Green Hydrogels Loaded with Extracts from Solanaceae for the Controlled Disinfection of Agricultural Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. HPLC-ESI-LTQ Quantification of Glycoalkaloids
2.3. Preparation and Loading of Hydrogel Microbeads
2.4. Drying Kinetics and Equilibrium Water Content
2.5. Differential Scanning Calorimetry
2.6. Thermogravimetric Analysis
2.7. ATR-FTIR Spectroscopy
2.8. Scanning Electron Microscopy (SEM)
2.9. Encapsulation Efficiency and Release of Bioactive Compound
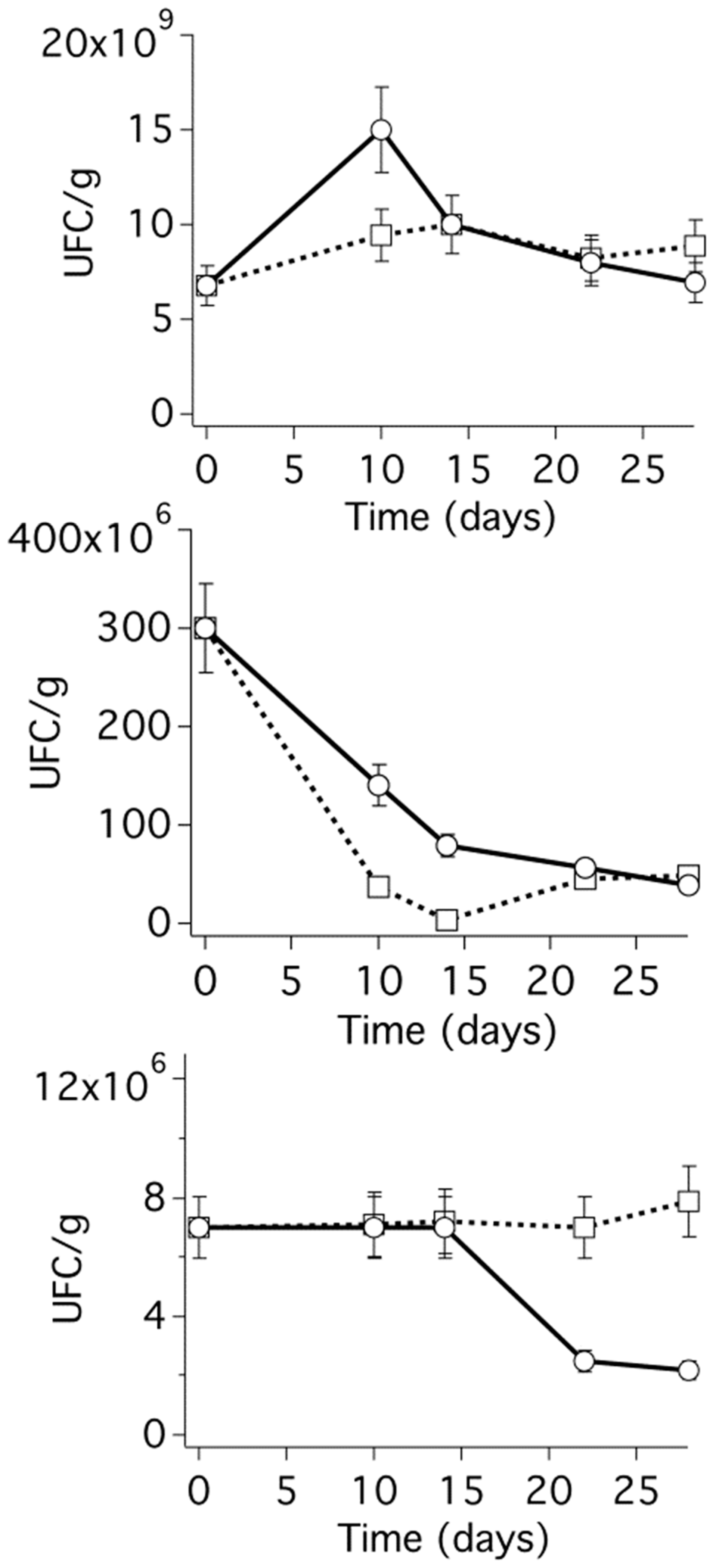
2.10. Laboratory Microbiological Tests
2.11. In Situ Tests: Treatment of a Model Soil
3. Results and Discussion
3.1. Synthesis and Characterization of the CRS
3.2. Assessment of the Efficiency of the Selected CRS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Song, S.; Wang, R.; Liu, Z.; Meng, J.; Sweetman, A.J.; Jenkins, A.; Ferrier, R.C.; Li, H.; Luo, W.; et al. Impacts of Soil and Water Pollution on Food Safety and Health Risks in China. Environ. Int. 2015, 77, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Kogan, V.; Shelton, J.F.; Delwiche, L.; Hansen, R.L.; Ozonoff, S.; Ma, C.C.; McCanlies, E.C.; Bennett, D.H.; Hertz-Picciotto, I.; et al. Combined Prenatal Pesticide Exposure and Folic Acid Intake in Relation to Autism Spectrum Disorder. Environ. Health Perspect. 2023, 125, 97007. [Google Scholar] [CrossRef] [PubMed]
- Neri-Badang, M.C.; Chakraborty, S. Carbohydrate Polymers as Controlled Release Devices for Pesticides. J. Carbohydr. Chem. 2019, 38, 67–85. [Google Scholar] [CrossRef]
- Sun, S.; Sidhu, V.; Rong, Y.; Zheng, Y. Pesticide Pollution in Agricultural Soils and Sustainable Remediation Methods: A Review. Curr. Pollut. Rep. 2018, 4, 240–250. [Google Scholar] [CrossRef]
- Huang, H.-S.; Hsu, C.-C.; Weng, S.-F.; Lin, H.-J.; Wang, J.-J.; Su, S.-B.; Huang, C.-C.; Guo, H.-R. Acute Anticholinesterase Pesticide Poisoning Caused a Long-Term Mortality Increase: A Nationwide Population-Based Cohort Study. Medicine 2015, 94, e1222. [Google Scholar] [CrossRef]
- Doolotkeldieva, T.; Konurbaeva, M.; Bobusheva, S. Microbial Communities in Pesticide-Contaminated Soils in Kyrgyzstan and Bioremediation Possibilities. Environ. Sci. Pollut. Res. 2018, 25, 31848–31862. [Google Scholar] [CrossRef]
