Preceramic Polymers for Additive Manufacturing of Silicate Ceramics
Abstract
:1. Preceramic Polymers
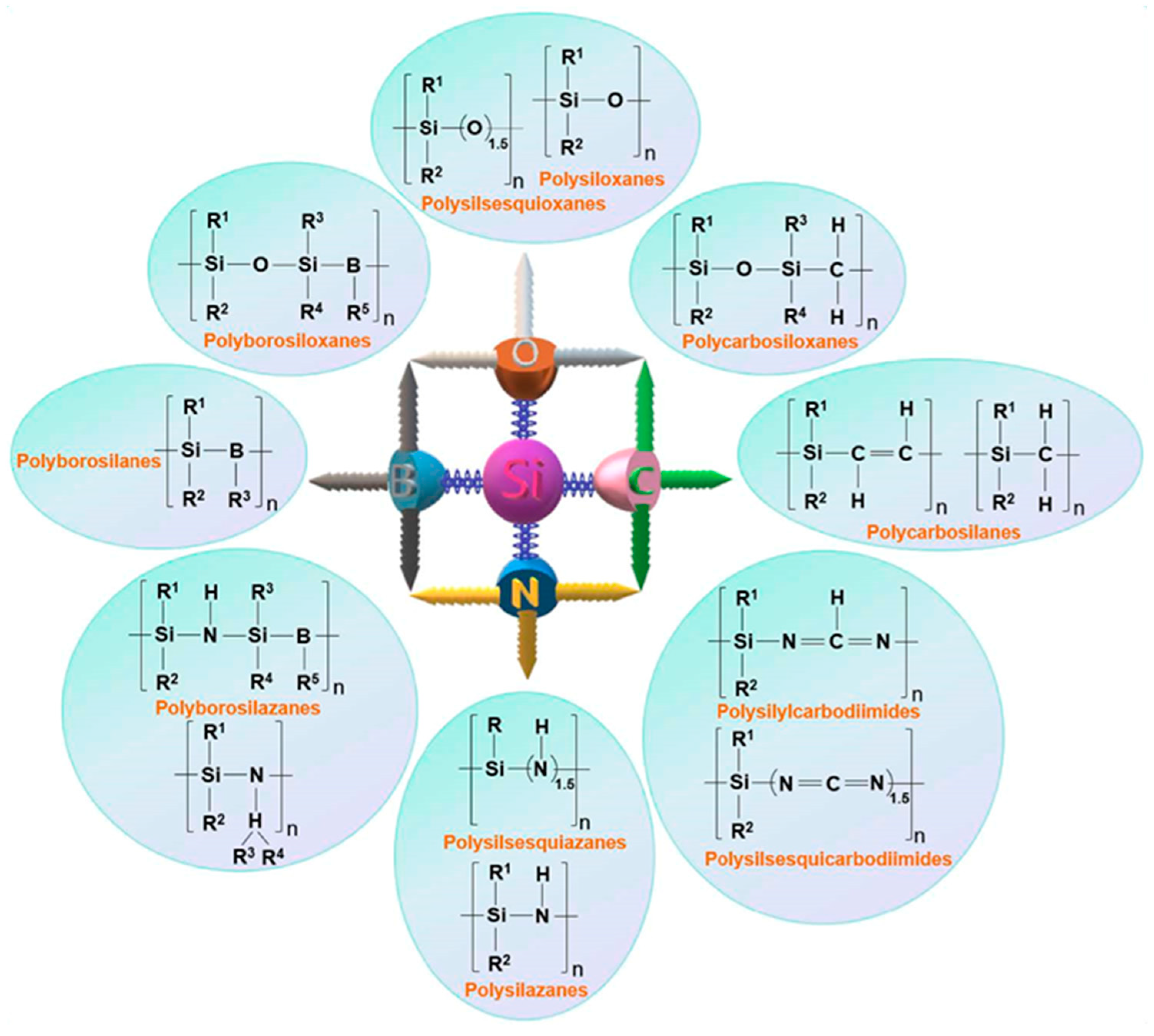
1.1. Si-Based Preceramic Polymers
1.1.1. Polysilanes
1.1.2. Polycarbosilanes
1.1.3. Polysilazanes
1.1.4. Polysiloxanes and Polysilsesquioxanes
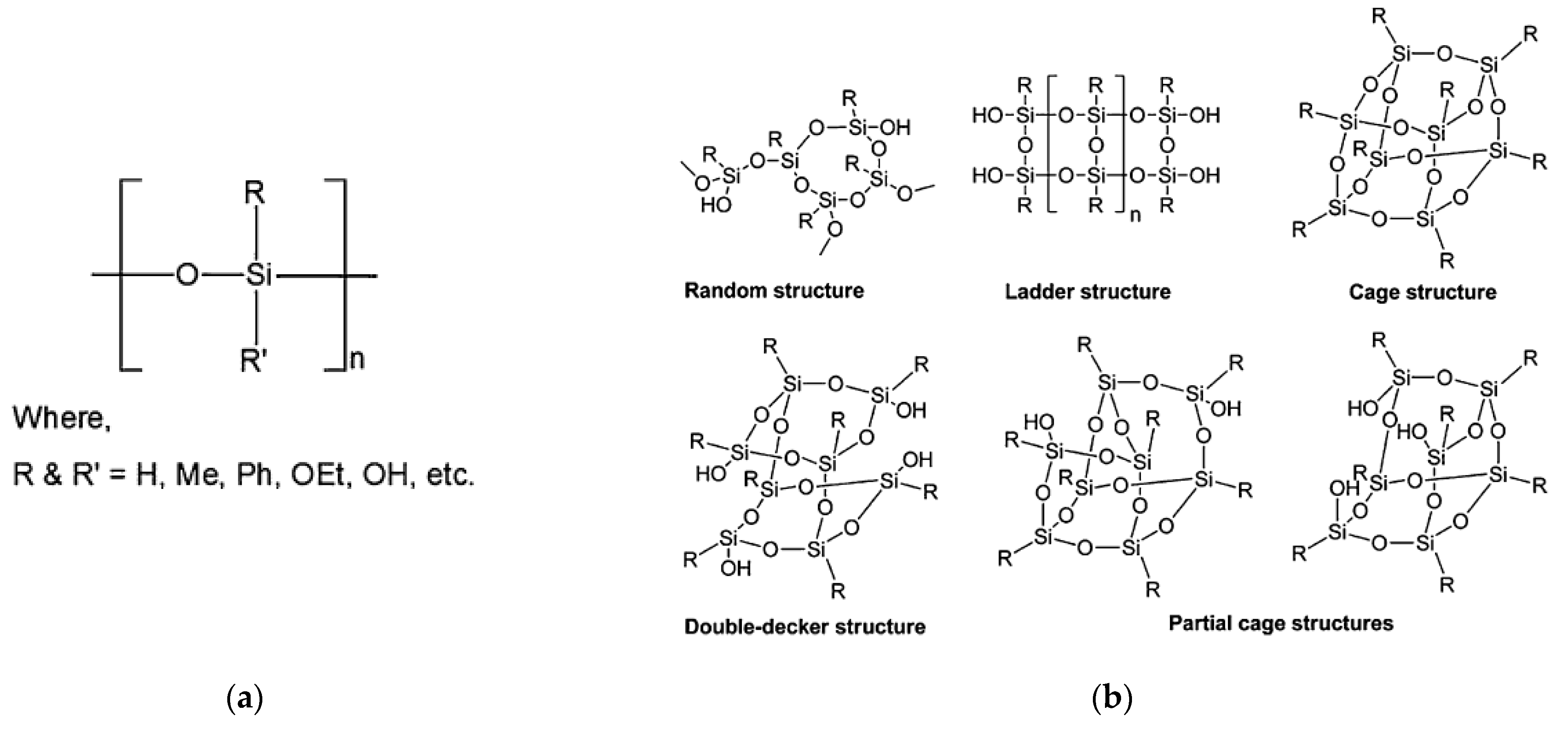
1.2. Properties of Si-Based Polymers
- -
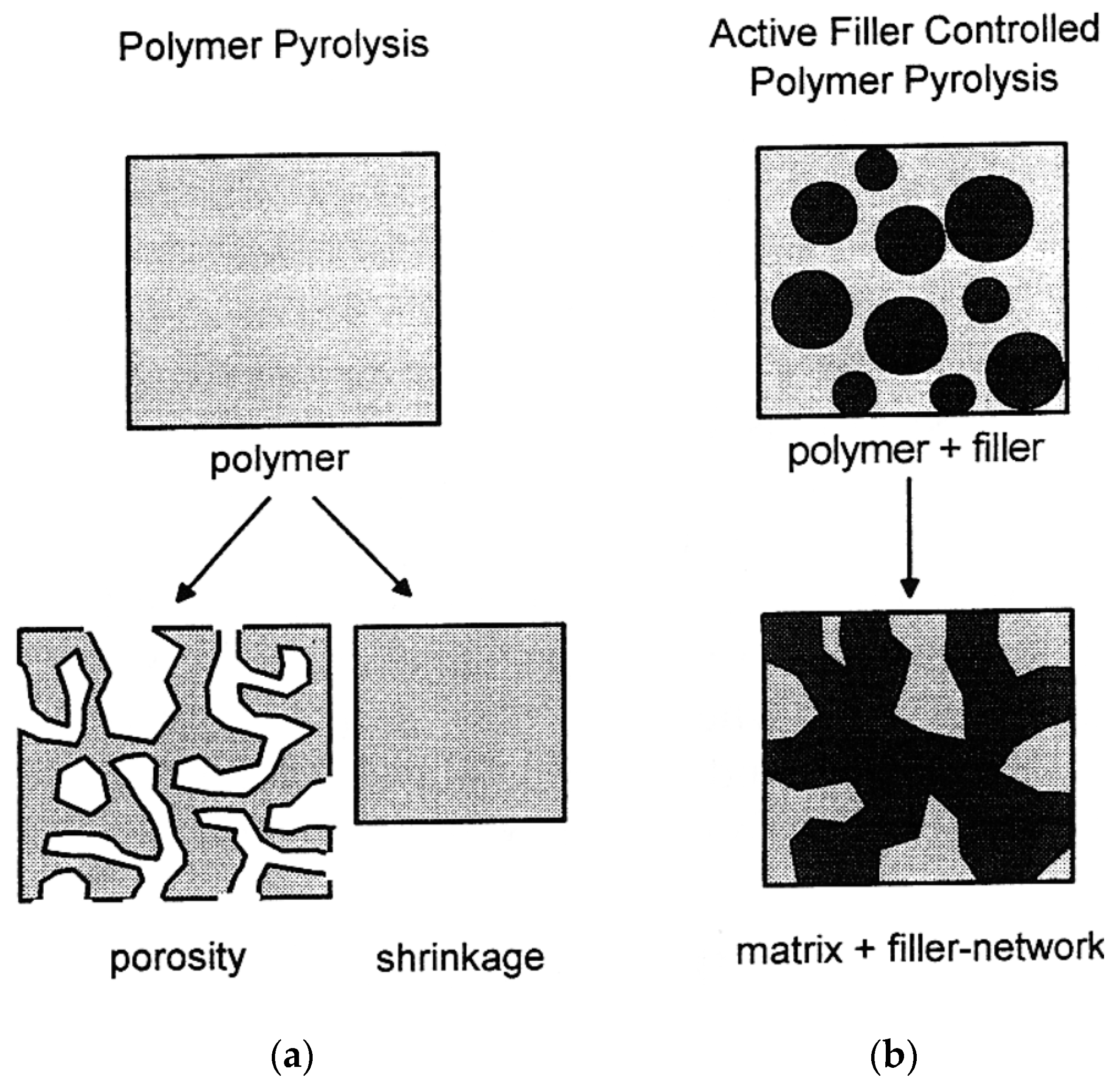
- passive fillers: this group of fillers is not reactive. They only control the shrinkage and presence of macro defects during pyrolysis. Typical examples are SiC and Si3N4.
