Advances in Hole Transport Materials for Layered Casting Solar Cells
Abstract
:1. Introduction
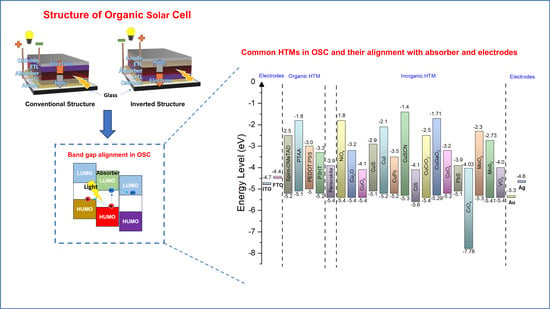
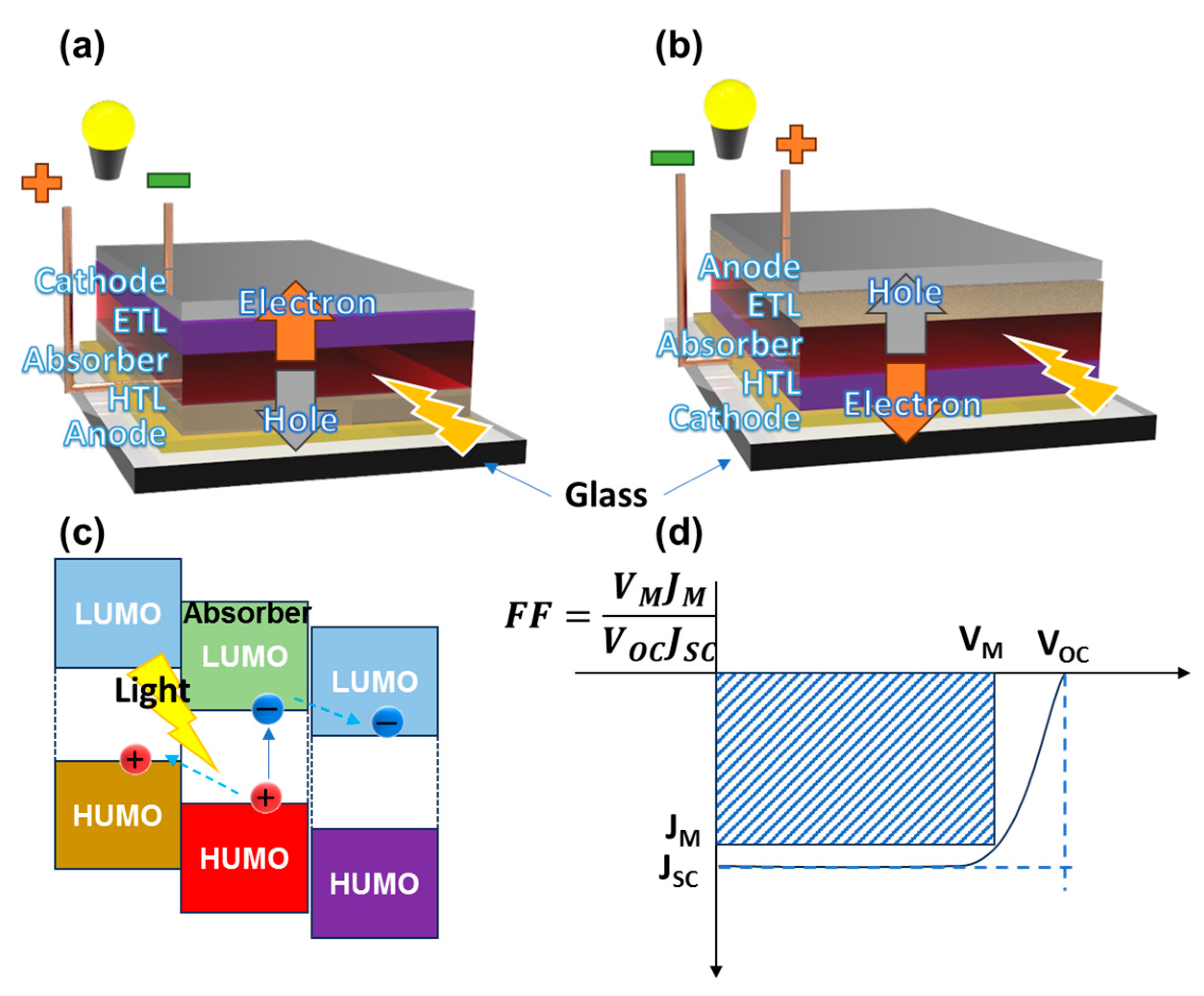
2. Solar Cell Factors
3. Organic Hole Transport Layer (OHTL)
3.1. Spiro-OMeTAD
3.2. PTAA
3.3. PEDOT:PSS
3.4. P3HT
3.5. Other OHTMs
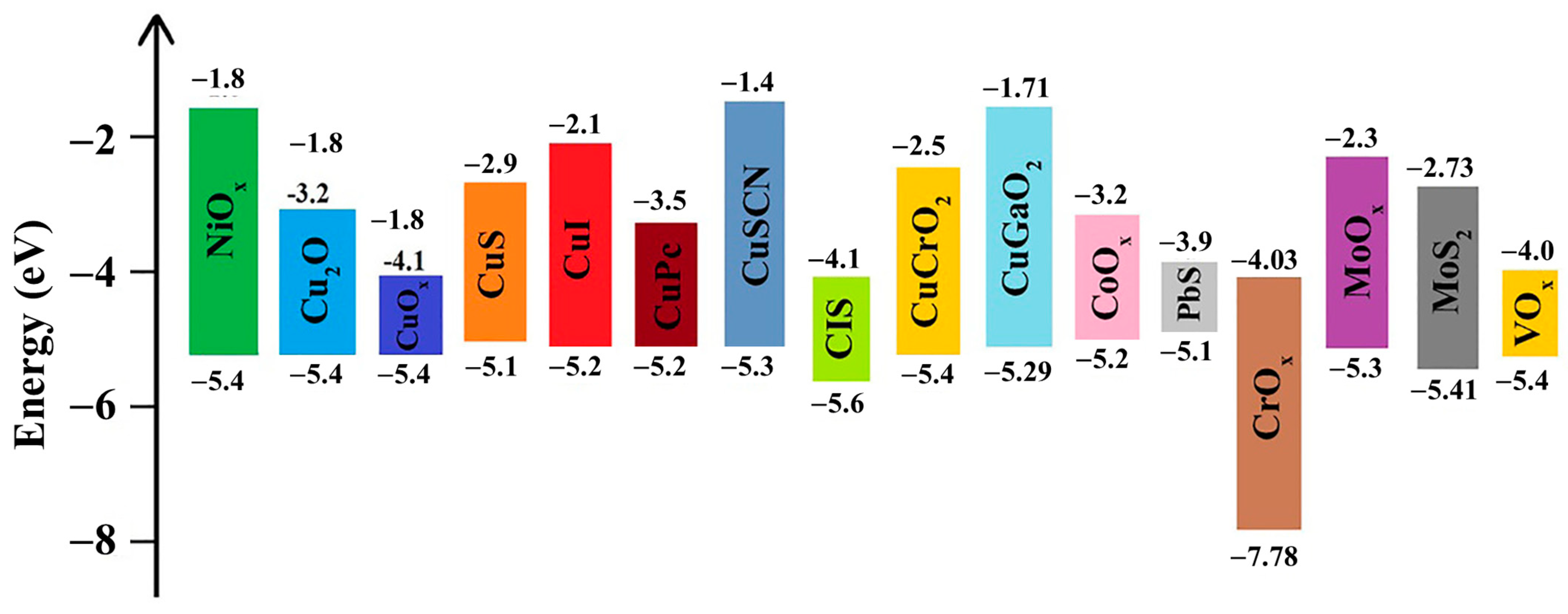
4. Inorganic Hole Transport Layer (IHTL)
4.1. Graphene Oxide and Carbon Derivatives
4.2. Metal Oxides
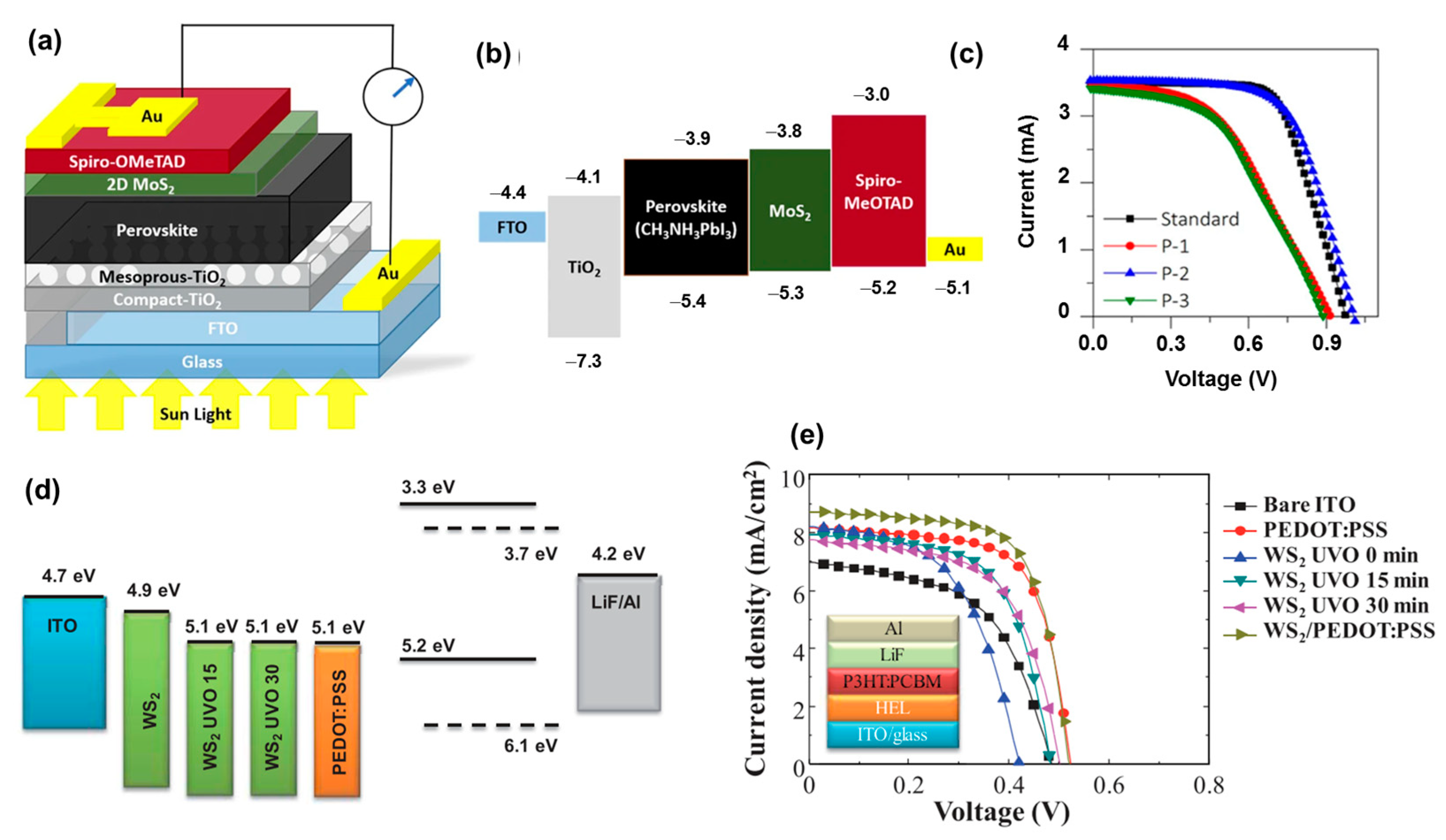
4.3. Transition Metal Sulfides
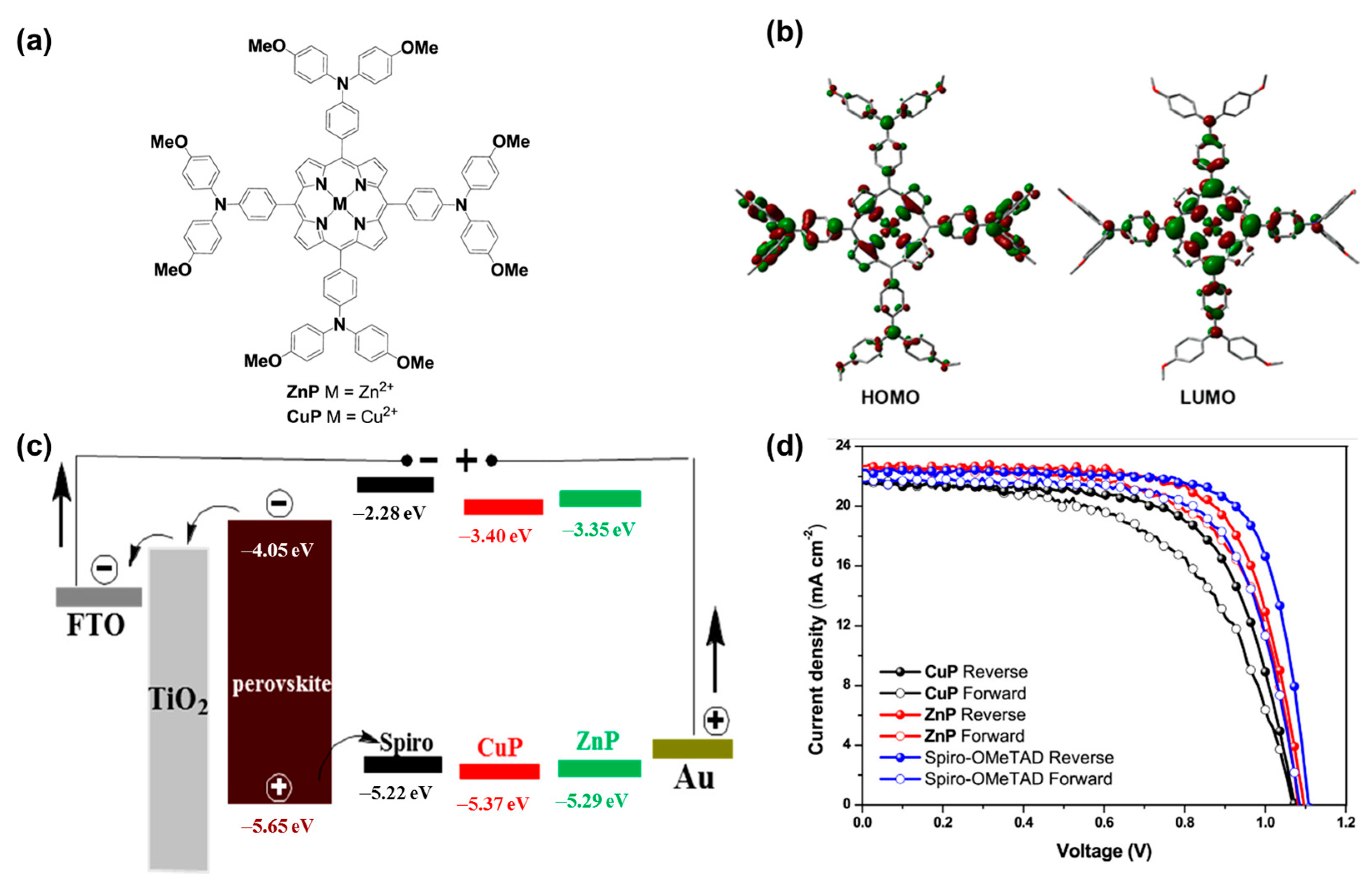
4.4. Organometallic Materials
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Aydin, E.; De Bastiani, M.; Xiao, C.; Isikgor, F.H.; Xue, D.-J.; Chen, B.; Chen, H.; Bahrami, B.; Chowdhury, A.H.; et al. Efficient tandem solar cells with solution-processed perovskite on textured crystalline silicon. Science 2020, 367, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Szabó, G.; Park, N.-G.; De Angelis, F.; Kamat, P.V. Are Perovskite Solar Cells Reaching the Efficiency and Voltage Limits? ACS Energy Lett. 2023, 8, 3829–3831. [Google Scholar] [CrossRef]
- Ikram, M.; Malik, R.; Raees, R.; Imran, M.; Wang, F.; Ali, S.; Khan, M.; Khan, Q.; Maqbool, M. Recent advancements and future insight of lead-free non-toxic perovskite solar cells for sustainable and clean energy production: A review. Sustain. Energy Technol. Assess. 2022, 53, 102433. [Google Scholar] [CrossRef]
- Sharma, D.; Mehra, R.; Raj, B. Comparative study of hole transporting layers commonly used in high-efficiency perovskite solar cells. J. Mater. Sci. 2022, 57, 21172–21191. [Google Scholar] [CrossRef]
- Brabec, C.J.; Gowrisanker, S.; Halls, J.J.M.; Laird, D.; Jia, S.; Williams, S.P. Polymer–Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2010, 22, 3839–3856. [Google Scholar] [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Dou, L.; You, J.; Hong, Z.; Xu, Z.; Li, G.; Street, R.A.; Yang, Y. 25th Anniversary Article: A Decade of Organic/Polymeric Photovoltaic Research. Adv. Mater. 2013, 25, 6642–6671. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, M.; Talebi, P.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; Hashmi, S.G. Advanced research trends in dye-sensitized solar cells. J. Mater. Chem. A 2021, 9, 10527–10545. [Google Scholar] [CrossRef]
- Li, S.; Cao, Y.-L.; Li, W.-H.; Bo, Z.-S. A brief review of hole transporting materials commonly used in perovskite solar cells. Rare Met. 2021, 40, 2712–2729. [Google Scholar] [CrossRef]
- Liao, K.; Li, C.; Xie, L.; Yuan, Y.; Wang, S.; Cao, Z.; Ding, L.; Hao, F. Hot-Casting Large-Grain Perovskite Film for Efficient Solar Cells: Film Formation and Device Performance. Nanomicro Lett. 2020, 12, 156. [Google Scholar] [CrossRef]
- Anrango-Camacho, C.; Pavón-Ipiales, K.; Frontana-Uribe, B.A.; Palma-Cando, A. Recent Advances in Hole-Transporting Layers for Organic Solar Cells. Nanomaterials 2022, 12, 443. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yuan, F.; Zhou, D.; Liao, X.; Chen, L.; Chen, Y. Hole transport layers for organic solar cells: Recent progress and prospects. J. Mater. Chem. A 2020, 8, 11478–11492. [Google Scholar] [CrossRef]
- Rombach, F.M.; Haque, S.A.; Macdonald, T.J. Lessons learned from spiro-OMeTAD and PTAA in perovskite solar cells. Energy Environ. Sci. 2021, 14, 5161–5190. [Google Scholar] [CrossRef]
- Yin, X.; Song, J.; Hu, L.; Jin, Y.; Lu, L.; Su, Z.; Li, Z. Novel dopant-free hole transport materials enabling 20.9% efficiency in perovskite solar cells. Chem. Commun. 2022, 58, 7940–7943. [Google Scholar] [CrossRef]
- Krishna, A.; Grimsdale, A.C. Hole transporting materials for mesoscopic perovskite solar cells—Towards a rational design? J. Mater. Chem. A 2017, 5, 16446–16466. [Google Scholar] [CrossRef]
- Park, G.; Kim, D.; Kim, G.; Jeong, U. High-Performance Indium–Tin Oxide (ITO) Electrode Enabled by a Counteranion-Free Metal–Polymer Complex. ACS Nanosci. Au 2022, 2, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Shrotriya, V.; Li, G.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. Accurate Measurement and Characterization of Organic Solar Cells. Adv. Funct. Mater. 2006, 16, 2016–2023. [Google Scholar] [CrossRef]
- Sun, L.; Fukuda, K.; Someya, T. Recent progress in solution-processed flexible organic photovoltaics. Npj Flex. Electron. 2022, 6, 89. [Google Scholar] [CrossRef]
- Nowsherwan, G.A.; Samad, A.; Iqbal, M.A.; Mushtaq, T.; Hussain, A.; Malik, M.; Haider, S.; Pham, P.V.; Choi, J.R. Performance Analysis and Optimization of a PBDB-T:ITIC Based Organic Solar Cell Using Graphene Oxide as the Hole Transport Layer. Nanomaterials 2022, 12, 1767. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- Hawash, Z.; Ono, L.K.; Qi, Y. Recent Advances in Spiro-MeOTAD Hole Transport Material and Its Applications in Organic–Inorganic Halide Perovskite Solar Cells. Adv. Mater. Interfaces 2018, 5, 1700623. [Google Scholar] [CrossRef]
- Schloemer, T.H.; Christians, J.A.; Luther, J.M.; Sellinger, A. Doping strategies for small molecule organic hole-transport materials: Impacts on perovskite solar cell performance and stability. Chem. Sci. 2019, 10, 1904–1935. [Google Scholar] [CrossRef]
- Cappel, U.B.; Daeneke, T.; Bach, U. Oxygen-Induced Doping of Spiro-MeOTAD in Solid-State Dye-Sensitized Solar Cells and Its Impact on Device Performance. Nano Lett. 2012, 12, 4925–4931. [Google Scholar] [CrossRef]
- Fantacci, S.; De Angelis, F.; Nazeeruddin, M.K.; Grätzel, M. Electronic and Optical Properties of the Spiro-MeOTAD Hole Conductor in Its Neutral and Oxidized Forms: A DFT/TDDFT Investigation. J. Phys. Chem. C 2011, 115, 23126–23133. [Google Scholar] [CrossRef]
- Schulz, P.; Edri, E.; Kirmayer, S.; Hodes, G.; Cahen, D.; Kahn, A. Interface energetics in organo-metal halide perovskite-based photovoltaic cells. Energy Environ. Sci. 2014, 7, 1377–1381. [Google Scholar] [CrossRef]
- Ono, L.K.; Raga, S.R.; Remeika, M.; Winchester, A.J.; Gabe, A.; Qi, Y. Pinhole-free hole transport layers significantly improve the stability of MAPbI3-based perovskite solar cells under operating conditions. J. Mater. Chem. A 2015, 3, 15451–15456. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Leyden, M.R.; Wang, S.; Ono, L.K.; Hawash, Z.; Qi, Y. Role of the Dopants on the Morphological and Transport Properties of Spiro-MeOTAD Hole Transport Layer. Chem. Mater. 2016, 28, 5702–5709. [Google Scholar] [CrossRef]
- Jung, E.H.; Jeon, N.J.; Park, E.Y.; Moon, C.S.; Shin, T.J.; Yang, T.-Y.; Noh, J.H.; Seo, J. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene). Nature 2019, 567, 511–515. [Google Scholar] [CrossRef]
- Urieta-Mora, J.; García-Benito, I.; Molina-Ontoria, A.; Martín, N. Hole transporting materials for perovskite solar cells: A chemical approach. Chem. Soc. Rev. 2018, 47, 8541–8571. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, B.; Shi, L.; Zhou, S.; Xu, J.; Liu, Z.; Yun, J.S.; Choi, E.; Zhang, M.; Lv, Y.; et al. Perovskite solar cells based on spiro-OMeTAD stabilized with an alkylthiol additive. Nat. Photonics 2023, 17, 96–105. [Google Scholar] [CrossRef]
- Pham, N.D.; Shang, J.; Yang, Y.; Hoang, M.T.; Tiong, V.T.; Wang, X.; Fan, L.; Chen, P.; Kou, L.; Wang, L.; et al. Alkaline-earth bis(trifluoromethanesulfonimide) additives for efficient and stable perovskite solar cells. Nano Energy 2020, 69, 104412. [Google Scholar] [CrossRef]
- Seo, J.-Y.; Kim, H.-S.; Akin, S.; Stojanovic, M.; Simon, E.; Fleischer, M.; Hagfeldt, A.; Zakeeruddin, S.M.; Grätzel, M. Novel p-dopant toward highly efficient and stable perovskite solar cells. Energy Environ. Sci. 2018, 11, 2985–2992. [Google Scholar] [CrossRef]
