Abstract
Rubbers are extensively applied in chemical protective clothing (CPC) due to their eye-catching anti-penetration of chemicals. However, their impermeability, particularly that of natural rubber (NR), is unsatisfactory. In this work, we demonstrate the facile construction of Ti3C2Tx MXene/NR interface using a plant-scale and feasible method combining latex mixing, emulsion flocculation, and flat-plate vulcanisation. The above crafts achieved a homogeneous dispersion of Ti3C2Tx MXene in the NR matrix in a single layer, thereby constructing a strong interfacial interaction between Ti3C2Tx MXene and NR, which induced the formation of a robust three-dimensional (3D) network in the composite. The anti-swelling capacity of the 3D cross-linked network structure and the layered structure of Ti3C2Tx MXene effectively prolonged the permeation path of toxic chemicals. Compared with pure NR, the nanocomposite with 1 wt% of Ti3C2Tx MXene showed substantially enhanced breakthrough times of toluene, dichloromethane, and concentrated sulfuric acid (increased by 140%, 178.6%, and 92.5%, respectively). Furthermore, its tensile strength, elongation at break, and shore hardness increased by 7.847 MPa, 194%, and 12 HA, respectively. Taken together with the satisfactory anti-permeability, tensile strength, elongation at break, and shore hardness, the resulting Ti3C2Tx MXene/NR nanocomposites hold promise for application to long-term and high-strength CPC in the chemical industry and military fields.
1. Introduction
Frequent chemical accidents [1,2] or chemical attacks [3,4,5] heavily emphasize the significance of chemical protective clothing (CPC) [6,7,8].Chemical protective equipment is divided into four categories based on the various levels of protection as defined by the United States Environmental Protection Agency (USEPA), ranging from minimal dermal and respiratory protection (level D) to maximal protection (level A) [9]. Due to their anti-penetration, flexibility, and processability [10,11,12], rubbers are generally applied to encapsulating outfits for high-level chemical protection or chemical-resistant gloves and boots against various levels of toxic chemicals. Compared to synthetic rubber (SR), renewable and environmentally friendly natural rubber (NR) can be separated from common rubber trees, such as Hevea brasiliensis [13]. The protection ability of CPC is mainly determined via permeation, penetration, and degradation parameters [11,14]. However, NR only works for the penetration of chemicals in the form of liquid, vapor, and aerosol [11], its behaviours in anti-permeability [15] and anti-degradation of acids [16] are unsatisfactory. Therefore, it is essential to improve the protective performance of NR against toxic chemicals. Furthermore, its mechanical property is another vital aspect to guarantee the practical applications of NR for CPC [11], mainly including tear strength, tensile strength, cutting resistance, puncture resistance, and abrasion resistance.
Filling NR with the proper types of nanomaterials, such as carbon-based, inorganic-based, and bio-based fillers [17], can optimize its barrier property, mechanical performance, and other desirable properties. For instance, George et al. [18] claimed that multiwalled carbon nanotubes (MWCNT) nanofillers have a positive impact on the solvent resistance and gas barrier properties, as well as on the mechanical properties of NR-MWCNTR nanocomposites. The considerable mechanical behaviours of NR-based composites armed with carbon black or Al2O3 nanoparticles were reported by Nouraei et al. [19]. Wongwat’s [20] group prepared the NR/poly(lactic acid)/thermoplastic starch/nano-precipitated calcium carbonate (NR/PLA/TPS/NPCC) nanocomposites and reported that the NPCC content and mixing order of constituents affected the multiple performances of NR/PLA/TPS/NPCC nanocomposites, such as tensile and water vapor barrier properties. Although these fillers clearly improved the barrier or mechanical characteristics of NR, they were equipped with few surface functional groups and interacted with NR chains weakly, causing their aggregation within the NR matrix [21], thus failing to fully improve the mechanical or barrier performances of NR.