- Feld, L.; Hjelmsø, M.H.; Nielsen, M.S.; Jacobsen, A.D.; Rønn, R.; Ekelund, F.; Krogh, P.H.; Strobel, B.W.; Jacobsen, C.S. Pesticide Side Effects in an Agricultural Soil Ecosystem as Measured by AmoA Expression Quantification and Bacterial Diversity Changes. PLoS ONE 2015, 10, e0126080. [Google Scholar] [CrossRef]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patnaik, S. Advances in Controlled Release Pesticide Formulations: Prospects to Safer Integrated Pest Management and Sustainable Agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef]
- Mun, A.; Simaan Yameen, H.; Edelbaum, G.; Seliktar, D. Alginate Hydrogel Beads Embedded with Drug-Bearing Polycaprolactone Microspheres for Sustained Release of Paclobutrazol. Sci. Rep. 2021, 11, 10877. [Google Scholar] [CrossRef]
- Clemente, I.; Menicucci, F.; Colzi, I.; Sbraci, L.; Benelli, C.; Giordano, C.; Gonnelli, C.; Ristori, S.; Petruccelli, R. Unconventional and Sustainable Nanovectors for Phytohormone Delivery: Insights on Olea Europaea. ACS Sustain. Chem. Eng. 2018, 6, 15022–15031. [Google Scholar] [CrossRef]
- Clemente, I.; Falsini, S.; Di Cola, E.; Fadda, G.C.; Gonnelli, C.; Spinozzi, F.; Bacia-Verloop, M.; Grillo, I.; Ristori, S. Green Nanovectors for Phytodrug Delivery: In-Depth Structural and Morphological Characterization. ACS Sustain. Chem. Eng. 2019, 7, 12838–12846. [Google Scholar] [CrossRef]
- Singh, G.; Ramadass, K.; Sooriyakumar, P.; Hettithanthri, O.; Vithange, M.; Bolan, N.; Tavakkoli, E.; Van Zwieten, L.; Vinu, A. Nanoporous Materials for Pesticide Formulation and Delivery in the Agricultural Sector. J. Control. Release 2022, 343, 187–206. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Akalin, G.O.; Pulat, M. Preparation and Characterization of Nanoporous Sodium Carboxymethyl Cellulose Hydrogel Beads. J. Nanomater. 2018, 2018, 9676949. [Google Scholar] [CrossRef]
- Bierhalz, A.C.K.; da Silva, M.A.; Braga, M.E.M.; Sousa, H.J.C.; Kieckbusch, T.G. Effect of Calcium and/or Barium Crosslinking on the Physical and Antimicrobial Properties of Natamycin-Loaded Alginate Films. LWT-Food Sci. Technol. 2014, 57, 494–501. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, S.J.; Gu, B.K.; Kim, C.-H. Ionically Crosslinked Alginate–Carboxymethyl Cellulose Beads for the Delivery of Protein Therapeutics. Appl. Surf. Sci. 2012, 262, 28–33. [Google Scholar] [CrossRef]
- Zhao, H.; Ouyang, X.-K.; Yang, L.-Y. Adsorption of Lead Ions from Aqueous Solutions by Porous Cellulose Nanofiber–Sodium Alginate Hydrogel Beads. J. Mol. Liq. 2021, 324, 115122. [Google Scholar] [CrossRef]
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of Cellulose-Based Superabsorbent Hydrogels as Water Reservoir in Agriculture. Int. J. Polym. Sci. 2013, 2013, 435073. [Google Scholar] [CrossRef]
