Reverse Engineering and 3D Printing of Medical Devices for Drug Delivery and Drug-Embedded Anatomic Implants
Abstract
:1. Introduction
- Acquisition of 3D geometrical data: This initial phase involves digitally capturing 3D geometric information. These data can be directly gleaned from the patient or extracted from their medical records. Advanced imaging techniques such as computed tomography (CT) or magnetic resonance imaging (MRI) play a pivotal role in this stage, precisely capturing patient-specific anatomical details.
- Modification and Adaptation: Following data acquisition, the subsequent step entails modification and adaptation procedures. This stage serves as a bridge between raw data and tailored solutions. It involves fine-tuning the acquired data to align with the unique requirements specific to the patient.
- Creation of a 3D model or final product: With refined data, the process advances to create a 3D model or the ultimate product. Employing cutting-edge 3D printing technology, these models or products are realized. This stage further encompasses meticulous control over various aspects, including material attributes, shape, dimensions, and, most importantly, patient comfort.
2. Medical 3D Printing
- Diverse filament options: FDM offers a broad selection of filament materials providing flexibility in design and functionality.
- Speed and efficiency: This technology allows for rapid production ensuring a quick turnaround time for finished parts and assemblies.
- Cost-effectiveness: It is more affordable compared to other 3D printing techniques, making it budget-friendly choice for various applications.
- Durability: It produces robust and sturdy models, making it ideal for parts intended for harsh environments or heavy use.
- Complex geometries: It excels in handling projects involving large pieces and intricate designs, often at a lower cost to size ratio than alternative methods.
- Precision and consistency: This method enables high precision production with consistent and repeatable results, ensuring quality across multiple iterations.
- Toolless manufacturing: All that is required to create a part is a 3D printer, eliminating the need for specialized tools or molds.
- Environmentally friendly: This technique is eco-friendly emphasizing sustainable practices in manufacturing processes.
2.1. Common Reverse Additive Manufacturing Techniques Used for Drug Delivery Applications
2.2. Biomaterials Used in 3D Printing
2.2.1. Introduction to Biomaterials:
2.2.2. Physical, Chemical and Biological Properties of Biomaterials
2.3. Research Relating Drug Release Properties When 3D Printing Is Used
2.3.1. Targeted Approach
2.3.2. Current Applications of 3D Printing in Drug Delivery
- Infuse Bone Graft: This implantable device, manufactured by Medtronic, delivers recombinant human bone morphogenetic protein-2 (rhBMP-2) to promote bone growth in spinal fusion procedures. 3D printing could create patient-specific implants with optimized geometry and drug release profiles, improving surgical outcomes and reducing complications.
- Zoladex: A biodegradable implant developed by AstraZeneca for treating prostate cancer and certain gynecological disorders. It releases the drug goserelin over time, which helps regulate hormone levels. 3D printing could enable the development of implants with customizable drug release rates and more precise control over hormone regulation.
- Norplant: A subdermal contraceptive implant that releases the hormone levonorgestrel over an extended period. It has been replaced by newer systems such as Nexplanon and Implanon. 3D printing could be used to develop patient-specific implants that deliver the optimal drug dose based on individual patient needs and characteristics, potentially reducing side effects and improving efficacy.
- Vitrasert: An ocular implant used to treat cytomegalovirus retinitis in patients with AIDS. The implant releases the antiviral drug ganciclovir over an extended period. 3D printing could be used to develop customized ocular implants that conform better to individual patient anatomy, improving drug delivery and reducing complications.
- Probuphine is a subdermal implant that delivers buprenorphine to treat opioid dependence. Titan Pharmaceuticals developed the implant, which provides continuous drug release for up to six months. 3D printing could create personalized implants that optimize drug release based on individual patient needs, potentially improving treatment outcomes, and reducing relapse rates.
- Customization: 3D printing enables the creation of patient-specific implants that match individual patient anatomy and clinical needs, potentially improving treatment outcomes and reducing complications.
