Improvement of Water Vapor Permeability in Polypropylene Composite Films by the Synergy of Carbon Nanotubes and β-Nucleating Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
3. Experimental Techniques
3.1. Characterization Techniques
3.2. In Vitro Cytotoxicity Studies
3.2.1. Cell Cultures and Conditions
3.2.2. Phase-Contrast Microscopy
3.2.3. MTT Cell Viability Assay
3.2.4. Statistical Analysis
4. Results and Discussion
4.1. Crystallization Behavior of PP Composite Films
4.1.1. Influence of MWCNT Loading
4.1.2. Influence of β-ΝA Loading
4.1.3. Influence of the Loading of Both β-NA and MWCNTs
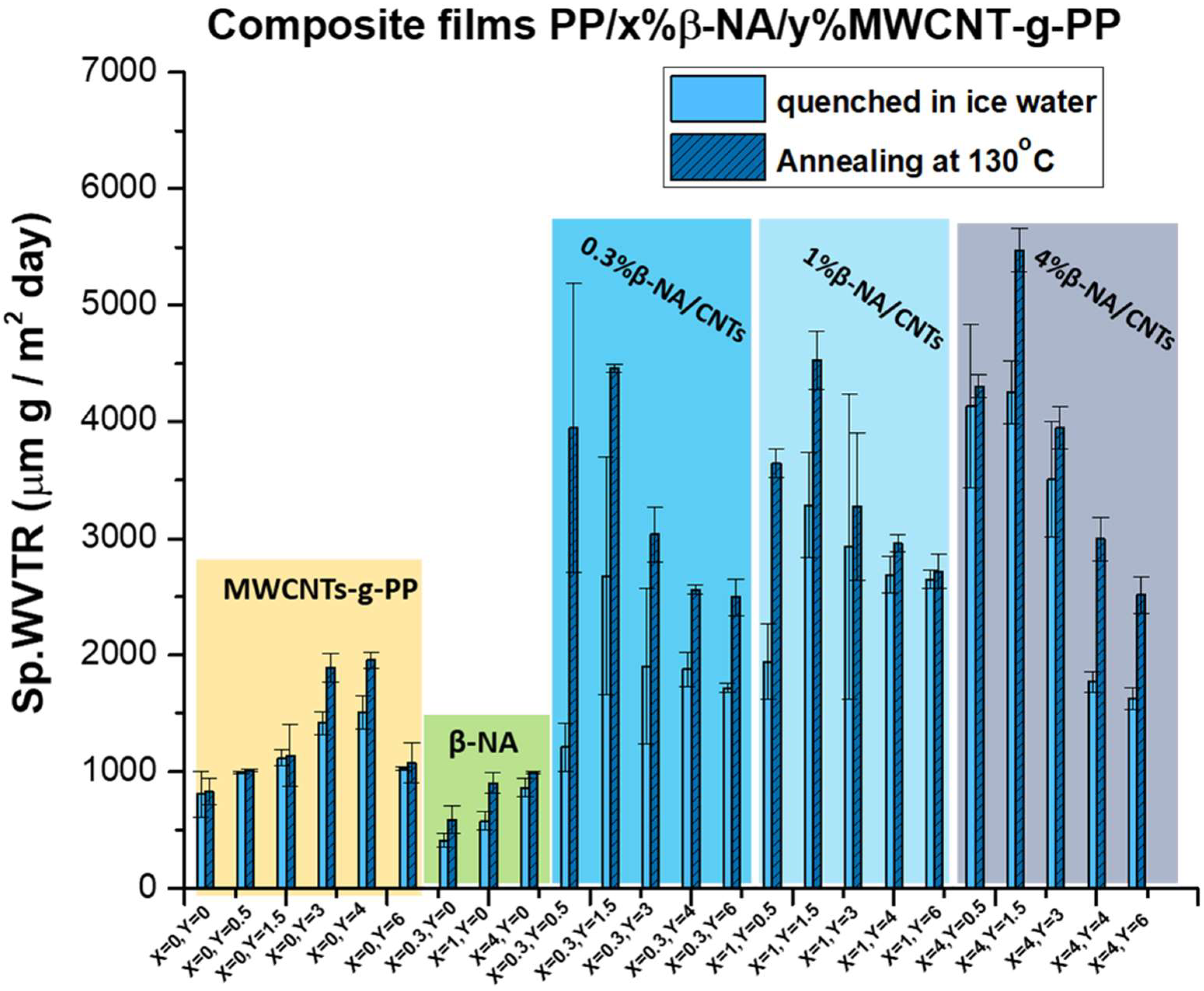
4.2. Water Vapor Transport of Hybrid PP/β-ΝA/MWCNT-g-PP Films
4.3. Cytotoxicity Evaluation of CNTs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, D.D.L. Applications of composite materials. In Composite Materials: Functional Materials for Modern Technologies; Derby, B., Ed.; Springer: Manchester, UK, 2004; pp. 1–12. [Google Scholar]
- Wu, P.C.; Jones, G.; Shelley, C.; Woefli, B. Novel microporous films and their composites. J. Eng. Fibers Fabr. 2007, 2, 49–58. [Google Scholar] [CrossRef]
- Liu, S.; Jun, S.C.; Zhang, S.; Wang, F.; Yu, J.; Ding, B. Advancements in Electrospun Nanofibrous Membranes for Improved Waterproofing and Breathability. In Macromolecular Materials and Engineering; Wiley: Hoboken, NJ, USA, 2023; pp. 2300312–2300337. [Google Scholar]
- Zuiderduin, W.C.J.; Westzaan, C.; Huétink, J.; Gaymans, R.J. Toughening of polypropylene with calcium carbonate particles. Polymer 2003, 44, 261–275. [Google Scholar] [CrossRef]
- Tay, J.Y.; Lim, S.; Mah, S.K.; Tan, Y.M. Development of Microporous Breathable Polyethylene Film Using Different Types of Linear Low-Density Polyethylene with Calcium Carbonate as Filler. Solid State Phenom. 2023, 349, 95–100. [Google Scholar] [CrossRef]
- Fang, W.; Liang, G.; Li, J.; Guo, S. Microporous Formation Mechanism of Biaxial Stretching PA6/PP Membranes with High Porosity and Uniform Pore Size Distribution. Polymers 2022, 14, 2291. [Google Scholar] [CrossRef]
- MohammadKarimi, S.; Morshedian, J.; Morshedian, H. Elaboration of porosity for the alumina particle surfaces/ bimodal PP composite cast films under continuous stretching. J. Appl. Polym. Sci. 2021, 138, e50842. [Google Scholar] [CrossRef]
- Alexiou, V.F.; Mathioudakis, G.N.; Andrikopoulos, K.S.; Beobide, A.S.; Voyiatzis, G.A. Poly(ethylene Terephthalate) Carbon-Based Nanocomposites: A Crystallization and Molecular Orientation Study. Polymers 2020, 12, 2626. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Salvetat, J.P.; Bonard, J.M.; Thomson, N.H.; Kulik, A.J.; Benoit, W.; Zuppiroli, L. Mechanical properties of carbon nanotubes. Appl. Phys. A 1999, 69, 255–260. [Google Scholar] [CrossRef]