- Michalik, R.; Wandzik, I. A Mini-Review on Chitosan-Based Hydrogels with Potential for Sustainable Agricultural Applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan Nanoparticle Based Delivery Systems for Sustainable Agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Gallagher, L.; Smith, A.; Kavanagh, K.; Devereux, M.; Colleran, J.; Breslin, C.; Richards, K.G.; McCann, M.; Rooney, A.D. Preparation and Antimicrobial Properties of Alginate and Serum Albumin/Glutaraldehyde Hydrogels Impregnated with Silver(I) Ions. Chemistry 2021, 3, 672–686. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ismail, S.; Dadrasnia, A. Encapsulation of Plant Growth Promoting Rhizobacteria—Prospects and Potential in Agricultural Sector: A Review. J. Plant Nutr. 2019, 42, 2600–2623. [Google Scholar] [CrossRef]
- Bravo Cadena, M.; Preston, G.M.; Van der Hoorn, R.A.L.; Flanagan, N.A.; Townley, H.E.; Thompson, I.P. Enhancing Cinnamon Essential Oil Activity by Nanoparticle Encapsulation to Control Seed Pathogens. Ind. Crops Prod. 2018, 124, 755–764. [Google Scholar] [CrossRef]
- Pacifico, D.; Lanzanova, C.; Pagnotta, E.; Bassolino, L.; Mastrangelo, A.M.; Marone, D.; Matteo, R.; Lo Scalzo, R.; Balconi, C. Sustainable Use of Bioactive Compounds from Solanum Tuberosum and Brassicaceae Wastes and By-Products for Crop Protection—A Review. Molecules 2021, 26, 2174. [Google Scholar] [CrossRef] [PubMed]
- Boulogne, I.; Petit, P.; Ozier-Lafontaine, H.; Desfontaines, L.; Loranger-Merciris, G. Insecticidal and Antifungal Chemicals Produced by Plants: A Review. Environ. Chem. Lett. 2012, 10, 325–347. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz, J.d.J.; López-Mata, M.A.; Del-Toro-Sánchez, C.L.; Ayala-Zavala, J.F.; Márquez-Ríos, E. Total Phenolic, Flavonoid, Tomatine, and Tomatidine Contents and Antioxidant and Antimicrobial Activities of Extracts of Tomato Plant. Int. J. Anal. Chem. 2015, 2015, 284071. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, M.; Mewis, I.; Huyskens-Keil, S.; Jansen, M.A.K.; Zrenner, R.; Winkler, J.B.; O’Brien, N.; Krumbein, A. UV-B-Induced Secondary Plant Metabolites—Potential Benefits for Plant and Human Health. CRC Crit. Rev. Plant Sci. 2012, 31, 229–240. [Google Scholar] [CrossRef]
- Friedman, M. Tomato Glycoalkaloids: Role in the Plant and in the Diet. J. Agric. Food Chem. 2002, 50, 5751–5780. [Google Scholar] [CrossRef]
