Recycling Compatible Organic Electrode Materials Containing Amide Bonds for Use in Rechargeable Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
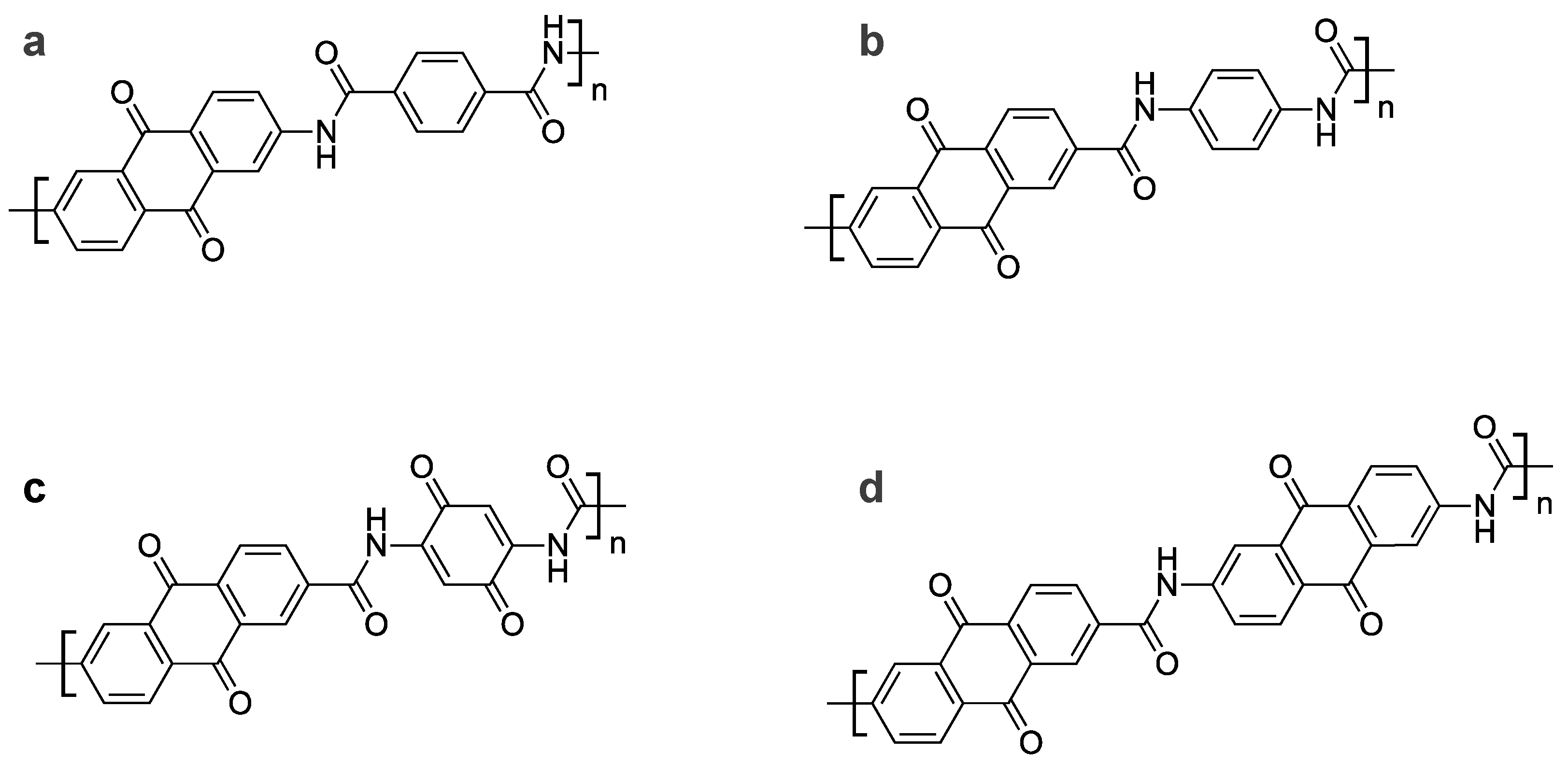
2.2.1. Anthraquinone Amide Dimer
2.2.2. Anthraquinone Amide Trimer
2.3. Battey Measurements
2.4. Hydrolysis Test
2.5. Theoretical Calculations
3. Results
3.1. Synthesis
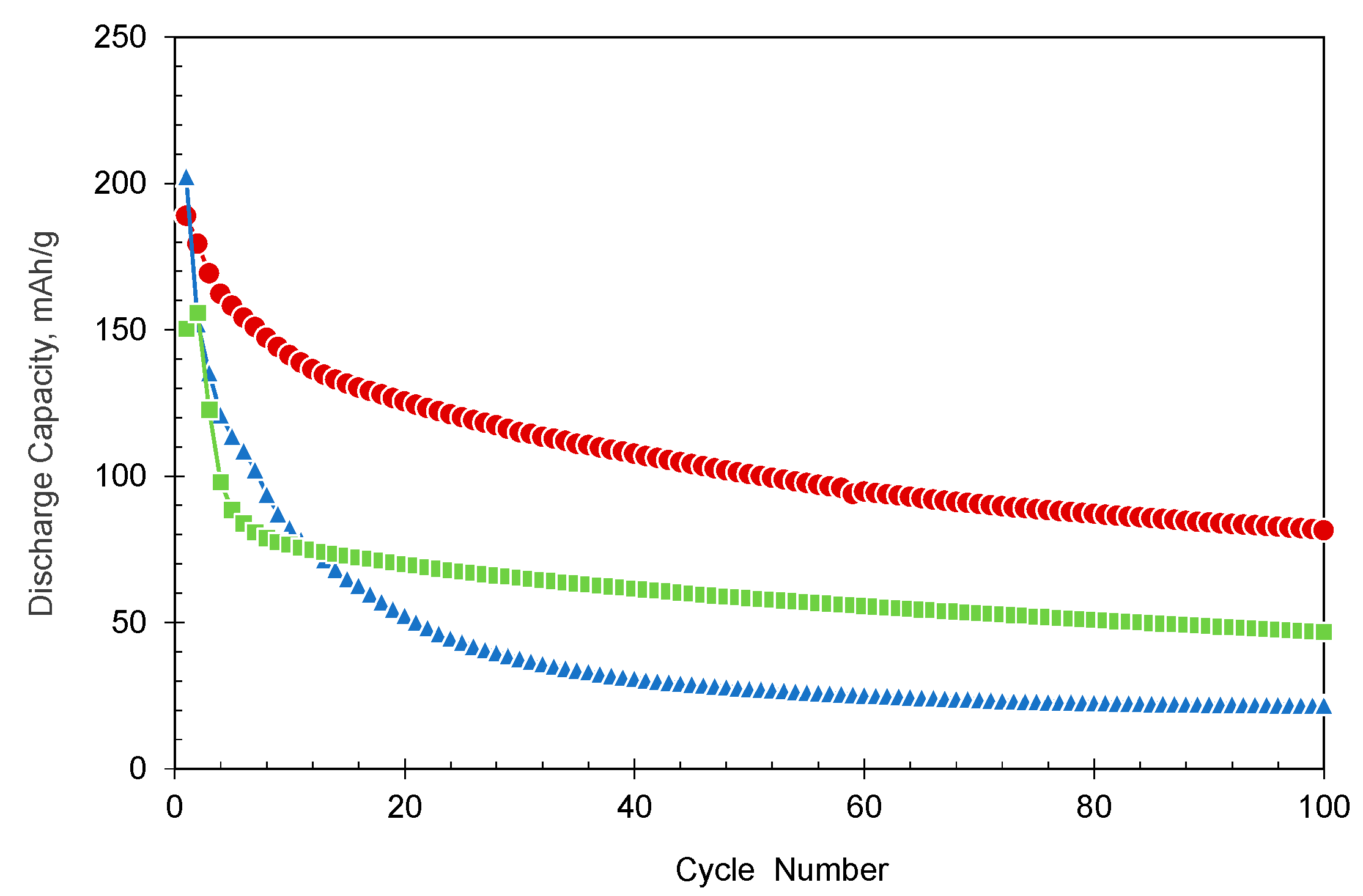
3.2. Electrochemical Tests
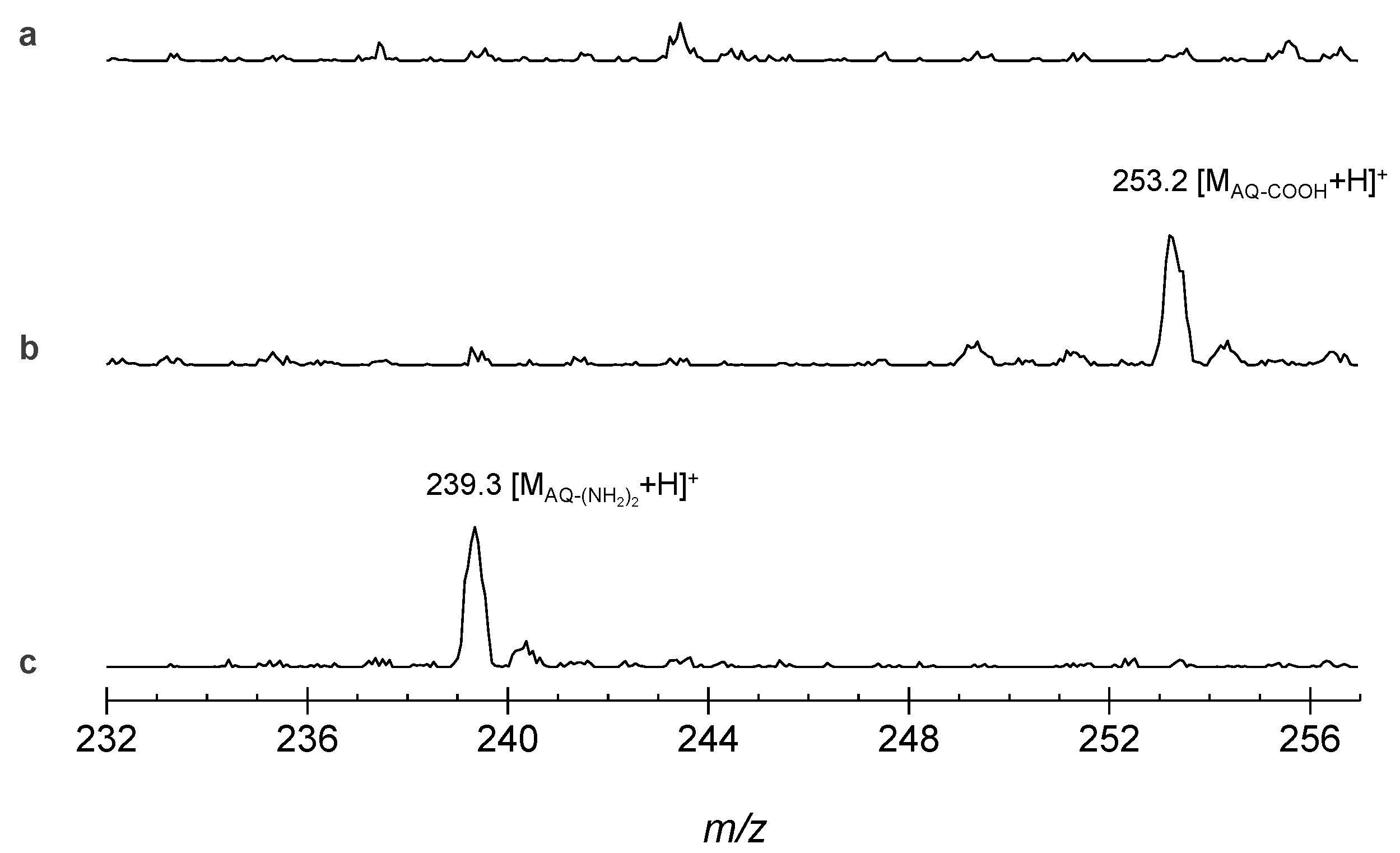
3.3. Recycling Ability Test: Hydrolysis Reaction
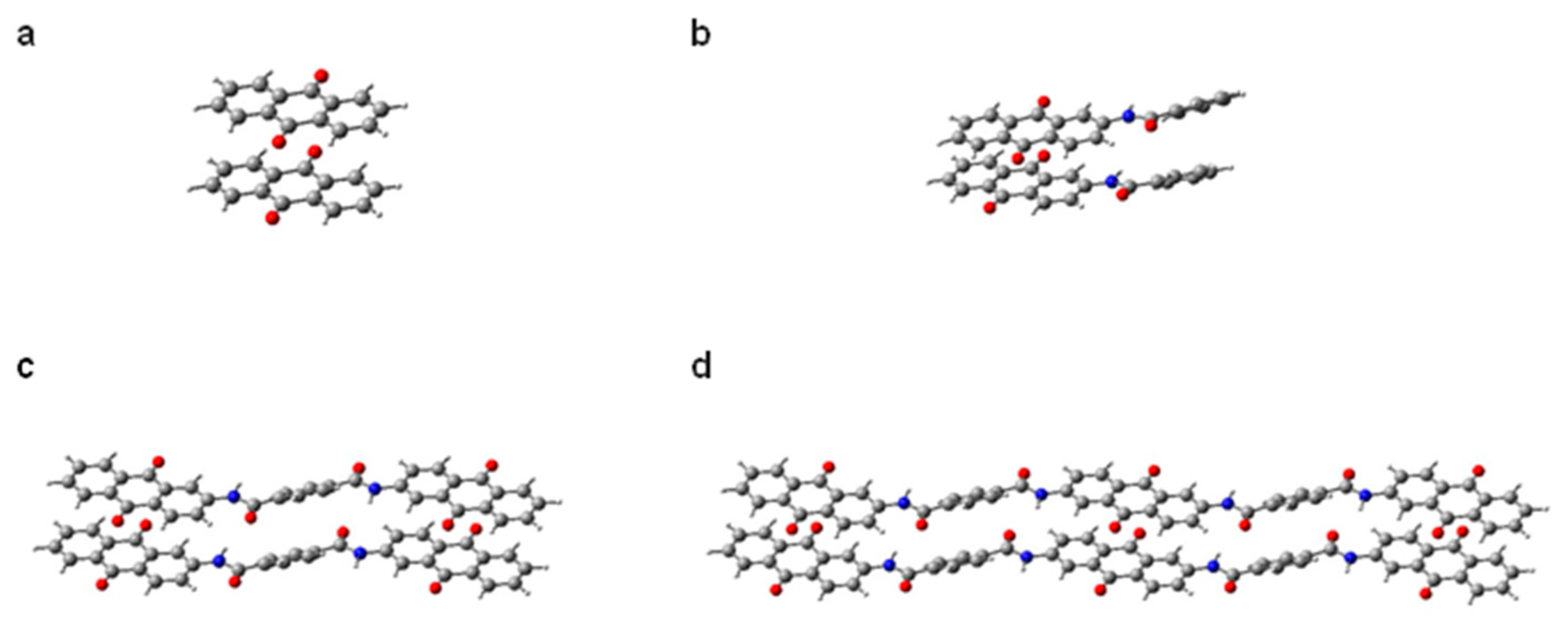
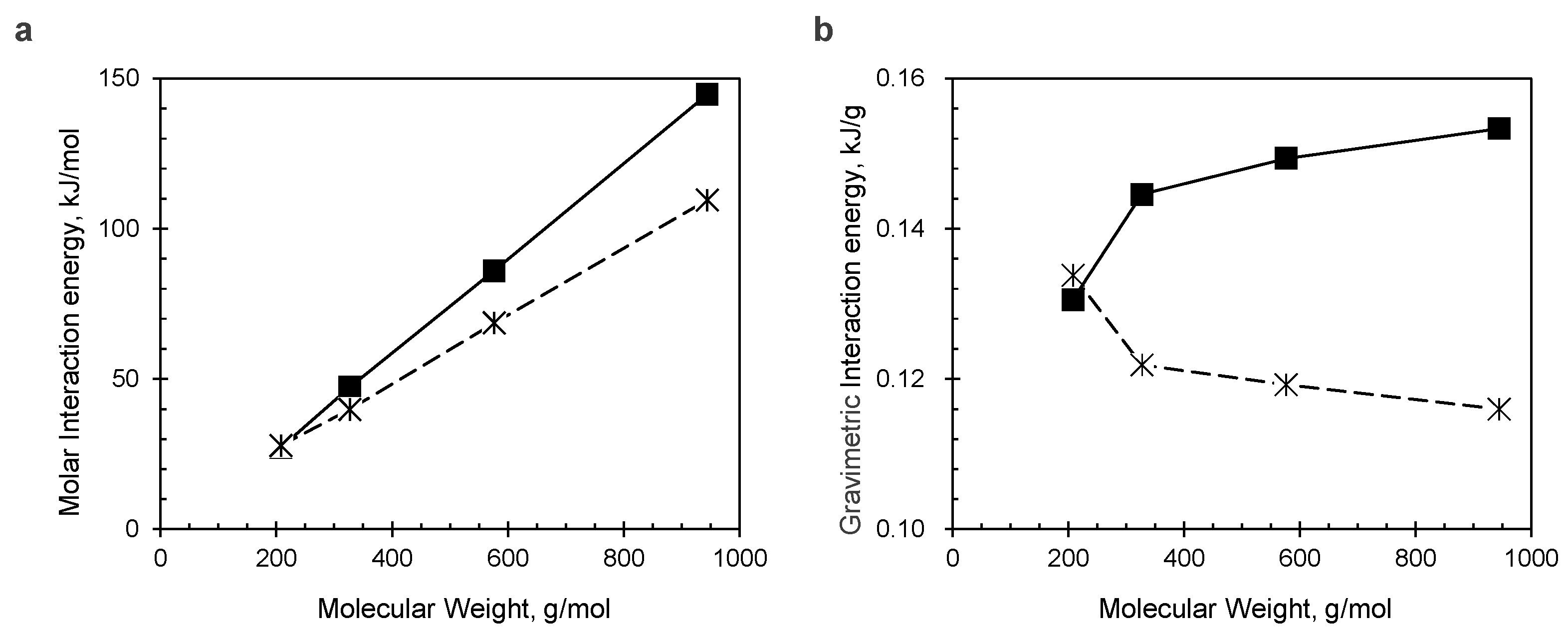
3.4. Quantum Chemistry Calculation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novák, P.; Müller, K.; Santhanam, K.S.V.; Hass, O. Electrochemically active polymers for rechargeable batteries. Chem. Rev. 1997, 97, 207–281. [Google Scholar] [PubMed]