- Viet, L.; Luo, N.V.; Umer, S.H.; Pawar, R.; Zheng, S.J. A new formula for the effective Young’s modulus and Shear modulus of multiwall carbon nanotubes. Acad. J. Biotechnol. 2017, 5, 147–155. [Google Scholar]
- Allaoui, A.; Bai, S.; Cheng, H.M.; Bai, J.B. Mechanical and electrical properties of a MWNT/epoxy composite. Compos. Sci. Technol. 2002, 62, 1993–1998. [Google Scholar] [CrossRef]
- Demczyk, B.G.; Wang, Y.M.; Cumings, J.; Hetman, M.; Han, W.; Zettl, A.; Ritchie, R.O. Direct mechanical measurement of the tensile strength and elastic modulus of multiwalled carbon nanotubes. Mater. Sci. Eng. A 2002, 334, 173–178. [Google Scholar] [CrossRef]
- Kim, S.; Jinschek, J.R.; Chen, H.; Sholl, D.S.; Marand, E. Scalable fabrication of carbon nanotube/polymer nanocomposite membranes for high flux gas transport. Nano Lett. 2007, 7, 2806–2811. [Google Scholar] [CrossRef]
- Baek, Y.; Kim, C.; Seo, D.K.; Kim, T.; Lee, J.S.; Kim, Y.H.; Ahn, K.H.; Bae, S.S.; Lee, S.C.; Lim, J.; et al. High performance and antifouling vertically aligned carbon nanotube membrane for water purification. J. Membr. Sci. 2014, 460, 171–177. [Google Scholar] [CrossRef]
- Yang, W.; Xu, H.; Chen, W.; Shen, Z.; Ding, M.; Lin, T.; Tao, H.; Kong, Q.; Yang, G.; Xie, Z. A polyamide membrane with tubular crumples incorporating carboxylated single-walled carbon nanotubes for high water flux. Desalination 2020, 479, 114–330. [Google Scholar] [CrossRef]
- Bounos, G.; Andrikopoulos, K.; Moschopoulou, H.; Lainioti, G.; Roilo, D.; Checchetto, R.; Ioannides, T.; Kallitsis, J.; Voyiatzis, G. Enhancing water vapor permeability in mixed matrix polypropylene membranes through carbon nanotubes dispersion. J. Membr. Sci. 2017, 524, 576–584. [Google Scholar] [CrossRef]
- Holt, J.; Park, H.G.; Wang, Y.; Stadermann, M.; Artyukhin, A.; Grigoropoulos, C.; Noy, A.; Bakajin, O. Fast Mass Transport Through Sub-2-Nanometer Carbon Nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef]
- Noy, A.; Park, H.G.; Fornasieroa, F.; Holt, J.K.; Grigoropoulos, C.; Bakajina, O. Nanofluidics in carbon nanotubes. Nanotoday 2007, 2, 22–29. [Google Scholar] [CrossRef]
- Kannam, S.K.; Todd, B.D.; Hansen, J.S.; Daivis, P.J. How fast does water flow in carbon nanotubes? J. Chem. Phys. 2013, 138, 094701–094709. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Q.; Wang, K.; Fu, Q.; Zhang, C. Water transport behavior of chitosan porous membrane containing MWCNTs. J. Membr. Sci. 2009, 337, 240–247. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically Functionalized Carbon Nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef]
- Jeon, I.Y.; Chang, D.W.; Kumar, N.A.; Baek, J.B. Functionalization of Carbon Nanotubes; Yellampalli, S., Ed.; TechOpen: London, UK, 2011. [Google Scholar]