- Precision: 3D printing allows for precise control over implant geometry, material properties, and drug release profiles, which could lead to more effective and safer drug delivery.
- Complex geometries and multi-component systems: 3D printing can produce implants with intricate structures and multiple drugs, allowing for more sophisticated drug delivery strategies.
- Rapid prototyping and production: 3D printing technologies enable faster development and production of implantable drug delivery devices, potentially speeding up bringing new devices to market.
2.3.3. Drug Release Rate
3. Limitations
4. Prospects and Research Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ballard, D.H.; Trace, A.P.; Ali, S.; Hodgdon, T.; Zygmont, M.E.; DeBenedectis, C.M.; Smith, S.E.; Richardson, M.L.; Patel, M.J.; Decker, S.J. Clinical applications of 3D printing: Primer for radiologists. Acad. Radiol. 2018, 25, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef] [PubMed]
- Mardis, N.J. Emerging Technology and Applications of 3D Printing in the Medical Field. MO Med. 2018, 115, 368–373. [Google Scholar] [PubMed]
- Ganapathy, A.; Chen, D.; Elumalai, A.; Albers, B.; Tappa, K.; Jammalamadaka, U.; Hoegger, M.J.; Ballard, D.H. Guide for starting or optimizing a 3D printing clinical service. Methods 2022, 206, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Mwema, F.M.; Akinlabi, E.T.; Mwema, F.M.; Akinlabi, E.T. Basics of fused deposition modelling (FDM). In Fused Deposition Modeling: Strategies for Quality Enhancement; Springer: Cham, Switzerland, 2020; pp. 1–15. [Google Scholar]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for extrusion-based 3D printing of pharmaceuticals: A holistic materials–process perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef]
- Zub, K.; Hoeppener, S.; Schubert, U.S. Inkjet printing and 3D printing strategies for biosensing, analytical, and diagnostic applications. Adv. Mater. 2022, 34, 2105015. [Google Scholar] [CrossRef]
- Castiaux, A.D.; Pinger, C.W.; Hayter, E.A.; Bunn, M.E.; Martin, R.S.; Spence, D.M. PolyJet 3D-printed enclosed microfluidic channels without photocurable supports. Anal. Chem. 2019, 91, 6910–6917. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J.; Kotta, S. 3D printing in medicine: Technology overview and drug delivery applications. Ann. 3D Print. Med. 2021, 4, 100037. [Google Scholar] [CrossRef]
