Investigation on AC and DC Breakdown Mechanism of Surface-Ozone-Treated LDPE Films under Varied Thicknesses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
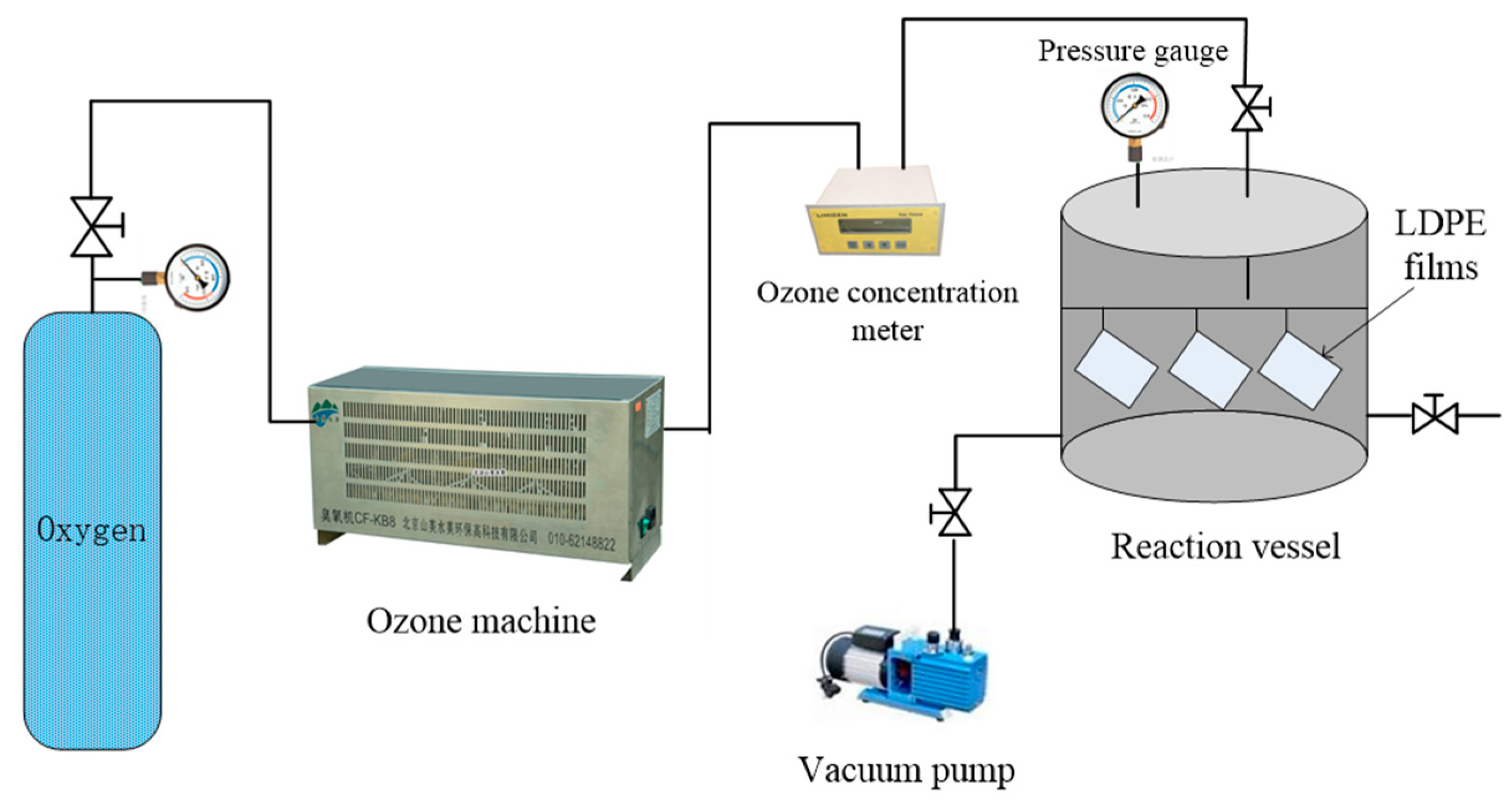
2.2. Sample Preparation
2.3. Characterizations
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Surface Potential Decay (SPD)
2.3.3. Volume Resistivity Tests
2.3.4. Pulsed Electro-Acoustic (PEA) Method