- Mallakpour, S.; Soltaniana, S. Surface functionalization of carbon nanotubes: Fabrication and applications. RSC Adv. 2016, 6, 109916–109935. [Google Scholar] [CrossRef]
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Shubhra, Q.T.; Alam, A.; Quaiyyum, M. Mechanical properties of polypropylene composites: A review. J. Thermoplast. Compos. Mater. 2013, 26, 362–391. [Google Scholar] [CrossRef]
- Nevalainen, K.; Auvinen, S.; Orell, O.; Etelaaho, P.; Suihkonen, R.; Vuorinen, J.; Jarvela, P. Characterization of melt-compounded and masterbatch-diluted polypropylene composites filled with several fillers. Polym. Compos. 2013, 34, 554–569. [Google Scholar] [CrossRef]
- Bruckner, S.; Meille, S.V.; Petraccone, V.; Pirozzi, B. Polymorphism in isotactic polypropylene. Prog. Polym. Sci. 1991, 16, 361–403. [Google Scholar] [CrossRef]
- Lotz, B.; Wittmann, J.C.; Lovinger, A.J. Structure and morphology of poly(propylenes): A molecular analysis. Polymer 1996, 37, 4979–4992. [Google Scholar] [CrossRef]
- Phillips, P.J.; Mezghani, K. Polypropylene Isotactic (Polymorphism). In The Polymeric Materials Encyclopedia; Salamone., J.C., Ed.; CRC Press: Boca Raton, FL, USA, 1996; Volume 9, pp. 6637–6642. [Google Scholar]
- Natta, G.; Corradini, P. General considerations on the structure of crystalline polyhydrocarbons. NuoVo Cimento 1960, 15 (Suppl. S1), 9–39. [Google Scholar] [CrossRef]
- Turner-Jones, A.; Aizlewood, J.M.; Beckett, D.R. Crystalline forms of isotactic polypropylene. Makromol. Chem. Phys. 1964, 75, 134–158. [Google Scholar] [CrossRef]
- Meille, S.V.; Ferro, D.R.; Brueckner, S.; Lovinger, A.J.; Padden, F.J. Structure of β-isotactic polypropylene: A long-standing structural puzzle. Macromolecules 1994, 27, 2615–2622. [Google Scholar] [CrossRef]
- Stocker, W.; Schumacher, M.; Graff, S.; Thierry, A.; Wittmann, J.-C.; Lotz, B. Epitaxial crystallization and AFM investigation of a frustrated polymer structure: Isotactic poly(propylene), β phase. Macromolecules 1998, 31, 807–814. [Google Scholar] [CrossRef]
- Padden, F.; Keith, J.R.H. Spherulitic Crystallization in Polypropylene. J. Appl. Phys. 1959, 30, 1479–1484. [Google Scholar] [CrossRef]
- Varga, J. β-Modification of isotactic polypropylene preparation, structure, processing, properties and application. J. Macro Sci. Phys. 2002, 41, 1121–1171. [Google Scholar] [CrossRef]
- Varga, J. Supermolecular structure of isotactic polypropylene. J. Mater. Sci. 1992, 27, 2557–2579. [Google Scholar] [CrossRef]
- Keith, H.D.; Padden, F.J.; Walter, N.M.; Wyckoff, H.W. Evidence for a Second Crystal Form of Polypropylene. J. Appl. Phys. 1959, 30, 1484–1488. [Google Scholar] [CrossRef]