- West, T.G.; Bradbury, T.J. 3D printing: A case of ZipDose® technology–World’s first 3D printing platform to obtain FDA approval for a pharmaceutical product. In 3D and 4D Printing in Biomedical Applications: Process Engineering and Additive Manufacturing; Wiley: Hoboken, NJ, USA, 2019; pp. 53–79. [Google Scholar]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D printed “Starmix” drug loaded dosage forms for paediatric applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef] [PubMed]
- Sadia, M.; Arafat, B.; Ahmed, W.; Forbes, R.T.; Alhnan, M.A. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J. Control. Release 2018, 269, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Öblom, H.; Zhang, J.; Pimparade, M.; Speer, I.; Preis, M.; Repka, M.; Sandler, N. 3D-printed isoniazid tablets for the treatment and prevention of tuberculosis—Personalized dosing and drug release. AAPS PharmSciTech 2019, 20, 52. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, Q.; Qiang, W.; Li, H.; Zhong, W.; Pan, S.; Yang, G. Hydrophilic excipient-independent drug release from SLA-printed pellets. Pharmaceutics 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D printing of a multi-layered polypill containing six drugs using a novel stereolithographic method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef]
- Economidou, S.N.; Pere, C.P.P.; Reid, A.; Uddin, M.J.; Windmill, J.F.; Lamprou, D.A.; Douroumis, D. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater. Sci. Eng. C 2019, 102, 743–755. [Google Scholar] [CrossRef]
- Xu, X.; Goyanes, A.; Trenfield, S.J.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Gaisford, S.; Basit, A.W. Stereolithography (SLA) 3D printing of a bladder device for intravesical drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111773. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advances in powder bed fusion 3D printing in drug delivery and healthcare. Adv. Drug Deliv. Rev. 2021, 174, 406–424. [Google Scholar] [CrossRef]
- Gueche, Y.A.; Sanchez-Ballester, N.M.; Bataille, B.; Aubert, A.; Leclercq, L.; Rossi, J.-C.; Soulairol, I. Selective laser sintering of solid oral dosage forms with copovidone and paracetamol using a CO2 laser. Pharmaceutics 2021, 13, 160. [Google Scholar] [CrossRef]
- Salmoria, G.; Vieira, F.; Muenz, E.; Gindri, I.; Marques, M.; Kanis, L. Additive Manufacturing of PE/fluorouracil/progesterone intrauterine device for endometrial and ovarian cancer treatments. Polym. Test. 2018, 71, 312–317. [Google Scholar] [CrossRef]
