Depolymerisation of Kraft Lignin by Tailor-Made Alkaliphilic Fungal Laccases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Flask Production and Purification of Laccases
2.3. Kraft Lignin Oxidation
2.3.1. Effect of pH and Enzyme Variant on Treatment of Lignin at Low Concentration
2.3.2. Demethylation Assay
2.3.3. Enzymatic Treatment of Lignin at High Concentration
2.4. Gel Permeation Chromatography (GPC) Analysis
2.5. Nuclear Magnetic Resonance Analyses
2.6. GC/MS
2.7. Membrane Separation System at Bench Scale
3. Results and Discussion
3.1. Effect of pH on Lignin Transformation by Three Alkaliphilic Fungal Laccase Variants
3.2. Lignin Transformation at High Concentration with Alkaliphilic Fungal Laccase
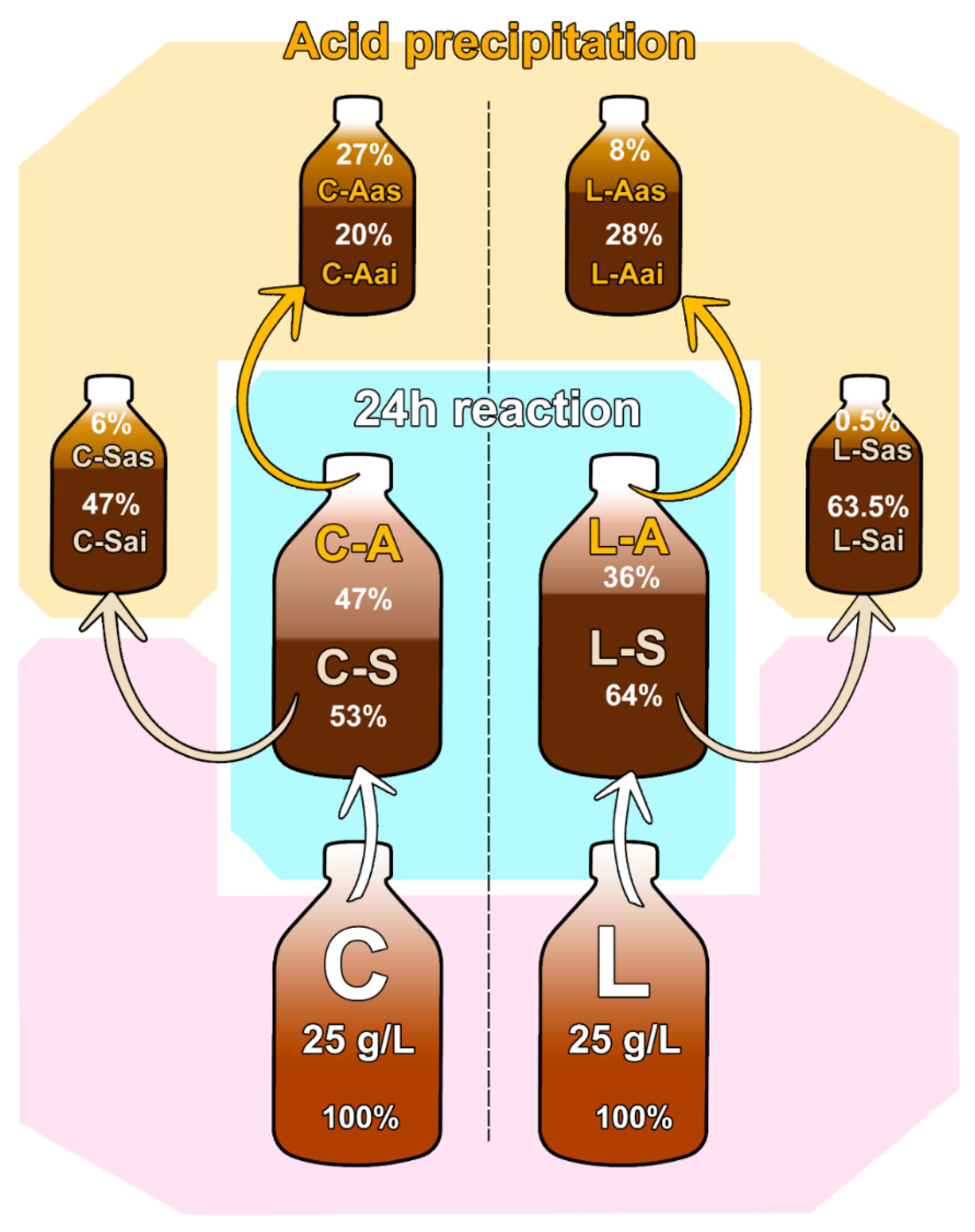
3.2.1. Lignin Mass Balance
3.2.2. Chemical Characterisation of the Lignin Fractions Obtained after Acid Resuspension and Precipitation
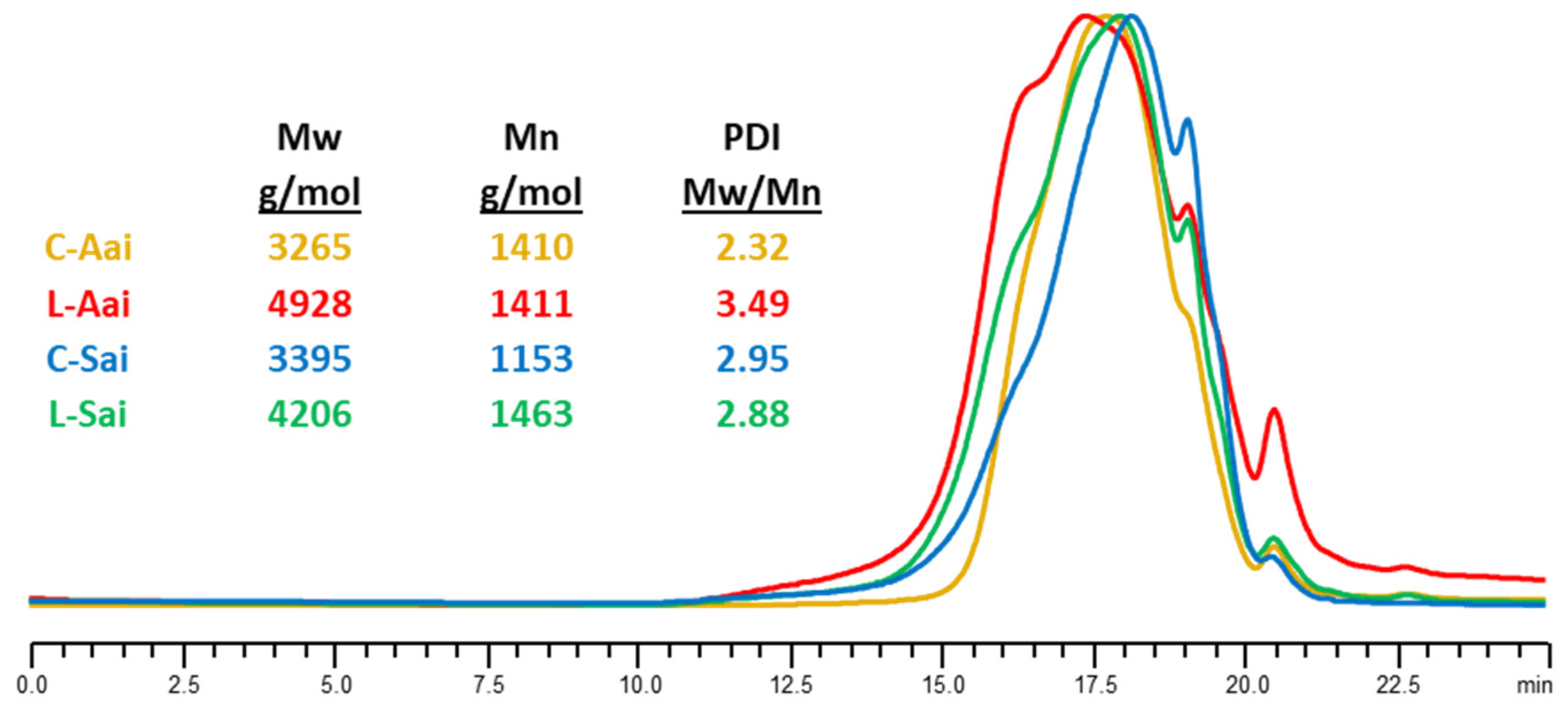
Determination of Molecular Weight
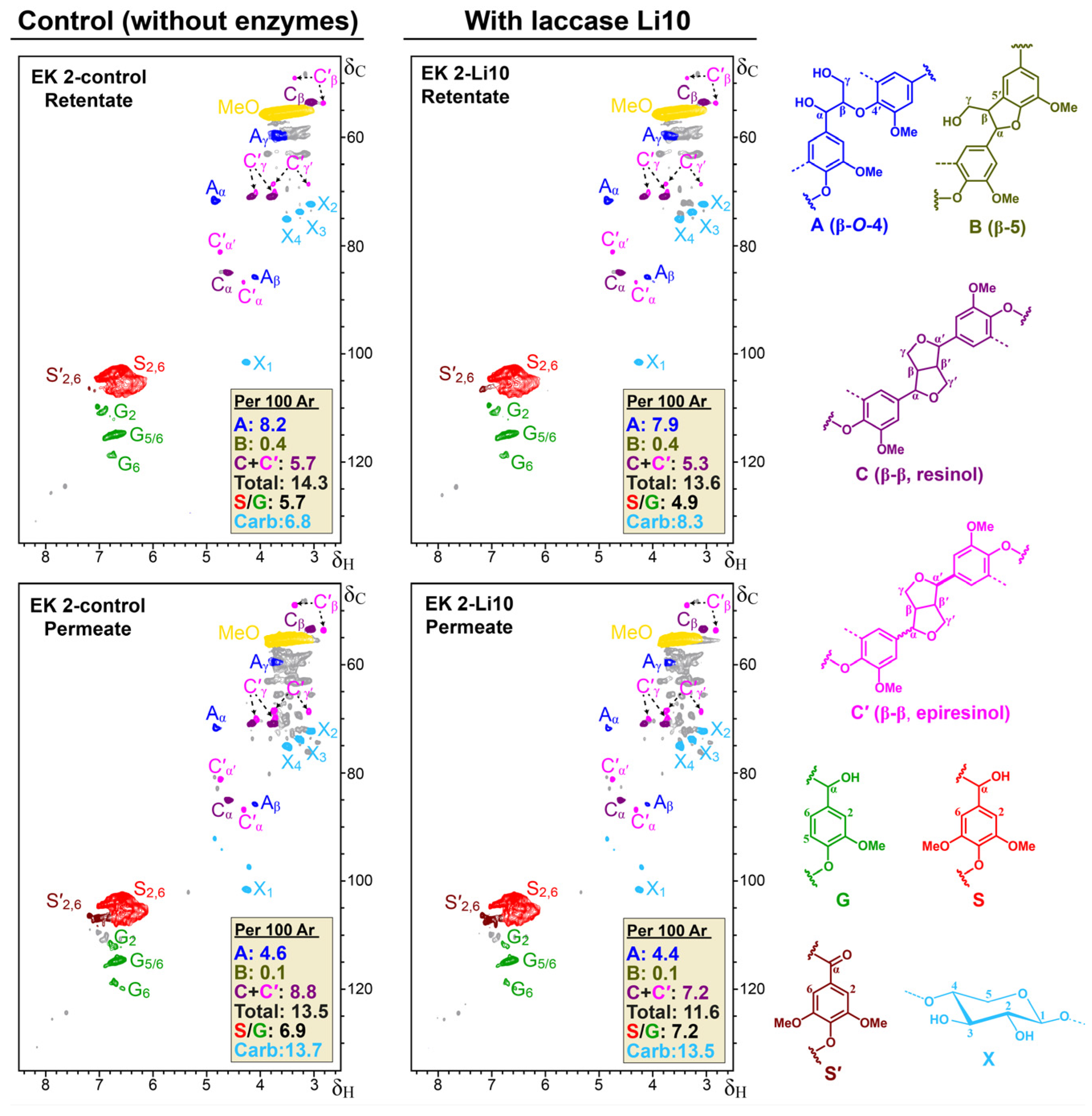
Changes in Lignin Substructures as Observed in 2D-NMR Analyses
NMR Determination of Phenolic Hydroxyl and Carbonyl Groups
3.2.3. Analysis of Simple Phenols in the Acid-Soluble Fractions
3.3. Bench-Scale Trials in Bioreactor Coupled to a Membrane Separation System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Takkellapati, S. The Current and Emerging Sources of Technical Lignins and Their Applications. Biofuels Bioprod. Biorefining 2018, 12, 756–787. [Google Scholar] [CrossRef] [PubMed]
- Bidlack, J.; Malone, M.; Benson, R. Molecular Structure and Component Integration of Secondary Cell Walls in Plants. Proc. Okla. Acad. Sci. 1992, 56, 51–56. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Lu, F. Lignins: Natural Polymers from Oxidative Coupling of 4-Hydroxyphenyl- Propanoids John. Phytochem. Rev. Biomass Valorization 2021, 12, 1341–1355. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Mastrolitti, S.; Borsella, E.; Guiliano, A.; Petrone, M.T.; De Bari, I.; Gosselink, R.; Van Erven, G.; Annevelink, E.; Triantafyllidis, K.S.; Stichnothe, H. Sustainable Lignin Valorization; LignoCOST; IEA Bioenergy Task 42: Denver, CO, USA, 2021; ISBN 9791280907011. [Google Scholar]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.; Mazumder, P.; Kalamdhad, A.S. Recent Advances in Removal of Lignin from Paper Industry Wastewater and Its Industrial Applications—A Review. Bioresour. Technol. 2020, 312, 123636. [Google Scholar] [CrossRef]
- Tomani, P. The Lignoboost Process. Cellul. Chem. Technol. 2010, 44, 53–58. [Google Scholar]
- Kouisni, L.; Holt-Hindle, P.; Maki, K.; Paleologou, M. The LignoForce System™: A New Process for the Production of High-Quality Lignin from Black Liquor. Pulp Pap. Can. 2014, 115, 18–22. [Google Scholar]
- Gellerstedt, G.; Gustafsson, K.; Northey, R.A. Structural Changes in Lignin during Kraft Cooking. Nord. Pulp Pap. Res. J. 1988, 3, 87–94. [Google Scholar] [CrossRef]
- Gierer, J. Chemical Aspects of Kraft Pulping. Wood Sci. Technol. 1980, 14, 241–266. [Google Scholar] [CrossRef]
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Pulkki, R.; Xu, C. Global Lignin Supply Overview and Kraft Lignin Potential as an Alternative for Petroleum-Based Polymers. Renew. Sustain. Energy Rev. 2020, 123, 109768. [Google Scholar] [CrossRef]
- Donmez Cavdar, A.; Kalaycioglu, H.; Hiziroglu, S. Some of the Properties of Oriented Strandboard Manufactured Using Kraft Lignin Phenolic Resin. J. Mater. Process. Technol. 2008, 202, 559–563. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal Laccases-Occurrence and Properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Escribano, D.; Pliego-Magán, R.; de Salas, F.; Aza, P.; Gentili, P.; Ihalainen, P.; Levée, T.; Meyer, V.; Petit-Conil, M.; Tapin-Lingua, S.; et al. Tailor-Made Alkaliphilic and Thermostable Fungal Laccases for Industrial Wood Processing. Biotechnol. Biofuels Bioprod. 2022, 15, 149. [Google Scholar] [CrossRef] [PubMed]