- -
- active fillers: using active fillers, on the other hand, a new phase compared to the starting PCP can be achieved. Carbides, nitrides, silicates, oxides, and silicides can be produced as a result of a reaction between the filler and atmosphere or PCP residue after pyrolysis or the gaseous products during pyrolysis itself.
- -
- meltable fillers: this category of fillers consists of meltable materials, typically glasses. When subjected to high temperatures, the glass phase melts or softens and effectively fills the available porosity which enhances the density. This approach protects the part against oxidation and corrosion. When meltable fillers are used in coatings, their softening at elevated temperatures reduces Young’s modulus, allowing for the relaxation of thermomechanical stresses arising from mismatches in thermal expansion between the substrate, coating, and fillers within the precursor matrix. They may also undergo chemical reactions with other components in the system, acting as active fillers.
- -
- sacrificial fillers: These organic compounds are mixed with the PCP and removed after crosslinking using thermal decomposition or dissolution in a solvent. Their main function is to create the porosity in the PDC parts.
1.3. Synthesis of Silicate Ceramics Using Polysiloxane and Polysilsesquioxanes
1.4. Processing of Preceramic Polymers
1.4.1. Shaping
1.4.2. Crosslinking
1.4.3. Pyrolysis
2. Additive Manufacturing of Preceramic Polymers
2.1. Light-Assisted AM (Vat Photopolymerization)
2.1.1. Stereolithography (SL)
2.1.2. Digital Light Processing (DLP)
2.1.3. Two-Photon Polymerization (TPP)
2.2. Selective Laser Sintering (SLS)
2.3. Laminated Object Manufacturing (LOM)
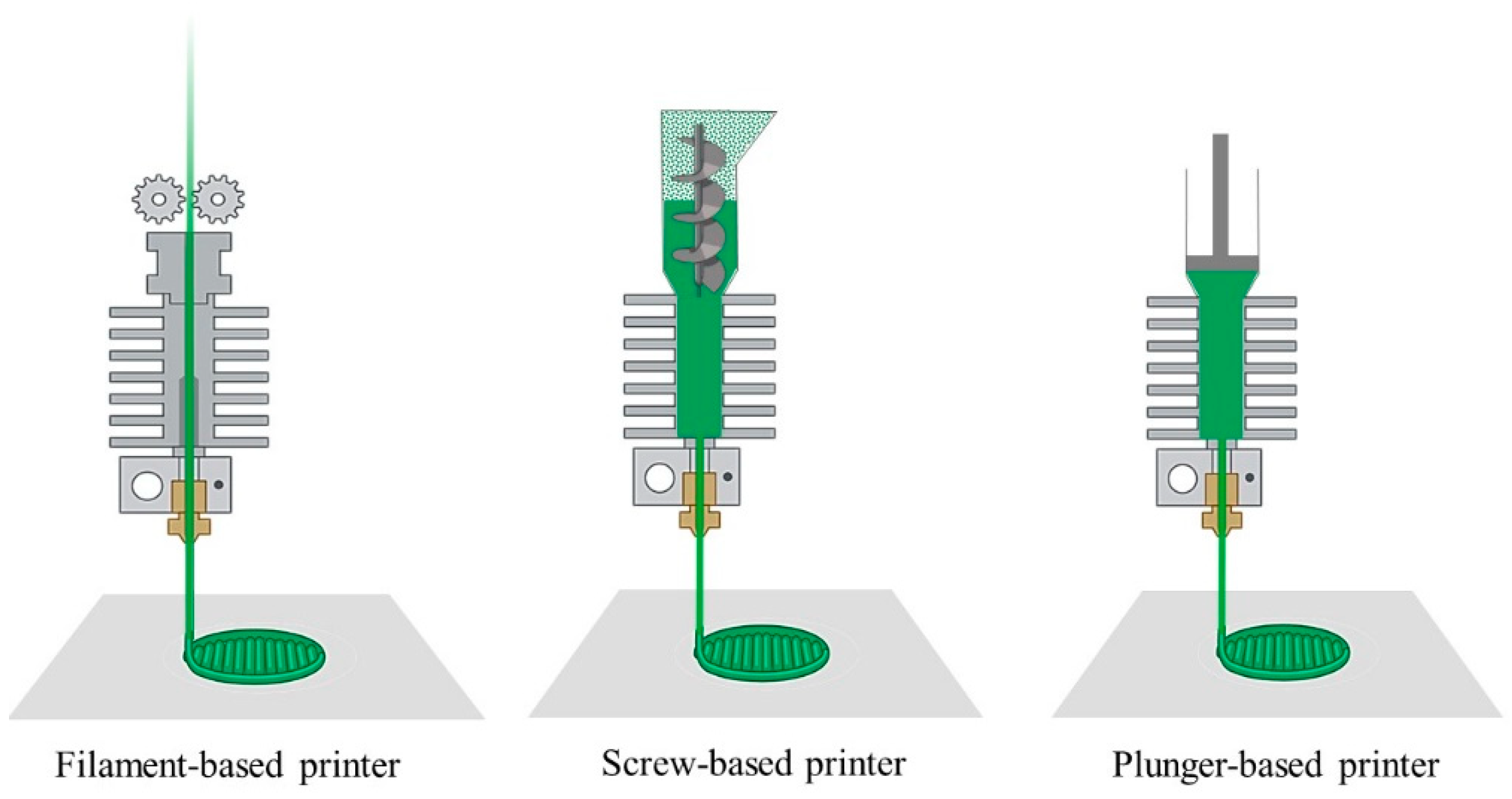
2.4. Extrusion-Based AM
2.4.1. Direct Ink Writing (DIW)
- -
- High solid loading of the ink/paste formulation: Using a high solid content, forming a network of the extruded material happens fast [124]. However, only nozzles with a diameter of approximately 500 μm are applicable to avoid clogging of the nozzle.
- -
- Addition of polymeric binder: Organic binders such as polyvinyl butyral (PVB) or polyethylene glycol (PEG) can be added to the ceramic phase (maximum of 23 wt%) [125]. In this way, the rheology of the ink can be justified without manipulating parameters such as pH.
- -
- Reversible gel transformation: In this approach, ink is extruded in a non-wetting bath, often oil [126]. To achieve a reversible gelling effect, ceramic suspension is flocculated in a controlled manner by introducing polyelectrolytes, manipulating pH or ionic strength of the solvent. Addition of a gelling aid, like inverse thermoreversible gels, can be used alternatively.
- -
- Use of preceramic polymers: Polysiloxanes and polysilsesquioxanes can be incorporated to control the rheology of the ink PCPs and offer a dual role [123]. The PCPs have the potential to serve as reactive binder additives, since they result in SiO2 and SiOC ceramics after pyrolysis in air or an inert atmosphere, respectively. When active fillers are added to the yielded SiO2, various silicate ceramics can be produced.