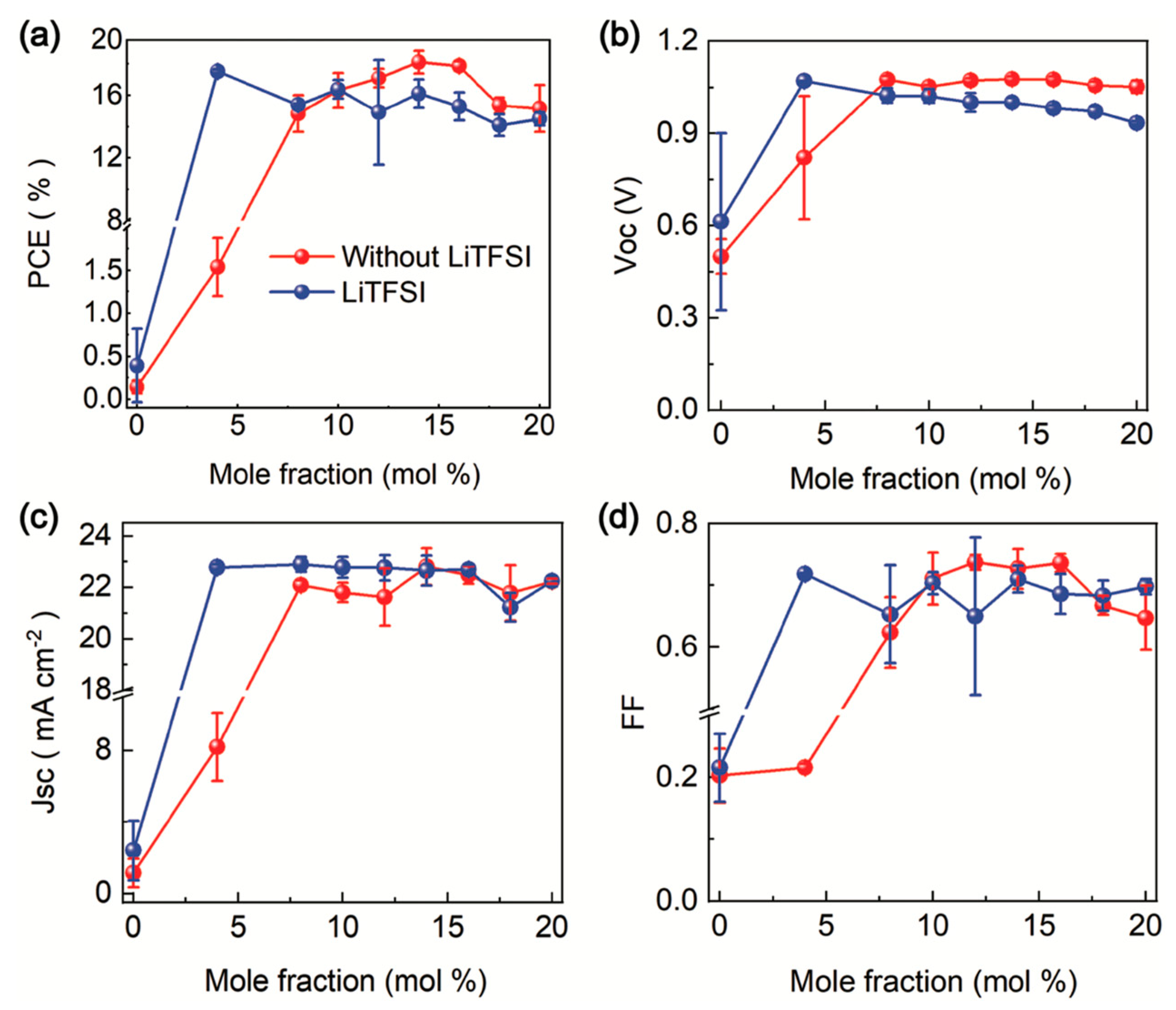
- Tan, B.; Raga, S.R.; Chesman, A.S.R.; Fürer, S.O.; Zheng, F.; McMeekin, D.P.; Jiang, L.; Mao, W.; Lin, X.; Wen, X.; et al. LiTFSI-Free Spiro-OMeTAD-Based Perovskite Solar Cells with Power Conversion Efficiencies Exceeding 19%. Adv. Energy Mater. 2019, 9, 1901519. [Google Scholar] [CrossRef]
- Kasparavicius, E.; Magomedov, A.; Malinauskas, T.; Getautis, V. Long-Term Stability of the Oxidized Hole-Transporting Materials used in Perovskite Solar Cells. Chem.—Eur. J. 2018, 24, 9910–9918. [Google Scholar] [CrossRef]
- Lamberti, F.; Gatti, T.; Cescon, E.; Sorrentino, R.; Rizzo, A.; Menna, E.; Meneghesso, G.; Meneghetti, M.; Petrozza, A.; Franco, L. Evidence of Spiro-OMeTAD De-doping by tert-Butylpyridine Additive in Hole-Transporting Layers for Perovskite Solar Cells. Chem 2019, 5, 1806–1817. [Google Scholar] [CrossRef]
- Malinauskas, T.; Tomkute-Luksiene, D.; Sens, R.; Daskeviciene, M.; Send, R.; Wonneberger, H.; Jankauskas, V.; Bruder, I.; Getautis, V. Enhancing Thermal Stability and Lifetime of Solid-State Dye-Sensitized Solar Cells via Molecular Engineering of the Hole-Transporting Material Spiro-OMeTAD. ACS Appl. Mater. Interfaces 2015, 7, 11107–11116. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Yun, J.H.; Son, H.J.; Kim, T.-S. Accelerated Degradation Due to Weakened Adhesion from Li-TFSI Additives in Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 7029–7035. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Lee, I.; Kim, T.-S.; Ko, M.J.; Kim, J.Y.; Son, H.J. Synergistic enhancement and mechanism study of mechanical and moisture stability of perovskite solar cells introducing polyethylene-imine into the CH3NH3PbI3/HTM interface. J. Mater. Chem. A 2015, 3, 22176–22182. [Google Scholar] [CrossRef]
- Wei, D.; Wang, T.; Ji, J.; Li, M.; Cui, P.; Li, Y.; Li, G.; Mbengue, J.M.; Song, D. Photo-induced degradation of lead halide perovskite solar cells caused by the hole transport layer/metal electrode interface. J. Mater. Chem. A 2016, 4, 1991–1998. [Google Scholar] [CrossRef]
- Lin, C.-T.; Ngiam, J.; Xu, B.; Chang, Y.-H.; Du, T.; Macdonald, T.J.; Durrant, J.R.; McLachlan, M.A. Enhancing the operational stability of unencapsulated perovskite solar cells through Cu–Ag bilayer electrode incorporation. J. Mater. Chem. A 2020, 8, 8684–8691. [Google Scholar] [CrossRef]
- Jeon, N.J.; Lee, H.G.; Kim, Y.C.; Seo, J.; Noh, J.H.; Lee, J.; Seok, S.I. o-Methoxy Substituents in Spiro-OMeTAD for Efficient Inorganic–Organic Hybrid Perovskite Solar Cells. J. Am. Chem. Soc. 2014, 136, 7837–7840. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Salehi-Abar, P. Design of two novel hole transport materials via replacing the core of spiro-OMeTAD with tetrathiafulvalene and tetraazafulvalene for application in perovskite solar cells. Sol. Energy 2018, 173, 132–138. [Google Scholar] [CrossRef]
- Kadi, Z.; Wang, R.; Berton, N.; Kobeissi, M.; Jiang, Y.; Gao, J.; Schmaltz, B. Interface compatibility: How to outperform classical spiro-OMeTAD in perovskite solar cells with carbazole derivatives. J. Mater. Chem. C 2022, 10, 7680–7689. [Google Scholar] [CrossRef]
- Wu, F.; Wang, B.; Wang, R.; Shan, Y.; Liu, D.; Wong, K.Y.; Chen, T.; Zhu, L. Investigation on a dopant-free hole transport material for perovskite solar cells. RSC Adv. 2016, 6, 69365–69369. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, J.; Hua, Y.; Liu, P.; Wang, L.; Ruan, C.; Li, Y.; Boschloo, G.; Johansson, E.M.J.; Kloo, L.; et al. Tailor-Making Low-Cost Spiro[fluorene-9,9′-xanthene]-Based 3D Oligomers for Perovskite Solar Cells. Chem 2017, 2, 676–687. [Google Scholar] [CrossRef]
- Fu, Y.; Li, Y.; Zeng, Q.; Wu, H.; Wang, L.; Tang, H.; Xing, G.; Cao, D. Influence of donor units on spiro[fluorene-9,9′-xanthene]-based dopant-free hole transporting materials for perovskite solar cells. Sol. Energy 2021, 216, 180–187. [Google Scholar] [CrossRef]
- Bi, D.; Xu, B.; Gao, P.; Sun, L.; Grätzel, M.; Hagfeldt, A. Facile synthesized organic hole transporting material for perovskite solar cell with efficiency of 19.8%. Nano Energy 2016, 23, 138–144. [Google Scholar] [CrossRef]
- Saliba, M.; Orlandi, S.; Matsui, T.; Aghazada, S.; Cavazzini, M.; Correa-Baena, J.-P.; Gao, P.; Scopelliti, R.; Mosconi, E.; Dahmen, K.-H.; et al. A molecularly engineered hole-transporting material for efficient perovskite solar cells. Nat. Energy 2016, 1, 15017. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, B.; Yang, L.; Ruan, C.; Wang, L.; Liu, P.; Zhang, W.; Vlachopoulos, N.; Kloo, L.; Boschloo, G.; et al. The Importance of Pendant Groups on Triphenylamine-Based Hole Transport Materials for Obtaining Perovskite Solar Cells with over 20% Efficiency. Adv. Energy Mater. 2018, 8, 1701209. [Google Scholar] [CrossRef]
- Jeong, M.; Choi, I.W.; Yim, K.; Jeong, S.; Kim, M.; Choi, S.J.; Cho, Y.; An, J.-H.; Kim, H.-B.; Jo, Y.; et al. Large-area perovskite solar cells employing spiro-Naph hole transport material. Nat. Photonics 2022, 16, 119–125. [Google Scholar] [CrossRef]
- Nakka, L.; Cheng, Y.; Aberle, A.G.; Lin, F. Analytical Review of Spiro-OMeTAD Hole Transport Materials: Paths Toward Stable and Efficient Perovskite Solar Cells. Adv. Energy Sustain. Res. 2022, 3, 2200045. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Liu, T.; Zeng, Q.; Cao, D.; Pan, H.; Xing, G. Interfacial Engineering of PTAA/Perovskites for Improved Crystallinity and Hole Extraction in Inverted Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 3284–3292. [Google Scholar] [CrossRef]
- Ko, Y.; Kim, Y.; Lee, C.; Kim, Y.; Jun, Y. Investigation of Hole-Transporting Poly(triarylamine) on Aggregation and Charge Transport for Hysteresisless Scalable Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 11633–11641. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical Management for Colorful, Efficient, and Stable Inorganic–Organic Hybrid Nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef]
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.-S.; Chang, J.A.; Lee, Y.H.; Kim, H.-j.; Sarkar, A.; Nazeeruddin, M.K.; et al. Efficient inorganic–organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photonics 2013, 7, 486–491. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, R.; Zhang, Z.; Xiong, J.; He, Z.; Fan, B.; Dai, Z.; Yang, B.; Xue, X.; Cai, P.; et al. Achieving efficient inverted planar perovskite solar cells with nondoped PTAA as a hole transport layer. Org. Electron. 2019, 71, 106–112. [Google Scholar] [CrossRef]
- Batmunkh, M.; Shearer, C.J.; Biggs, M.J.; Shapter, J.G. Nanocarbons for mesoscopic perovskite solar cells. J. Mater. Chem. A 2015, 3, 9020–9031. [Google Scholar] [CrossRef]
- Lee, I.; Rolston, N.; Brunner, P.-L.; Dauskardt, R.H. Hole-Transport Layer Molecular Weight and Doping Effects on Perovskite Solar Cell Efficiency and Mechanical Behavior. ACS Appl. Mater. Interfaces 2019, 11, 23757–23764. [Google Scholar] [CrossRef] [PubMed]
- Brauer, J.C.; Lee, Y.H.; Nazeeruddin, M.K.; Banerji, N. Charge Transfer Dynamics from Organometal Halide Perovskite to Polymeric Hole Transport Materials in Hybrid Solar Cells. J. Phys. Chem. Lett. 2015, 6, 3675–3681. [Google Scholar] [CrossRef] [PubMed]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Ryan, J.W.; Miyano, K. Exploring the effects of interfacial carrier transport layers on device performance and optoelectronic properties of planar perovskite solar cells. J. Mater. Chem. C 2017, 5, 8819–8827. [Google Scholar] [CrossRef]