- Varga, J. Crystallization, melting and supermolecular structure of isotactic polypropylene. In Polypropylene: Structure, Blends and Composites, Structure and Morphology; Karger-Kocsis, J., Ed.; Chapman and Hall: London, UK, 1995; Volume 1, p. 56. [Google Scholar]
- Varga, J.; Ehrenstein, G.W. Beta-modification of isotactic polypropylene. In Polypropylene; Karger-Kocsis, J., Ed.; Kluwer: Dordrecht, The Netherlands; London, UK, 1999; Volume 2, p. 51. [Google Scholar]
- Papagerogiou, D.; Chrisafis, K.; Bikiaris, D. β-Nucleated Polypropylene: Processing, Properties and Nanocomposites. Polymer Rev. 2015, 55, 1–34. [Google Scholar]
- Sauer, J.A.; Pae, K.D. Structure and thermal behavior of pressure-crystallized polypropylene. J. Appl. Phys. 1968, 39, 4959–4968. [Google Scholar] [CrossRef]
- Turner-Jones, A. Development of the γ-crystal form in random copolymers of propylene and their analysis by DSC and X-ray methods. Polymer 1971, 12, 487–508. [Google Scholar] [CrossRef]
- Xu, M.; Hu, S.; Guan, J.; Sun, X.; Wu, W.; Zhu, W. Polypropylene Microporous Film. US Patent US5134174A, 28 July 1992. [Google Scholar]
- Chu, F.; Yamaoka, T.; Ide, H.; Kimura, Y. Microvoid formation process during the plastic deformation of β-form polypropylene. Polymer 1994, 35, 3442–3448. [Google Scholar] [CrossRef]
- Offord, G.T.; Armstrong, S.R.; Freeman, B.D.; Baer, E.; Hiltner, A.; Paul, D.R. Influence of processing strategies on porosity and permeability of β nucleated isotactic polypropylene stretched films. Polymer 2013, 54, 2796–2807. [Google Scholar] [CrossRef]
- Ran, S.; Zong, X.; Fang, D.; Hsiao, B.S.; Chu, B. Structural and Morphological Studies of Isotactic Polypropylene Fibers during Heat/Draw Deformation by in-Situ Synchrotron SAXS/WAXD. Macromolecules 2001, 34, 2569–2578. [Google Scholar] [CrossRef]
- Shi, G.; Chu, F.; Zhou, G.; Hang, Z. Plastic deformation and solid-phase transformation in β-phase polypropylene. Makromol. Chem. 1989, 190, 907–913. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, G.G.; Ding, C.; Zhang, Y.; Yang, W.; Yang, M. Formation of oriented β-transcrystals induced by self-assembly of nucleating agent and its micropores formation during uniaxial stretching. Polym. Cryst. 2020, 3, 10129–10136. [Google Scholar] [CrossRef]
- Wu, T.; Xiang, M.; Cao, Y.; Kang, J.; Yang, F. Pore formation mechanism of b nucleated polypropylene stretched membranes. RSC Adv. 2014, 4, 36689–36701. [Google Scholar] [CrossRef]
- Kang, J.; He, J.; Chen, Z.; Yu, H.; Chen, J.; Yang, F.; Cao, Y.; Xiang, M. Investigation on the crystallization behavior and polymorphic composition of isotactic polypropylene/multi-walled carbon nanotube composites nucleated with β-nucleating agent. J. Therm. Anal. Calorim. 2015, 119, 1769–1780. [Google Scholar] [CrossRef]