2.3.5. AC and DC Breakdown Tests
3. Results
3.1. Chemical Structure
3.2. Trap Distributions
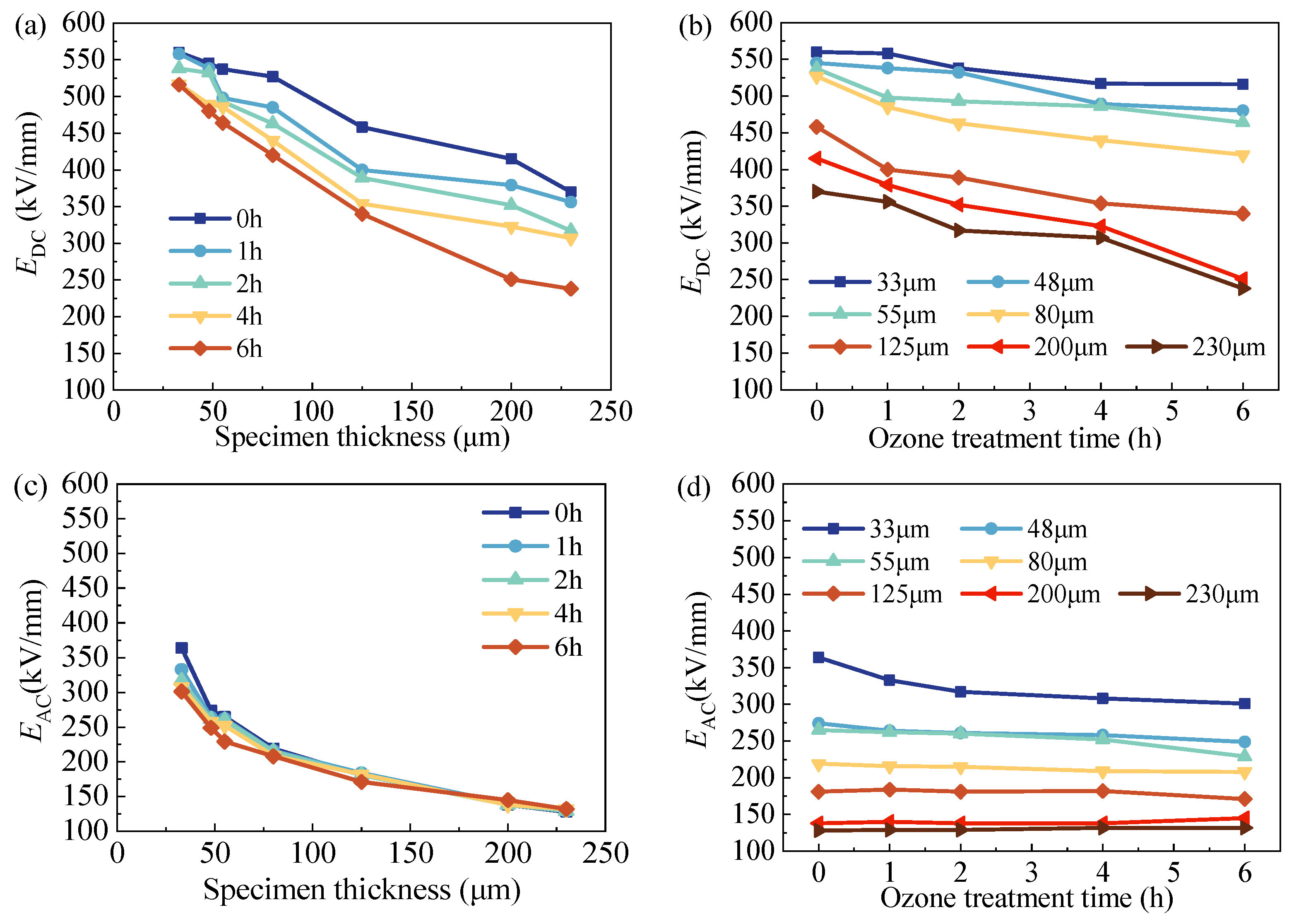
3.3. Breakdown Behavior
4. Discussion
4.1. Thickness-Dependent Electrical Breakdown
4.2. Surface-Oxidation-Dependent Electrical Breakdown
5. Conclusions
- With the extension of the oxidation duration, the shallow trap density for electrons and holes increased, but the average trap level reduced.
