material for MEMS Magnetic Devices: A Mössbauer Study. Micromachines 2023, 14, 925. [Google Scholar] [CrossRef]
- Liu, K.; Peng, W.; Han, D.; Liang, Y.; Fan, B.; Lu, H.; Wang, H.; Xu, H.; Zhang, R.; Shao, G. Enhanced Piezoresistivity of Polymer-Derived SiCN Ceramics by Regulating Divinylbenzene-Induced Free Carbon. Ceram. Int. 2023, 49, 2296–2301. [Google Scholar] [CrossRef]
- Velu, R.; Calais, T.; Jayakumar, A.; Raspall, F. A Comprehensive Review on Bio-Nanomaterials for Medical Implants and Feasibility Studies on Fabrication of Such Implants by Additive Manufacturing Technique. Materials 2019, 13, 92. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Domingos, M.A.N.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive Manufacturing of Tissues and Organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Leal, R.; Barreiros, F.M.; Alves, L.; Romeiro, F.; Vasco, J.C.; Santos, M.; Marto, C. Additive Manufacturing Tooling for the Automotive Industry. Int. J. Adv. Manuf. Technol. 2017, 92, 1671–1676. [Google Scholar] [CrossRef]
- Jahromi, F.T.; Nikzad, M.; Prasad, K.; Norén, J.; Isaksson, M.; Arian, A.; Sbarski, I. Additive Manufacturing of Polypropylene Micro and Nano Composites through Fused Filament Fabrication for Automotive Repair Applications. Polym. Adv. Technol. 2023, 34, 1059–1074. [Google Scholar] [CrossRef]
- Najmon, J.C.; Raeisi, S.; Tovar, A. Review of Additive Manufacturing Technologies and Applications in the Aerospace Industry. In Additive Manufacturing for the Aerospace Industry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 7–31. [Google Scholar] [CrossRef]
- Careri, F.; Khan, R.H.U.; Todd, C.; Attallah, M.M. Additive Manufacturing of Heat Exchangers in Aerospace Applications: A Review. Appl. Therm. Eng. 2023, 235, 121387. [Google Scholar] [CrossRef]
- Wang, M.; Xie, C.; He, R.; Ding, G.; Zhang, K.; Wang, G.; Fang, D. Polymer-Derived Silicon Nitride Ceramics by Digital Light Processing Based Additive Manufacturing. J. Am. Ceram. Soc. 2019, 102, 5117–5126. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Zhao, T.; Han, W.; Duan, W.; Wang, L.; Dou, R.; Wang, G. Additive Manufacturing of SiBCN/Si 3 N 4 w Composites from Preceramic Polymers by Digital Light Processing. RSC Adv. 2020, 10, 5681–5689. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Colombo, P. Digital Light Processing of Ceramic Components from Polysiloxanes. J. Eur. Ceram. Soc. 2018, 38, 57–66. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, H.; Xi, X.; Deng, Y.; Zhang, H.; Wang, C.; Lin, H. Vinyl Polysilazane Based Photo-Curable Resin Derived Ceramics Manufactured by Digital Light Processing with Al Flake as the Active Filler. J. Eur. Ceram. Soc. 2023, 43, 332–340. [Google Scholar] [CrossRef]
- Wang, X.; Schmidt, F.; Li, D.; Gurlo, S.; Wang, X.; Schmidt, F.; Hanaor, D.; Kamm, P.H.; Li, S.; Gurlo, A. Additive Manufacturing of Ceramics from Preceramic Polymers: A Versatile Stereolithographic Approach Assisted by Thiol-Ene Click Chemistry. Addit. Manuf. 2019, 27, 80–90. [Google Scholar] [CrossRef]