- Nakahara, K.; Iwasa, S.; Satoh, M.; Morioka, Y.; Iriyama, J.; Suguro, M.; Hasegawa, E. Rechargeable batteries with organic radical cathode. Chem. Phys. Lett. 2002, 359, 351–354. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Matsunaga, T.; Kubota, T.; Sugimoto, T.; Satoh, M. High-performance lithium secondary batteries using cathode active materials of triquinoxalinylenes exhibiting six electron migration. Chem. Lett. 2011, 40, 750–752. [Google Scholar] [CrossRef]
- Liang, Y.L.; Tao, Z.L.; Chen, J. Organic electrode materials for rechargeable lithium batteries. Adv. Energy Mater. 2012, 2, 742–769. [Google Scholar] [CrossRef]
- Ogihara, N.; Yasuda, T.; Kishida, Y.; Ohsuna, T.; Miyamoto, K.; Ohba, N. Organic dicarboxylate negative electrode materials with remarkably small strain for high-voltage bipolar batteries. Angew. Chem. Int. Ed. 2014, 126, 11651–11656. [Google Scholar] [CrossRef]
- Muench, S.; Wild, A.; Friebe, C.; Häupler, B.; Janoschka, T.; Schubert, U.S. Polymer-based organic batteries. Chem. Rev. 2016, 116, 9438–9484. [Google Scholar]