- Boehm, R.D.; Miller, P.R.; Daniels, J.; Stafslien, S.; Narayan, R.J. Inkjet printing for pharmaceutical applications. Mater. Today 2014, 17, 247–252. [Google Scholar] [CrossRef]
- Pollard, T.D.; Seoane-Viaño, I.; Ong, J.J.; Januskaite, P.; Awwad, S.; Orlu, M.; Bande, M.F.; Basit, A.W.; Goyanes, A. Inkjet drug printing onto contact lenses: Deposition optimisation and non-destructive dose verification. Int. J. Pharm. X 2023, 5, 100150. [Google Scholar] [CrossRef]
- Nasiri, G.; Ahmadi, S.; Shahbazi, M.-A.; Nosrati-Siahmazgi, V.; Fatahi, Y.; Dinarvand, R.; Rabiee, M.; Haftlang, F.; Kim, H.S.; Rabiee, N. 3D printing of bioactive materials for drug delivery applications. Expert Opin. Drug Deliv. 2022, 19, 1061–1080. [Google Scholar] [CrossRef] [PubMed]
- Damiati, L.A.; El-Yaagoubi, M.; Damiati, S.A.; Kodzius, R.; Sefat, F.; Damiati, S. Role of Polymers in Microfluidic Devices. Polymers 2022, 14, 5132. [Google Scholar] [CrossRef] [PubMed]
- Mancilla-De-la-Cruz, J.; Rodriguez-Salvador, M.; An, J.; Chua, C.K. Three-Dimensional Printing Technologies for Drug Delivery Applications: Processes, Materials, and Effects. Int. J. Bioprint. 2022, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Boccaccini, A.R. Nature-derived and synthetic additives to poly (ɛ-caprolactone) nanofibrous systems for biomedicine; an updated overview. Front. Chem. 2022, 9, 809676. [Google Scholar] [CrossRef]
- Wasti, S.; Adhikari, S. Use of biomaterials for 3D printing by fused deposition modeling technique: A review. Front. Chem. 2020, 8, 315. [Google Scholar] [CrossRef]
- Alabduladhem, T.O.; Bordoni, B. Physiology, Krebs Cycle; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Davison, N.L.; Barrère-de Groot, F.; Grijpma, D.W. Degradation of biomaterials. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 177–215. [Google Scholar]
- Li, W.; Mille, L.S.; Robledo, J.A.; Uribe, T.; Huerta, V.; Zhang, Y.S. Recent advances in formulating and processing biomaterial inks for vat polymerization-based 3D printing. Adv. Healthc. Mater. 2020, 9, 2000156. [Google Scholar] [CrossRef]
- Bose, S.; Jo, Y.; Majumdar, U.; Bandyopadhyay, A. Binder jet additive manufacturing of biomaterials. In Additive Manufacturing in Biomedical Applications; ASM International: Novelty, OH, USA, 2022. [Google Scholar]
- Ponni, R.T.; Swamivelmanickam, M.; Sivakrishnan, S. 3D Printing in Pharmaceutical Technology—A Review. Int. J. Pharm. Investig. 2020, 10. [Google Scholar] [CrossRef]