Because of their rich surface chemistry and high mechanical properties [22,23,24,25], two-dimensional (2D) transition metal carbide/nitride/carbonitride (MXene) sheets have been intensively and extensively reported as nanofillers to improve the mechanical performances of SR and chemical resistances of P84 copolyimide [26,27]. However, there has been no investigation related to MXene/NR in the chemical protective field to date. Considering that the NR obtained from rubber trees exists in the form of latex, which is an aqueous colloidal dispersion of rubber particles, Ti3C2Tx MXene nanofillers with strong hydrophilicity can be easily distributed in the NR matrix [21]. In this research, a Ti3C2Tx MXene/NR nanocomposite (MNx) was prepared via a feasible method combining latex mixing, emulsion flocculation, and flat-plate vulcanisation technologies, which induced the birth of homogeneous liquid mixing of NR and single-layer Ti3C2Tx MXene. The mixture rapidly co-coagulated during emulsion flocculation, thereby constructing the filler network. The final vulcanisation process promoted the formation of sulphide cross-links between the rubber chains, further strengthening the network in MNx. After filling the NR with 1 wt% Ti3C2Tx MXene, the breakthrough time (BTT) of the composite to toluene, dichloromethane, and concentrated sulfuric acid increased by 140%, 178.6%, and 92.5%, respectively. Moreover, a striking improvement in the mechanical properties was also realized. The substantial optimization of the toxic chemical barrier and mechanical behaviours of NR is mainly due to the interface constructed between the Ti3C2Tx filler and NR matrix, which induced the formation of a robust 3D network in the composite.
2. Materials and Methods
2.1. Materials
Ti3AlC2 was provided by Jilin Yiyi Technology Co. (Jilin, China). A total of 38% hydrochloric acid (HCl) and lithium fluoride (LiF) were obtained from Beijing J&K Scientific Technology Co., Ltd. (Beijing, China) and Sinopharm Chemical Reagent Co. (Shanghai, China), respectively. NR emulsion (60 wt%) (solid content: 60 wt %) was purchased from Guangzhou Songbai Chemical Co., Ltd., (Changzhou, China). Calcium chloride (CaCl2, AR) was purchased from the Beijing Chemical Factory (Beijing, China). Rubber additives were commercial products. Other reagents were chemically pure and used as received.
2.2. Preparation of Ti3C2Tx MXene Nanosheets
As shown in Figure 1, Ti3C2Tx MXene sheets were synthesized by etching the Ti3AlC2 MAX phase with HCl/LiF solvents followed by ultrasonic delamination [28]. Then, the resulting suspension was rigorously washed with ultra-pure water. After freeze-drying for 6 h under a vacuum, the Ti3C2Tx MXene powder was dispersed in the aqueous phase via sonication to prepare a suspension of Ti3C2Tx MXene at a concentration of 20 mg/mL. Details are shown in the supporting information.
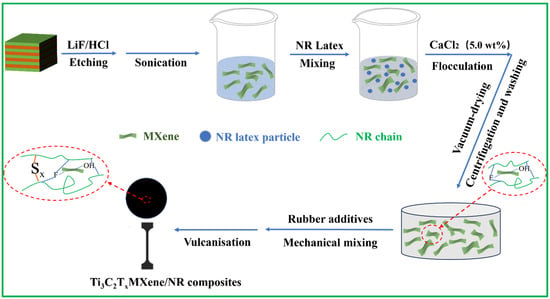
Figure 1.
Schematic of the preparation of Ti3C2Tx MXene nanosheets and MNx composites.
2.3. Preparation of MNx Composites
As described in Figure 1, the NR emulsion (60 wt%) was mixed with different contents of the above Ti3C2Tx MXene suspension (20 mg/mL) via continuous magnetic stirring for 4 h to obtain a uniform mixture. The detailed proportions of Ti3C2Tx and NR are shown in Table S1. Then, a CaCl2 solution (5 wt%) was added dropwise to the mixture for flocculation. Next, the completely flocculated mixture was precipitated with deionized water and filtered 3 times. Finally, the Ti3C2Tx MXene/NR masterbatch was obtained by freeze-drying the mixture under a vacuum for subsequent use.