- Liang, Y.; Chen, Z.; Jing, Y.; Rong, Y.; Facchetti, A.; Yao, Y. Heavily n-Dopable π-Conjugated Redox Polymers with Ultrafast Energy Storage Capability. J. Am. Chem. Soc. 2015, 137, 4956–4959. [Google Scholar] [CrossRef]
- Poizot, P.; Gaubicher, J.; Renault, S.; Dubois, L.; Liang, Y.; Yao, Y. Opportunities and challenges for organic electrodes in electrochemical energy storage. Chem. Rev. 2020, 120, 6490–6557. [Google Scholar] [CrossRef]
- Yao, M.; Ando, H.; Kataoka, R.; Kiyobayashi, T.; Takeichi, N. Improvement in the thermal safety of rechargeable lithium batteries: Endothermic behavior of organic positive-electrode materials. In ECS Meeting Abstracts; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2016; Volume MA2016-02, p. 303. [Google Scholar]
- Yao, M.; Senoh, H.; Yamazaki, S.; Siroma, Z.; Sakai, T.; Yasuda, K. High-capacity organic positive-electrode material based on a benzoquinone derivative for use in rechargeable lithium batteries. J. Power Sources 2010, 195, 8336–8340. [Google Scholar] [CrossRef]
- Song, Z.; Zhan, H.; Zhou, Y. Anthraquinone based polymer as high performance cathode material for rechargeable lithium batteries. Chem. Commun. 2009, 4, 448–450. [Google Scholar] [CrossRef]
- Nokami, T.; Matsuo, T.; Inatomi, Y.; Hojo, N.; Tsukagoshi, T.; Yoshizawa, H.; Shimizu, A.; Kuramoto, H.; Komae, K.; Tsuyama, H.; et al. Polymer-bound pyrene-4,5,9,10-tetraone for fast-charge and -discharge lithium-ion batteries with high capacity. J. Am. Chem. Soc. 2012, 134, 19694–19700. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liang, Y.; Gheytani, S.; Yao, Y. Cross-conjugated oligomeric quinones for high performance organic batteries. Nano Energy 2017, 37, 46–52. [Google Scholar] [CrossRef]
- Yokoji, T.; Kameyama, Y.; Maruyama, N.; Matsubara, H. High-capacity organic cathode active materials of 2,2′-bis-p-benzoquinone derivatives for rechargeable batteries. J. Mater. Chem. A 2016, 4, 5457–5466. [Google Scholar] [CrossRef]