- Space charge injection from the electrode–dielectric interface was significantly enhanced after surface oxidation, and the injection strength gradually became stronger with the increase in oxidation treatment duration.
- The DC and AC breakdown strengths of LDPE films decreased with the increase in film thickness during the oxidation treatment due to the stronger homo charge injection.
- For DC breakdown, surface oxidation introduced shallow traps, resulting in more involved charges in electrical conduction, which more easily gained energy and accelerated to trigger charge multiplication, which finally resulted in a lower breakdown strength.
- The effect of oxidation on AC breakdown is closely related to film thickness. For thick films, the breakdown of the surface-modified layer cannot have a significant effect on the rest of the untreated film, leading to a barely changed breakdown strength with changes in film thickness.
- For surface oxidation, an ozone concentration up to 110 mg/L and an oxidation time up to 6 h are recommended, and it is optimal to stabilize the pressure in a reaction kettle at 0.04 MPa. A minimum thickness of 10 μm is recommended for polymeric insulating materials, since thinner films might be vertically penetrated during surface oxidation.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Papailiou, K.O. Composite insulators are gaining ground-25 years of Swiss experience. In Proceedings of the 1999 IEEE Transmission and Distribution Conference, New Orleans, LA, USA, 11–16 April 1999; Volume 822, pp. 827–833. [Google Scholar]
- Tu, Y.P.; Tong, Y.L.; Wang, Q.; Li, M. Influence of humidity on ozone aging performance of HTV silicon rubber. Gaodianya Jishu/High Volt. Eng. 2011, 37, 841–847. [Google Scholar]
- Fujimoto, K.; Takebayashi, Y.; Inoue, H.; Ikada, Y. Ozone-induced graft polymerization onto polymer surface. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1035–1043. [Google Scholar] [CrossRef]
- Laifaoui, A.; Herzine, M.S.; Zebboudj, Y.; Reboul, J.M.; Nedjar, M. Breakdown Strength Measurements on Cylindrical Polyvinyl Chloride Sheaths under AC and DC Voltages. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 2267–2273. [Google Scholar] [CrossRef]