- Wang, S.W.; Yang, W.; Bao, R.Y.; Wang, B.; Xie, B.H.; Yang, M.B. The enhanced nucleating ability of carbon nanotube-supported β-nucleating agent in isotactic polypropylene. Colloid Polym. Sci. 2010, 288, 681–688. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Yang, S.; Ren, P.; Zhang, Q.; Li, Z. Polypropylene films with high barrier performance via crystal morphology manipulation. J. Mater. Sci. 2017, 52, 5449–5461. [Google Scholar] [CrossRef]
- Martin, J.; Bourson, P.; Dahoun, A.; Hiver, J. The β-Spherulite Morphology of Isotactic Polypropylene Investigated by Raman Spectroscopy. Appl. Spectrosc. 2009, 63, 1377–1381. [Google Scholar] [CrossRef]
- Kumaran, M.K. Interlaboratory Comparison of the ASTM Standard Test Methods for Water Vapor Transmission of Materials (E96-95). J. Test. Eval. 1998, 26, 83–88. [Google Scholar]
- Andrikopoulos, K.; Bounos, G.; Tasis, D.; Sygellou, L.; Drakopoulos, V.; Voyiatzis, G. The Effect of Thermal Reduction on the Water Vapor Permeation in Graphene Oxide Membranes. Adv. Mater. Interfaces 2014, 1, 1400250–1400258. [Google Scholar] [CrossRef]
- Bhuiyan, K.H.; Rahman, M.; Mina, F.; Islam, M.R.; Gafur, A.; Begum, A. Crystalline morphology and properties of multi-walled carbon nanotube filled isotactic polypropylene nanocomposites: Influence of filler size and loading. Compos. Part A Appl. Sci. Manuf. 2013, 52, 70–79. [Google Scholar] [CrossRef]
- Rybnikar, F. Orientation in composite of polypropylene and talc. J. Appl. Polym. Sci. 1989, 38, 1479–1490. [Google Scholar] [CrossRef]
- Li, J.; Cheung, W. On the deformation mechanisms of β-polypropylene: 1. Effect of necking on β-phase PP crystals. Polymer 1998, 39, 6935–6940. [Google Scholar] [CrossRef]
- Li, J.; Cheung, W.; Jia, D. A study on the heat of fusion of β-polypropylene. Polymer 1999, 40, 1219–1222. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Q.; Wang, K.; Deng, H.; Fu, Q. Combined effect of β-nucleating agent and multi-walled carbon nanotubes on polymorphic composition and morphology of isotactic polypropylene. J. Therm. Anal. Calorim. 2012, 107, 733–743. [Google Scholar] [CrossRef]
- Varga, J.; Menyhrd, A. Effect of Solubility and Nucleating Duality of N,N′-Dicyclohexyl-2,6-naphthalenedicarboxamide on the Supermolecular Structure of Isotactic Polypropylene. Macromolecules 2007, 40, 2422–2431. [Google Scholar] [CrossRef]
- Samuels, R.J.; Yee, R.Y. Characterization of the structure and organization of β-form crystals in type III and type IV isotactic polypropylene spherulites. J. Polym. Sci. 1972, 10, 385–432. [Google Scholar] [CrossRef]





| PP Composite Samples | ||
|---|---|---|
| MWCNT-g-PP Loading (wt.%) | β-ΝA Loading (wt.%) | β-ΝA and MWCNT-g-PP Loading |
| 0.5, 1.5, 3, 4, 6 | 0.3, 1, 4 | All possible combinations |