- Chen, S.; Wan, X.; Li, J.; Gao, B.; Chen, X.; Meng, S.; Lu, J. Fabrication of Electrical Semi-Conductive SiCN Ceramics by Vat Photopolymerization. J. Mater. Sci. Technol. 2023, 165, 123–131. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Xiong, H.; Zhou, K.; Zhang, D. Optimized Preceramic Polymer for 3D Structured Ceramics via Fused Deposition Modeling. J. Eur. Ceram. Soc. 2021, 41, 5066–5074. [Google Scholar] [CrossRef]
- Gorjan, L.; Tonello, R.; Sebastian, T.; Colombo, P.; Clemens, F. Fused Deposition Modeling of Mullite Structures from a Preceramic Polymer and γ-Alumina. J. Eur. Ceram. Soc. 2019, 39, 2463–2471. [Google Scholar] [CrossRef]
- Pierin, G.; Grotta, C.; Colombo, P.; Mattevi, C. Direct Ink Writing of Micrometric SiOC Ceramic Structures Using a Preceramic Polymer. J. Eur. Ceram. Soc. 2016, 36, 1589–1594. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Xue, F.; Huang, Y.; Zhou, K.; Zhang, D. 3D Printing of SiC Ceramic: Direct Ink Writing with a Solution of Preceramic Polymers. J. Eur. Ceram. Soc. 2018, 38, 5294–5300. [Google Scholar] [CrossRef]
- Mott, M.; Evans, J.R.G. Solid Freeforming of Silicon Carbide by Inkjet Printing Using a Polymeric Precursor. J. Am. Ceram. Soc. 2004, 84, 307–313. [Google Scholar] [CrossRef]
- Han, J.; Liu, C.; Bradford-Vialva, R.L.; Klosterman, D.A.; Cao, L. Additive Manufacturing of Advanced Ceramics Using Preceramic Polymers. Materials 2023, 16, 4636. [Google Scholar] [CrossRef]
- Lacelle, T.; Sampson, K.L.; Yazdani Sarvestani, H.; Rahimizadeh, A.; Barroeta Robles, J.; Mirkhalaf, M.; Rafiee, M.; Jakubinek, M.B.; Paquet, C.; Ashrafi, B. Additive Manufacturing of Polymer Derived Ceramics: Materials, Methods, and Applications. APL Mater. 2023, 11, 070602. [Google Scholar] [CrossRef]
- Cummins, G.; Desmulliez, M.P.Y. Inkjet Printing of Conductive Materials: A Review. Circuit World 2012, 38, 193–213. [Google Scholar] [CrossRef]
- Vaezi, M.; Chianrabutra, S.; Mellor, B.; Yang, S. Multiple Material Additive Manufacturing—Part 1: A Review. Virtual Phys. Prototyp. 2013, 8, 19–50. [Google Scholar] [CrossRef]
- Magdassi, S. The Chemistry of Inkjet Inks; World Scientific: Singapore, 2009; ISBN 978-981-281-821-8. [Google Scholar]
- Graf, D.; Qazzazie, A.; Hanemann, T. Investigations on the Processing of Ceramic Filled Inks for 3D InkJet Printing. Materials 2020, 13, 2587. [Google Scholar] [CrossRef]
- Riedel, R.; Mera, G.; Hauser, R.; Klonczynski, A. Silicon-Based Polymer-Derived Ceramics: Synthesis Properties and Applications—A Review. J. Ceram. Soc. Jpn. 2006, 114, 425–444. [Google Scholar] [CrossRef]
- Günthner, M.; Kraus, T.; Krenkel, W.; Motz, G.; Dierdorf, A.; Decker, D. Particle-Filled PHPS Silazane-Based Coatings on Steel. Int. J. Appl. Ceram. Technol. 2009, 6, 373–380. [Google Scholar] [CrossRef]
- Konegger, T.; Prochaska, T.; Obmann, R. Tubular Ceramic Structures from Polymer Precursors with Controlled Porosity, Strength, and Permeability Characteristics. J. Ceram. Soc. Jpn. 2016, 124, 1030–1034. [Google Scholar] [CrossRef]
- Huh, T.; Lee, T.S.; Kwark, Y. Fabrication of Hierarchically Porous Silicon Carbonitride Monolith Using Branched Polysilazane and Freeze Casting Method without Crosslinking Step: Effect of Polymer Additive to Aligned Macropore Structure. J. Am. Ceram. Soc. 2022, 105, 7785–7794. [Google Scholar] [CrossRef]