- Li, W.G.; Liu, Z.K.; Wei, B.; Qiu, M.; Gao, X.J.; Gao, C.; Zhao, Y.Q.; Li, S.; Hou, J.Z.; Chen, P.P. Comparison Between the DC and AC Breakdown Characteristics of Dielectric Sheets in Liquid Nitrogen. IEEE Trans. Appl. Supercond. 2014, 24, 8800606. [Google Scholar] [CrossRef]
- Koo, J.H.; Hwang, J.S.; Shin, W.J.; Sakamoto, K.; Oh, D.H.; Lee, B.W. Comparison of DC and AC Surface Breakdown Characteristics of GFRP and Epoxy Nanocomposites in Liquid Nitrogen. IEEE Trans. Appl. Supercond. 2016, 26, 7700804. [Google Scholar] [CrossRef]
- Hayden, J.L.R.; Eddy, W.N.E. Dielectric Strength Ratio Between Alternating and Direct Voltages. J. Am. Inst. Electr. Eng. 1923, 42, 706–712. [Google Scholar] [CrossRef]
- Wagner, K.W. The physical nature of the electrical breakdown of solid dielectrics. J. Am. Inst. Electr. Eng. 1922, 41, 1034–1044. [Google Scholar] [CrossRef]
- Von Hippel, A. Electric breakdown of solid and liquid insulators. J. Appl. Phys. 1937, 8, 815–832. [Google Scholar] [CrossRef]
- Fröhlich, H. Theory of electrical breakdown in ionic crystals. II. Proc. R. Soc. A Maof Lond. Ser. A-Math. Phys. Sci. 1939, 172, 94–106. [Google Scholar] [CrossRef]
- Stark, K.H.; Garton, C.G. Electric Strength of Irradiated Polythene. Nature 1955, 176, 1225–1226. [Google Scholar] [CrossRef]
- Artbauer, J. Elektrische Festigkeit von Polymeren. Kolloid-Z. Z. Für Polym. 1965, 202, 15–25. [Google Scholar] [CrossRef]
- Balpinarli, M.; Dai, J.J.; Gela, G. Ac and Dc Breakdown Versus Thickness Characteristics for Rubber Gloves. IEEE Trans. Power Deliv. 1988, 3, 384–391. [Google Scholar] [CrossRef]
- Grzybowski, S.; Fan, J. Electrical breakdown characteristics of the XLPE cables under AC, DC, and pulsating voltages. In Proceedings of the 5th International Conference on Properties and Applications of Dielectric Materials, Seoul, Republic of Korea, 25–30 May 1997; Volumes 1 and 2, pp. 389–393. [Google Scholar]
- Jow, T.R.; Cygan, P.J. Investigation of dielectric breakdown of polyvinylidene fluoride using AC and DC methods. In Proceedings of the Conference Record of the 1992 IEEE International Symposium on Electrical Insulation, Baltimore, MD, USA, 7–10 June 1992; pp. 181–184. [Google Scholar]