2.4.2. Fused Deposition Modeling (FDM)
3. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bernardo, E.; Fiocco, L.; Parcianello, G.; Storti, E.; Colombo, P. Advanced Ceramics from Preceramic Polymers Modified at the Nano-Scale: A Review. Materials 2014, 7, 1927–1956. [Google Scholar] [CrossRef]
- Chaudhary, R.P.; Parameswaran, C.; Idrees, M.; Rasaki, A.S.; Liu, C.; Chen, Z.; Colombo, P. Additive manufacturing of polymer-derived ceramics: Materials, technologies, properties and potential applications. Prog. Mater. Sci. 2022, 128, 100969. [Google Scholar]
- Yajima, S.; Okamura, K.; Hayashi, J. Structural analysis in continuous silicon carbide fiber of high tensile strength. Chem. Lett. 1975, 4, 1209–1212. [Google Scholar] [CrossRef]
- Monthioux, M.; Delverdier, O. Thermal behavior of (organosilicon) polymer-derived ceramics. V Main Facts Trends. J. Eur. Ceram. Soc. 1996, 16, 721–737. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, M.; Zhu, Y. Organosilicon polymer-derived ceramics: An overview. J. Adv. Ceram. 2019, 8, 457–478. [Google Scholar] [CrossRef]
- Wen, Q.; Qu, F.; Yu, Z.; Graczyk-Zajac, M.; Xiong, X.; Riedel, R. Si-based polymer-derived ceramics for energy conversion and storage. J. Adv. Ceram. 2022, 11, 197–246. [Google Scholar] [CrossRef]
- Pivin, J.; Colombo, P.; Martucci, A.; Soraru, G.; Pippel, E.; Sendova-Vassileva, M. Ion beam induced conversion of Si-based polymers and gels layers into ceramics coatings. J. Sol-Gel Sci. Technol. 2003, 26, 251–255. [Google Scholar] [CrossRef]
- El Chawich, G.; El Hayek, J.; Rouessac, V.; Cot, D.; Rebière, B.; Habchi, R.; Garay, H.; Bechelany, M.; Zakhour, M.; Miele, P.; et al. Design and Manufacturing of Si-Based Non-Oxide Cellular Ceramic Structures through Indirect 3D Printing. Materials 2022, 15, 471. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.L.; Parvulescu, M.J.; Patel, T.A.; Mogilevsky, P.; Key, T.S.; Thompson, C.M.; Dickerson, M.B. Bioinspired cross-linking of preceramic polymers via metal ion coordination bonding. J. Eur. Ceram. Soc. 2021, 41, 6366–6376. [Google Scholar] [CrossRef]
- Narisawa, M. Silicone Resin Applications for Ceramic Precursors and Composites. Materials 2010, 3, 3518–3536. [Google Scholar] [CrossRef]
- Koo, J.; Miller, M.; Weispfenning, J.; Blackmon, C. Silicone polymer composites for thermal protection system: Fiber reinforcements and microstructures. J. Compos. Mater. 2010, 45, 1363–1380. [Google Scholar] [CrossRef]
- Jones, R.G.; Holder, S.J. High-yield controlled syntheses of polysilanes by the Wurtz-type reductive coupling reaction. Polym. Int. 2005, 55, 711–718. [Google Scholar] [CrossRef]
- Okamura, K. Ceramic fibres from polymer precursors. Composites 1987, 18, 107–120. [Google Scholar] [CrossRef]
- Yajima, S.; Hasegawa, Y.; Okamura, K.; Matsuzawa, T. Development of high tensile strength silicon carbide fibre using an organosilicon polymer precursor. Nature 1978, 273, 525–527. [Google Scholar] [CrossRef]
- Kleebe, H.J.; Störmer, H.; Trassl, S.; Ziegler, G. Thermal stability of SiCN ceramics studied by spectroscopy and electron microscopy. Appl. Organomet. Chem. 2001, 15, 858–866. [Google Scholar] [CrossRef]
- Hörz, M.; Zern, A.; Berger, F.; Haug, J.; Müller, K.; Aldinger, F.; Weinmann, M. Novel polysilazanes as precursors for silicon nitride/silicon carbide composites without “free” carbon. J. Eur. Ceram. Soc. 2005, 25, 99–110. [Google Scholar] [CrossRef]
- Günthner, M.; Kraus, T.; Dierdorf, A.; Decker, D.; Krenkel, W.; Motz, G. Advanced coatings on the basis of Si(C)N precursors for protection of steel against oxidation. J. Eur. Ceram. Soc. 2009, 29, 2061–2068. [Google Scholar] [CrossRef]
- Abe, Y.; Gunji, T. Oligo-and polysiloxanes. Prog. Polym. Sci. 2004, 29, 149–182. [Google Scholar] [CrossRef]
- Fan, L.; Wang, X.; Wu, D. Polyhedral oligomeric silsesquioxanes (POSS)-based hybrid materials: Molecular design, solution self-assembly and biomedical applications. Chin. J. Chem. 2021, 39, 757–774. [Google Scholar] [CrossRef]
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Packirisamy, S.; Sreejith, K.; Devapal, D.; Swaminathan, B. Polymer-derived ceramics and their space applications. In Handbook of Advanced Ceramics and Composites: Defense, Security, Aerospace and Energy Applications; Springer: Cham, Switzerland, 2020; pp. 975–1080. [Google Scholar]
- Stabler, C.; Ionescu, E.; Graczyk-Zajac, M.; Gonzalo-Juan, I.; Riedel, R. Silicon oxycarbide glasses and glass-ceramics: “All-Rounder” materials for advanced structural and functional applications. J. Am. Ceram. Soc. 2018, 101, 4817–4856. [Google Scholar] [CrossRef]
- Soraru, G.D.; Dallapiccola, E.; D’Andrea, G. Mechanical Characterization of Sol-Gel-Derived Silicon Oxycarbide Glasses. J. Am. Ceram. Soc. 1996, 79, 2074–2080. [Google Scholar] [CrossRef]
- Melcher, R.; Cromme, P.; Scheffler, M.; Greil, P. Greil, Centrifugal casting of thin-walled ceramic tubes from preceramic polymers. J. Am. Ceram. Soc. 2003, 86, 1211–1213. [Google Scholar] [CrossRef]
- Colombo, P.; Bernardo, E.; Parcianello, G. Multifunctional advanced ceramics from preceramic polymers and nano-sized active fillers. J. Eur. Ceram. Soc. 2013, 33, 453–469. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-Derived Ceramics: 40 Years of Research and Innovation in Advanced Ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Rocha, R.M.D.; Greil, P.; Bressiani, J.C.; Bressiani, A.H.D.A. Complex-shaped ceramic composites obtained by machining compact polymer-filler mixtures. Mater. Res. 2005, 8, 191–196. [Google Scholar] [CrossRef]
- Seyferth, D. Preceramic Polymers: Past, Present and Future; Massachusetts Institute of Technology, Cambridge Department of Chemistry: Cambridge, MA, USA, 1992. [Google Scholar]
- Lewinsohn, C.A.; Colombo, P.; Reimanis, I.; Ünal, Ö. Ünal, Stresses occurring during joining of ceramics using preceramic polymers. J. Am. Ceram. Soc. 2004, 84, 2240–2244. [Google Scholar] [CrossRef]
- Greil, P. Polymer derived engineering ceramics. Adv. Eng. Mater. 2000, 2, 339–348. [Google Scholar] [CrossRef]