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- Okafor-Muo, O.L.; Hassanin, H.; Kayyali, R.; ElShaer, A. 3D printing of solid oral dosage forms: Numerous challenges with unique opportunities. J. Pharm. Sci. 2020, 109, 3535–3550. [Google Scholar] [CrossRef] [PubMed]
- Matijašić, G.; Gretić, M.; Vinčić, J.; Poropat, A.; Cuculić, L.; Rahelić, T. Design and 3D printing of multi-compartmental PVA capsules for drug delivery. J. Drug Deliv. Sci. Technol. 2019, 52, 677–686. [Google Scholar] [CrossRef]
- Sandler, N.; Salmela, I.; Fallarero, A.; Rosling, A.; Khajeheian, M.; Kolakovic, R.; Genina, N.; Nyman, J.; Vuorela, P. Towards fabrication of 3D printed medical devices to prevent biofilm formation. Int. J. Pharm. 2014, 459, 62–64. [Google Scholar] [CrossRef]
- Weisman, J.A.; Nicholson, J.C.; Tappa, K.; Jammalamadaka, U.; Wilson, C.G.; Mills, D.K. Antibiotic and chemotherapeutic enhanced three-dimensional printer filaments and constructs for biomedical applications. Int. J. Nanomed. 2015, 10, 357–370. [Google Scholar] [CrossRef]
- Boyer, C.J.; Woerner, J.E.; Galea, C.; Gatlin, C.A.; Ghali, G.E.; Mills, D.K.; Weisman, J.A.; McGee, D.J.; Alexander, J.S. Personalized bioactive nasal supports for postoperative cleft rhinoplasty. J. Oral Maxillofac. Surg. 2018, 76, 1562.e1–1562.e5. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.; Tappa, K.; Jammalamadaka, U.; Weisman, J.; Woerner, J. The use of 3D printing in the fabrication of nasal stents. Inventions 2017, 3, 1. [Google Scholar] [CrossRef]
- Weisman, J.A.; Ballard, D.H.; Jammalamadaka, U.; Tappa, K.; Sumerel, J.; D’Agostino, H.B.; Mills, D.K.; Woodard, P.K. 3D Printed Antibiotic and Chemotherapeutic Eluting Catheters for Potential Use in Interventional Radiology: In Vitro Proof of Concept Study. Acad. Radiol. 2019, 26, 270–274. [Google Scholar] [CrossRef]
- Bilian, X. Intrauterine devices. Best Pract. Res. Clin. Obstet. Gynaecol. 2002, 16, 155–168. [Google Scholar] [CrossRef]
- Genina, N.; Holländer, J.; Jukarainen, H.; Mäkilä, E.; Salonen, J.; Sandler, N. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. Eur. J. Pharm. Sci. 2016, 90, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Mancinelli, C.; Mancuso, E.; García-Romero, I.; Gilmore, B.F.; Casettari, L.; Larrañeta, E.; Lamprou, D.A. 3D printing of drug-loaded thermoplastic polyurethane meshes: A potential material for soft tissue reinforcement in vaginal surgery. Pharmaceutics 2020, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, Z.; Hua, C.; Ji, J.; Zhou, Z.; Fang, Y.; Weng, D.; Lu, L.; Pang, Y.; Sun, W. Design, modeling and 3D printing of a personalized cervix tissue implant with protein release function. Biomed. Mater. 2020, 15, 045005. [Google Scholar] [CrossRef]