- Zhu, Y.; Qiao, N.; Dong, S.; Qu, G.; Chen, Y.; Lu, W.; Qin, Z.; Li, D.; Wu, K.; Nie, Y.; et al. Side-Chain Engineering of Polystyrene Dielectrics Toward High-Performance Photon Memories and Artificial Synapses. Chem. Mater. 2022, 34, 6505–6517. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, Y.; Li, S.; Wei, P.; Li, D.; Liu, B.; Cui, D.; Zhang, Z.; Li, G.; Nie, Y.; et al. Soluble poly(4-fluorostyrene): A high-performance dielectric electret for organic transistors and memories. Mater. Horiz. 2020, 7, 1861–1871. [Google Scholar] [CrossRef]
- Zha, J.W.; Dang, Z.M.; Song, H.T.; Yin, Y.; Chen, G. Dielectric properties and effect of electrical aging on space charge accumulation in polyimide/TiO nanocomposite films. J. Appl. Phys. 2010, 108, 094113. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, G. Space charge behaviours in polyethylene under combined AC and DC electric fields. In Proceedings of the 2014 IEEE Conference on Electrical Insulation and Dielectric Phenomena, Des Moines, IA, USA, 19–22 October 2014; pp. 848–851. [Google Scholar]
- Li, S.; Zhu, Y.; Min, D.; Chen, G. Space Charge Modulated Electrical Breakdown. Sci. Rep. 2016, 6, 32588. [Google Scholar] [CrossRef] [PubMed]
- Riffaud, J.; Griseri, V.; Berquez, L. New design of the pulsed electro-acoustic upper electrode for space charge measurements during electronic irradiation. Rev. Sci. Instrum. 2016, 87, 073901. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.; Borrelli, E.; Travagli, V.; Zanardi, I. The ozone paradox: Ozone is a strong oxidant as well as a medical drug. Med. Res. Rev. 2009, 29, 646–682. [Google Scholar] [CrossRef]
- Cataldo, F. The action of ozone on polymers having unconjugated and cross or linearly conjugated unsaturation chemistry and technological aspects. Polym. Degrad. Stab. 2001, 73, 511–520. [Google Scholar] [CrossRef]
- Walzak, M.J.; Flynn, S.; Foerch, R.; Hill, J.M.; Karbashewski, E.; Lin, A.; Strobel, M. UV and ozone treatment of polypropylene and poly(ethylene terephthalate). J. Adhes. Sci. Technol. 1995, 9, 1229–1248. [Google Scholar] [CrossRef]