- Yang, W.; Zhong, D.; Shi, M.; Qu, S.; Chen, H. Toward Highly Thermal Stable Perovskite Solar Cells by Rational Design of Interfacial Layer. iScience 2019, 22, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, Z.; Matteocci, F.; Lamanna, E.; Di Girolamo, D.; Marrani, A.G.; Zanoni, R.; Di Carlo, A.; Moshaii, A. Light-induced improvement of dopant-free PTAA on performance of inverted perovskite solar cells. Sol. Energy Mater. Sol. Cells 2020, 215, 110606. [Google Scholar] [CrossRef]
- Xia, Y.; Dai, S. Review on applications of PEDOTs and PEDOT:PSS in perovskite solar cells. J. Mater. Sci. Mater. Electron. 2021, 32, 12746–12757. [Google Scholar] [CrossRef]
- Reza, K.M.; Gurung, A.; Bahrami, B.; Mabrouk, S.; Elbohy, H.; Pathak, R.; Chen, K.; Chowdhury, A.H.; Rahman, M.T.; Letourneau, S.; et al. Tailored PEDOT:PSS hole transport layer for higher performance in perovskite solar cells: Enhancement of electrical and optical properties with improved morphology. J. Energy Chem. 2020, 44, 41–50. [Google Scholar] [CrossRef]
- Chin, Y.-C.; Daboczi, M.; Henderson, C.; Luke, J.; Kim, J.-S. Suppressing PEDOT:PSS Doping-Induced Interfacial Recombination Loss in Perovskite Solar Cells. ACS Energy Lett. 2022, 7, 560–568. [Google Scholar] [CrossRef]
- Ha, S.R.; Park, S.; Oh, J.T.; Kim, D.H.; Cho, S.; Bae, S.Y.; Kang, D.-W.; Kim, J.-M.; Choi, H. Water-resistant PEDOT:PSS hole transport layers by incorporating a photo-crosslinking agent for high-performance perovskite and polymer solar cells. Nanoscale 2018, 10, 13187–13193. [Google Scholar] [CrossRef]
- Xu, D.; Gong, Z.; Jiang, Y.; Feng, Y.; Wang, Z.; Gao, X.; Lu, X.; Zhou, G.; Liu, J.-M.; Gao, J. Constructing molecular bridge for high-efficiency and stable perovskite solar cells based on P3HT. Nat. Commun. 2022, 13, 7020. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, F.; Renno, G.; Taheri, B.; Yaghoobi Nia, N.; Ilieva, V.; Fin, A.; Di Carlo, A.; Bonomo, M.; Barolo, C.; Brunetti, F. Modified P3HT materials as hole transport layers for flexible perovskite solar cells. J. Power Sources 2021, 494, 229735. [Google Scholar] [CrossRef]
- Nia, N.Y.; Matteocci, F.; Cina, L.; Di Carlo, A. High-Efficiency Perovskite Solar Cell Based on Poly(3-Hexylthiophene): Influence of Molecular Weight and Mesoscopic Scaffold Layer. ChemSusChem 2017, 10, 3854–3860. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, Q.; Li, M.; Shan, H.; Feng, Y.; Xu, Z.-X. Poly(3-hexylthiophene)/Gold Nanorod Composites as Efficient Hole-Transporting Materials for Perovskite Solar Cells. Sol. RRL 2020, 4, 2000109. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wu, B.; Pang, S.; Yuan, X.; Duan, C.; Huang, F.; Cao, Y. High-Efficiency P3HT-Based All-Polymer Solar Cells with a Thermodynamically Miscible Polymer Acceptor. Sol. RRL 2022, 6, 2200073. [Google Scholar] [CrossRef]
- Song, J.; Xie, H.; Lim, E.L.; Li, Y.; Kong, T.; Zhang, Y.; Zhou, X.; Duan, C.; Bi, D. Multistrategy Toward Highly Efficient and Stable CsPbI2Br Perovskite Solar Cells Based on Dopant-Free Poly(3-Hexylthiophene). Sol. RRL 2022, 6, 2100880. [Google Scholar] [CrossRef]
- Nayana, V.; Kandasubramanian, B. Polycarbazole and its derivatives: Progress, synthesis, and applications. J. Polym. Res. 2020, 27, 285. [Google Scholar] [CrossRef]
- Magaldi, D.; Ulfa, M.; Péralta, S.; Goubard, F.; Pauporté, T.; Bui, T.-T. Carbazole-based material: Synthesis, characterization, and application as hole transporting material in perovskite solar cells. J. Mater. Sci. Mater. Electron. 2021, 32, 12856–12861. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Rahman, S.; Pasanen, H.P.; Tian, J.; Li, J.; Deng, Z.; Zhang, H.; Vivo, P. Hydrogen bonding drives the self-assembling of carbazole-based hole-transport material for enhanced efficiency and stability of perovskite solar cells. Nano Energy 2022, 101, 107604. [Google Scholar] [CrossRef]
- He, L.; Wang, D.; Zhao, Y.; Zhang, Y.; Wei, W.; Shen, L. Efficient hole transport layers based on cross-linked poly(N-vinylcarbazole) for high-performance perovskite photodetectors. J. Mater. Chem. C 2021, 9, 11722–11728. [Google Scholar] [CrossRef]
- Kim, G.-W.; Kang, G.; Kim, J.; Lee, G.-Y.; Kim, H.I.; Pyeon, L.; Lee, J.; Park, T. Dopant-free polymeric hole transport materials for highly efficient and stable perovskite solar cells. Energy Environ. Sci. 2016, 9, 2326–2333. [Google Scholar] [CrossRef]
- Li, M.; Ni, W.; Wan, X.; Zhang, Q.; Kan, B.; Chen, Y. Benzo[1,2-b:4,5-b′]dithiophene (BDT)-based small molecules for solution processed organic solar cells. J. Mater. Chem. A 2015, 3, 4765–4776. [Google Scholar] [CrossRef]
- Yin, X.; Zhou, J.; Song, Z.; Dong, Z.; Bao, Q.; Shrestha, N.; Bista, S.S.; Ellingson, R.J.; Yan, Y.; Tang, W. Dithieno[3,2-b:2′,3′-d]pyrrol-Cored Hole Transport Material Enabling Over 21% Efficiency Dopant-Free Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1904300. [Google Scholar] [CrossRef]
- Zhao, B.X.; Yao, C.; Gu, K.; Liu, T.; Xia, Y.; Loo, Y.-L. A hole-transport material that also passivates perovskite surface defects for solar cells with improved efficiency and stability. Energy Environ. Sci. 2020, 13, 4334–4343. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, L.; Mu, X.; Chen, C.-C.; Wu, Y.; Cao, J.; Tang, Y. Intramolecular Electric Field Construction in Metal Phthalocyanine as Dopant-Free Hole Transporting Material for Stable Perovskite Solar Cells with >21 % Efficiency. Angew. Chem. Int. Ed. 2021, 60, 6294–6299. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Xu, Z.; Tang, X.; Liu, T.; Dong, X.; Zhang, X.; Zheng, N.; Xie, Z.; Liu, Y. Multifunctional Two-Dimensional Conjugated Materials for Dopant-Free Perovskite Solar Cells with Efficiency Exceeding 22%. ACS Energy Lett. 2021, 6, 1521–1532. [Google Scholar] [CrossRef]
- Lin, H.-S.; Doba, T.; Sato, W.; Matsuo, Y.; Shang, R.; Nakamura, E. Triarylamine/Bithiophene Copolymer with Enhanced Quinoidal Character as Hole-Transporting Material for Perovskite Solar Cells. Angew. Chem. Int. Ed. 2022, 61, e202203949. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Dar, M.I.; Hinderhofer, A.; Pellet, N.; Schreiber, F.; Zakeeruddin, S.M.; Grätzel, M. Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%. Science 2017, 358, 768–771. [Google Scholar] [CrossRef]
- Li, R.; Wang, P.; Chen, B.; Cui, X.; Ding, Y.; Li, Y.; Zhang, D.; Zhao, Y.; Zhang, X. NiOx/Spiro Hole Transport Bilayers for Stable Perovskite Solar Cells with Efficiency Exceeding 21%. ACS Energy Lett. 2020, 5, 79–86. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Noel, N.K.; Larson, B.W.; Reid, O.G.; Blackburn, J.L. Rapid Charge-Transfer Cascade through SWCNT Composites Enabling Low-Voltage Losses for Perovskite Solar Cells. ACS Energy Lett. 2019, 4, 1872–1879. [Google Scholar] [CrossRef]
- Duong, T.; Peng, J.; Walter, D.; Xiang, J.; Shen, H.; Chugh, D.; Lockrey, M.; Zhong, D.; Li, J.; Weber, K.; et al. Perovskite Solar Cells Employing Copper Phthalocyanine Hole-Transport Material with an Efficiency over 20% and Excellent Thermal Stability. ACS Energy Lett. 2018, 3, 2441–2448. [Google Scholar] [CrossRef]
- Lou, Q.; Lou, G.; Guo, H.; Sun, T.; Wang, C.; Chai, G.; Chen, X.; Yang, G.; Guo, Y.; Zhou, H. Enhanced Efficiency and Stability of n-i-p Perovskite Solar Cells by Incorporation of Fluorinated Graphene in the Spiro-OMeTAD Hole Transport Layer. Adv. Energy Mater. 2022, 12, 2201344. [Google Scholar] [CrossRef]
- Yin, X.; Song, Z.; Li, Z.; Tang, W. Toward ideal hole transport materials: A review on recent progress in dopant-free hole transport materials for fabricating efficient and stable perovskite solar cells. Energy Environ. Sci. 2020, 13, 4057–4086. [Google Scholar] [CrossRef]
- Zhang, B.-W.; Lin, H.-S.; Qiu, X.-Y.; Shui, Q.-J.; Zheng, Y.-J.; Almesfer, M.; Kauppinen, E.I.; Matsuo, Y.; Maruyama, S. Spiro-OMeTAD versus PTAA for single-walled carbon nanotubes electrode in perovskite solar cells. Carbon 2023, 205, 321–327. [Google Scholar] [CrossRef]