Ti3C2Tx MXene/NR masterbatch was combined with additives on an open refining machine in accordance with the specific proportions, as depicted in Table S2. Vulcanised MNx with a thickness of 1 mm was achieved by plate vulcanisation at 143 °C for 10 min. The final nanocomposites with 0, 0.5, 1, and 3 wt% of Ti3C2Tx were named MN0, MN 0.5, MN1, and MN3, respectively.
2.4. Characterization
Morphological information was collected from a scanning electron microscope (SEM, ZEISS Gemini SEM 300, Jena, Germany). The crystal information was determined using an X-ray diffractometer (Bruker D8 Advance TXS) with Cu-Kα radiation. X-ray photoelectron spectroscopy (XPS) spectra were recorded using a Thermo Scientific K-Alpha (Thermo Electron Corporation, Waltham, MA, USA) X-ray photoelectron spectrometer with monochromatic Al-Kα (1486.6 eV) radiation. A rubber processing analyser (RPA) (RPA 2000, Alpha Technologies Corporation, Howell, MI, USA) was employed to monitor the relationship between storage modulus and the strain of uncured rubber compounds. TEM measurement was conducted on a JEOL JEM-2100 Plus transmission electron microscope (Japan Electronics Corporation, Tokyo, Japan) operated at 300 kV. And samples were embedded and sectioned using a Leica EM UC7 frozen ultrathin sectioning machine before TEM characterization. Atomic Force microscopy (AFM) test was carried out on a Bruker Multimode 8 with a trapping mode. Tensile testing was operated on a UCAN UT-2060 according to ASTM D 412. Dynamic mechanical analysis (DMA, Frequency: 1 Hz.) was performed on a TAQ 800 (TA Corporation, Santa Fe Springs, CA, USA) instrument in a tensile mode. Fourier transform infrared spectroscopy (FTIR) spectra were recorded using Nicolet iS20 Fourier transform infrared spectrometer (FT-IR, Thermo Scientific, Waltham, MA, USA). The cross-linking densities (CDs) of the vulcanisates were determined via nuclear magnetic resonance (NMR) spectroscopy (VTMR20-010V-1, Suzhou Niumag Corporation, Suzhou, China) at a frequency of 15 MHz, magnetic induction intensity of 0.5 ± 0.05 T and temperature of 90 °C. The bound rubber content in the MNx compounds was measured using a method reported by Leblanc [29]. Toxic chemical permeability tests were conducted at 25 °C using an apparatus for measuring chemical permeability in accordance with ISO 1817:2022. The tensile test and tear test were performed following ISO 37:2005 and ISO 34-1:2004 standards, respectively.
3. Results and Discussion
3.1. Microstructure of Ti3C2Tx MXene Nanosheets
The SEM image in Figure 2a implies the tightly packed structure of the Ti3AlC2 MAX. After being etched by the LiF/HCl system, Ti3AlC2 MAX transformed into a loose accordion-like structure (Figure S1). The final ultrasonication step peeled off the loose Ti3AlC2 MAX into ultra-thin Ti3C2Tx Mxene sheets [Figure 2b,c]. The AFM image in Figure 2d suggests that the Ti3C2Tx MXene is 4 nm thick. The XPS survey spectra of Ti3AlC2 MAX and Ti3C2Tx MXene in Figure 2e show the appearance of C 1s, O 1s, and Ti 2p peaks in both. Of note, the enhancement of the Ti 2p signal, disappearance of the Al 2p peak, and occurrence of the F 1s peak in Ti3C2Tx MXene preliminarily confirmed the successful fabrication of Ti3C2Tx MXene. The XRD patterns in Figure 2f further confirmed the successful transformation of Ti3AlC2 MAX (JCPDS No. 52-0875) into Ti3C2Tx MXene [30,31]. Obviously, after the removal of the Al layer, peaks of the (002) pattern shifted from 9.68° to 7.03°. The disappearance of the strong and distinguished peak at 38.8° indicates that the accordion-like structure was separated into single-layer sheets.