- Simmons, J.G.; Wei, L.S. Theory of dynamic charge current and capacitance characteristics in MIS systems containing distributed surface traps. Solid-State Electron. 1973, 16, 53–66. [Google Scholar] [CrossRef]
- Nie, Y.; Yang, L.; Zhao, N.; Min, D.; Li, S. Effect of surface state on DC breakdown of LDPE films. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2522–2530. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, J.W.; Li, S.T.; Zhong, L.S. Origin of thickness dependent dc electrical breakdown in dielectrics. Appl. Phys. Lett. 2012, 100, 222904. [Google Scholar] [CrossRef]
- Angalane, S.K.; Kasinathan, E. A review on polymeric insulation for high-voltage application under various stress conditions. Polym. Compos. 2022, 43, 4803–4834. [Google Scholar] [CrossRef]
- Ieda, M. Dielectric Breakdown Process of Polymers. IEEE Trans. Electr. Insul. 1980, EI-15, 206–224. [Google Scholar] [CrossRef]
- Montanari, G.C. Bringing an insulation to failure: The role of space charge. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 339–364. [Google Scholar] [CrossRef]
- Mazzanti, G.; Montanari, G.C. Electrical aging and life models: The role of space charge. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 876–890. [Google Scholar] [CrossRef]
- Yuan, M.; Zou, L.; Li, Z.; Pang, L.; Zhao, T.; Zhang, L.; Zhou, J.; Xiao, P.; Akram, S.; Wang, Z.; et al. A review on factors that affect surface charge accumulation and charge-induced surface flashover. Nanotechnology 2021, 32, 262001. [Google Scholar] [CrossRef]
- Liu, P.; Xie, Z.; Pang, X.; Xu, T.; Zhang, S.; Morshuis, P.H.F.; Li, H.; Peng, Z. Space Charge Behavior in Epoxy-Based Dielectrics: Progress and Perspective. Adv. Electron. Mater. 2022, 8, 2200259. [Google Scholar] [CrossRef]
- Damamme, G.; Gressus, C.L.; Reggi, A.S.D. Space charge characterization for the 21th century. IEEE Trans. Dielectr. Electr. Insul. 1997, 4, 558–584. [Google Scholar] [CrossRef]
- Blaise, G.; Sarjeant, W.J. Space charge in dielectrics. Energy storage and transfer dynamics from atomistic to macroscopic scale. IEEE Trans. Dielectr. Electr. Insul. 1998, 5, 779–808. [Google Scholar] [CrossRef]