- Greil, P.; Seibold, M. Modelling of dimensional changes during polymer-ceramic conversion for bulk component fabrication. J. Mater. Sci. 1992, 27, 1053–1060. [Google Scholar] [CrossRef]
- Greil, P. Active-filler-controlled pyrolysis of preceramic polymers. J. Am. Ceram. Soc. 1995, 78, 835–848. [Google Scholar] [CrossRef]
- Barroso, G.; Li, Q.; Bordia, R.K.; Motz, G. Polymeric and ceramic silicon-based coatings—A review. J. Mater. Chem. A 2019, 7, 1936–1963. [Google Scholar] [CrossRef]
- Colombo, P.; Gambaryan-Roisman, T.; Scheffler, M.; Buhler, P.; Greil, P. Conductive ceramic foams from preceramic polymers. J. Am. Ceram. Soc. 2001, 84, 2265–2268. [Google Scholar] [CrossRef]
- Mirkhalaf, M.; Sarvestani, H.Y.; Yang, Q.; Jakubinek, M.B.; Ashrafi, B. A comparative study of nano-fillers to improve toughness and modulus of polymer-derived ceramics. Sci. Rep. 2021, 11, 6951. [Google Scholar] [CrossRef]
- Suttor, D.; Kleebe, H.J.; Ziegler, G. Formation of mullite from filled siloxanes. J. Am. Ceram. Soc. 1997, 80, 2541–2548. [Google Scholar] [CrossRef]
- Bernardo, E.; Colombo, P.; Pippel, E.; Woltersdorf, J. Woltersdorf, Novel mullite synthesis based on alumina nanoparticles and a preceramic polymer. J. Am. Ceram. Soc. 2006, 89, 1577–1583. [Google Scholar] [CrossRef]
- Gorjan, L.; Tonello, R.; Sebastian, T.; Colombo, P.; Clemens, F. Fused deposition modeling of mullite structures from a preceramic polymer and γ-alumina. J. Eur. Ceram. Soc. 2019, 39, 2463–2471. [Google Scholar] [CrossRef]
- Lehman, R.L. Ceramic Matrix Fiber Composites, Treatise on Materials Science & Technology; Elsevier: Amsterdam, The Netherlands, 1989; pp. 229–291. [Google Scholar]
- Ionescu, E.; Francis, A.; Riedel, R. Dispersion assessment and studies on AC percolative conductivity in polymer-derived Si–C–N/CNT ceramic nanocomposites. J. Mater. Sci. 2009, 44, 2055–2062. [Google Scholar] [CrossRef]
- Cordelair, J.; Greil, P. Electrical characterization of polymethylsiloxane/MoSi2-derived composite ceramics. J. Am. Ceram. Soc. 2004, 84, 2256–2259. [Google Scholar] [CrossRef]
- Windsheimer, H.; Travitzky, N.; Hofenauer, A.; Greil, P. Laminated object manufacturing of preceramic-paper-derived Si? SiC composites. Adv. Mater. 2007, 19, 4515–4519. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, H.D.; Park, C.B. Processing of microcellular mullite. J. Am. Ceram. Soc. 2005, 88, 3311–3315. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, Z.; Yu, J.; Ren, Z. Preparation of silica ceramic cores by the preceramic pyrolysis technology using silicone resin as precursor and binder. Mater. Chem. Phys. 2019, 223, 676–682. [Google Scholar] [CrossRef]
- Schmidt, J.; Altun, A.A.; Schwentenwein, M.; Colombo, P. Complex mullite structures fabricated via digital light processing of a preceramic polysiloxane with active alumina fillers. J. Eur. Ceram. Soc. 2018, 39, 1336–1343. [Google Scholar] [CrossRef]
- Schmidt, J.; Colombo, P. Digital light processing of ceramic components from polysiloxanes. J. Eur. Ceram. Soc. 2018, 38, 57–66. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, Z.; Zhao, Z.; Yu, J.; Ren, Z.; Zhang, G.; Yu, G. Microstructure and properties of SiO2-based ceramic cores with ball-shaped powders by the preceramic polymer technique in N2 atmosphere. Mater. Chem. Phys. 2020, 243, 122609. [Google Scholar] [CrossRef]
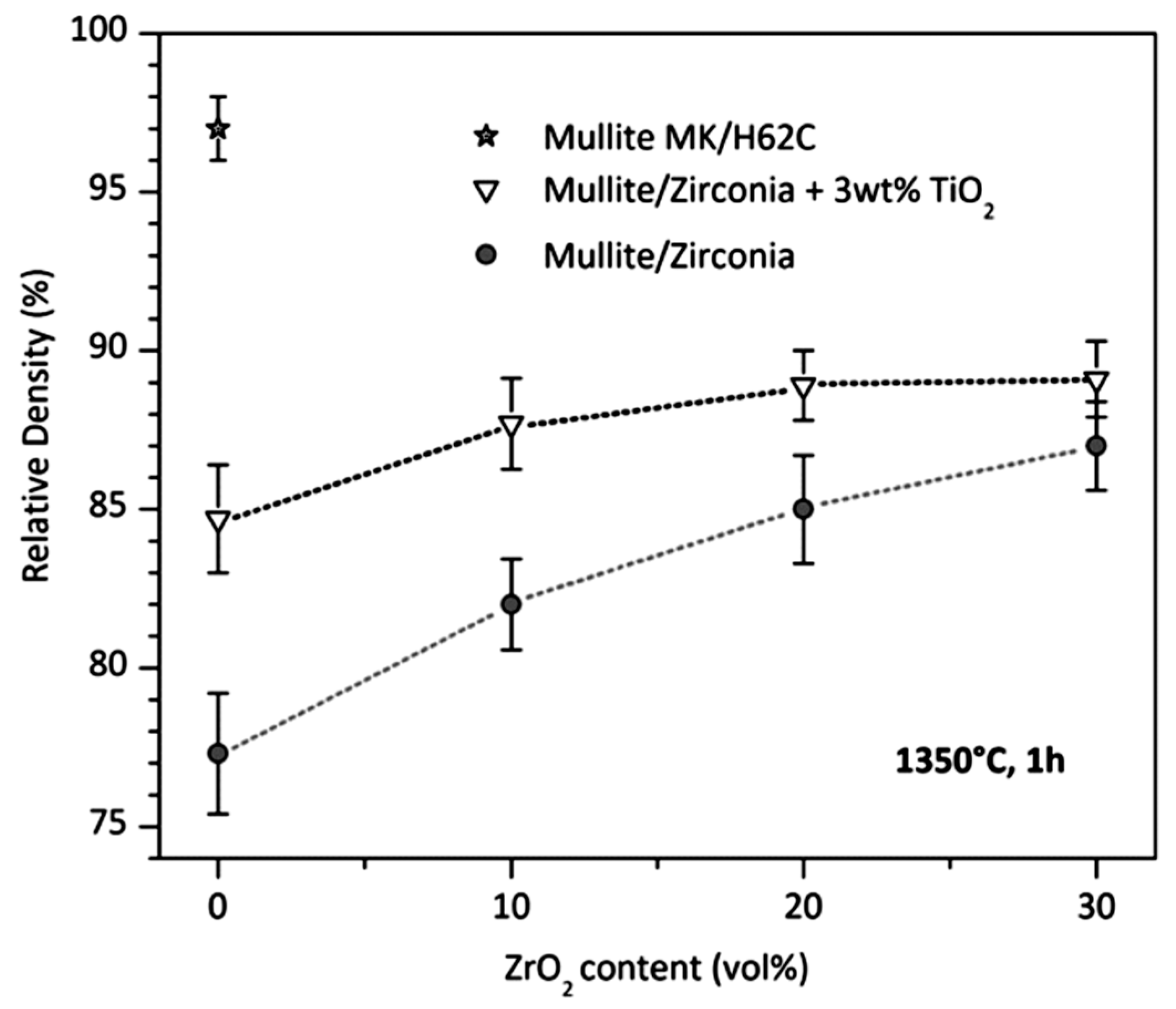
- Parcianello, G.; Bernardo, E.; Colombo, P. Mullite/zirconia nanocomposites from a preceramic polymer and nanosized fillers. J. Am. Ceram. Soc. 2011, 94, 1357–1362. [Google Scholar] [CrossRef]
- Bernardo, E.; Tomasella, E.; Colombo, P. Development of multiphase bioceramics from a filler-containing preceramic polymer. Ceram. Int. 2009, 35, 1415–1421. [Google Scholar] [CrossRef]
- Bernardo, E.; Colombo, P.; Cacciotti, I.; Bianco, A.; Bedini, R.; Pecci, R.; Pardun, K.; Treccani, L.; Rezwan, K. Porous wollastonite–hydroxyapatite bioceramics from a preceramic polymer and micro- or nano-sized fillers. J. Eur. Ceram. Soc. 2012, 32, 399–408. [Google Scholar] [CrossRef]
- Bernardo, E.; Colombo, P.; Dainese, E.; Lucchetta, G.; Bariani, P.F. Novel 3D wollastonite-based scaffolds from preceramic polymers containing micro-and nano-sized reactive particles. Adv. Eng. Mater. 2012, 14, 269–274. [Google Scholar] [CrossRef]
- Bernardo, E.; Parcianello, G.; Colombo, P. Novel synthesis and applications of yttrium silicates from a silicone resin containing oxide nano-particle fillers. Ceram. Int. 2012, 38, 5469–5474. [Google Scholar] [CrossRef]
- Parcianello, G.; Bernardo, E.; Colombo, P. Low temperature synthesis of zircon from silicone resins and oxide nano-sized particles. J. Eur. Ceram. Soc. 2012, 32, 2819–2824. [Google Scholar] [CrossRef]
- Bernardo, E.; Fiocco, L.; Giffin, G.A.; Di Noto, V.; Colombo, P. Microstructure Development and Dielectric Characterization of Forsterite-Based Ceramics from Silicone Resins and Oxide Fillers. Adv. Eng. Mater. 2014, 16, 806–813. [Google Scholar] [CrossRef]
- Parcianello, G.; Bernardo, E.; Colombo, P. Cordierite ceramics from silicone resins containing nano-sized oxide particle fillers. Ceram. Int. 2013, 39, 8893–8899. [Google Scholar] [CrossRef]
- Bernardo, E.; Fiocco, L.; Prnová, A.; Klement, R.; Galusek, D. Gehlenite: Eu3+ phosphors from a silicone resin and nano-sized fillers. Opt. Mater. 2014, 36, 1243–1249. [Google Scholar] [CrossRef]
- Bernardo, E.; Carlotti, J.-F.; Dias, P.M.; Fiocco, L.; Colombo, P.; Treccani, L.; Hess, U.; Rezwan, K. Novel akermanite-based bioceramics from preceramic polymers and oxide fillers. Ceram. Int. 2014, 40, 1029–1035. [Google Scholar] [CrossRef]
- Bernardo, E.; Colombo, P.; Hampshire, S. SiAlON-based ceramics from filled preceramic polymers. J. Am. Ceram. Soc. 2006, 89, 3839–3842. [Google Scholar] [CrossRef]
- Bernardo, E.; Colombo, P.; Hampshire, S. Advanced ceramics from a preceramic polymer and nano-fillers. J. Eur. Ceram. Soc. 2009, 29, 843–849. [Google Scholar] [CrossRef]