- Wassei, J.K.; Kaner, R.B. Graphene, a promising transparent conductor. Mater. Today 2010, 13, 52–59. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Naghdi, S.; Sanchez-Arriaga, G.; Rhee, K.Y. Tuning the work function of graphene toward application as anode and cathode. J. Alloys Compd. 2019, 805, 1117–1134. [Google Scholar] [CrossRef]
- Sygellou, L.; Paterakis, G.; Galiotis, C.; Tasis, D. Work Function Tuning of Reduced Graphene Oxide Thin Films. J. Phys. Chem. C 2016, 120, 281–290. [Google Scholar] [CrossRef]
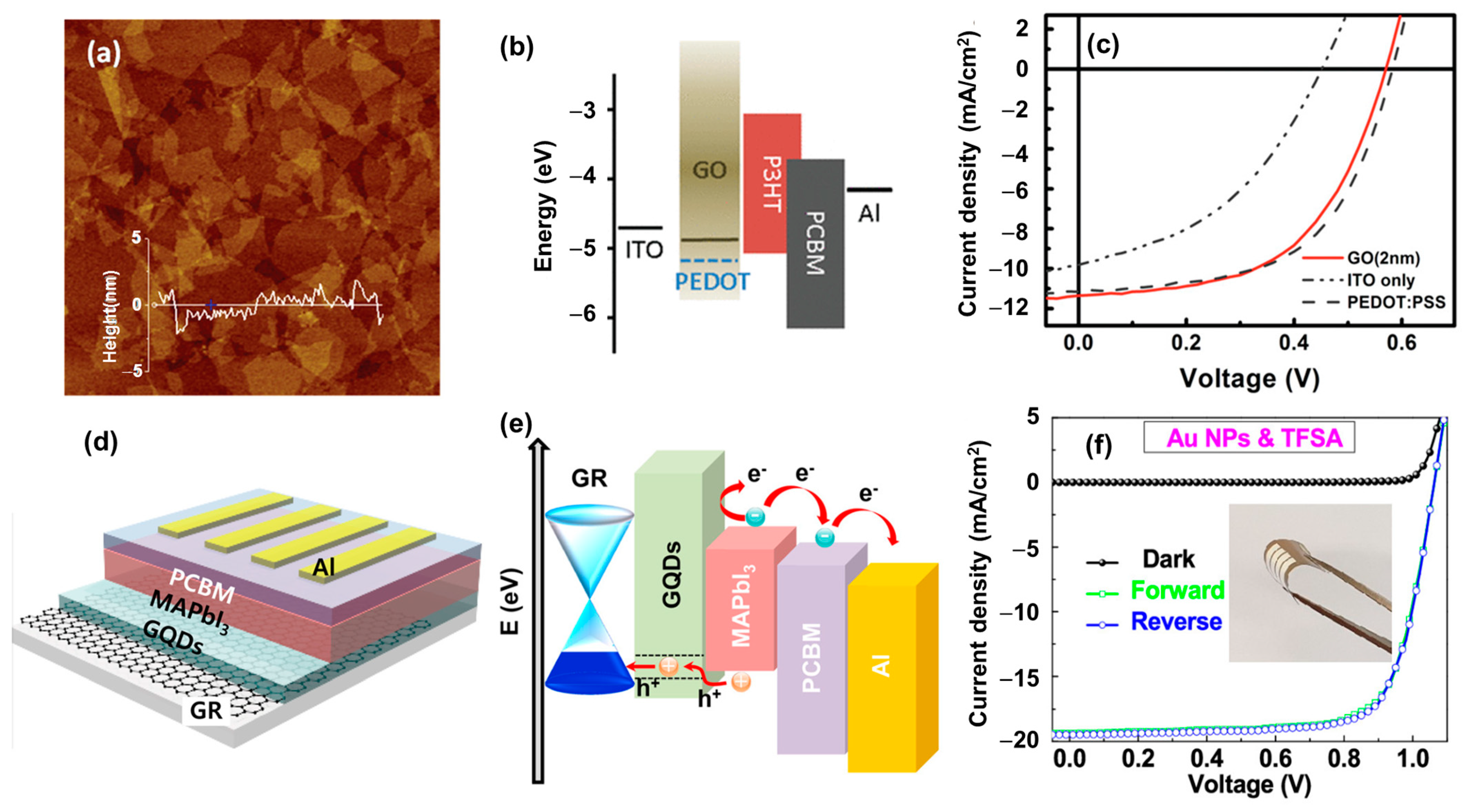
- Li, S.-S.; Tu, K.-H.; Lin, C.-C.; Chen, C.-W.; Chhowalla, M. Solution-Processable Graphene Oxide as an Efficient Hole Transport Layer in Polymer Solar Cells. ACS Nano 2010, 4, 3169–3174. [Google Scholar] [CrossRef] [PubMed]
- Rafique, S.; Abdullah, S.M.; Shahid, M.M.; Ansari, M.O.; Sulaiman, K. Significantly improved photovoltaic performance in polymer bulk heterojunction solar cells with graphene oxide/PEDOT:PSS double decked hole transport layer. Sci. Rep. 2017, 7, 39555. [Google Scholar] [CrossRef]
- Yu, J.C.; Jang, J.I.; Lee, B.R.; Lee, G.-W.; Han, J.T.; Song, M.H. Highly Efficient Polymer-Based Optoelectronic Devices Using PEDOT:PSS and a GO Composite Layer as a Hole Transport Layer. ACS Appl. Mater. Interfaces 2014, 6, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xue, Y.; Dai, L. Sulfated Graphene Oxide as a Hole-Extraction Layer in High-Performance Polymer Solar Cells. J. Phys. Chem. Lett. 2012, 3, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, E.; Savva, K.; Konios, D.; Petridis, C.; Kymakis, E. Improving the efficiency of organic photovoltaics by tuning the work function of graphene oxide hole transporting layers. Nanoscale 2014, 6, 6925–6931. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Yun, J.-M.; Kim, D.-Y.; Na, S.-I.; Kim, S.-S. Moderately reduced graphene oxide as hole transport layer in polymer solar cells via thermal assisted spray process. Appl. Surf. Sci. 2014, 296, 140–146. [Google Scholar] [CrossRef]
- Jung, J.W.; Son, S.H.; Choi, J. Polyaniline/Reduced Graphene Oxide Composites for Hole Transporting Layer of High-Performance Inverted Perovskite Solar Cells. Polymers 2021, 13, 1281. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.A.; Jung, E.D.; Yu, J.C.; Kim, D.W.; Nam, Y.S.; Oh, I.; Lee, E.; Yoo, J.-W.; Cho, S.; Song, M.H. Improved Efficiency of Perovskite Solar Cells Using a Nitrogen-Doped Graphene-Oxide-Treated Tin Oxide Layer. ACS Appl. Mater. Interfaces 2020, 12, 2417–2423. [Google Scholar] [CrossRef]
- Soltan, M.S.; Ismail, N.; Hassan, H.M.A.; Shawky, A.; El-Sharkawy, E.A.; Youssef, R.M.; Maruyama, S.; Elshaarawy, R.F.M. Copper nanoparticle-decorated RGO electrodes as hole transport layer of perovskite solar cells enhancing efficiency and shelf stability. J. Mater. Res. Technol. 2021, 14, 631–638. [Google Scholar] [CrossRef]
- Nicasio-Collazo, J.; Maldonado, J.-L.; Salinas-Cruz, J.; Barreiro-Argüelles, D.; Caballero-Quintana, I.; Vázquez-Espinosa, C.; Romero-Borja, D. Functionalized and reduced graphene oxide as hole transport layer and for use in ternary organic solar cell. Opt. Mater. 2019, 98, 109434. [Google Scholar] [CrossRef]
- Cheng, X.; Long, J.; Wu, R.; Huang, L.; Tan, L.; Chen, L.; Chen, Y. Fluorinated Reduced Graphene Oxide as an Efficient Hole-Transport Layer for Efficient and Stable Polymer Solar Cells. ACS Omega 2017, 2, 2010–2016. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.-S.; Kang, R.; Lee, S.; Jeon, Y.-J.; Myoung, N.; Lee, C.-L.; Kim, D.-Y.; Yun, J.-M.; Seo, Y.-H.; Kim, S.-S.; et al. Highly efficient and stable planar perovskite solar cells with reduced graphene oxide nanosheets as electrode interlayer. Nano Energy 2015, 12, 96–104. [Google Scholar] [CrossRef]
- Luo, H.; Lin, X.; Hou, X.; Pan, L.; Huang, S.; Chen, X. Efficient and Air-Stable Planar Perovskite Solar Cells Formed on Graphene-Oxide-Modified PEDOT:PSS Hole Transport Layer. Nano-Micro Lett. 2017, 9, 39. [Google Scholar] [CrossRef]
- Shin, S.H.; Shin, D.H.; Choi, S.-H. Enhancement of Stability of Inverted Flexible Perovskite Solar Cells by Employing Graphene-Quantum-Dots Hole Transport Layer and Graphene Transparent Electrode Codoped with Gold Nanoparticles and Bis(trifluoromethanesulfonyl)amide. ACS Sustain. Chem. Eng. 2019, 7, 13178–13185. [Google Scholar] [CrossRef]
- Li, W.; Cheng, N.; Cao, Y.; Zhao, Z.; Xiao, Z.; Zi, W.; Sun, Z. Boost the performance of inverted perovskite solar cells with PEDOT:PSS/Graphene quantum dots composite hole transporting layer. Org. Electron. 2020, 78, 105575. [Google Scholar] [CrossRef]
- Magne, T.M.; de Oliveira Vieira, T.; Alencar, L.M.R.; Junior, F.F.M.; Gemini-Piperni, S.; Carneiro, S.V.; Fechine, L.M.U.D.; Freire, R.M.; Golokhvast, K.; Metrangolo, P.; et al. Graphene and its derivatives: Understanding the main chemical and medicinal chemistry roles for biomedical applications. J. Nanostruct. Chem. 2022, 12, 693–727. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shu, H.; Wang, S.; Wang, J. Electronic and Optical Properties of Graphene Quantum Dots: The Role of Many-Body Effects. J. Phys. Chem. C 2015, 119, 4983–4989. [Google Scholar] [CrossRef]
- Maheswaran, R.; Shanmugavel, B.P. A Critical Review of the Role of Carbon Nanotubes in the Progress of Next-Generation Electronic Applications. J. Electron. Mater. 2022, 51, 2786–2800. [Google Scholar] [CrossRef]
- Kalluri, A.; Debnath, D.; Dharmadhikari, B.; Patra, P. Chapter Twelve—Graphene Quantum Dots: Synthesis and Applications. In Methods Enzymol.; Kumar, C.V., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 609, pp. 335–354. [Google Scholar]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Vagena, I.-A.; Pippa, N.; Gazouli, M.; Pavlatou, E.A.; Lagopati, N. The Use of Crystalline Carbon-Based Nanomaterials (CBNs) in Various Biomedical Applications. Crystals 2023, 13, 1236. [Google Scholar] [CrossRef]
- Li, M.; Ni, W.; Kan, B.; Wan, X.; Zhang, L.; Zhang, Q.; Long, G.; Zuo, Y.; Chen, Y. Graphene quantum dots as the hole transport layer material for high-performance organic solar cells. Phys. Chem. Chem. Phys. 2013, 15, 18973–18978. [Google Scholar] [CrossRef]
- Ali, A.; Kazici, M.; Bozar, S.; Asghar, M.A.; Alwadai, N.; Kahveci, C.; Iqbal, M.; Ahmad, A.; Keskin, B.; Shahbaz, M.; et al. Super aligned carbon nanotubes for interfacial modification of hole transport layer in polymer solar cells. Sustain. Mater. Technol. 2023, 35, e00569. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Bhattacharya, A.; Stepanova, D.; Mikhaylov, A.; Grilli, M.L.; Khosravy, M.; Senjyu, T. A Systematic Review of Metal Oxide Applications for Energy and Environmental Sustainability. Metals 2020, 10, 1604. [Google Scholar] [CrossRef]
- Guo, T.; Yao, M.-S.; Lin, Y.-H.; Nan, C.-W. A comprehensive review on synthesis methods for transition-metal oxide nanostructures. CrystEngComm 2015, 17, 3551–3585. [Google Scholar] [CrossRef]
- Reed, J.; Ceder, G. Role of Electronic Structure in the Susceptibility of Metastable Transition-Metal Oxide Structures to Transformation. Chem. Rev. 2004, 104, 4513–4534. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; He, Q.; Ouyang, D.; Fang, G.; Choy, W.C.H.; Li, G. Transition metal oxides as hole-transporting materials in organic semiconductor and hybrid perovskite based solar cells. Sci. China Chem. 2017, 60, 472–489. [Google Scholar] [CrossRef]