- Couto, S.R.; Toca-herrera, J.L. Lacasses in the Textile Industry. Biotechnol. Mol. Biol. Rev. 2006, 1, 115–120. [Google Scholar]
- Yin, Q.; Zhou, G.; Peng, C.; Zhang, Y.; Kües, U.; Liu, J.; Xiao, Y.; Fang, Z. The First Fungal Laccase with an Alkaline PH Optimum Obtained by Directed Evolution and Its Application in Indigo Dye Decolorization. AMB Express 2019, 9, 151. [Google Scholar] [CrossRef]
- Otsuka Saito, K.; Ikeda, R.; Endo, K.; Tsujino, Y.; Takagi, M.; Tamiya, E. Isolation of a Novel Alkaline-Induced Laccase from Flammulina Velutipes and Its Application for Hair Coloring. J. Biosci. Bioeng. 2012, 113, 575–579. [Google Scholar] [CrossRef]
- Vicente, A.I.; Viña-Gonzalez, J.; Santos-Moriano, P.; Marquez-Alvarez, C.; Ballesteros, A.O.; Alcalde, M. Evolved Alkaline Fungal Laccase Secreted by Saccharomyces Cerevisiae as Useful Tool for the Synthesis of C–N Heteropolymeric Dye. J. Mol. Catal. B Enzym. 2016, 134, 323–330. [Google Scholar] [CrossRef]
- Munk, L.; Sitarz, A.K.; Kalyani, D.C.; Mikkelsen, J.D.; Meyer, A.S. Can Laccases Catalyze Bond Cleavage in Lignin? Biotechnol. Adv. 2015, 33, 13–24. [Google Scholar] [CrossRef]
- Roth, S.; Spiess, A.C. Laccases for Biorefinery Applications: A Critical Review on Challenges and Perspectives. Bioprocess Biosyst. Eng. 2015, 38, 2285–2313. [Google Scholar] [CrossRef]
- Hämäläinen, V.; Grönroos, T.; Suonpää, A.; Heikkilä, M.W.; Romein, B.; Ihalainen, P.; Malandra, S.; Birikh, K.R. Enzymatic Processes to Unlock the Lignin Value. Front. Bioeng. Biotechnol. 2018, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Camarero, S.; Pardo, I.; Cañas, A.I.; Molina, P.; Record, E.; Martínez, A.T.; Martínez, M.J.; Alcalde, M. Engineering Platforms for Directed Evolution of Laccase from Pycnoporus Cinnabarinus. Appl. Environ. Microbiol. 2012, 78, 1370–1384. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Escribano, D.; de Salas, F.; Pardo, I.; Camarero, S. High-Throughput Screening Assay for Laccase Engineering toward Lignosulfonate Valorization. Int. J. Mol. Sci. 2017, 18, 1793. [Google Scholar] [CrossRef] [PubMed]