- Martin, V.; Ribeiro, I.A.; Alves, M.M.; Gonçalves, L.; Claudio, R.A.; Grenho, L.; Fernandes, M.H.; Gomes, P.; Santos, C.F.; Bettencourt, A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater. Sci. Eng. C 2019, 101, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, K.; Zhu, Y.; Zhang, J.; Ye, X. 3D-printed hierarchical scaffold for localized isoniazid/rifampin drug delivery and osteoarticular tuberculosis therapy. Acta Biomater. 2015, 16, 145–155. [Google Scholar] [CrossRef]
- Li, K.; Zhu, M.; Xu, P.; Xi, Y.; Cheng, Z.; Zhu, Y.; Ye, X. Three-dimensionally plotted MBG/PHBHHx composite scaffold for antitubercular drug delivery and tissue regeneration. J. Mater. Sci. Mater. Med. 2015, 26, 102. [Google Scholar] [CrossRef]
- Han, C.; Yao, Y.; Cheng, X.; Luo, J.; Luo, P.; Wang, Q.; Yang, F.; Wei, Q.; Zhang, Z. Electrophoretic deposition of gentamicin-loaded silk fibroin coatings on 3D-printed porous cobalt–chromium–molybdenum bone substitutes to prevent orthopedic implant infections. Biomacromolecules 2017, 18, 3776–3787. [Google Scholar] [CrossRef]
- Costa, P.F.; Puga, A.M.; Díaz-Gomez, L.; Concheiro, A.; Busch, D.H.; Alvarez-Lorenzo, C. Additive manufacturing of scaffolds with dexamethasone controlled release for enhanced bone regeneration. Int. J. Pharm. 2015, 496, 541–550. [Google Scholar] [CrossRef]
- Farto-Vaamonde, X.; Auriemma, G.; Aquino, R.P.; Concheiro, A.; Alvarez-Lorenzo, C. Post-manufacture loading of filaments and 3D printed PLA scaffolds with prednisolone and dexamethasone for tissue regeneration applications. Eur. J. Pharm. Biopharm. 2019, 141, 100–110. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, Q.; Guo, X.; Sun, J.; Liu, Y. A programmed release multi-drug implant fabricated by three-dimensional printing technology for bone tuberculosis therapy. Biomed. Mater. 2009, 4, 065005. [Google Scholar] [CrossRef]
- Poudel, I.; Annaji, M.; Zhang, C.; Panizzi, P.R.; Arnold, R.D.; Kaddoumi, A.; Amin, R.H.; Lee, S.; Shamsaei, N.; Babu, R.J. Gentamicin Eluting 3D-Printed Implants for Preventing Post-Surgical Infections in Bone Fractures. Mol. Pharm. 2023, 20, 4236–4255. [Google Scholar] [CrossRef] [PubMed]