- Barroso, G.; Kraus, T.; Degenhardt, U.; Scheffler, M.; Motz, G. Functional Coatings Based on Preceramic Polymers. Adv. Eng. Mater. 2016, 18, 746–753. [Google Scholar] [CrossRef]
- Jana, P.; Santoliquido, O.; Ortona, A.; Colombo, P.; Sorarù, G.D. Polymer-Derived SiCN Cellular Structures from Replica of 3D Printed Lattices. J. Am. Ceram. Soc. 2018, 101, 2732–2738. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Liu, J.; Fu, Y.; Liu, C.; Wang, P.; Jiang, M.; Lao, C. Additive Manufacturing of Lightweight and High-Strength Polymer-Derived SiOC Ceramics. Virtual Phys. Prototyp. 2020, 15, 163–177. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, X.; Elsayed, H.; Huang, K.; Franchin, G.; Motz, G.; Tong, Y.; Gong, H.; Colombo, P. Enhanced Electromagnetic Microwave Absorption Properties of SiCN(Fe) Ceramics Produced by Additive Manufacturing via in-Situ Reaction of Ferrocene. Ceram. Int. 2023, 49, 25051–25062. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Shi, S.; Wang, K.; Lu, B. Polysilazane-Derived SiCN Ceramics-Based Layerless Printing. Addit. Manuf. 2022, 55, 102845. [Google Scholar] [CrossRef]
- D’Elia, R.; Dusserre, G.; Del Confetto, S.; Eberling-Fux, N.; Descamps, C.; Cutard, T. Cure Kinetics of a Polysilazane System: Experimental Characterization and Numerical Modelling. Eur. Polym. J. 2016, 76, 40–52. [Google Scholar] [CrossRef]
- D’Elia, R.; Dusserre, G.; Del Confetto, S.; Eberling-Fux, N.; Descamps, C.; Cutard, T. Effect of Dicumyl Peroxide Concentration on the Polymerization Kinetics of a Polysilazane System. Polym. Eng. Sci. 2018, 58, 859–869. [Google Scholar] [CrossRef]
- Qazzazie-Hauser, A.; Honnef, K.; Hanemann, T. Crosslinking Behavior of UV-Cured Polyorganosilazane as Polymer-Derived Ceramic Precursor in Ambient and Nitrogen Atmosphere. Polymers 2021, 13, 2424. [Google Scholar] [CrossRef]
- Mera, G.; Ionescu, E. Polymer-Derived Ceramics. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 1–26. [Google Scholar]
- Hagelüken, L.; Sasikumar, P.V.W.; Lee, H.Y.; Di Stadio, D.; Chandorkar, Y.; Rottmar, M.; Maniura-Weber, K.; Blugan, G.; Brugger, J. Multiscale 2D/3D Microshaping and Property Tuning of Polymer-Derived SiCN Ceramics. J. Eur. Ceram. Soc. 2022, 42, 1963–1970. [Google Scholar] [CrossRef]
- Schulz, M.; Börner, M.; Göttert, J.; Hanemann, T.; Haußelt, J.; Motz, G. Cross Linking Behavior of Preceramic Polymers Effected by UV- and Synchrotron Radiation. Adv. Eng. Mater. 2004, 6, 676–680. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Miccoli, I.; Edler, F.; Pfnür, H.; Tegenkamp, C. The 100th Anniversary of the Four-Point Probe Technique: The Role of Probe Geometries in Isotropic and Anisotropic Systems. J. Phys. Condens. Matter 2015, 27, 223201. [Google Scholar] [CrossRef]
- Andrade, E.N.D.C. XLI. A Theory of the Viscosity of Liquids—Part I. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1934, 17, 497–511. [Google Scholar] [CrossRef]
- Divinylbenzene Experimental Properties. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Divinylbenzene#section=Other-Experimental-Properties (accessed on 31 October 2023).