- Lancefield, C.S.; Wienk, H.J.; Boelens, R.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Identification of a Diagnostic Structural Motif Reveals a New Reaction Intermediate and Condensation Pathway in Kraft Lignin Formation. Chem. Sci. 2018, 9, 6348–6360. [Google Scholar] [CrossRef] [PubMed]
- Rencoret, J.; Kim, H.; Evaristo, A.B.; Gutiérrez, A.; Ralph, J.; Del Río, J.C. Variability in Lignin Composition and Structure in Cell Walls of Different Parts of Macaúba (Acrocomia aculeata) Palm Fruit. J. Agric. Food Chem. 2018, 66, 138–153. [Google Scholar] [CrossRef]
- Giummarella, N.; Pylypchuk, I.V.; Sevastyanova, O.; Lawoko, M. New Structures in Eucalyptus Kraft Lignin with Complex Mechanistic Implications. ACS Sustain. Chem. Eng. 2020, 8, 10983–10994. [Google Scholar] [CrossRef]
- Agustin, M.B.; de Carvalho, D.M.; Lahtinen, M.H.; Hilden, K.; Lundell, T.; Mikkonen, K.S. Laccase as a Tool in Building Advanced Lignin-Based Materials. ChemSusChem 2021, 14, 4615–4635. [Google Scholar] [CrossRef]
- Pang, T.; Wang, G.; Sun, H.; Sui, W.; Si, C. Lignin Fractionation: Effective Strategy to Reduce Molecule Weight Dependent Heterogeneity for Upgraded Lignin Valorization. Ind. Crops Prod. 2021, 165, 113442. [Google Scholar] [CrossRef]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and Analysis of the Molecular Weight of Lignin for Biorefining Studies. Biofuels Bioprod. Biorefin. 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Y.; Gao, J.; Li, H. Coloring Characteristics of in Situ Lignin during Heat Treatment. Wood Sci. Technol. 2012, 46, 33–40. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Dillies, J.; Vivien, C.; Chevalier, M.; Rulence, A.; Châtaigné, G.; Flahaut, C.; Senez, V.; Froidevaux, R. Enzymatic Depolymerization of Industrial Lignins by Laccase-Mediator Systems in 1,4-Dioxane/Water. Biotechnol. Appl. Biochem. 2020, 67, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Vignali, E.; Gigli, M.; Cailotto, S.; Pollegioni, L.; Rosini, E.; Crestini, C. The Laccase-Lig Multienzymatic Multistep System in Lignin Valorization. ChemSusChem 2022, 15, e202201147. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Fakayode, O.A.; Ren, M.; Li, H.; Liang, J.; Yagoub, A.E.G.A.; Fan, Z.; Zhou, C. Laccase-Catalyzed Lignin Depolymerization in Deep Eutectic Solvents: Challenges and Prospects. Bioresour. Bioprocess. 2023, 10, 21. [Google Scholar] [CrossRef]
- Steinmetz, V.; Villain-gambier, M.; Klem, A.; Ziegler, I.; Dumarcay, S.; Trebouet, D. In-Situ Extraction of Depolymerization Products by Membrane Filtration against Lignin Condensation. Bioresour. Technol. 2020, 311, 123530. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, R.; Romero, R.A.; Redondo, A.; Gnanakaran, S. Theoretical Study of the Remarkably Diverse Linkages in Lignin. J. Phys. Chem. Lett. 2011, 2, 2660–2666. [Google Scholar] [CrossRef]
- Berlin, A.; Balakshin, M. Industrial Lignins: Analysis, Properties, and Applications; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780444595614. [Google Scholar]
- Suota, M.J.; da Silva, T.A.; Zawadzki, S.F.; Sassaki, G.L.; Hansel, F.A.; Paleologou, M.; Ramos, L.P. Chemical and Structural Characterization of Hardwood and Softwood LignoForce™ Lignins. Ind. Crops Prod. 2021, 173, 114138. [Google Scholar] [CrossRef]
- Gellerstedt, G. Softwood Kraft Lignin: Raw Material for the Future. Ind. Crops Prod. 2015, 77, 845–854. [Google Scholar] [CrossRef]




| Sample | Ph-OH | Carboxylic-OH | ||
|---|---|---|---|---|
| Ch | NH | Ch | NH | |
| C-Aai | 4.12 | 3.60 | 0.65 | 0.55 |
| L-Aai | 2.81 | 2.50 | 0.50 | 0.38 |
| C-Sai | 4.28 | 3.60 | 0.25 | 0.20 |
| L-Sai | 3.98 | 3.19 | 0.27 | 0.21 |
| Sample | Mw | PDI | PhOH a | COOH a | AliphOH a | Total-OH a |
|---|---|---|---|---|---|---|
| Lignin | 2324 | 2.27 | 3.94 (3.77) | 0.41 (0.41) | 1.97 (1.93) | 6.32 (6.10) |
| Fungal laccase | ||||||
| Retentate C | 5124 | 2.78 | 2.59 (2.98) | 0.44 (0.54) | 1.40 (1.60) | 4.43 (5.12) |
| Retentate L | 4940 | 3.07 | 2.55 (2.75) | 0.43 (0.48) | 1.43 (1.55) | 4.40 (4.78) |