- Lau, K.Y.; Vaughan, A.S.; Chen, G.; Hosier, I.L.; Holt, A.F.; Ching, K.Y. On the space charge and DC breakdown behavior of polyethylene/silica nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 340–351. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, G. Space charge and AC electrical breakdown strength in polyethylene. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 559–566. [Google Scholar] [CrossRef]
- Neelmani; Suematsu, H.; Sarathi, R. Investigation on space charge dynamics and mechanical properties of Epoxy Alumina nanocomposites. Mater. Res. Express 2020, 7, 025037. [Google Scholar] [CrossRef]
- Neelmani; Thangabalan, B.; Vasa, N.J.; Srinivasan, B.; Suematsu, H.; Sarathi, R. Investigation on Surface Condition of the Corona-Aged Silicone Rubber Nanocomposite Adopting Wavelet and LIBS Technique. IEEE Trans. Plasma Sci. 2021, 49, 2294–2304. [Google Scholar] [CrossRef]
- Panda, P.; Chakroborty, S.; Modi, A.; Moharana, S. Metal Oxide Nanofiller-Introduced Polymer-Based Nanocomposite Dielectrics for Advanced Energy Storage Devices. In Emerging Nanodielectric Materials for Energy Storage: From Bench to Field; Moharana, S., Gregory, D.H., Mahaling, R.N., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 415–431. [Google Scholar]
- Tohluebaji, N.; Thainiramit, P.; Putson, C.; Muensit, N. Phase and Structure Behavior vs. Electromechanical Performance of Electrostrictive P(VDF-HFP)/ZnO Composite Nanofibers. Polymers 2021, 13, 2565. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Polymer Nanocomposites: Synthesis and Characterization. In Environmental Nanotechnology Volume 4; Dasgupta, N., Ranjan, S., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 265–315. [Google Scholar]
- Adnan, M.; Abdul-Malek, Z.; Lau, K.Y.; Tahir, M. Polypropylene-based nanocomposites for HVDC cable insulation. IET Nanodielectr. 2021, 4, 84–97. [Google Scholar] [CrossRef]
- Marwat, M.A.; Yasar, M.; Ma, W.; Fan, P.; Liu, K.; Lu, D.; Tian, Y.; Samart, C.; Ye, B.; Zhang, H. Significant Energy Density of Discharge and Charge–Discharge Efficiency in Ag@BNN Nanofillers-Modified Heterogeneous Sandwich Structure Nanocomposites. ACS Appl. Energy Mater. 2020, 3, 6591–6601. [Google Scholar] [CrossRef]