- Milner, S.E.; Brunton, N.P.; Jones, P.W.; O’Brien, N.M.; Collins, S.G.; Maguire, A.R. Bioactivities of Glycoalkaloids and Their Aglycones from Solanum Species. J. Agric. Food Chem. 2011, 59, 3454–3484. [Google Scholar] [CrossRef]
- Tamasi, G.; Pardini, A.; Croce, R.; Consumi, M.; Leone, G.; Bonechi, C.; Rossi, C.; Magnani, A. Combined Experimental and Multivariate Model Approaches for Glycoalkaloid Quantification in Tomatoes. Molecules 2021, 26, 3068. [Google Scholar] [CrossRef]
- Chowański, S.; Adamski, Z.; Marciniak, P.; Rosiński, G.; Büyükgüzel, E.; Büyükgüzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F.; et al. A Review of Bioinsecticidal Activity of Solanaceae Alkaloids. Toxins 2016, 8, 60. [Google Scholar] [PubMed]
- Pardini, A.; Consumi, M.; Leone, G.; Bonechi, C.; Tamasi, G.; Sangiorgio, P.; Verardi, A.; Rossi, C.; Magnani, A. Effect of Different Post-Harvest Storage Conditions and Heat Treatment on Tomatine Content in Commercial Varieties of Green Tomatoes. J. Food Compos. Anal. 2021, 96, 103735. [Google Scholar] [CrossRef]
- Cangeloni, L.; Bonechi, C.; Leone, G.; Consumi, M.; Andreassi, M.; Magnani, A.; Rossi, C.; Tamasi, G. Characterization of Extracts of Coffee Leaves (Coffea arabica L.) by Spectroscopic and Chromatographic/Spectrometric Techniques. Foods 2022, 11, 2495. [Google Scholar] [CrossRef] [PubMed]
- Łabowska, M.B.; Skrodzka, M.; Sicińska, H.; Michalak, I.; Detyna, J. Influence of Cross-Linking Conditions on Drying Kinetics of Alginate Hydrogel. Gels 2023, 9, 63. [Google Scholar] [PubMed]
- Mikula, K.; Skrzypczak, D.; Ligas, B.; Witek-Krowiak, A. Preparation of Hydrogel Composites Using Ca2+ and Cu2+ Ions as Crosslinking Agents. SN Appl. Sci. 2019, 1, 643. [Google Scholar] [CrossRef]
- Zhang, Q.; Litchfield, J.B. An Optimization of Intermittent Corn Drying in a Laboratory Scale Thin Layer Dryer. Dry. Technol. 1991, 9, 383–395. [Google Scholar] [CrossRef]
- Toğrul, İ.T.; Pehlivan, D. Modelling of Drying Kinetics of Single Apricot. J. Food Eng. 2003, 58, 23–32. [Google Scholar] [CrossRef]
- Domingues, J.A.L.; Bonelli, N.; Giorgi, R.; Fratini, E.; Gorel, F.; Baglioni, P. Innovative Hydrogels Based on Semi-Interpenetrating p(HEMA)/PVP Networks for the Cleaning of Water-Sensitive Cultural Heritage Artifacts. Langmuir 2013, 29, 2746–2755. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic Hydrocolloids for the Intestinal Delivery of Protein Drugs: Alginate and Chitosan—A Review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef]