- Manders, J.R.; Tsang, S.-W.; Hartel, M.J.; Lai, T.-H.; Chen, S.; Amb, C.M.; Reynolds, J.R.; So, F. Solution-Processed Nickel Oxide Hole Transport Layers in High Efficiency Polymer Photovoltaic Cells. Adv. Funct. Mater. 2013, 23, 2993–3001. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Feng, X.; Guo, X.; Lu, C.; Li, X.; Fang, J. Photoconductive NiOx hole transport layer for efficient perovskite solar cells. Chem. Eng. J. 2022, 435, 135140. [Google Scholar] [CrossRef]
- Di Girolamo, D.; Matteocci, F.; Piccinni, M.; Di Carlo, A.; Dini, D. Anodically electrodeposited NiO nanoflakes as hole selective contact in efficient air processed p-i-n perovskite solar cells. Sol. Energy Mater. Sol. Cells 2020, 205, 110288. [Google Scholar] [CrossRef]
- Sun, J.; Lu, J.; Li, B.; Jiang, L.; Chesman, A.S.R.; Scully, A.D.; Gengenbach, T.R.; Cheng, Y.-B.; Jasieniak, J.J. Inverted perovskite solar cells with high fill-factors featuring chemical bath deposited mesoporous NiO hole transporting layers. Nano Energy 2018, 49, 163–171. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Fan, J.; Djurišić, A.B.; Liu, F.; Tam, H.W.; Ng, A.; Surya, C.; Chan, W.K.; Wang, D.; et al. Understanding the Doping Effect on NiO: Toward High-Performance Inverted Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1703519. [Google Scholar] [CrossRef]
- Li, G.; Jiang, Y.; Deng, S.; Tam, A.; Xu, P.; Wong, M.; Kwok, H.-S. Overcoming the Limitations of Sputtered Nickel Oxide for High-Efficiency and Large-Area Perovskite Solar Cells. Adv. Sci. 2017, 4, 1700463. [Google Scholar] [CrossRef] [PubMed]
- Yates, H.M.; Meroni, S.M.P.; Raptis, D.; Hodgkinson, J.L.; Watson, T.M. Flame assisted chemical vapour deposition NiO hole transport layers for mesoporous carbon perovskite cells. J. Mater. Chem. C 2019, 7, 13235–13242. [Google Scholar] [CrossRef]
- Parthiban, S.; Kim, S.; Tamilavan, V.; Lee, J.; Shin, I.; Yuvaraj, D.; Jung, Y.K.; Hyun, M.H.; Jeong, J.H.; Park, S.H. Enhanced efficiency and stability of polymer solar cells using solution-processed nickel oxide as hole transport material. Curr. Appl. Phys. 2017, 17, 1232–1237. [Google Scholar] [CrossRef]
- Kim, J.K. PEG-assisted Sol-gel Synthesis of Compact Nickel Oxide Hole-Selective Layer with Modified Interfacial Properties for Organic Solar Cells. Polymers 2019, 11, 120. [Google Scholar] [CrossRef]
- Xu, L.; Chen, X.; Jin, J.; Liu, W.; Dong, B.; Bai, X.; Song, H.; Reiss, P. Inverted perovskite solar cells employing doped NiO hole transport layers: A review. Nano Energy 2019, 63, 103860. [Google Scholar] [CrossRef]
- Liu, Z.; Chang, J.; Lin, Z.; Zhou, L.; Yang, Z.; Chen, D.; Zhang, C.; Liu, S.; Hao, Y. High-Performance Planar Perovskite Solar Cells Using Low Temperature, Solution–Combustion-Based Nickel Oxide Hole Transporting Layer with Efficiency Exceeding 20%. Adv. Energy Mater. 2018, 8, 1703432. [Google Scholar] [CrossRef]
- Boyd, C.C.; Shallcross, R.C.; Moot, T.; Kerner, R.; Bertoluzzi, L.; Onno, A.; Kavadiya, S.; Chosy, C.; Wolf, E.J.; Werner, J.; et al. Overcoming Redox Reactions at Perovskite-Nickel Oxide Interfaces to Boost Voltages in Perovskite Solar Cells. Joule 2020, 4, 1759–1775. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.; Shen, S.; Tang, Y.; Yu, A.; Kang, B.; Silva, S.R.P.; Lu, G. Enhancing the performance of polymer solar cells using solution-processed copper doped nickel oxide nanoparticles as hole transport layer. J. Colloid Interface Sci. 2019, 535, 308–317. [Google Scholar] [CrossRef]
- Ouyang, D.; Zheng, J.; Huang, Z.; Zhu, L.; Choy, W.C.H. An efficacious multifunction codoping strategy on a room-temperature solution-processed hole transport layer for realizing high-performance perovskite solar cells. J. Mater. Chem. A 2021, 9, 371–379. [Google Scholar] [CrossRef]
- Wan, X.; Jiang, Y.; Qiu, Z.; Zhang, H.; Zhu, X.; Sikandar, I.; Liu, X.; Chen, X.; Cao, B. Zinc as a New Dopant for NiOx-Based Planar Perovskite Solar Cells with Stable Efficiency near 20%. ACS Appl. Energy Mater. 2018, 1, 3947–3954. [Google Scholar] [CrossRef]
- Saki, Z.; Sveinbjörnsson, K.; Boschloo, G.; Taghavinia, N. The Effect of Lithium Doping in Solution-Processed Nickel Oxide Films for Perovskite Solar Cells. ChemPhysChem 2019, 20, 3322–3327. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xiang, Y.; Zhang, F.; Lian, J.; Hu, R.; Zeng, P.; Song, J.; Qu, J. Improvement of red light harvesting ability and open circuit voltage of Cu:NiOx based p-i-n planar perovskite solar cells boosted by cysteine enhanced interface contact. Nano Energy 2018, 45, 471–479. [Google Scholar] [CrossRef]
- Chavhan, S.D.; Hansson, R.; Ericsson, L.K.E.; Beyer, P.; Hofmann, A.; Brütting, W.; Opitz, A.; Moons, E. Low temperature processed NiOx hole transport layers for efficient polymer solar cells. Org. Electron. 2017, 44, 59–66. [Google Scholar] [CrossRef]
- Jiang, F.; Choy, W.C.H.; Li, X.; Zhang, D.; Cheng, J. Post-treatment-Free Solution-Processed Non-stoichiometric NiOx Nanoparticles for Efficient Hole-Transport Layers of Organic Optoelectronic Devices. Adv. Mater. 2015, 27, 2930–2937. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Zhang, S.-W.; Li, Y.-X.; Li, S.-L.; Huang, L.-Q.; Jing, Y.-N.; Cheng, Q.; Xiao, L.-G.; Wang, B.-X.; Han, B.; et al. Solution-processed Molybdenum Oxide Hole Transport Layer Stabilizes Organic Solar Cells. Chin. J. Polym. Sci. 2023, 41, 202–211. [Google Scholar] [CrossRef]
- Pan, W.; Tian, R.; Jin, H.; Guo, Y.; Zhang, L.; Wu, X.; Zhang, L.; Han, Z.; Liu, G.; Li, J.; et al. Structure, Optical, and Catalytic Properties of Novel Hexagonal Metastable h-MoO3 Nano- and Microrods Synthesized with Modified Liquid-Phase Processes. Chem. Mater. 2010, 22, 6202–6208. [Google Scholar] [CrossRef]
- Jasieniak, J.J.; Seifter, J.; Jo, J.; Mates, T.; Heeger, A.J. A Solution-Processed MoOx Anode Interlayer for Use within Organic Photovoltaic Devices. Adv. Funct. Mater. 2012, 22, 2594–2605. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Cui, Y.; Liu, D.; Xu, B.; Hou, J. Over 100-nm-Thick MoOx Films with Superior Hole Collection and Transport Properties for Organic Solar Cells. Adv. Energy Mater. 2018, 8, 1800698. [Google Scholar] [CrossRef]
- Han, S.; Shin, W.S.; Seo, M.; Gupta, D.; Moon, S.-J.; Yoo, S. Improving performance of organic solar cells using amorphous tungsten oxides as an interfacial buffer layer on transparent anodes. Org. Electron. 2009, 10, 791–797. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, Y.; Wu, Y.; Guo, J.; Kong, X.; Wu, X.; Li, Y.; Zheng, D.; Wu, S.; Gao, X.; et al. Low-Temperature-Processed WOx as Electron Transfer Layer for Planar Perovskite Solar Cells Exceeding 20% Efficiency. Sol. RRL 2020, 4, 1900499. [Google Scholar] [CrossRef]
- Hussain, S.; Liu, H.; Hussain, M.; Mehran, M.T.; Kim, H.-S.; Jung, J.; Vikraman, D.; Kang, J. Development of MXene/WO3 embedded PEDOT:PSS hole transport layers for highly efficient perovskite solar cells and X-ray detectors. Int. J. Energy Res. 2022, 46, 12485–12497. [Google Scholar] [CrossRef]
- Shen, W.; Tang, J.; Wang, D.; Yang, R.; Chen, W.; Bao, X.; Wang, Y.; Jiao, J.; Wang, Y.; Huang, Z.; et al. Enhanced efficiency of polymer solar cells by structure-differentiated silver nano-dopants in solution-processed tungsten oxide layer. Mater. Sci. Eng. B 2016, 206, 61–68. [Google Scholar] [CrossRef]
- Liu, P.; Wang, C.; Zhou, D.; Yuan, Q.; Wang, Y.; Hu, Y.; Han, D.; Feng, L. WOx@PEDOT Core–Shell Nanorods: Hybrid Hole-Transporting Materials for Efficient and Stable Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 1742–1752. [Google Scholar] [CrossRef]
- Ali, F.; Pham, N.D.; Fan, L.; Tiong, V.; Ostrikov, K.; Bell, J.M.; Wang, H.; Tesfamichael, T. Low Hysteresis Perovskite Solar Cells Using an Electron-Beam Evaporated WO3–x Thin Film as the Electron Transport Layer. ACS Appl. Energy Mater. 2019, 2, 5456–5464. [Google Scholar] [CrossRef]