- Lei, Z.; Men, R.; Wang, F.; Li, Y.; Song, J.; Shahsavarian, T.; Li, C.; Fabiani, D. Surface modified nano-SiO2 enhances dielectric properties of stator coil insulation for HV motors. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1029–1037. [Google Scholar] [CrossRef]
- Neelmani; Sarathi, R.; Suematsu, H. Understanding the impact of space charge variations with UV- and water-aged epoxy alumina nanocomposites adopting pulsed electroacoustic techniques. Micro Nano Lett. 2020, 15, 1059–1064. [Google Scholar] [CrossRef]
- Zhao, N.; Nie, Y.; Li, S. Space charge characteristics of fluorinated polyethylene: Different effects of fluorine and oxygen. AIP Adv. 2018, 8, 045103. [Google Scholar] [CrossRef]
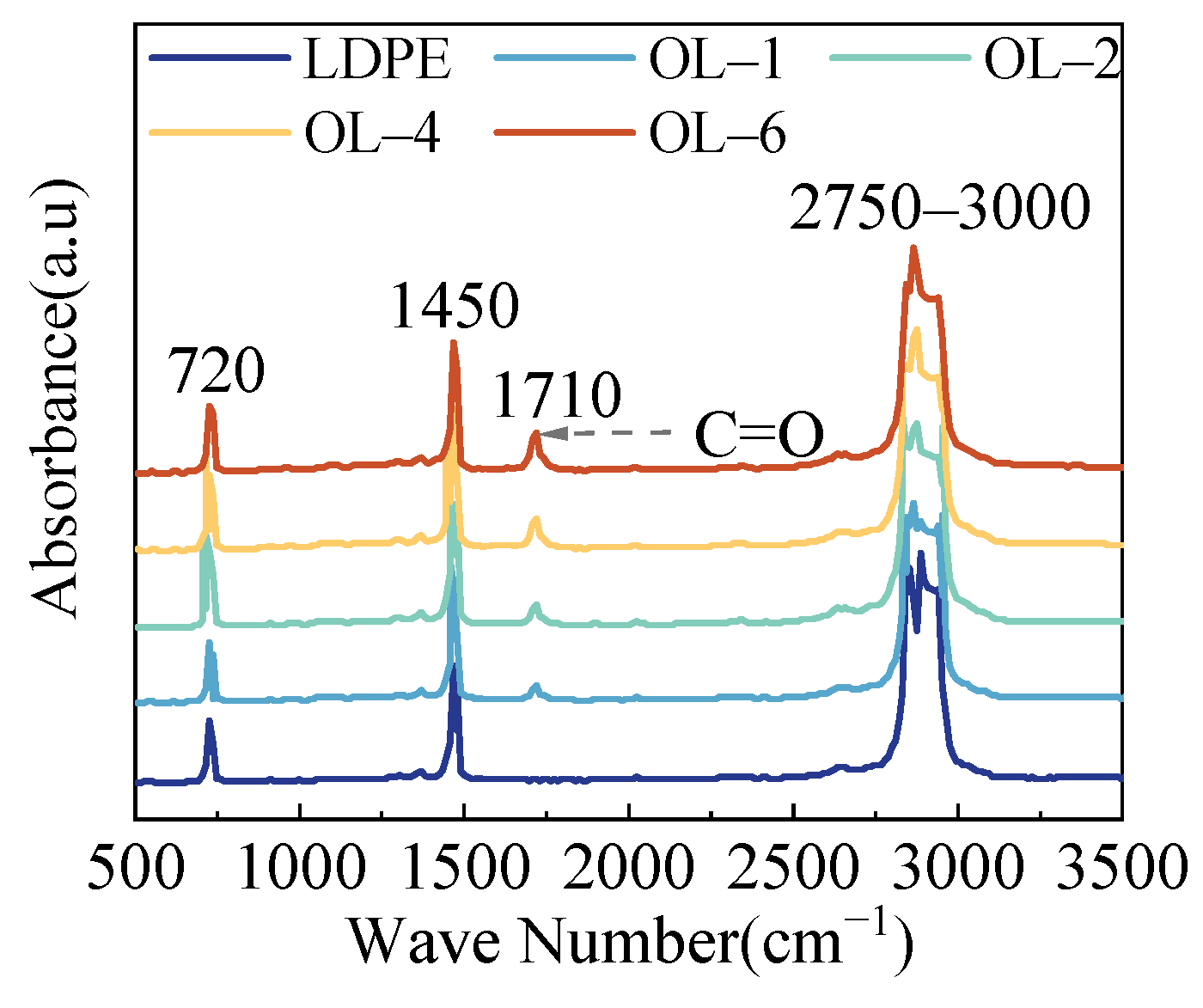

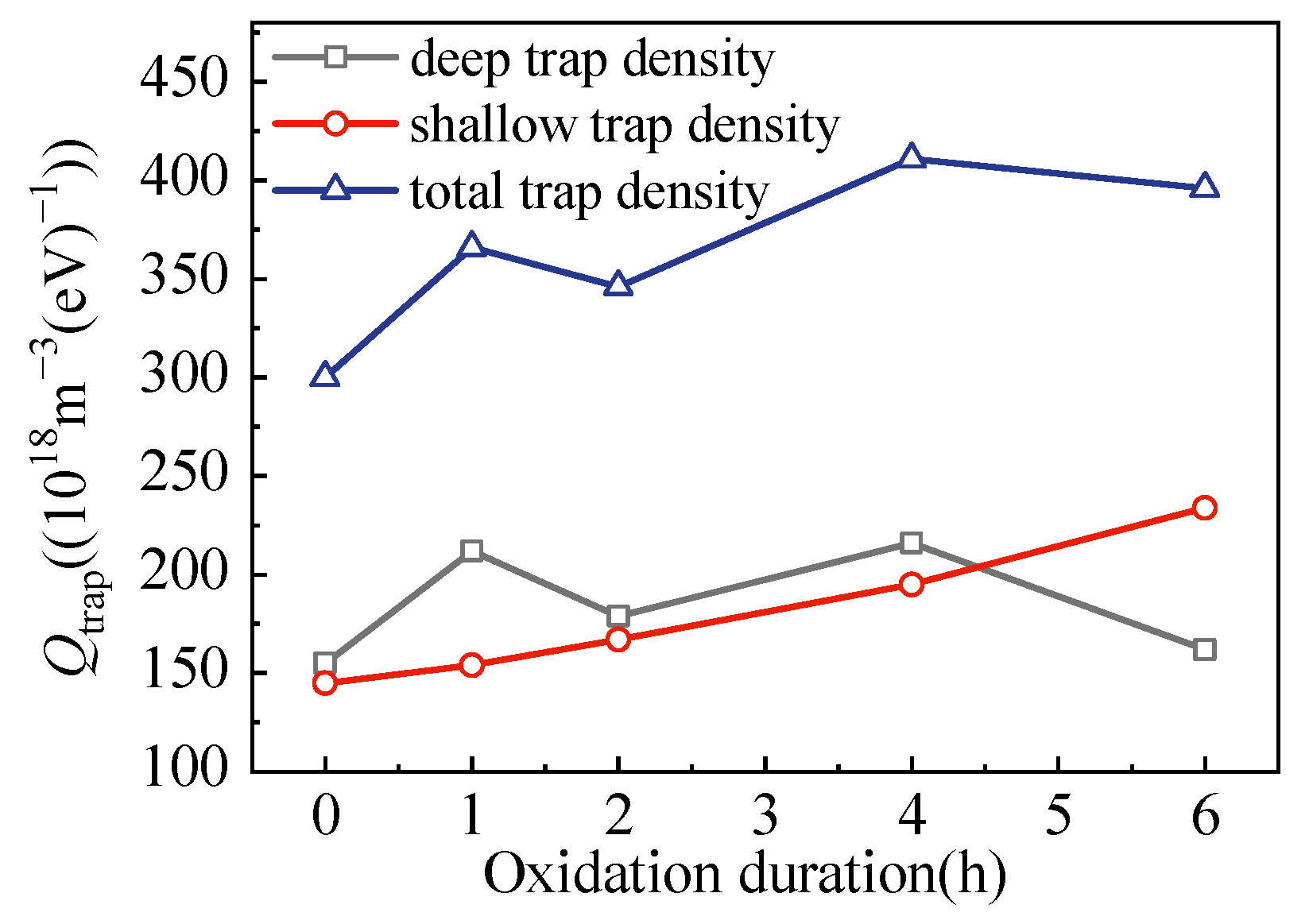


| Films | Trap Depth (eV) | Trap Density (×1018 m−3(eV)−1) | ||
|---|---|---|---|---|
| Shallow | Deep | Shallow | Deep | |
| LDPE | 0.88 | 0.93 | 142 | 280 |
| OL-1 | 0.87 | 0.92 | 168 | 206 |
| OL-2 | 0.86 | 0.91 | 216 | 224 |
| OL-4 | 0.86 | 0.91 | 289 | 193 |
| OL-6 | 0.86 | 0.90 | 342 | 141 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, Y.; Liu, J.; Ke, J.; Zhao, X.; Li, S.; Zhu, Y. Investigation on AC and DC Breakdown Mechanism of Surface-Ozone-Treated LDPE Films under Varied Thicknesses. Polymers 2023, 15, 4490. https://doi.org/10.3390/polym15234490
Nie Y, Liu J, Ke J, Zhao X, Li S, Zhu Y. Investigation on AC and DC Breakdown Mechanism of Surface-Ozone-Treated LDPE Films under Varied Thicknesses. Polymers. 2023; 15(23):4490. https://doi.org/10.3390/polym15234490
Chicago/Turabian StyleNie, Yongjie, Jie Liu, Junxin Ke, Xianping Zhao, Shengtao Li, and Yuanwei Zhu. 2023. "Investigation on AC and DC Breakdown Mechanism of Surface-Ozone-Treated LDPE Films under Varied Thicknesses" Polymers 15, no. 23: 4490. https://doi.org/10.3390/polym15234490
APA StyleNie, Y., Liu, J., Ke, J., Zhao, X., Li, S., & Zhu, Y. (2023). Investigation on AC and DC Breakdown Mechanism of Surface-Ozone-Treated LDPE Films under Varied Thicknesses. Polymers, 15(23), 4490. https://doi.org/10.3390/polym15234490