- Bernardo, E.; Parcianello, G.; Colombo, P.; Adair, J.; Barnes, A.; Hellmann, J.; Jones, B.; Kruise, J.; Swab, J. SiAlON ceramics from preceramic polymers and nano-sized fillers: Application in ceramic joining. J. Eur. Ceram. Soc. 2012, 32, 1329–1335. [Google Scholar] [CrossRef]
- Zocca, A.; Elsayed, H.; Bernardo, E.; Gomes, C.M.; A Lopez-Heredia, M.; Knabe, C.; Colombo, P.; Günster, J. 3D-printed silicate porous bioceramics using a non-sacrificial preceramic polymer binder. Biofabrication 2015, 7, 025008. [Google Scholar] [CrossRef]
- Elsayed, H.; Zocca, A.; Bernardo, E.; Gomes, C.M.; Günster, J.; Colombo, P. Development of bioactive silicate-based glass-ceramics from preceramic polymer and fillers. J. Eur. Ceram. Soc. 2015, 35, 731–739. [Google Scholar] [CrossRef]
- Fiocco, L.; Elsayed, H.; Ferroni, L.; Gardin, C.; Zavan, B.; Bernardo, E. Bioactive Wollastonite-Diopside Foams from Preceramic Polymers and Reactive Oxide Fillers. Materials 2015, 8, 2480–2494. [Google Scholar] [CrossRef]
- Choudhary, A.; Sahoo, S.P.; Behera, S.K. Lithium orthosilicate ceramics with preceramic polymer as silica source. Ceram. Int. 2017, 43, 7951–7957. [Google Scholar] [CrossRef]
- Elsayed, H.; Rebesan, P.; Crovace, M.C.; Zanotto, E.D.; Colombo, P.; Bernardo, E. Biosilicate® scaffolds produced by 3D-printing and direct foaming using preceramic polymers. J. Am. Ceram. Soc. 2018, 102, 1010–1020. [Google Scholar] [CrossRef]
- Michalet, T.; Parlier, M.; Beclin, F.; Duclos, R.; Crampon, J. Elaboration of low shrinkage mullite by active filler controlled pyrolysis of siloxanes. J. Eur. Ceram. Soc. 2002, 22, 143–152. [Google Scholar] [CrossRef]
- Bernardo, E.; Colombo, P.; Manias, E. SiOC glass modified by montmorillonite clay. Ceram. Int. 2006, 32, 679–686. [Google Scholar] [CrossRef]
- Hong, S.-H.; Cermignani, W.; Messing, G. Anisotropic grain growth in seeded and B2O3-doped diphasic mullite gels. J. Eur. Ceram. Soc. 1996, 16, 133–141. [Google Scholar] [CrossRef]
- Pyzik, A.J.; Todd, C.S.; Han, C. Formation mechanism and microstructure development in acicular mullite ceramics fabricated by controlled decomposition of fluorotopaz. J. Eur. Ceram. Soc. 2008, 28, 383–391. [Google Scholar] [CrossRef]
- Riedel, R.; Toma, L.; Fasel, C.; Miehe, G. Polymer-derived mullite–SiC-based nanocomposites. J. Eur. Ceram. Soc. 2009, 29, 3079–3090. [Google Scholar] [CrossRef]
- Toma, L.; Fasel, C.; Lauterbach, S.; Kleebe, H.-J.; Riedel, R. Influence of nano-aluminum filler on the microstructure of SiOC ceramics. J. Eur. Ceram. Soc. 2011, 31, 1779–1789. [Google Scholar] [CrossRef]
- Mazzucato, E.; Gualtieri, A. Wollastonite polytypes in the CaO-SiO2 system. Part I. Cryst. Kinet. Phys. Chem. Miner. 2000, 27, 565–574. [Google Scholar] [CrossRef]
- Ni, S.; Chou, L.; Chang, J. Preparation and characterization of forsterite (Mg2SiO4) bioceramics. Ceram. Int. 2007, 33, 83–88. [Google Scholar] [CrossRef]
- Song, K.; Chen, X. Phase evolution and microwave dielectric characteristics of Ti-substituted Mg2SiO4 forsterite ceramics. Mater. Lett. 2008, 62, 520–522. [Google Scholar] [CrossRef]
- Emadi, R.; Tavangarian, F.; Esfahani, S.I.R.; Sheikhhosseini, A.; Kharaziha, M. Nanostructured forsterite coating strengthens porous hydroxyapatite for bone tissue engineering. J. Am. Ceram. Soc. 2010, 93, 2679–2683. [Google Scholar] [CrossRef]
- Ramesh, S.; Yaghoubi, A.; Lee, K.S.; Chin, K.C.; Purbolaksono, J.; Hamdi, M.; Hassan, M. Nanocrystalline forsterite for biomedical applications: Synthesis, microstructure and mechanical properties. J. Mech. Behav. Biomed. Mater. 2013, 25, 63–69. [Google Scholar] [CrossRef]
- Sebdani, M.M.; Fathi, M. Novel hydroxyapatite–forsterite–bioglass nanocomposite coatings with improved mechanical prop-erties. J. Alloys Compd. 2011, 509, 2273–2276. [Google Scholar] [CrossRef]
- Ohsato, H.; Tsunooka, T.; Sugiyama, T.; Kakimoto, K.-I.; Ogawa, H. Forsterite ceramics for millimeterwave dielectrics. J. Electroceramics 2006, 17, 445–450. [Google Scholar] [CrossRef]
- Sano, S.; Saito, N.; Matsuda, S.I.; Ohashi, N.; Haneda, H.; Arita, Y.; Takemoto, M. Synthesis of high density and transparent forsterite ceramics using nano-sized precursors and their dielectric properties. J. Am. Ceram. Soc. 2006, 89, 568–574. [Google Scholar] [CrossRef]
- Song, K.X.; Chen, X.M.; Fan, X.C. Effects of Mg/Si ratio on microwave dielectric characteristics of forsterite ceramics. J. Am. Ceram. Soc. 2007, 90, 1808–1811. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, Y.; Wang, J.; Li, M. γ-Y2Si2O7, a machinable silicate ceramic: Mechanical properties and machinability. J. Am. Ceram. Soc. 2007, 90, 2535–2541. [Google Scholar] [CrossRef]
- Dıaz, M.; Garcia-Cano, I.; Mello-Castanho, S.; Moya, J.; Rodrıguez, M. Synthesis of nanocrystalline yttrium disilicate powder by a sol–gel method. J. Non-Cryst. Solids 2001, 289, 151–154. [Google Scholar] [CrossRef]
- Moya, J.; Díaz, M.; Serna, C.; Mello-Castanho, S. Formation of nanocrystalline yttrium disilicate powder by an oxalate gel method. J. Eur. Ceram. Soc. 1998, 18, 1381–1384. [Google Scholar] [CrossRef]
- Becerro, A.I.; Naranjo, M.; Perdigon, A.C.; Trillo, J.M. Hydrothermal chemistry of silicates: Low-temperature synthesis of y-yttrium disilicate. ChemInform 2003, 86, 1592–1594. [Google Scholar] [CrossRef]
- Elsayed, H.; Secco, M.; Zorzi, F.; Schuhladen, K.; Detsch, R.; Boccaccini, A.R.; Bernardo, E. Highly porous polymer-derived bioceramics based on a complex hardystonite solid solution. Materials 2019, 12, 3970. [Google Scholar] [CrossRef] [PubMed]
- Taki, T.; Maeda, S.; Okamura, K.; Sato, M.; Matsuzawa, T. Oxidation curing mechanism of polycarbosilane fibres by solid-state 29 Si high-resolution NMR. J. Mater. Sci. Lett. 1987, 6, 826–828. [Google Scholar] [CrossRef]
- Ly, H.; Taylor, R.; Day, R.; Heatley, F. Conversion of polycarbosilane (PCS) to SiC-based ceramic Part 1. Characterisation of PCS and curing products. J. Mater. Sci. 2001, 36, 4037–4043. [Google Scholar] [CrossRef]