- Inatomi, Y.; Hojo, N.; Yamamoto, T.; Watanabe, S.; Misaki, Y. Construction of rechargeable batteries using multifused tetrathiafulvalene systems as cathode materials. ChemPlusChem 2012, 77, 973–976. [Google Scholar] [CrossRef]
- Yao, M.; Sano, H.; Ando, H.; Kiyobayashi, T.; Takeichi, N. Anthraquinone-based oligomer as a long cycle-life organic electrode material for use in rechargeable batteries. ChemPhysChem 2019, 20, 967–971. [Google Scholar] [CrossRef]
- Hashimoto, K.; Hamano, T.; Okada, M. Degradation of several polyamides in soils. J. Appl. Polym. Sci. 1994, 54, 1579–1583. [Google Scholar] [CrossRef]
- Oppermann, F.B.; Pickartz, S.; Steinbüchel, A. Biodegradation of polyamides. Polym. Degrad. Stabil. 1998, 59, 337–344. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. 1988, A38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. 1988, B37, 785–789. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 6.1.1; Semichem Inc.: Shawnee Mission, KS, USA, 2016.






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, M.; Sano, H.; Ando, H. Recycling Compatible Organic Electrode Materials Containing Amide Bonds for Use in Rechargeable Batteries. Polymers 2023, 15, 4395. https://doi.org/10.3390/polym15224395
Yao M, Sano H, Ando H. Recycling Compatible Organic Electrode Materials Containing Amide Bonds for Use in Rechargeable Batteries. Polymers. 2023; 15(22):4395. https://doi.org/10.3390/polym15224395
Chicago/Turabian StyleYao, Masaru, Hikaru Sano, and Hisanori Ando. 2023. "Recycling Compatible Organic Electrode Materials Containing Amide Bonds for Use in Rechargeable Batteries" Polymers 15, no. 22: 4395. https://doi.org/10.3390/polym15224395
APA StyleYao, M., Sano, H., & Ando, H. (2023). Recycling Compatible Organic Electrode Materials Containing Amide Bonds for Use in Rechargeable Batteries. Polymers, 15(22), 4395. https://doi.org/10.3390/polym15224395