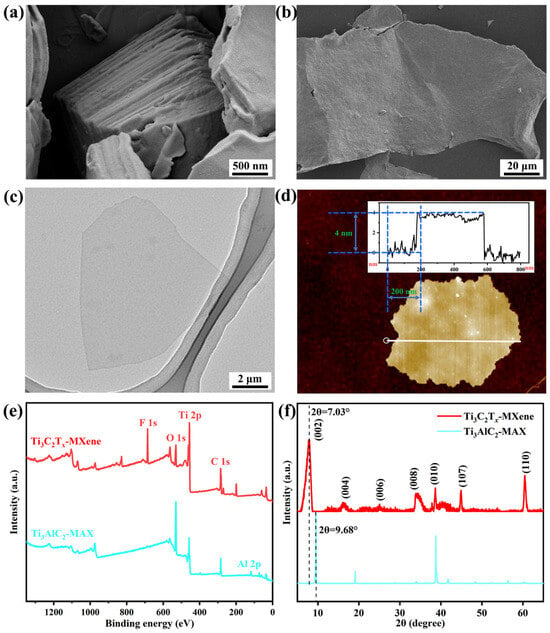
Figure 2.
(a) SEM images of Ti3AlC2 MAX and (b) monolayer Ti3C2Tx MXene. (c) TEM and (d) AFM images of monolayer Ti3C2Tx MXene. (e) XPS survey spectra and (f) XRD patterns of Ti3AlC2 MAX and Ti3C2Tx MXene.
3.2. Filler Dispersion of MNx Composites
Glue liquid mixing and subsequent flocculation solidification technologies were employed to manufacture the Ti3C2Tx MXene/NR (MNx, x = 0~3) composite. XRD was employed to examine the chemical compositions of MNx composites as well as confirm the uniformity of the Ti3C2Tx MXene in the NR matrix [Figure 3a]. Broden diffraction peaks at about 20° of all samples indicate the amorphous structure of the NR. The diffraction peaks between 30° and 70° were attributed to vulcanising auxiliary ZnO [32]. In the XRD patterns of MN0.5 and MN1, the lack of obvious characteristic peaks related to Ti3C2Tx MXene confirmed the uniform dispersion of Ti3C2Tx MXene throughout the entire NR matrix [33,34]. In contrast, the appearance of evident peaks of (002) and (004) patterns in the XRD spectrum of MN3 implied the aggregation of Ti3C2Tx Mxene sheets.
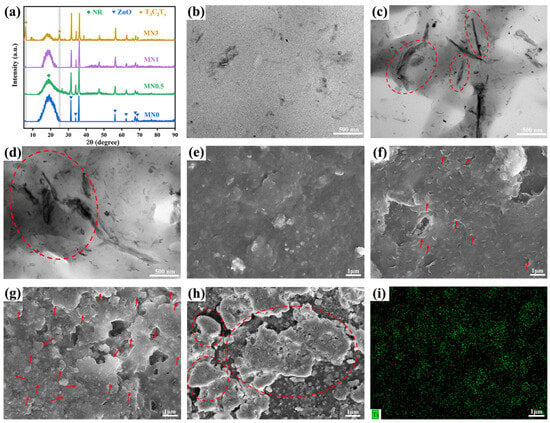
Figure 3.
(a) XRD patterns of the MNx nanocomposites (x = 0, 0.5, 1, 3). TEM images of (b) MN0, (c) MN1, and (d) MN3. SEM images of MNx, (e) x = 0, (f) 0.5, (g) 1, (h) 3, and (i) SEM EDS elemental mapping of Ti elements for MN1.
The uniform dispersion of the filler and interfacial interaction between filler and rubber are the key factors in determining the final properties of rubber composites [35]. Figure 3e–h show the SEM images of the MNx composites with different contents of Ti3C2Tx MXene. Actually, the surface of NR was flat and smooth [Figure 3e]. After being filled with Ti3C2Tx MXene, an obvious contour of the layered material was observed on the surface of MN0.5 to MN1 [Figure 3f,g]. Furthermore, Ti3C2Tx MXene sheet fillers were uniformly dispersed in the NR matrix with fewer defects on their surfaces. The SEM EDS elemental mapping of Ti elements in Figure 3i further demonstrates the uniform distribution of Ti3C2Tx MXene sheets. As x further increased to 3, large Ti3C2Tx MXene lamellar aggregates appeared and the number of defects increased significantly on the surface of the composites [Figure 3h], indicating the enrichment of Ti3C2Tx within the MNx composite.