- Wang, K.; Shi, Y.; Dong, Q.; Li, Y.; Wang, S.; Yu, X.; Wu, M.; Ma, T. Low-Temperature and Solution-Processed Amorphous WOX as Electron-Selective Layer for Perovskite Solar Cells. J. Phys. Chem. Lett. 2015, 6, 755–759. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, X.; Chen, S.; Zhao, X.; Dai, S.; Xu, B. Hydrophobic Cu2O Quantum Dots Enabled by Surfactant Modification as Top Hole-Transport Materials for Efficient Perovskite Solar Cells. Adv. Sci. 2019, 6, 1801169. [Google Scholar] [CrossRef]
- Tan, W.; Hendricks, O.L.; Meng, A.C.; Braun, M.R.; McGehee, M.D.; Chidsey, C.E.D.; McIntyre, P.C. Atomic Layer Deposited TiO2–IrOx Alloy as a Hole Transport Material for Perovskite Solar Cells. Adv. Mater. Interfaces 2018, 5, 1800191. [Google Scholar] [CrossRef]
- Yang, B.; Ouyang, D.; Huang, Z.; Ren, X.; Zhang, H.; Choy, W.C.H. Multifunctional Synthesis Approach of In:CuCrO2 Nanoparticles for Hole Transport Layer in High-Performance Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1902600. [Google Scholar] [CrossRef]
- Huang, Z.; Ouyang, D.; Ma, R.; Wu, W.; Roy, V.A.L.; Choy, W.C.H. A General Method: Designing a Hypocrystalline Hydroxide Intermediate to Achieve Ultrasmall and Well-Dispersed Ternary Metal Oxide for Efficient Photovoltaic Devices. Adv. Funct. Mater. 2019, 29, 1904684. [Google Scholar] [CrossRef]
- Papadas, I.T.; Ioakeimidis, A.; Armatas, G.S.; Choulis, S.A. Low-Temperature Combustion Synthesis of a Spinel NiCo2O4 Hole Transport Layer for Perovskite Photovoltaics. Adv. Sci. 2018, 5, 1701029. [Google Scholar] [CrossRef]
- Bashir, A.; Shukla, S.; Lew, J.H.; Shukla, S.; Bruno, A.; Gupta, D.; Baikie, T.; Patidar, R.; Akhter, Z.; Priyadarshi, A.; et al. Spinel Co3O4 nanomaterials for efficient and stable large area carbon-based printed perovskite solar cells. Nanoscale 2018, 10, 2341–2350. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, W.; Fu, W.; Zhang, Z.; Yang, W.; Wang, S.; Li, H.; Xu, M.; Chen, H. An aqueous solution-processed CuOX film as an anode buffer layer for efficient and stable organic solar cells. J. Mater. Chem. A 2016, 4, 5130–5136. [Google Scholar] [CrossRef]
- Yu, R.-S.; Tasi, C.-P. Structure, composition and properties of p-type CuCrO2 thin films. Ceram. Int. 2014, 40, 8211–8217. [Google Scholar] [CrossRef]
- Han, M.; Jiang, K.; Zhang, J.; Yu, W.; Li, Y.; Hu, Z.; Chu, J. Structural, electronic band transition and optoelectronic properties of delafossite CuGa1−xCrxO2 (0 ≤ x ≤ 1) solid solution films grown by the sol–gel method. J. Mater. Chem. 2012, 22, 18463–18470. [Google Scholar] [CrossRef]
- Xiong, D.; Xu, Z.; Zeng, X.; Zhang, W.; Chen, W.; Xu, X.; Wang, M.; Cheng, Y.-B. Hydrothermal synthesis of ultrasmall CuCrO2 nanocrystal alternatives to NiO nanoparticles in efficient p-type dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 24760–24768. [Google Scholar] [CrossRef]
- Wang, J.; Daunis, T.B.; Cheng, L.; Zhang, B.; Kim, J.; Hsu, J.W.P. Combustion Synthesis of p-Type Transparent Conducting CuCrO2+x and Cu:CrOx Thin Films at 180 °C. ACS Appl. Mater. Interfaces 2018, 10, 3732–3738. [Google Scholar] [CrossRef]
- Zhang, B.; Thampy, S.; Dunlap-Shohl, W.A.; Xu, W.; Zheng, Y.; Cao, F.-Y.; Cheng, Y.-J.; Malko, A.V.; Mitzi, D.B.; Hsu, J.W.P. Mg Doped CuCrO2 as Efficient Hole Transport Layers for Organic and Perovskite Solar Cells. Nanomaterials 2019, 9, 1311. [Google Scholar] [CrossRef]
- Wang, Y.; Djurišić, A.B.; Chen, W.; Liu, F.; Cheng, R.; Ping Feng, S.; Ng, A.M.C.; He, Z. Metal oxide charge transport layers in perovskite solar cells—Optimising low temperature processing and improving the interfaces towards low temperature processed, efficient and stable devices. J. Phys. Energy 2021, 3, 012004. [Google Scholar] [CrossRef]
- Yang, X.; Liang, H.-J.; Yu, H.-Y.; Wang, M.-Y.; Zhao, X.-X.; Wang, X.-T.; Wu, X.-L. Recent progresses and challenges of metal sulfides as advanced anode materials in rechargeable sodium-ion batteries. J. Phys. Mater. 2020, 3, 042004. [Google Scholar] [CrossRef]
- Chen, J.; Chua, D.H.C.; Lee, P.S. The Advances of Metal Sulfides and In Situ Characterization Methods beyond Li Ion Batteries: Sodium, Potassium, and Aluminum Ion Batteries. Small Methods 2020, 4, 1900648. [Google Scholar] [CrossRef]
- Pariari, D.; Sarma, D.D. Nature and origin of unusual properties in chemically exfoliated 2D MoS2. APL Mater. 2020, 8, 040909. [Google Scholar] [CrossRef]
- Ray, S.C. Structure and optical properties of molybdenum disulphide (MoS2) thin film deposited by the dip technique. J. Mater. Sci. Lett. 2000, 19, 803–804. [Google Scholar] [CrossRef]
- Frey, G.L.; Tenne, R.; Matthews, M.J.; Dresselhaus, M.S.; Dresselhaus, G. Optical Properties of MS2 (M = Mo, W) Inorganic Fullerenelike and Nanotube Material Optical Absorption and Resonance Raman Measurements. J. Mater. Res. 1998, 13, 2412–2417. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Z.; Zhang, H. Metal dichalcogenide nanosheets: Preparation, properties and applications. Chem. Soc. Rev. 2013, 42, 1934–1946. [Google Scholar] [CrossRef]
- Kumar, N.; Tomar, R.; Wadehra, N.; Devi, M.M.; Prakash, B.; Chakraverty, S. Growth of Highly Crystalline and Large Scale Monolayer MoS2 by CVD: The Role of substrate Position. Cryst. Res. Technol. 2018, 53, 1800002. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.-L.; Araujo, C.M.; Luo, W.; Ahuja, R. Single-layer MoS2 as an efficient photocatalyst. Catal. Sci. Technol. 2013, 3, 2214–2220. [Google Scholar] [CrossRef]
- Hu, X.; Zeng, X.; Liu, Y.; Lu, J.; Yuan, S.; Yin, Y.; Hu, J.; McCarthy, D.T.; Zhang, X. Nano-layer based 1T-rich MoS2/g-C3N4 co-catalyst system for enhanced photocatalytic and photoelectrochemical activity. Appl. Catal. B 2019, 268, 118466. [Google Scholar] [CrossRef]
- He, Q.; Wang, L.; Yin, K.; Luo, S. Vertically Aligned Ultrathin 1T-WS2 Nanosheets Enhanced the Electrocatalytic Hydrogen Evolution. Nanoscale Res. Lett. 2018, 13, 167. [Google Scholar] [CrossRef]
- Liu, H.; Wu, R.; Tian, L.; Kong, Y.; Sun, Y. Synergetic photocatalytic effect between 1 T@2H-MoS2 and plasmon resonance induced by Ag quantum dots. Nanotechnology 2018, 29, 285402. [Google Scholar] [CrossRef]
- Yang, D.; Jimenez Sandoval, S.; Divigalpitiya, W.M.R.; Frindt, R.F. Structure of single-molecular-layer MoS2. Phys. Rev. B 1991, 43, 12053. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H. Two-dimensional MoS2: Properties, preparation, and applications. J. Mater. 2015, 1, 33–44. [Google Scholar] [CrossRef]
- Liang, M.; Ali, A.; Belaidi, A.; Hossain, M.I.; Ronan, O.; Downing, C.; Tabet, N.; Sanvito, S.; Ei-Mellouhi, F.; Nicolosi, V. Improving stability of organometallic-halide perovskite solar cells using exfoliation two-dimensional molybdenum chalcogenides. NPJ 2D Mater. Appl. 2020, 4, 40. [Google Scholar] [CrossRef]
- Wang, D.; Elumalai, N.K.; Mahmud, M.A.; Yi, H.; Upama, M.B.; Lee Chin, R.A.; Conibeer, G.; Xu, C.; Haque, F.; Duan, L.; et al. MoS2 incorporated hybrid hole transport layer for high performance and stable perovskite solar cells. Synth. Met. 2018, 246, 195–203. [Google Scholar] [CrossRef]
- Le, Q.V.; Nguyen, T.P.; Jang, H.W.; Kim, S.Y. The use of UV/ozone-treated MoS2 nanosheets for extended air stability in organic photovoltaic cells. Phys. Chem. Chem. Phys. 2014, 16, 13123–13128. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.V.; Nguyen, T.P.; Kim, S.Y. UV/ozone-treated WS2 hole-extraction layer in organic photovoltaic cells. Phys. Status Solidi-Rapid Res. Lett. 2014, 8, 390–394. [Google Scholar] [CrossRef]