- Ballard, D.H.; Weisman, J.A.; Jammalamadaka, U.; Tappa, K.; Alexander, J.S.; Griffen, F.D. Three-dimensional printing of bioactive hernia meshes: In vitro proof of principle. Surgery 2017, 161, 1479–1481. [Google Scholar] [CrossRef] [PubMed]
- Holländer, J.; Hakala, R.; Suominen, J.; Moritz, N.; Yliruusi, J.; Sandler, N. 3D printed UV light cured polydimethylsiloxane devices for drug delivery. Int. J. Pharm. 2018, 544, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Ballard, D.H.; Jammalamadaka, U.; Tappa, K.; Weisman, J.A.; Boyer, C.J.; Alexander, J.S.; Woodard, P.K. 3D printing of surgical hernia meshes impregnated with contrast agents: In vitro proof of concept with imaging characteristics on computed tomography. 3D Print. Med. 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Mei, Z.; Zhang, F.; He, M.; Fletcher, C.; Wang, F.; Yang, J.; Bi, D.; Jiang, Y.; et al. 3D printed biodegradable implants as an individualized drug delivery system for local chemotherapy of osteosarcoma. Mater. Des. 2020, 186, 108336. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, X.; Huang, R.; Chen, H.; Shi, X.; Wang, J.; Tan, W.; Tan, Z. E-jet 3D printed drug delivery implants to inhibit growth and metastasis of orthotopic breast cancer. Biomaterials 2020, 230, 119618. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Stefaniak, D.; Arafat, B.; Isreb, A.; Wan, K.-W.; Alhnan, M.A. A lower temperature FDM 3D printing for the manufacture of patient-specific immediate release tablets. Pharm. Res. 2016, 33, 2704–2712. [Google Scholar] [CrossRef]
- Bhatt, U.; Malakar, T.K.; Murty, U.S.; Banerjee, S. 3D printing of immediate-release tablets containing olanzapine by filaments extrusion. Drug Dev. Ind. Pharm. 2021, 47, 1200–1208. [Google Scholar] [CrossRef]
- Giri, B.R.; Maniruzzaman, M. Fabrication of Sustained-Release Dosages Using Powder-Based Three-Dimensional (3D) Printing Technology. AAPS PharmSciTech 2022, 24, 4. [Google Scholar] [CrossRef]
- Wu, J.; Miao, G.; Zheng, Z.; Li, Z.; Ren, W.; Wu, C.; Li, Y.; Huang, Z.; Yang, L.; Guo, L. 3D printing mesoporous bioactive glass/sodium alginate/gelatin sustained release scaffolds for bone repair. J. Biomater. Appl. 2019, 33, 755–765. [Google Scholar] [CrossRef] [PubMed]
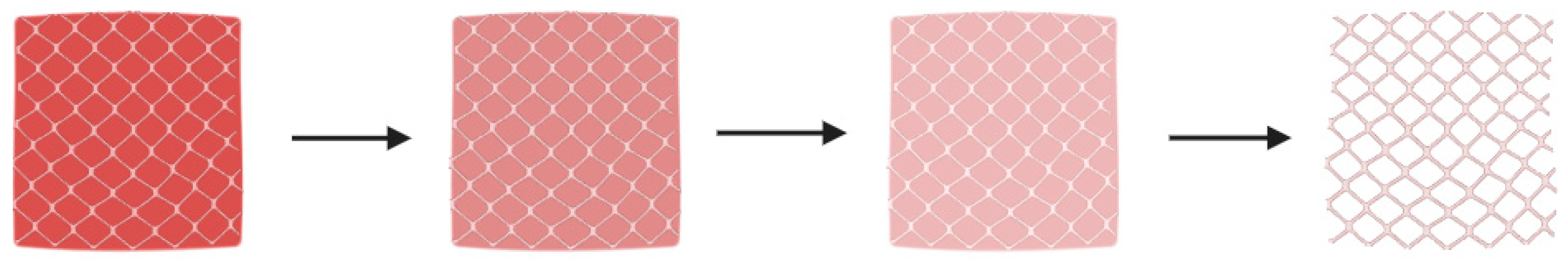

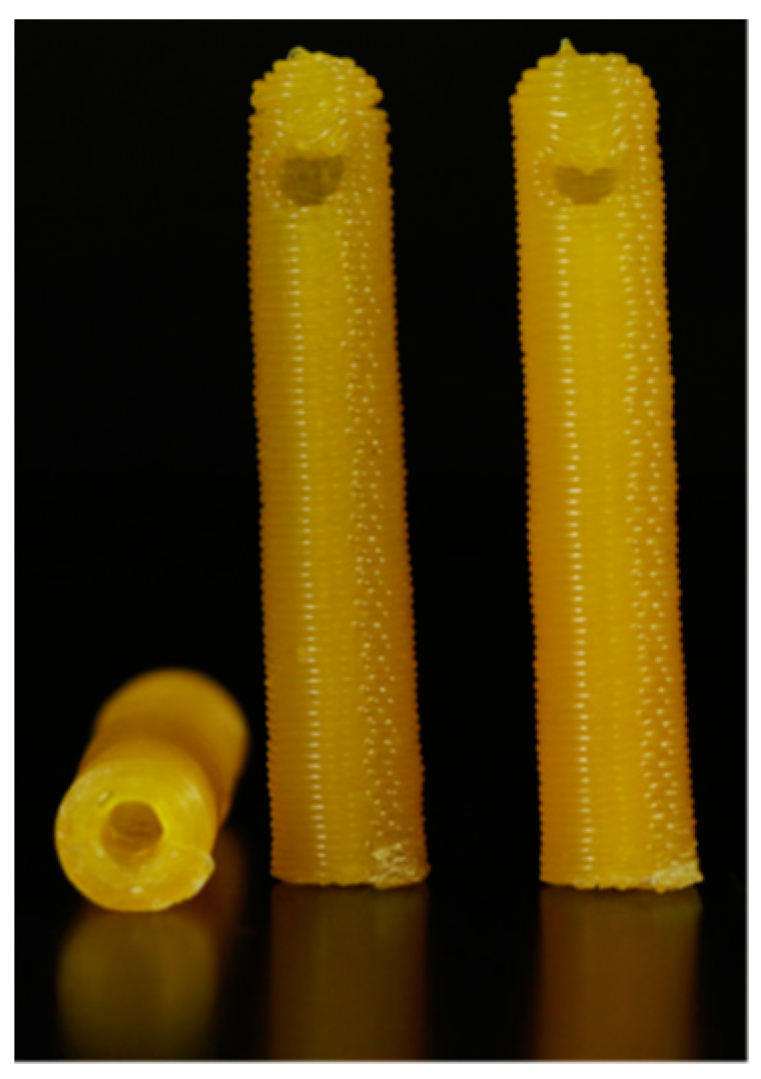
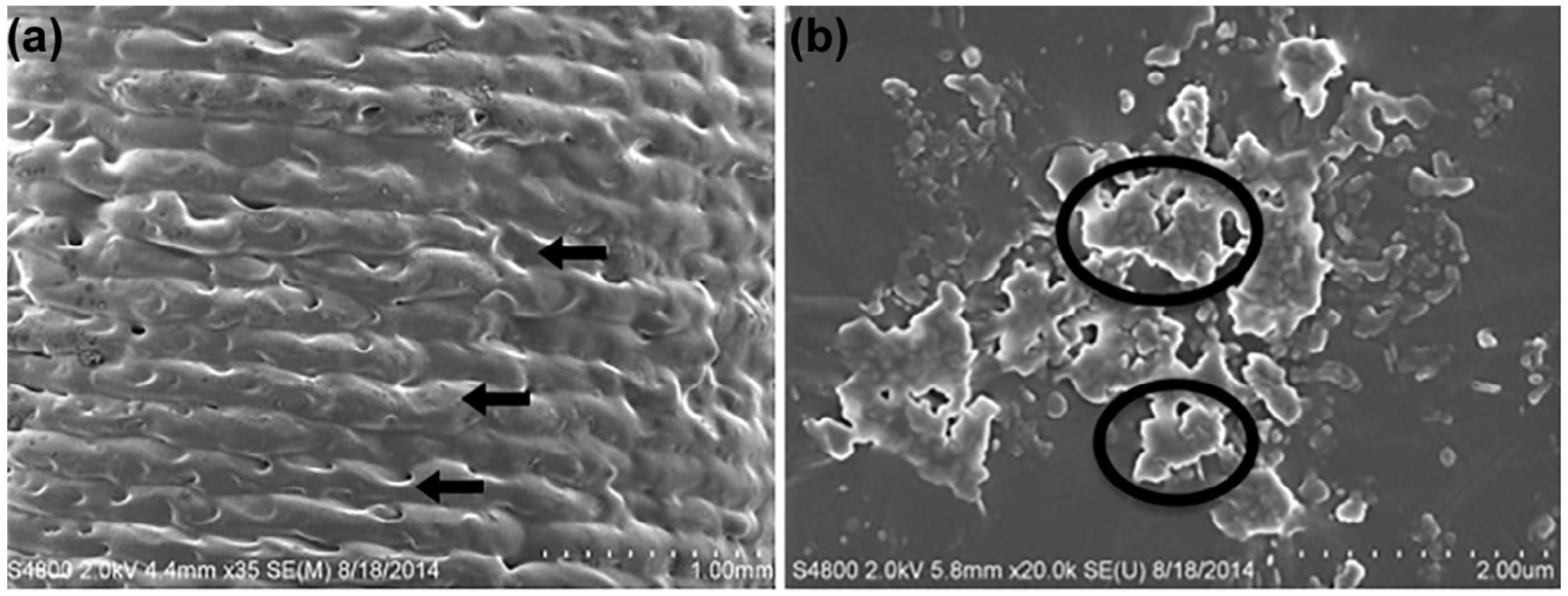