- Arkles, B.; Larson, G.L. Silicon Compounds: Silanes and Silicones: A Survey of Properties and Chemistry; Gelest. Inc.: Morrisville, PA, USA, 2008; ISBN 9780578122359. [Google Scholar]
- Chavez, R.; Ionescu, E.; Balan, C.; Fasel, C.; Riedel, R. Effect of Ambient Atmosphere on Crosslinking of Polysilazanes. J. Appl. Polym. Sci. 2011, 119, 794–802. [Google Scholar] [CrossRef]
- Adigun, O.D.; Ricohermoso, E.; Daniyan, A.A.; Umoru, L.E.; Ionescu, E. Structure and Electrical Properties of Carbon-Rich Polymer Derived Silicon Carbonitride (SiCN). Ceramics 2022, 5, 690–705. [Google Scholar] [CrossRef]





| Sample | OPSZ (wt.%) | DVB (wt.%) | Photo Initiator (wt.%) | Thermal Initiator (wt.%) |
|---|---|---|---|---|
| DVB0 | 100 | 0 | 3 | 3 |
| DVB15 | 85 | 15 | 3 | 3 |
| DVB30 | 70 | 30 | 3 | 3 |
| DVB45 | 55 | 45 | 3 | 3 |
| DVB60 | 40 | 60 | 3 | 3 |
| DVB75 | 25 | 75 | 3 | 3 |
| Sample | Surface Tension (mN/m) | Contact Angle on Silicon Wafer (°) | Viscosity at 500 s−1 (mPa∙s) |
|---|---|---|---|
| DVB0 | 21 | 5 | 30 |
| DVB15 | 24.7 | 5 | 21 |
| DVB30 | 24.2 | 3 | 9.5 |
| DVB45 | 24.9 | Spreading | 7 |
| DVB60 | 24.6 | 3 | 3.7 |
| DVB75 | 26.4 | Spreading | 2.5 |
| DVB100 | 30.55 | - | 1.6 |
| Sample | Degree of Conversion (Vinyl Group at 1595 cm−1) (%) |
|---|---|
| DVB0 | - |
| DVB15 | 82 ± 3 |
| DVB30 | 66 ± 7 |
| DVB45 | 75 ± 7 |
| DVB60 | 81 ± 3 |
| DVB75 | 83 ± 9 |
| Sample | Residual Mass at 1100 °C (%) |
|---|---|
| DVB0 | 75 |
| DVB15 | 69 |
| DVB30 | 65 |
| DVB45 | 61 |
| DVB60 | 55 |
| DVB75 | 42 |
| DVB100 | 17 |
| Sample | Electrical Conductivity (S/cm) |
|---|---|
| DVB45 | 1.1 × 10−1 |
| DVB60 | 2.3 × 10−1 |
| DVB75 | 1.2 |
| D-Band (cm−1) | G-Band (cm−1) | ID/IG - | La (nm) | |
|---|---|---|---|---|
| DVB0 | 1338 | 1563 | 1.0 | 1.2 |
| DVB15 | 1329 | 1541 | 1.3 | 1.5 |
| DVB30 | 1320 | 1539 | 1.9 | 1.7 |
| DVB45 | 1330 | 1546 | 2.0 | 1.8 |
| DVB60 | 1327 | 1535 | 1.5 | 1.6 |
| DVB75 | 1322 | 1547 | 1.8 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qazzazie-Hauser, A.; Honnef, K.; Hanemann, T. Development of Inkjet Printable Formulations Based on Polyorganosilazane and Divinylbenzene. Polymers 2023, 15, 4512. https://doi.org/10.3390/polym15234512
Qazzazie-Hauser A, Honnef K, Hanemann T. Development of Inkjet Printable Formulations Based on Polyorganosilazane and Divinylbenzene. Polymers. 2023; 15(23):4512. https://doi.org/10.3390/polym15234512
Chicago/Turabian StyleQazzazie-Hauser, Afnan, Kirsten Honnef, and Thomas Hanemann. 2023. "Development of Inkjet Printable Formulations Based on Polyorganosilazane and Divinylbenzene" Polymers 15, no. 23: 4512. https://doi.org/10.3390/polym15234512
APA StyleQazzazie-Hauser, A., Honnef, K., & Hanemann, T. (2023). Development of Inkjet Printable Formulations Based on Polyorganosilazane and Divinylbenzene. Polymers, 15(23), 4512. https://doi.org/10.3390/polym15234512