- Le, Q.V.; Nguyen, T.P.; Choi, K.S.; Cho, Y.H.; Hong, Y.J.; Kim, S.Y. Dual use of tantalum disulfides as hole and electron extraction layers in organic photovoltaic cells. Phys. Chem. Chem. Phys. 2014, 16, 25468–25472. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rojas, F.; Hssein, M.; El Jouad, Z.; Armijo, F.; Cattin, L.; Louarn, G.; Stephant, N.; del Valle, M.A.; Addou, M.; Soto, J.P.; et al. Mo(SxOy) thin films deposited by electrochemistry for application in organic photovoltaic cells. Mater. Chem. Phys. 2017, 201, 331–338. [Google Scholar] [CrossRef]
- Xing, W.; Chen, Y.; Wang, X.; Lv, L.; Ouyang, X.; Ge, Z.; Huang, H. MoS2 Quantum Dots with a Tunable Work Function for High-Performance Organic Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 26916–26923. [Google Scholar] [CrossRef]
- Adilbekova, B.; Lin, Y.; Yengel, E.; Faber, H.; Harrison, G.; Firdaus, Y.; El-Labban, A.; Anjum, D.H.; Tung, V.; Anthopoulos, T.D. Liquid phase exfoliation of MoS2 and WS2 in aqueous ammonia and their application in highly efficient organic solar cells. J. Mater. Chem. C 2020, 8, 5259–5264. [Google Scholar] [CrossRef]
- Van Le, Q.; Nguyen, T.P.; Park, M.; Sohn, W.; Jang, H.W.; Kim, S.Y. Bottom-Up Synthesis of MeSxNanodots for Optoelectronic Device Applications. Adv. Opt. Mater. 2016, 4, 1796–1804. [Google Scholar] [CrossRef]
- Le, Q.V.; Kim, C.M.; Nguyen, T.P.; Park, M.; Kim, T.-Y.; Han, S.M.; Kim, S.Y. (NH4)2WS4 precursor as a hole-injection layer in organic optoelectronic devices. Chem. Eng. J. 2016, 284, 285–293. [Google Scholar] [CrossRef]
- Rao, H.; Sun, W.; Ye, S.; Yan, W.; Li, Y.; Peng, H.; Liu, Z.; Bian, Z.; Huang, C. Solution-Processed CuS NPs as an Inorganic Hole-Selective Contact Material for Inverted Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 7800–7805. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zang, S.; Pan, D.; Zhang, X.; Liu, Y. CuSx hole transport layer for PbS quantum dot solar cell. Sol. Energy 2020, 209, 118–122. [Google Scholar] [CrossRef]
- Tirado, J.; Roldán-Carmona, C.; Muñoz-Guerrero, F.A.; Bonilla-Arboleda, G.; Ralaiarisoa, M.; Grancini, G.; Queloz, V.I.E.; Koch, N.; Nazeeruddin, M.K.; Jaramillo, F. Copper sulfide nanoparticles as hole-transporting-material in a fully-inorganic blocking layers n-i-p perovskite solar cells: Application and working insights. Appl. Surf. Sci. 2019, 478, 607–614. [Google Scholar] [CrossRef]
- Peng, Z.; Luo, W.; Long, C.; Wang, Y.; Fu, Y. A CuxS/GO composite hole transport layer for photovoltaic performance enhancement on CuInS2 quantum dot-sensitized solar cells. Appl. Phys. A 2022, 129, 54. [Google Scholar] [CrossRef]
- Kumar, P.; You, S.; Vomiero, A. CuSCN as a hole transport layer in an inorganic solution-processed planar Sb2S3 solar cell, enabling carbon-based and semitransparent photovoltaics. J. Mater. Chem. C 2022, 10, 16273–16282. [Google Scholar] [CrossRef]
- Suresh Kumar, M.; Mohanta, K.; Batabyal, S.K. Solution processed Cu2CdSnS4 as a low-cost inorganic hole transport material for polymer solar cells. Sol. Energy Mater. Sol. Cells 2017, 161, 157–161. [Google Scholar] [CrossRef]
- Yu, Z.; Li, W.; Cheng, N.; Liu, Z.; Lei, B.; Xiao, Z.; Zi, W.; Zhao, Z.; Tu, Y. Cu2SnS3 Nanocrystal-Based Hole-Transport Layer for Carbon Electrode-Based Perovskite Solar Cells. ACS Appl. Nano Mater. 2022, 5, 10755–10762. [Google Scholar] [CrossRef]
- Molla, A.; Sahu, M.; Hussain, S. Synthesis of Tunable Band Gap Semiconductor Nickel Sulphide Nanoparticles: Rapid and Round the Clock Degradation of Organic Dyes. Sci. Rep. 2016, 6, 26034. [Google Scholar] [CrossRef]
- Hamed, M.S.G.; Oseni, S.O.; Kumar, A.; Sharma, G.; Mola, G.T. Nickel sulphide nano-composite assisted hole transport in thin film polymer solar cells. Sol. Energy 2020, 195, 310–317. [Google Scholar] [CrossRef]
- Hilal, M.; Han, J.I. Preparation of hierarchical flower-like nickel sulfide as hole transporting material for organic solar cells via a one-step solvothermal method. Sol. Energy 2019, 188, 403–413. [Google Scholar] [CrossRef]
- Pitchaiya, S.; Natarajan, M.; Santhanam, A.; Ramakrishnan, V.M.; Asokan, V.; Palanichamy, P.; Rangasamy, B.; Sundaram, S.; Velauthapillai, D. Nickel sulphide-carbon composite hole transporting material for (CH3NH3PbI3) planar heterojunction perovskite solar cell. Mater. Lett. 2018, 221, 283–288. [Google Scholar] [CrossRef]
- He, Z.; Zhou, Y.; Liu, A.; Gao, L.; Zhang, C.; Wei, G.; Ma, T. Recent progress in metal sulfide-based electron transport layers in perovskite solar cells. Nanoscale 2021, 13, 17272–17289. [Google Scholar] [CrossRef]
- Arumugam, G.M.; Karunakaran, S.K.; Liu, C.; Zhang, C.; Guo, F.; Wu, S.; Mai, Y. Inorganic hole transport layers in inverted perovskite solar cells: A review. Nano Sel. 2021, 2, 1081–1116. [Google Scholar] [CrossRef]
- Guo, J.-J.; Bai, Z.-C.; Meng, X.-F.; Sun, M.-M.; Song, J.-H.; Shen, Z.-S.; Ma, N.; Chen, Z.-L.; Zhang, F. Novel dopant-free metallophthalocyanines based hole transporting materials for perovskite solar cells: The effect of core metal on photovoltaic performance. Sol. Energy 2017, 155, 121–129. [Google Scholar] [CrossRef]
- Wang, J.-M.; Wang, Z.-K.; Li, M.; Zhang, C.-C.; Jiang, L.-L.; Hu, K.-H.; Ye, Q.-Q.; Liao, L.-S. Doped Copper Phthalocyanine via an Aqueous Solution Process for Normal and Inverted Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1701688. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z.; Sun, B.; Tan, X.; Ye, H.; Tu, Y.; Shi, T.; Tang, Z.; Liao, G. 17.46% efficient and highly stable carbon-based planar perovskite solar cells employing Ni-doped rutile TiO2 as electron transport layer. Nano Energy 2018, 50, 201–211. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, Y.; Chen, Y.; Chen, X.; Deng, X.; Chen, W.; Shi, C. Soluble tetra-methoxyltriphenylamine substituted zinc phthalocyanine as dopant-free hole transporting materials for perovskite solar cells. Org. Electron. 2019, 69, 248–254. [Google Scholar] [CrossRef]
- Cheng, M.; Li, Y.; Safdari, M.; Chen, C.; Liu, P.; Kloo, L.; Sun, L. Efficient Perovskite Solar Cells Based on a Solution Processable Nickel(II) Phthalocyanine and Vanadium Oxide Integrated Hole Transport Layer. Adv. Energy Mater. 2017, 7, 1602556. [Google Scholar] [CrossRef]
- Chen, S.; Liu, P.; Hua, Y.; Li, Y.; Kloo, L.; Wang, X.; Ong, B.; Wong, W.-K.; Zhu, X. Study of Arylamine-Substituted Porphyrins as Hole-Transporting Materials in High-Performance Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 13231–13239. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Lv, X.; Zhang, P.; Chuong, T.T.; Wu, B.; Feng, X.; Shan, C.; Liu, J.; Tang, Y. Plant Sunscreen and Co(II)/(III) Porphyrins for UV-Resistant and Thermally Stable Perovskite Solar Cells: From Natural to Artificial. Adv. Mater. 2018, 30, 1800568. [Google Scholar] [CrossRef]
- Chen, R.; Feng, Y.; Zhang, C.; Wang, M.; Jing, L.; Ma, C.; Bian, J.; Shi, Y. Carbon-based HTL-free modular perovskite solar cells with improved contact at perovskite/carbon interfaces. J. Mater. Chem. C 2020, 8, 9262–9270. [Google Scholar] [CrossRef]
- Stubhan, T.; Li, N.; Luechinger, N.A.; Halim, S.C.; Matt, G.J.; Brabec, C.J. High Fill Factor Polymer Solar Cells Incorporating a Low Temperature Solution Processed WO3 Hole Extraction Layer. Adv. Energy Mater. 2012, 2, 1433–1438. [Google Scholar] [CrossRef]
- Kim, G.; Min, H.; Lee, K.S.; Lee, D.Y.; Yoon, S.M.; Seok, S.I. Impact of strain relaxation on performance of α-formamidinium lead iodide perovskite solar cells. Science 2020, 370, 108–112. [Google Scholar] [CrossRef]