- Janakiraman, N.; Aldinger, F. Fabrication and characterization of fully dense Si–C–N ceramics from a poly(ureamethylvinyl)silazane precursor. J. Eur. Ceram. Soc. 2009, 29, 163–173. [Google Scholar] [CrossRef]
- Schulz, M.; Börner, M.; Göttert, J.; Hanemann, T.; Haußelt, J.; Motz, G. Cross linking behavior of preceramic polymers effected by UV-and synchrotron radiation. Adv. Eng. Mater. 2004, 6, 676–680. [Google Scholar] [CrossRef]
- Eick, B.M.; Youngblood, J.P. SiC nanofibers by pyrolysis of electrospun preceramic polymers. J. Mater. Sci. 2009, 44, 160–165. [Google Scholar] [CrossRef]
- Danko, G.A.; Silberglitt, R.; Colombo, P.; Pippel, E.; Woltersdorf, J. Comparison of microwave hybrid and conventional heating of preceramic polymers to form silicon carbide and silicon oxycarbide ceramics. J. Am. Ceram. Soc. 2004, 83, 1617–1625. [Google Scholar] [CrossRef]
- Higginbotham, A.L.; Moloney, P.G.; Waid, M.C.; Duque, J.G.; Kittrell, C.; Schmidt, H.K.; Stephenson, J.J.; Arepalli, S.; Yowell, L.L.; Tour, J.M. Carbon nanotube composite curing through absorption of microwave radiation. Compos. Sci. Technol. 2008, 68, 3087–3092. [Google Scholar] [CrossRef]
- Banik, S.; Iqbal, I.; Nath, R.; Bora, L.; Singh, B.; Mandal, N.; Sankar, M. State of the art on zirconia toughened alumina cutting tools. Mater. Today Proc. 2019, 18, 2632–2641. [Google Scholar] [CrossRef]
- Greil, P. Near net shape manufacturing of polymer derived ceramics. J. Eur. Ceram. Soc. 1998, 18, 1905–1914. [Google Scholar] [CrossRef]
- Zhou, S.; Mei, H.; Chang, P.; Lu, M.; Cheng, L. Molecule ediTable 3D printed polymer-derived ceramics. Coord. Chem. Rev. 2020, 422, 213486. [Google Scholar] [CrossRef]
- Hull, C.W.; Spence, S.T.; Albert, D.J.; Smalley, D.R.; Harlow, R.A.; Steinbaugh, P.; Tarnoff, H.L.; Nguyen, H.D.; Lewis, C.W.; Vorgitch, T.J. Methods and Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent US4999143A, 12 March 1991. [Google Scholar]
- Nohut, S.; Schwentenwein, M. Vat photopolymerization additive manufacturing of functionally graded materials: A review. J. Manuf. Mater. Process. 2022, 6, 17. [Google Scholar] [CrossRef]
- Al Rashid, A.; Ahmed, W.; Khalid, M.Y.; Koç, M. Vat photopolymerization of polymers and polymer composites: Processes and applications. Addit. Manuf. 2021, 47, 102279. [Google Scholar] [CrossRef]
- Huang, J.; Qin, Q.; Wang, J. A review of stereolithography: Processes and systems. Processes 2020, 8, 1138. [Google Scholar] [CrossRef]
- Tian, X.; Li, D.; Chen, Z.; Zhou, W. Study on the fabrication accuracy of ceramic parts by direct stereolithography: Ceramic parts can be prepared using stereolithography by building composite parts from ceramic powder-loaded resins, followed by simultaneous polymer pyrolysis and ceramic sintering. This paper describes a systematic study into the influence of several parameters on the accuracy of such parts. Virtual Phys. Prototyp. 2012, 7, 195–202. [Google Scholar]
- Ożóg, P.; Elsayed, H.; Grigolato, L.; Savio, G.; Kraxner, J.; Galusek, D.; Bernardo, E. Engineering of silicone-based blends for the masked stereolithography of biosilicate/carbon composite scaffolds. J. Eur. Ceram. Soc. 2022, 42, 6192–6198. [Google Scholar] [CrossRef]
- Rosado, E.; Moreno, R. Mullite-silica scaffolds obtained by stereolithography and reaction sintering. Open Ceram. 2023, 14, 100361. [Google Scholar] [CrossRef]
- Eckel, Z.C.; Zhou, C.; Martin, J.H.; Jacobsen, A.J.; Carter, W.B.; Schaedler, T.A. Additive manufacturing of polymer-derived ceramics. Science 2016, 351, 58–62. [Google Scholar] [CrossRef]
- Arif, K.M.; Murakami, T. Slant beam rotation UV scanning to eliminate stair-steps in stereolithography fabrications. Int. J. Adv. Manuf. Technol. 2008, 41, 527–537. [Google Scholar] [CrossRef]
- Hornbeck, L.J. Digital light processing update: Status and future applications. In Projection Displays V; SPIE: Bellingham, WA, USA, 1999; pp. 158–170. [Google Scholar]
- Dasan, A.; Elsayed, H.; Kraxner, J.; Galusek, D.; Colombo, P.; Bernardo, E. Engineering of silicone-based mixtures for the digital light processing of Åkermanite scaffolds. J. Eur. Ceram. Soc. 2019, 40, 2566–2572. [Google Scholar] [CrossRef]
- No, Y.J.; Li, J.J.; Zreiqat, H. Doped calcium silicate ceramics: A new class of candidates for synthetic bone substitutes. Materials 2017, 10, 153. [Google Scholar] [CrossRef]
- Wang, J.; Dai, X.; Peng, Y.; Liu, M.; Lu, F.; Yang, X.; Gou, Z.; Ye, J. Digital light processing strength-strong ultra-thin bioceramic scaffolds for challengeable orbital bone regeneration and repair in situ. Appl. Mater. Today 2020, 22, 100889. [Google Scholar] [CrossRef]
- He, Z.; Jiao, C.; Zhang, H.; Xie, D.; Ge, M.; Yang, Y.; Wu, G.; Liang, H.; Shen, L.; Wang, C. Fabrication of a zirconia/calcium silicate composite scaffold based on digital light processing. Ceram. Int. 2022, 48, 25923–25932. [Google Scholar] [CrossRef]
- Maruo, S.; Nakamura, O.; Kawata, S. Three-dimensional microfabrication with two-photon-absorbed photopolymerization. Opt. Lett. 1997, 22, 132–134. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, S.; Pandit, A.; Heise, A.; Kellett, A. Two-Photon Polymerization: Fundamentals, Materials, and Chemical Modification Strategies. Adv. Sci. 2023, 10, 2204072. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Schmidt, F.; Hanaor, D.; Kamm, P.H.; Li, S.; Gurlo, A. Additive manufacturing of ceramics from preceramic polymers: A versatile stereolithographic approach assisted by thiol-ene click chemistry. Addit. Manuf. 2019, 27, 80–90. [Google Scholar] [CrossRef]
- Zanchetta, E.; Cattaldo, M.; Franchin, G.; Schwentenwein, M.; Homa, J.; Brusatin, G.; Colombo, P. Stereolithography of SiOC ceramic microcomponents. Adv. Mater. 2016, 28, 370–376. [Google Scholar] [CrossRef]
- Schmidt, J.; Brigo, L.; Gandin, A.; Schwentenwein, M.; Colombo, P.; Brusatin, G. Multiscale ceramic components from preceramic polymers by hybridization of vat polymerization-based technologies. Addit. Manuf. 2019, 30, 100913. [Google Scholar] [CrossRef]