| Biomaterial | Printing Method | Drug Delivery Application | Reference(s) |
|---|---|---|---|
| Polyethylene Glycol Polymer (PEG) | FDM | Indomethacin loaded chewable tablets shaped as animals to appeal to pediatric patients | [13] |
| Cellulose-Based (HPMC, HPC, Eudragit) | FDM | Isoniazid loaded drugs to treat tuberculosis | [15] |
| Polyethylene Glycol Diacrylate (PEGDA) | SLA | Ibuprofen-loaded mini-sized pellets for enhanced PO intake | [16] |
| Polyethylene Glycol Diacrylate (PEGda) | SLA | Personalized Polypills (multiple drugs in one drug product) to reduce patient non-adherence | [17] |
| Dental SG Resin | SLA | Microneedle arrays for transdermal insulin delivery | [18] |
| Copovidone | SLS (PBF) | Solid dosage forms without need for additional absorbance enhancers | [21] |
| High Density Polyethylene (HDPE) | SLS (PBF) | Intrauterine (IUD) drug delivery systems containing female sex hormones | [22] |
| Poly (methyl vinyl ether-co-maleic anhydride)) | Inkjet (SLA) | Transdermal microneedles for cutaneous fungal infection treatment | [23] |
| Timolol-loaded Ink | Inkjet with Near Infrared Spectroscopy (NIR) | Extended release of glaucoma therapy drug (timolol maleate) from contact lenses | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elumalai, A.; Nayak, Y.; Ganapathy, A.K.; Chen, D.; Tappa, K.; Jammalamadaka, U.; Bishop, G.; Ballard, D.H. Reverse Engineering and 3D Printing of Medical Devices for Drug Delivery and Drug-Embedded Anatomic Implants. Polymers 2023, 15, 4306. https://doi.org/10.3390/polym15214306
Elumalai A, Nayak Y, Ganapathy AK, Chen D, Tappa K, Jammalamadaka U, Bishop G, Ballard DH. Reverse Engineering and 3D Printing of Medical Devices for Drug Delivery and Drug-Embedded Anatomic Implants. Polymers. 2023; 15(21):4306. https://doi.org/10.3390/polym15214306
Chicago/Turabian StyleElumalai, Anusha, Yash Nayak, Aravinda K. Ganapathy, David Chen, Karthik Tappa, Udayabhanu Jammalamadaka, Grace Bishop, and David H. Ballard. 2023. "Reverse Engineering and 3D Printing of Medical Devices for Drug Delivery and Drug-Embedded Anatomic Implants" Polymers 15, no. 21: 4306. https://doi.org/10.3390/polym15214306
APA StyleElumalai, A., Nayak, Y., Ganapathy, A. K., Chen, D., Tappa, K., Jammalamadaka, U., Bishop, G., & Ballard, D. H. (2023). Reverse Engineering and 3D Printing of Medical Devices for Drug Delivery and Drug-Embedded Anatomic Implants. Polymers, 15(21), 4306. https://doi.org/10.3390/polym15214306