- da Silva Fernandes, R.; de Moura, M.R.; Glenn, G.M.; Aouada, F.A. Thermal, Microstructural, and Spectroscopic Analysis of Ca2+ Alginate/Clay Nanocomposite Hydrogel Beads. J. Mol. Liq. 2018, 265, 327–336. [Google Scholar] [CrossRef]
- Kim, J.-W.; Lee, Y.-D.; Lee, H.-G. Decomposition of Na2CO3 by Interaction with SiO2 in Mold Flux of Steel Continuous Casting. ISIJ Int. 2001, 41, 116–123. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal–Carboxylate Interactions in Metal–Alginate Complexes Studied with FTIR Spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef]
- Hua, S.; Ma, H.; Li, X.; Yang, H.; Wang, A. PH-Sensitive Sodium Alginate/Poly(Vinyl Alcohol) Hydrogel Beads Prepared by Combined Ca2+ Crosslinking and Freeze-Thawing Cycles for Controlled Release of Diclofenac Sodium. Int. J. Biol. Macromol. 2010, 46, 517–523. [Google Scholar] [CrossRef]
- Grasel, F.d.S.; Ferrão, M.F.; Wolf, C.R. Development of Methodology for Identification the Nature of the Polyphenolic Extracts by FTIR Associated with Multivariate Analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 94–101. [Google Scholar] [CrossRef]
- Lü, S.; Liu, M.; Ni, B.; Gao, C. A Novel PH- and Thermo-Sensitive PVP/CMC Semi-IPN Hydrogel: Swelling, Phase Behavior, and Drug Release Study. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1749–1756. [Google Scholar] [CrossRef]
- Supramaniam, J.; Adnan, R.; Mohd Kaus, N.H.; Bushra, R. Magnetic Nanocellulose Alginate Hydrogel Beads as Potential Drug Delivery System. Int. J. Biol. Macromol. 2018, 118, 640–648. [Google Scholar] [CrossRef] [PubMed]
- McKEE, R.K. Factors Affecting the Toxicity of Solanine and Related Alkaloids to Fusarium Caeruleum. J. Gen. Microbiol. 1959, 20, 686–696. [Google Scholar] [CrossRef]
- Fewell, A.M.; Roddick, J.G.; Weissenberg, M. Interactions between the Glycoalkaloids Solasonine and Solamargine in Relation to Inhibition of Fungal Growth. Phytochemistry 1994, 37, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Keukens, E.A.J.; de Vrije, T.; van den Boom, C.; de Waard, P.; Plasman, H.H.; Thiel, F.; Chupin, V.; Jongen, W.M.F.; de Kruijff, B. Molecular Basis of Glycoalkaloid Induced Membrane Disruption. Biochim. Biophys. Acta -Biomembr. 1995, 1240, 216–228. [Google Scholar] [CrossRef]
- Costa, S.D.; Gaugler, R.R. Sensitivity OfBeauveria Bassiana to Solanine and Tomatine. J. Chem. Ecol. 1989, 15, 697–706. [Google Scholar] [CrossRef]
- Virág, D.; Naár, Z.; Kiss, A. Microbial Toxicity of Pesticide Derivatives Produced with UV-Photodegradation. Bull. Environ. Contam. Toxicol. 2007, 79, 356–359. [Google Scholar] [CrossRef] [PubMed]