- Mazzoli, A. Selective laser sintering in biomedical engineering. Med. Biol. Eng. Comput. 2013, 51, 245–256. [Google Scholar] [CrossRef]
- Friedel, T.; Travitzky, N.; Niebling, F.; Scheffler, M.; Greil, P. Fabrication of polymer derived ceramic parts by selective laser curing. J. Eur. Ceram. Soc. 2005, 25, 193–197. [Google Scholar] [CrossRef]
- Dermeik, B.; Travitzky, N. Laminated object manufacturing of ceramic-based materials. Adv. Eng. Mater. 2020, 22, 2000256. [Google Scholar] [CrossRef]
- Sieber, H.; Friedrich, H.; Zeschky, J.; Greil, P. Light weight ceramic composites from laminated paper structures. In Proceedings of the 24th Annual Conference on Composites, Advanced Ceramics, Materials, and Structures: B: Ceramic Engineering and Science Proceedings; Wiley Online Library: Hoboken, NJ, USA, 2000; pp. 129–134. [Google Scholar]
- Wolff, F.; Münstedt, H. Continuous direct melt foaming of a preceramic polymer using carbon dioxide: Extrusion device and first results. J. Mater. Sci. 2011, 46, 6162–6167. [Google Scholar] [CrossRef]
- Balan, C.; Riedel, R. Rheological investigations of a polymeric precursor for ceramic materials: Experiments and theoretical modeling. J. Optoelectron. Adv. Mater. 2006, 8, 561. [Google Scholar]
- Cesarano, J.; Segalman, R.; Calvert, P. Robocasting provides MOULDLESS fabrication from slurry deposition. Ceram. Ind. 1998, 148, 94–96. [Google Scholar]
- Saadi, M.A.S.R.; Maguire, A.; Pottackal, N.T.; Thakur, S.H.; Ikram, M.M.; Hart, A.J.; Ajayan, P.M.; Rahman, M.M. Direct ink writing: A 3D printing technology for diverse materials. Adv. Mater. 2022, 34, e2108855. [Google Scholar] [CrossRef]
- Zocca, A.; Franchin, G.; Elsayed, H.; Gioffredi, E.; Bernardo, E.; Colombo, P. Direct ink writing of a preceramic polymer and fillers to produce hardystonite (Ca2ZnSi2O7) bioceramic scaffolds. J. Am. Ceram. Soc. 2016, 99, 1960–1967. [Google Scholar] [CrossRef]
- Lewis, J.A.; Smay, J.E.; Stuecker, J.; Cesarano, J. Direct ink writing of three-dimensional ceramic structures. J. Am. Ceram. Soc. 2006, 89, 3599–3609. [Google Scholar] [CrossRef]
- de Sousa, F.C.G.; Evans, J.R. Sintered hydroxyapatite latticework for bone substitute. J. Am. Ceram. Soc. 2003, 86, 517–519. [Google Scholar] [CrossRef]
- Fu, Q.; Saiz, E.; Tomsia, A.P. Direct ink writing of highly porous and strong glass scaffolds for load-bearing bone defects repair and regeneration. Acta Biomater. 2011, 7, 3547–3554. [Google Scholar] [CrossRef]
- Wei, L.; Li, J.; Zhang, S.; Li, B.; Liu, Y.; Wang, F.; Dong, S. Fabrication of SiOC ceramic with cellular structure via UV-Assisted direct ink writing. Ceram. Int. 2019, 46, 3637–3643. [Google Scholar] [CrossRef]
- Fiocco, L.; Elsayed, H.; Badocco, D.; Pastore, P.; Bellucci, D.; Cannillo, V.; Detsch, R.; Boccaccini, A.R.; Bernardo, E. Direct ink writing of silica-bonded calcite scaffolds from preceramic polymers and fillers. Biofabrication 2017, 9, 025012. [Google Scholar] [CrossRef]
- Dasan, A.; Elsayed, H.; Kraxner, J.; Galusek, D.; Bernardo, E. Hierarchically porous 3D-printed akermanite scaffolds from silicones and engineered fillers. J. Eur. Ceram. Soc. 2019, 39, 4445–4449. [Google Scholar] [CrossRef]
- Elsayed, H.; Picicco, M.; Dasan, A.; Kraxner, J.; Galusek, D.; Bernardo, E. Glass powders and reactive silicone binder: Interactions and application to additive manufacturing of bioactive glass-ceramic scaffolds. Ceram. Int. 2019, 45, 13740–13746. [Google Scholar] [CrossRef]
- Huang, K.; Elsayed, H.; Franchin, G.; Colombo, P. 3D printing of polymer-derived SiOC with hierarchical and tunable porosity. Addit. Manuf. 2020, 36, 101549. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Z.; Li, J.; Li, X.; Cheng, L. From materials to devices using fused deposition modeling: A state-of-art review. Nanotechnol. Rev. 2020, 9, 1594–1609. [Google Scholar] [CrossRef]
- Clemens, F.; Hadian, A.; Fricke, F. Material extrusion-based additive manufacturing for ceramics using thermoplastic feedstocks, cfi Ceram. Forum Int. Bd. 2022, 99, 96–100. [Google Scholar]
- Gonzalez-Gutierrez, J.; Cano, S.; Schuschnigg, S.; Kukla, C.; Sapkota, J.; Holzer, C. Additive manufacturing of metallic and ceramic components by the material extrusion of highly-filled polymers: A review and future perspectives. Materials 2018, 11, 840. [Google Scholar] [CrossRef]
- Clemens, F.; Sarraf, F.; Borzì, A.; Neels, A.; Hadian, A. Material extrusion additive manufacturing of advanced ceramics: Towards the production of large components. J. Eur. Ceram. Soc. 2023, 43, 2752–2760. [Google Scholar] [CrossRef]
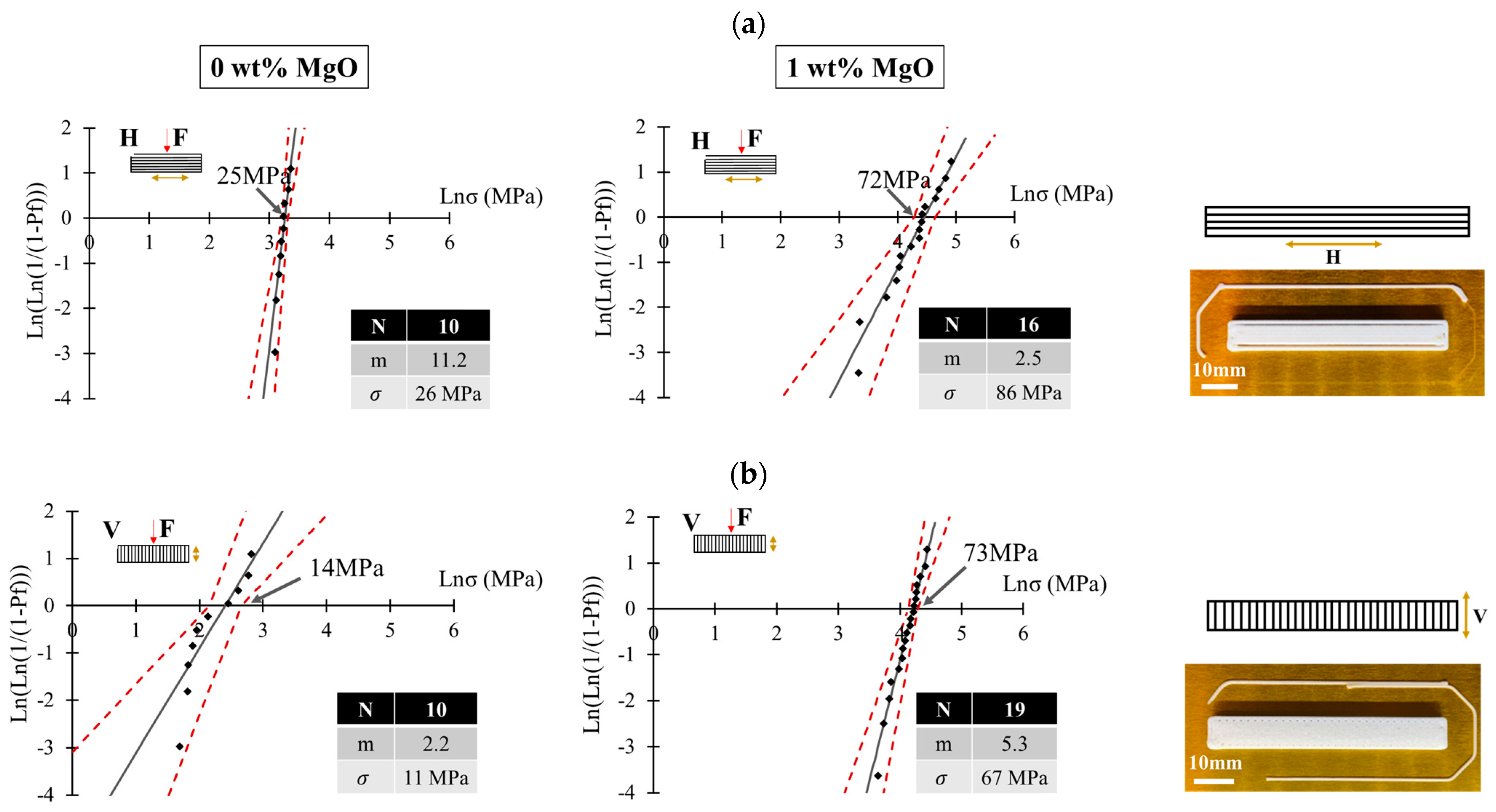
- Sarraf, F.; Abbatinali, E.; Gorjan, L.; Sebastian, T.; Colombo, P.; Churakov, S.V.; Clemens, F. Effect of MgO sintering additive on mullite structures manufactured by fused deposition modeling (FDM) technology. J. Eur. Ceram. Soc. 2021, 41, 6677–6686. [Google Scholar] [CrossRef]
- Sarraf, F.; Hadian, A.; Gfeller, F.; Churakov, S.V.; Clemens, F. Crosslinking and pyrolysis of a methyl-silsesquioxane: Effect of heating rate and atmosphere. Mater. Des. 2023; under review. [Google Scholar]
- Sarraf, F.; Hadian, A.; Churakov, S.V.; Clemens, F. EVA-PVA binder system for polymer derived mullite made by material extrusion based additive manufacturing. J. Eur. Ceram. Soc. 2023, 43, 530–541. [Google Scholar] [CrossRef]



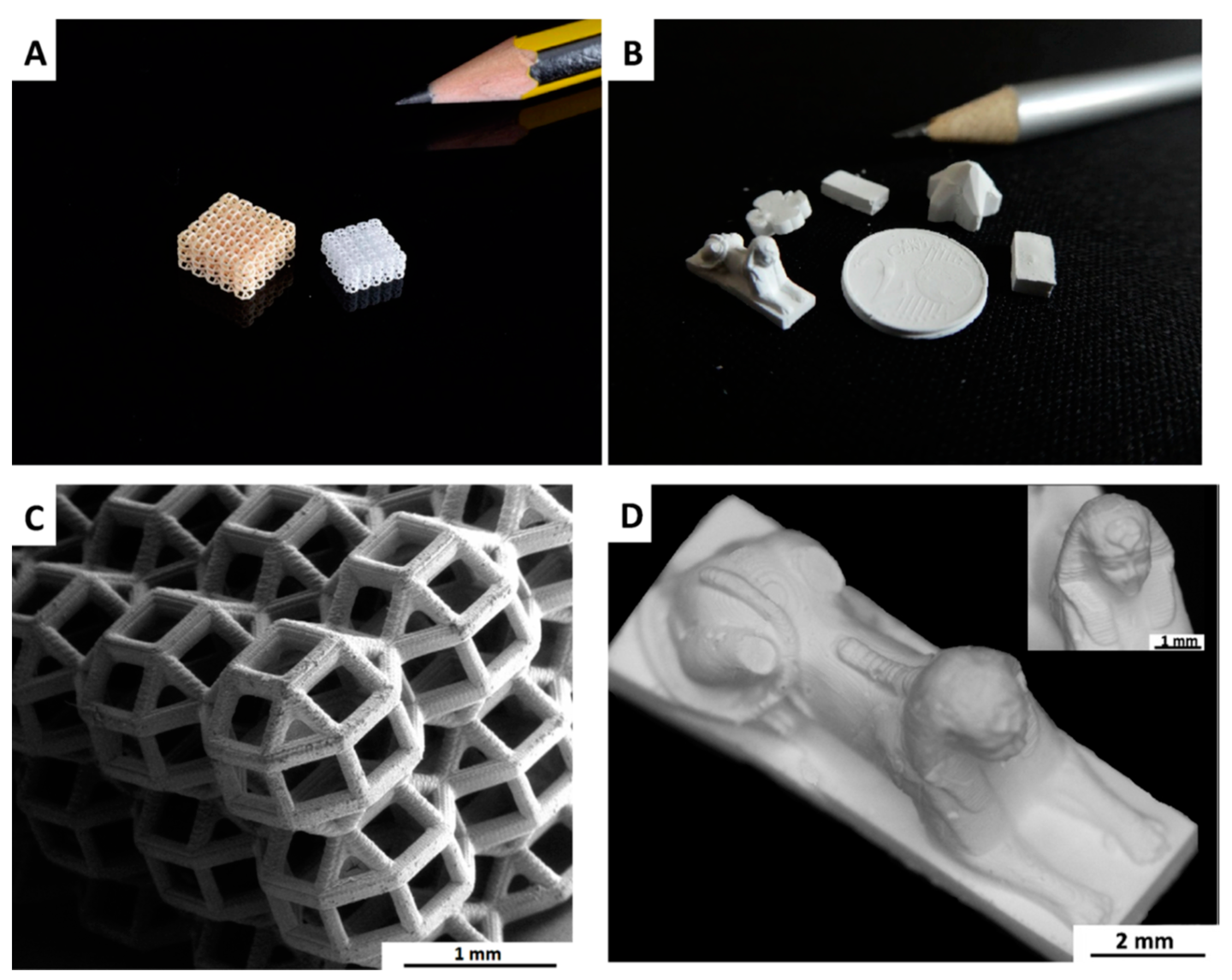
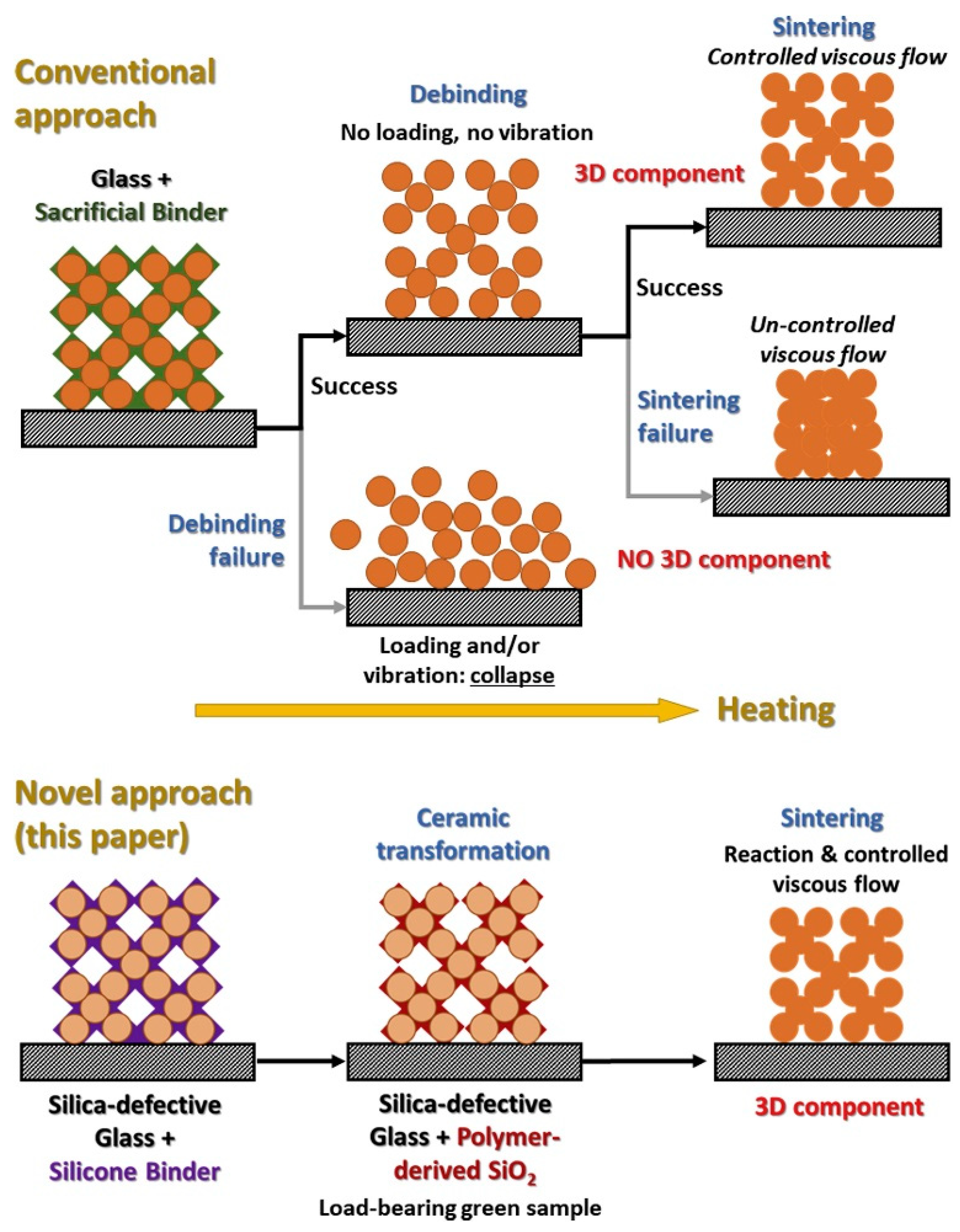

| Organosilicon Polymer | Backbone Structure | Synthesis Methods | Applications |
|---|---|---|---|
| Polysilane | -R1R2Si- | Wurtz-type coupling of halosilanes, anionic polymerization of masked disilenes, catalytic dehydrogenation of silanes, reduction of dichlorosilanes | Photoresists, photo conductors, semiconductors, and precursors for synthesis of polycarbosilane |
| Polycarbosilane | -R1R2Si-C- | Kumada rearrangement of polysilane, ring opening polymerization, dehydrocoupling reaction of trimethylsilane, hydrosilylation of vinylhydridosilanes, grignard coupling reaction of (chloromethyl)- triethoxysilane and vinylmagnesium bromide | Precursors for preparation of SiC, electric- or photoconductors, photoresist nonlinear optical materials |