| Permeate C | 1875 | 2.05 | 3.98 (2.57) | 1.09 (0.81) | 2.56 (1.78) | 7.63 (5.16) |
| Permeate L | 1595 | 1.92 | 2.58 (1.67) | 0.82 (0.56) | 1.92 (1.28) | 5.32 (3.51) |
| Bacterial laccase | ||||||
| Run 1 | ||||||
| Retentate C | 10,482 | 4.0 | 2.95 | 0.63 | 1.82 | 5.4 |
| Retentate L-1 | 20,383 | 7.6 | 2.62 | 0.73 | 1.68 | 5.03 |
| Permeate C | 3313 | 2.7 | 3.40 | 0.86 | 1.84 | 6.1 |
| Permeate L-1 | 4935 | 3.5 | 2.47 | 1.00 | 1.61 | 5.08 |
| Run 2 | ||||||
| Retentate C | 10,482 | 4.0 | 2.95 | 0.63 | 1.82 | 5.4 |
| Retentate L-2 | 37,498 | 13.6 | 1.95 | 0.67 | 1.41 | 4.03 |
| Permeate C | 3313 | 2.7 | 3.40 | 0.86 | 1.84 | 6.1 |
| Permeate L-2 | 3892 | 3.1 | 1.71 | 0.97 | 1.32 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Escribano, D.; de Salas, F.; Pliego, R.; Marques, G.; Levée, T.; Suonpää, A.; Gutiérrez, A.; Martínez, Á.T.; Ihalainen, P.; Rencoret, J.; et al. Depolymerisation of Kraft Lignin by Tailor-Made Alkaliphilic Fungal Laccases. Polymers 2023, 15, 4433. https://doi.org/10.3390/polym15224433
Rodríguez-Escribano D, de Salas F, Pliego R, Marques G, Levée T, Suonpää A, Gutiérrez A, Martínez ÁT, Ihalainen P, Rencoret J, et al. Depolymerisation of Kraft Lignin by Tailor-Made Alkaliphilic Fungal Laccases. Polymers. 2023; 15(22):4433. https://doi.org/10.3390/polym15224433
Chicago/Turabian StyleRodríguez-Escribano, David, Felipe de Salas, Rocío Pliego, Gisela Marques, Thomas Levée, Anu Suonpää, Ana Gutiérrez, Ángel T. Martínez, Petri Ihalainen, Jorge Rencoret, and et al. 2023. "Depolymerisation of Kraft Lignin by Tailor-Made Alkaliphilic Fungal Laccases" Polymers 15, no. 22: 4433. https://doi.org/10.3390/polym15224433
APA StyleRodríguez-Escribano, D., de Salas, F., Pliego, R., Marques, G., Levée, T., Suonpää, A., Gutiérrez, A., Martínez, Á. T., Ihalainen, P., Rencoret, J., & Camarero, S. (2023). Depolymerisation of Kraft Lignin by Tailor-Made Alkaliphilic Fungal Laccases. Polymers, 15(22), 4433. https://doi.org/10.3390/polym15224433