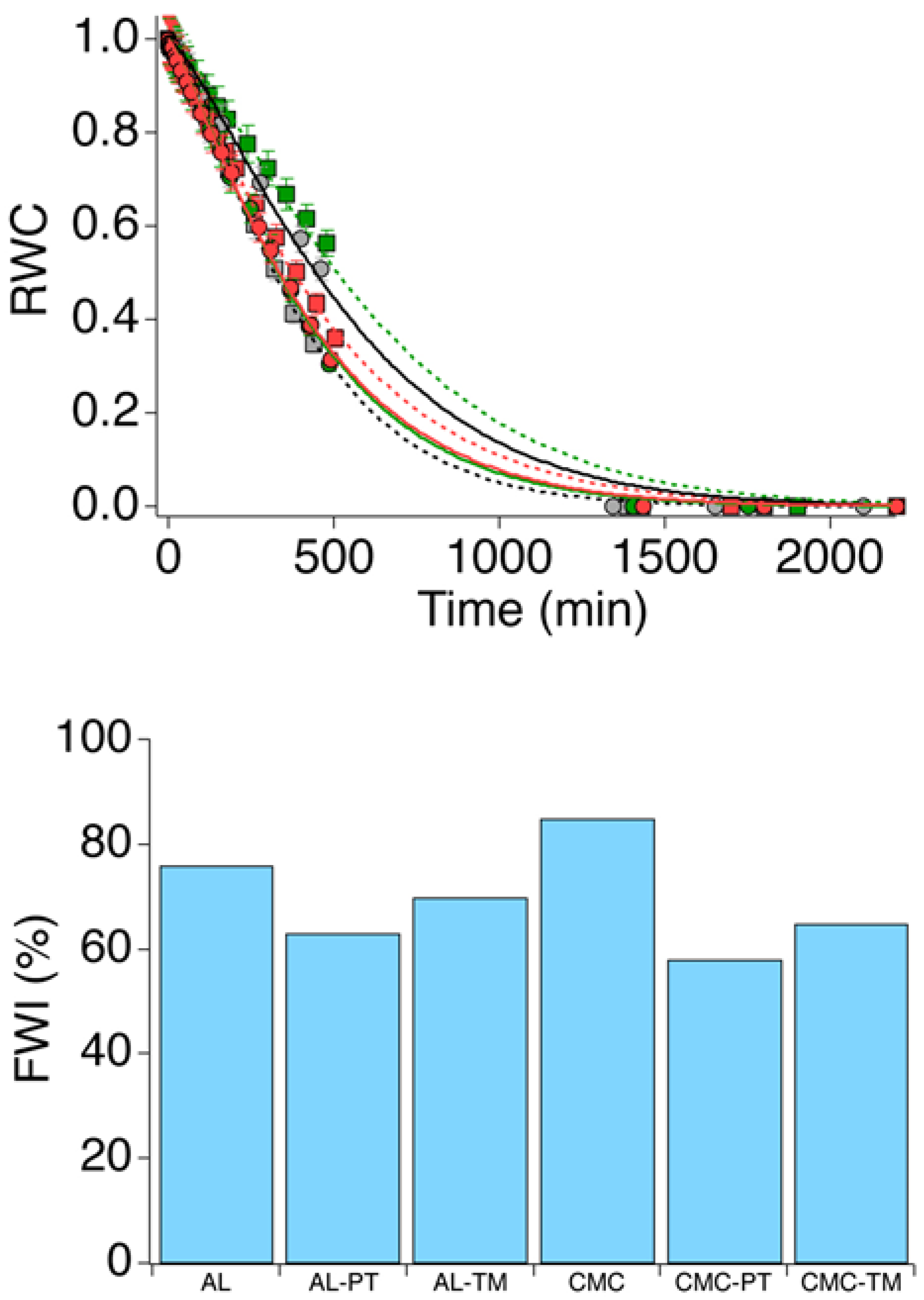

| Samples | Acronyms |
|---|---|
| Alginate-Ca2+ | AL |
| Alginate-Ca2++ potato leaf extract | AL-PT |
| Alginate-Ca2++ tomato leaf extract | AL-TM |
| CMC-Fe3+ | CMC |
| CMC-Fe3++ potato leaf extract | CMC-PT |
| CMC-Fe3++ tomato leaf extract | CMC-TM |
| Assignment | Wavenumber (cm−1) | |||
|---|---|---|---|---|
| poly-AL | AL | poly-CMC | CMC | |
| Stretching –OH | 3650–3000 | 3650–3000 | 3625–3000 | 3700–2650 |
| Stretching –CH | 2943 | 2930 | 2898 | - |
| Stretching –COO– (asymm.) | 1605 | 1627 | 1596 | 1620 |
| Stretching –COO– (symm.) | 1410 | 1434 | 1417 | 1417 |
| Stretching –C–O–C– | 1067 | 1086 | 1058 | 1062 |
| Assignment | Wavenumber (cm−1) | |
|---|---|---|
| PT | TM | |
| Stretching –OH/–NH | 3407 | 3419 |
| Stretching –CH3/–CH2 | 3010–2850 | 3010–2850 |
| Stretching C=O esters | 1729 | 1738 |
| Stretching C=O amides | 1650 | 1650 |
| Stretching C=C–C aromatic | 1627 | 1626 |
| 1530 | 1530 | |
| Bending –OH | 1240 | 1230 |
| Stretching C–O | 1169 | 1164 |
| Stretching C–O–C glycosidic | 1070 | 1069 |
| Microorganism | CMC | CMC−TM | |
|---|---|---|---|
| Bacteria | Escherichia coli | 0 mm | 8 mm |
| Pseudomonas aeruginosa | 0 mm | 22 mm | |
| Enterococcus faecalis | 0 mm | 13 mm | |
| Fungi | Aspergillus brasiliensis | 0 mm | 20 mm |
| Aspergillus fumigatus | 0 mm | 10 mm | |
| Fusarium oxysporum | 0 mm | 15 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente, I.; Baglioni, M.; Bonechi, C.; Bisozzi, F.; Rossi, C.; Tamasi, G. Green Hydrogels Loaded with Extracts from Solanaceae for the Controlled Disinfection of Agricultural Soils. Polymers 2023, 15, 4455. https://doi.org/10.3390/polym15224455
Clemente I, Baglioni M, Bonechi C, Bisozzi F, Rossi C, Tamasi G. Green Hydrogels Loaded with Extracts from Solanaceae for the Controlled Disinfection of Agricultural Soils. Polymers. 2023; 15(22):4455. https://doi.org/10.3390/polym15224455
Chicago/Turabian StyleClemente, Ilaria, Michele Baglioni, Claudia Bonechi, Flavia Bisozzi, Claudio Rossi, and Gabriella Tamasi. 2023. "Green Hydrogels Loaded with Extracts from Solanaceae for the Controlled Disinfection of Agricultural Soils" Polymers 15, no. 22: 4455. https://doi.org/10.3390/polym15224455
APA StyleClemente, I., Baglioni, M., Bonechi, C., Bisozzi, F., Rossi, C., & Tamasi, G. (2023). Green Hydrogels Loaded with Extracts from Solanaceae for the Controlled Disinfection of Agricultural Soils. Polymers, 15(22), 4455. https://doi.org/10.3390/polym15224455