| Film | Χα% | |
|---|---|---|
| Samples Quenched | Samples Annealed 130 °C | |
| PP | 48.0 | 54.3 |
| 0.5% MWCNT-g-PP | 39.3 | 43.7 |
| 1.5% MWCNT-g-PP | 44.0 | 50.0 |
| 3% MWCNT-g-PP | 49.0 | 50.4 |
| 4% MWCNT-g-PP | 38.3 | 44.5 |
| 6% MWCNT-g-PP | 40.5 | 44.7 |
| Film | Thermal History | Χβ% | Χα% | Χ% |
|---|---|---|---|---|
| PP | Tann = 130 °C | 54.3 | 54.3 | |
| quench in ice | 48.0 | 48.0 | ||
| 0.3% β-NA | Tann = 130 °C | 26.0 | 37.0 | 63.0 |
| quench in ice | 11.0 | 34.0 | 45.0 | |
| 1% β-NA | Tann = 130 °C | 26.7 | 30.0 | 56.7 |
| quench in ice | 11.0 | 31.7 | 42.7 | |
| 4% β-NA | Tann = 130 °C | 29.6 | 30.7 | 60.3 |
| quench in ice | 21.3 | 36.0 | 57.3 |
| Film | Thermal History | Χβ% | Χα% | Χ% |
|---|---|---|---|---|
| y = 0 | Tann = 130 °C | 29.6 (38) | 30.7 | 60.3 |
| quench in ice | 21.3 | 36.0 | 57.3 | |
| y = 0.5 | Tann = 130 °C | 31.7 (49) | 19.0 | 50.6 |
| quench in ice | 13.7 | 27.4 | 41.0 | |
| y = 1.5 | Tann = 130 °C | 33.6 (55) | 17.4 | 51.0 |
| quench in ice | 16.8 | 33.0 | 49.8 | |
| y = 3 | Tann = 130 °C | 31.6 (55) | 17.9 | 49.5 |
| quench in ice | 25.8 | 26.0 | 51.8 | |
| y = 4 | Tann = 130 °C | 17.4 (36) | 36.0 | 53.4 |
| quench in ice | 9.7 | 38.2 | 47.9 | |
| y = 6 | Tann = 130 °C | 17.4 (36) | 31.9 | 49.3 |
| quench in ice | 4.7 | 36.5 | 41.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visvini, G.A.; Mathioudakis, G.N.; Soto Beobide, A.; Piperigkou, Z.; Giannakas, A.E.; Messaritakis, S.; Sotiriou, G.; Voyiatzis, G.A. Improvement of Water Vapor Permeability in Polypropylene Composite Films by the Synergy of Carbon Nanotubes and β-Nucleating Agents. Polymers 2023, 15, 4432. https://doi.org/10.3390/polym15224432
Visvini GA, Mathioudakis GN, Soto Beobide A, Piperigkou Z, Giannakas AE, Messaritakis S, Sotiriou G, Voyiatzis GA. Improvement of Water Vapor Permeability in Polypropylene Composite Films by the Synergy of Carbon Nanotubes and β-Nucleating Agents. Polymers. 2023; 15(22):4432. https://doi.org/10.3390/polym15224432
Chicago/Turabian StyleVisvini, Glykeria A., Georgios N. Mathioudakis, Amaia Soto Beobide, Zoi Piperigkou, Aris E. Giannakas, Stavros Messaritakis, Giannis Sotiriou, and George A. Voyiatzis. 2023. "Improvement of Water Vapor Permeability in Polypropylene Composite Films by the Synergy of Carbon Nanotubes and β-Nucleating Agents" Polymers 15, no. 22: 4432. https://doi.org/10.3390/polym15224432
APA StyleVisvini, G. A., Mathioudakis, G. N., Soto Beobide, A., Piperigkou, Z., Giannakas, A. E., Messaritakis, S., Sotiriou, G., & Voyiatzis, G. A. (2023). Improvement of Water Vapor Permeability in Polypropylene Composite Films by the Synergy of Carbon Nanotubes and β-Nucleating Agents. Polymers, 15(22), 4432. https://doi.org/10.3390/polym15224432