| Polysilazane | -R1R2Si-N= | Ammonolysis reactions of chlorosilanes with ammonia or with aminolysis, ring opening polymerization of cyclic polysilanane | Precursors for preparation of Si3N4 or SiCN, barrier for heat exchanger or on steel against oxidation |
| Polysiloxane | -R1R2Si-O- | Ring-open polymerization of cyclic silaethers, polycondensation of linear silanes | Precursors for preparation of SiOC, medicine electronics, textile chemistry |
| Polysilylcarbodiimides | -R1R2Si-N=C=N- | Pyridine-catalyzed polycondensation reaction of chlorosilanes with bis(trimethylsilylcarbodiimide) | Precursors for preparation of SiCN |
| Polyborosilazane | -R1R2Si-N(R3R4B)- | Co-condensation reaction of boron trichloride, organodichlorosilanes, and hexamethyldisilazane | Precursors for preparation of SiCBN |
| Silicate Ceramic | Preceramic Polymer | Active Filler | Additive | Ref. |
|---|---|---|---|---|
| Mullite (3Al2O3·2SiO2) | MK | γ-Al2O3 | - | [1,37,38,43] |
| YR3370 | - | |||
| MK+H62C | - | |||
| H62C | Borax | |||
| ZTM (Zirconia Toughened Mullite) | MK | γ-Al2O3 | ZrO2 and TiO2 | [48] |
| Wollastonite (CaO·SiO2) | - | Ca-acetate | [49,50,51] | |
| MK | CaO | - | ||
| CaCO3 | Hap | |||
| Mk+H62C | CaCO3 | TEOS | ||
| Yttrium mono-silicate (Y2O3·SiO2) Yttrium di-silicate (Y2O3·2SiO2) | MK | Y2O3 | Eu2O3 | [1,52] |
| - | - | |||
| - | - | |||
| Zircon (ZrO2·SiO2) | MK, H62C | ZrO2 | TiO2 Zircon seeds | [53] |
| Forsterite (2MgO·SiO2) | MK, H62C | MgO | TiO2 | [54] |
| Willemite (2ZnO·SiO2) | MK | ZnO | Mn-acetate | [1] |
| Cordierite (2MgO·2Al2O3·5SiO2) | MK, H62C | γ-Al2O3, MgO | - | [55] |
| Gehlenite (2CaO·Al2O3·SiO2) | MK | γ-Al2O3, CaCO3 | Eu2O3, CeO2 | [56] |
| Akermanite | MK, H62C | MgO, CaCO3 | m-Hap, Borax | [57] |
| Hardystonite (2CaO·ZnO·2SiO2) | MK | γ-Al2O3, ZnO | Eu2O3 | [1] |
| β′-SiAlON | MK, H44 | γ-Al2O3 | Si3N4, AlN, SiC | [58,59,60] |
| Y-Si-O-Ns | MK | Y2O3 | Eu2O3, CeO2 | [25] |
| Wollastonite-based silicate bioceramic | MK | CaCO3 | AP40 glass (apatite–wollastonite system) | [61] |
| Wollastonite- and hardystonite-based ceramics | MK | ZnO, CaCO3 | AP40 glass (apatite–wollastonite system) | [62] |
| Wollastonite–diopside foam | H62C, MK | Mg(OH)2, CaCO3, Na2 HPO4·7H2O | Ca/Mg-rich silicate glass | [63] |
| Lithium orthosilicate (Li4SiO4) | PMS | Lithium carbonate (Li2CO3) | - | [64] |
| Biosilicate glass–ceramic | MK+H62C | CaCO3, Na2CO3, and anhydrous sodium phosphate | Biosilicate® glass frit powder | [65] |
| AM Technique | Features | Feedstock Form | Forming Method | Printing Requirements | Resolution |
|---|---|---|---|---|---|
| Direct ink writing (DIW) | Easy operation, low cost, wide choice of materials, highly accurate and complex 3D architectures | Slurry | Extrusion | Appropriate viscosity and elastic properties | Few hundred micrometers to mm |
| Fused deposition modelling | Low operating cost, high speed and large size capability, reuse waste, low printing precision, limited extrusion temperature range | Filament | Extrusion | In filament/pellet state | mm |
| Stereolithography (SL)/ Digital light Processing (DLP) | Moderate cost, high efficiency, good surface quality and ease of processability | Slurry | Polymerization | Dissolvable and possesses sufficient photocurable moieties | |
| Two-photon polymerization (TPP) | High surface quality, high printing precision, low speed | Slurry | Non-linear polymerization | Dissolvable, crosslinkable with two-photon absorption moieties | sub μm |
| Selective laser sintering (SLS) | Complex 3D structures, slow speed, low shrinkage, low curing temperature, high dimensional accuracy | Powder | Powder fusion | Meltable with laser and curable via reactive groups | μm to mm |
| Binder jetting | Complex 3D structures, limited strength, and rough surfaces | Powder and slurry | Binder bonding | Dissolvable in solvents and act as binders | μm to mm |
| Inkjet printing | High printing resolution, low material waste, limited by printable inks, low printing speed | Slurry | Binder bonding | Low viscosity, rapid crosslinking, and high ceramic yield | Few hundred micrometers to mm |
| Laminated object manufacturing | Large-scale production, no complicated chemical/physical processes, low speed, low precision, high anisotropy | Sheet | Binder bonding and laser cutting | In sheet state | mm |
| PCPs | Fillers | Obtained Ceramic | Print Resolution (μm) | Porosity (vol%) | Compressive Strength (MPa) |
|---|---|---|---|---|---|
| MK | ZnO and CaCO3 | hardystonite (Ca2ZnSi2O7) | 300–500 | Up to 80% | 0.6 ± 0.2 |
| MK | CaCO3 | silica-bonded calcite | 450 | 56–64% | 2.9–5.5 |
| MK | Active: CaCO3, Na2CO3, and anhydrous sodium phosphate Passive: Biosilicate® glass frit powder (<5 μm) | Biosilicate glass–ceramic | 600 | 60% | Average of 6.7 |
| MK | CaCO3, MgO | Akermanite (Ca2MgSi2O7) | - | 72.4 ± 2.9% | 3.3 ± 0.6 |
| MK and H62C | Active: ZnO, CaCO3, SrCO3, Mg(OH)2 Passive: Glass powder | Sr/Mg-doped hardystonite | 840 | 73 ± 1 | 2.3 ± 0.7 |
| MK | PMMA sacrificial microbeads | SiOC | 400 | 74.9 ± 3.2 | 8.19 ± 3.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarraf, F.; Churakov, S.V.; Clemens, F. Preceramic Polymers for Additive Manufacturing of Silicate Ceramics. Polymers 2023, 15, 4360. https://doi.org/10.3390/polym15224360
Sarraf F, Churakov SV, Clemens F. Preceramic Polymers for Additive Manufacturing of Silicate Ceramics. Polymers. 2023; 15(22):4360. https://doi.org/10.3390/polym15224360
Chicago/Turabian StyleSarraf, Fateme, Sergey V. Churakov, and Frank Clemens. 2023. "Preceramic Polymers for Additive Manufacturing of Silicate Ceramics" Polymers 15, no. 22: 4360. https://doi.org/10.3390/polym15224360
APA StyleSarraf, F., Churakov, S. V., & Clemens, F. (2023). Preceramic Polymers for Additive Manufacturing of Silicate Ceramics. Polymers, 15(22), 4360. https://doi.org/10.3390/polym15224360