- Gu, X.; Lai, X.; Zhang, Y.; Wang, T.; Tan, W.L.; McNeill, C.R.; Liu, Q.; Sonar, P.; He, F.; Li, W.; et al. Organic Solar Cell With Efficiency Over 20% and VOC Exceeding 2.1 V Enabled by Tandem With All-Inorganic Perovskite and Thermal Annealing-Free Process. Adv. Sci. 2022, 9, 2200445. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, J.; Bi, P.; Ren, J.; Wang, Y.; Yang, Y.; Liu, X.; Zhang, S.; Hou, J. Tandem Organic Solar Cell with 20.2% Efficiency. Joule 2022, 6, 171–184. [Google Scholar] [CrossRef]
- Ullah, F.; Chen, C.-C.; Choy, W.C.H. Recent Developments in Organic Tandem Solar Cells toward High Efficiency. Adv. Energy Sustain. Res. 2021, 2, 2000050. [Google Scholar] [CrossRef]
- Solak, E.K.; Irmak, E. Advances in organic photovoltaic cells: A comprehensive review of materials, technologies, and performance. RSC Adv. 2023, 13, 12244–12269. [Google Scholar] [CrossRef]
- Gebremichael, Z.T.; Ugokwe, C.; Alam, S.; Stumpf, S.; Diegel, M.; Schubert, U.S.; Hoppe, H. How varying surface wettability of different PEDOT:PSS formulations and their mixtures affects perovskite crystallization and the efficiency of inverted perovskite solar cells. RSC Adv. 2022, 12, 25593–25604. [Google Scholar] [CrossRef]
- Wang, S.; Cabreros, A.; Yang, Y.; Hall, A.S.; Valenzuela, S.; Luo, Y.; Correa-Baena, J.-P.; Kim, M.-c.; Fjeldberg, Ø.; Fenning, D.P.; et al. Impacts of the Hole Transport Layer Deposition Process on Buried Interfaces in Perovskite Solar Cells. Cell Rep. Phys. Sci. 2020, 1, 100103. [Google Scholar] [CrossRef]
- Chaudhary, N.; Naqvi, S.; Rathore, D.; Rathi, S.; Patra, A. Solvent influenced morphology control of hole transport layer of CuSCN on performance of organic solar cells. Mater. Chem. Phys. 2022, 282, 125898. [Google Scholar] [CrossRef]
- Wang, S.; Sina, M.; Parikh, P.; Uekert, T.; Shahbazian, B.; Devaraj, A.; Meng, Y.S. Role of 4-tert-Butylpyridine as a Hole Transport Layer Morphological Controller in Perovskite Solar Cells. Nano Lett. 2016, 16, 5594–5600. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.H.; Trinh, T.K.; Shaikh, H.M.; Al-Zahrani, S.M.; Alhamidi, A.; Bin Dahman, S.; Tamboli, M.S.; Truong, N.T.N. Controlling of Conductivity and Morphological Properties of Hole-Transport Layer Using Ionic Liquid for Vacuum-Free Planar Hybrid Solar Cells. Energies 2023, 16, 467. [Google Scholar] [CrossRef]



| HTMs | ABSORBER | VOC | JSC | FF | PCE | Ref. |
|---|---|---|---|---|---|---|
| Spiro-OMeTAD | ||||||
| Pristine Spiro-OMeTAD | CH3NH3PbI3 | 1.0 | 18.30 | 73.64 | 13.48 | [43] |
| Mg-TFSI2-doped Spiro-OMeTAD | Triple-cation perovskite (Cs0.06FA0.79MA0.15PbI2.55Br0.75) | 1.091 | 22.62 | 76.70 | 18.93 | [36] |
| Ca-TFSI2-doped Spiro-OMeTAD | 1.074 | 22.54 | 76.50 | 18.69 | ||
| Zn-TFSI2-doped Spiro-OMeTAD | Triple-cation perovskite | 1.162 | 23.78 | 78.80 | 22.00 | [37] |
| Spiro-OMeTAD(TFSI)2 | Rb0.05Cs0.05FA0.8MA0.07PbBr0.4I2.57 | 1.08 | 23.9 | 75.00 | 19.30 | [38] |
| PTAA | ||||||
| Pristine PTAA | CH3NH3PbI3 | 1.08 | 22.44 | 71.00 | 18.11 | [61] |
| UV-Treated dopant-free PTAA | Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3 | 1.08 | 22.74 | 78.00 | 19.17 | [67] |
| PTAA-MA | Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3 | 1.13 | 22.86 | 78.16 | 20.23 | [57] |
| PEDOT:PSS | ||||||
| Solvent-treated PEDOT:PSS (ethylene glycol + methanol) | MAPbI3 | 1.04 | 22.21 | 79.00 | 18.18 | [69] |
| PEDOT:PSS + PCDSA (MeOH treatment) | MAPbI3 | 0.90 | 18.88 | 72.00 | 13.01 | [71] |
| PTB7-Th:PC70BM | 0.72 | 17.40 | 59.00 | 7.71 | ||
| P3HT:PC60BM | 0.56 | 9.07 | 61.00 | 3.18 | ||
| P3HT | ||||||
| Pristine P3HT | (FAPbI3)0.95(MAPbBr3)0.05 + wide-bandgap halide perovskite (WBH) | 1.144 | 24.92 | 79.50 | 22.70 | [33] |
| P3HT-MDN | Cs0.05FA0.85MA0.10Pb(Br0.03I0.97)3 | 1.15 | 24.58 | 75.02 | 22.87 | [72] |
| Other OHTMs | ||||||
| PCz1 | Cs0.08FA0.80MA0.12Pb(I0.88Br0.12)3 | 1.04 | 22.41 | 77.55 | 18.04 | [79] |
| Hydrogen bonding PCz | Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3 | 1.03 | 22.6 | 64.7 | 15.1 | [80] |
| Benzothiophene CDs | MAPbI3 | 1.04 | 19.57 | 64.79 | 13.22 | [81] |
| RCP of BDT and BT | CH3NH3PbI3 | 1.08 | 21.9 | 75 | 17.3 | [82] |
| BTT | MA0.7 FA0.3PbI3 | 1.14 | 23.4 | 78.9 | 20.9 | [19] |
| BDD | 1.23 | 23.2 | 62.8 | 16.3 | ||
| HTM | ABSORBER | VOC | JSC | FF | PCE | Ref. |
|---|---|---|---|---|---|---|
| GO- and carbon derivative-based HTLs | ||||||
| GO | P3HT:PC61BM | 0.57 | 11.40 | 54.30 | 3.50 | [101] |
| PEDOT:PSS/GO | PTB7:PC71BM | 0.76 | 16.42 | 65.80 | 8.21 | [103] |
| PEDOT:PSS/GO | PCDTBT:PC71BM | 0.82 | 10.44 | 50.00 | 4.28 | [102] |
| S-GO | P3HT:PC61BM | 0.61 | 10.15 | 71.00 | 4.37 | [104] |
| CL-GO | PCDTBT:PC71BM | 0.88 | 13.65 | 54.70 | 6.56 | [105] |
| rGO | P3HT:PC61BM | 0.58 | 9.87 | 65.00 | 3.71 | [106] |
| N-GO/SnO2 | Rb-doped-FA-MA-Br-mixed perovskite | 1.17 | 18.87 | 74.93 | 16.54 | [108] |
| Cu@RGO | MAPbI3 | 0.97 | 19.20 | 69.80 | 13.23 | [109] |
| F5-GO/PEDOT:PSS | PTB7:PC71BM | 0.78 | 15.31 | 63.00 | 7.52 | [110] |
| F-rGO | PTB7:Th:PC71BM | 0.78 | 16.89 | 64.80 | 8.6 | [111] |
| GQDs | Dr3TBDT:PC71BM | 0.92 | 11.36 | 65.20 | 6.82 | [122] |
| CNT/PEDOT:PSS | P3HT:PC61BM | 0.55 | 11.58 | 58.00 | 3.69 | [123] |
| O-MWCNTs | Cs-FA-MAPbIBr | 0.99 | 21.96 | 41.09 | 8.99 | [215] |
| Metal oxide HTLs | ||||||
| NiO | PDTG-TPD:PC71BM | 0.82 | 13.90 | 68.40 | 7.82 | [128] |
| NiO | MAPbI | 0.98 | 21.10 | 78.00 | 16.1 | [130] |
| NiO | CsFA-MA-Pb-I-Br | 1.02 | 21.00 | 85.00 | 16.7 | [131] |
| Cu doped NiO | MAPbI | 1.10 | 21.73 | 75.30 | 18.02 | [132] |
| NiMgOx | MAPbI | 1.07 | 21.30 | 79.00 | 18.20 | [133] |
| NiOx | FA-MA-Pb-I-Cl | 1.12 | 23.70 | 76.00 | 20.20 | [138] |
| NiO | AVA-MAPI | 0.83 | 20.90 | 65.50 | 10.91 | [134] |
| S-NiOx | RP(BDT-PDBT):PC71BM | 0.71 | 9.85 | 63.00 | 4.45 | [135] |
| MoOx | PM6:Y6:PC71BM | 0.84 | 26.71 | 74.22 | 17.21 | [147] |
| MoOx | PB3T2:IT-M | 0.96 | 16.20 | 68.00 | 10.50 | [150] |
| s-MoOx | P3HT:PC61BM | 0.59 | 9.50 | 68.00 | 3.80 | [149] |
| WO3 | P3HT:PC71BM | 0.61 | 12.80 | 60.40 | 4.80 | [216] |
| WOx-PEDOT:PSS | MAPbICl | 0.97 | 20.76 | 70.90 | 14.30 | [155] |
| Ti3C2Tx/WO3/PEDOT:PSS | MAPbI3 | 0.90 | 22.47 | 60.02 | 12.26 | [153] |
| O-CuOx | PTB7:PC71BM | 0.74 | 16.44 | 71.00 | 8.52 | [164] |
| Metal sulfide HTLs | ||||||
| MoS2 | MAPbI3 | 0.95 | 20.70 | 72.30 | 14.2 | [186] |
| O-MoS2 QDs | PTB7:Th:PC71BM | 0.79 | 16.90 | 65.00 | 8.66 | [191] |
| MoS2 Ns | 0.63 | 12.50 | 53.20 | 4.18 | ||
| MoS2 | PBDB-T-2F:Y6:PC71BM | 0.81 | 25.30 | 71.00 | 14.9 | [192] |
| WS2 | 0.83 | 26.00 | 72.00 | 15.6 | ||
| CuS | MAPbI3 | 1.02 | 22.30 | 71.20 | 16.2 | [195] |
| CuxS-GO | TiO2/CuInS2 | 0.60 | 16.06 | 62.90 | 6.07 | [198] |
| Cu2SnS3 | FAPbI3 | 1.04 | 24.14 | 64.92 | 16.33 | [201] |
| Organometallic HTLs | ||||||
| Cop | CsFAMAPbIBr | 1.13 | 23.62 | 76.66 | 20.47 | [214] |
| Ni-Pc | FAPbI3@MAPbBr3 | 0.895 | 18.50 | 63.80 | 10.6 | [212] |
| Ni-Pc/V2O5 | 1.08 | 23.10 | 73.40 | 18.3 | ||
| Zn-Pc | MAPbIBr | 1.02 | 22.36 | 71.43 | 16.23 | [211] |
| CuPc | FAPbI3@MAPbBr3 | 1.07 | 20.52 | 66.30 | 16.36 | [213] |
| ZnPc | ~1.10 | 21.75 | 71.30 | 17.78 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, V.K.H.; Nguyen, T.P. Advances in Hole Transport Materials for Layered Casting Solar Cells. Polymers 2023, 15, 4443. https://doi.org/10.3390/polym15224443
Bui VKH, Nguyen TP. Advances in Hole Transport Materials for Layered Casting Solar Cells. Polymers. 2023; 15(22):4443. https://doi.org/10.3390/polym15224443
Chicago/Turabian StyleBui, Vu Khac Hoang, and Thang Phan Nguyen. 2023. "Advances in Hole Transport Materials for Layered Casting Solar Cells" Polymers 15, no. 22: 4443. https://doi.org/10.3390/polym15224443
APA StyleBui, V. K. H., & Nguyen, T. P. (2023). Advances in Hole Transport Materials for Layered Casting Solar Cells. Polymers, 15(22), 4443. https://doi.org/10.3390/polym15224443