The local dispersion of the Ti3C2Tx MXene nanosheets in the NR composites was analysed using TEM. The TEM images of MN0 and MN1 [Figure 3b,c] further illustrate the uniform distribution of Ti3C2Tx Mxene sheets in the NR, which may be due to the hydrogen bonds between the functional groups on the surface of Ti3C2Tx MXene and the molecular chains of NR. However, coarse dark lines in the NR matrix of MN3 [Figure 3d] imply the accumulation of Ti3C2Tx MXene nanosheets, attributed to the significant difference between the surface energy of Ti3C2Tx MXene and NR [36].
3.3. The Filler Network and Interfacial Interaction in MNx Composites
The interfacial interaction between the Ti3C2Tx MXene sheets and NR was studied using DMA. The loss factor (tan δ) at the glass transition temperature (Tg) was inversely proportional to the volume of the constrained polymer chains [37]. Figure 4a shows tan δ as a function of temperature curves for the MNx composites obtained from DMA. As x increased, the tan δ at Tg first decreased and then increased and the inflection point appeared when x = 1. The preliminary downshift implied that more rubber chains were constrained by Ti3C2Tx. This is reasonably explained by the 3D network constructed by the filler of Ti3C2Tx MXene restricting the mobility of NR molecules because of the hydrogen bond between Ti3C2Tx and the rubber chains. Such limitations enhanced the mechanical properties of MNx composite and its resistance against toxic chemicals [38,39]. When x further increased, Ti3C2Tx MXene aggregated and the limitation effect decreased, eventually resulting in an increase in tan δ at Tg. The structure of the filler in the composites was analysed via dynamic rheological measurement. Figure 4b shows the strain-dependent storage modulus (G’) plots for uncured MNx (x = 0, 0.5, 1, and 3). As x increased to 1, the initial G’ value of Ti3C2Tx MXene/NR first slowly and then rapidly increased, which was attributed to the hydrodynamic effects resulting from the stiff fillers in the rubber matrix [40,41]. The failure to obtain a higher initial G’ when x = 3 may have resulted from the accumulation of Ti3C2Tx MXene in the composites. Such aggregation destructs the partial network of the Ti3C2Tx MXene nanosheets and thus weakens the interaction between Ti3C2Tx MXene and NR. The inverse relationship between the G’ value and strain is defined as the ‘‘Payne effect”, which is largely attributed to the damage of the filler network and the liberation of the constrained rubber chains in the filler network during oscillatory shear. The “Payne effect” of MN1 is the most obvious among all components, suggesting the formation of a highly interconnected 3D network, which would enhance the overall performance of MNx, particularly in terms of barrier properties [21].
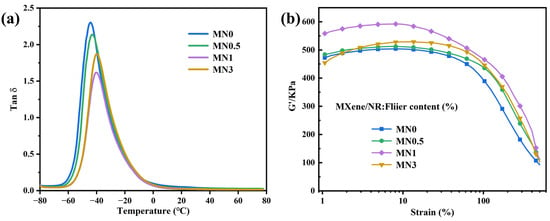
Figure 4.
(a) Tan δ versus temperature curves and (b) plots of strain-dependent G’ for MN0, MN0.5, MN1.0, and MN3.
3.4. Mechanism of Interfacial Interaction between Ti3C2Tx MXene Nanosheets and NR Molecules
The intense interaction between the NR matrix and Ti3C2Tx MXene filler is the key to establishing a continuous and stable network in the composite. Figure 5a exhibits the FT-IR spectra of NR, Ti3C2Tx Mxene, and Ti3C2Tx Mxene/NR. The typical signals of Ti3C2Tx Mxene are situated at 3434 cm−1 (–OH), 1632 cm−1 (=O), and 539 cm−1 (–OH) [42,43,44]. Three peaks related to the NR material located at 1454 cm−1, 841 cm−1, and 2949 cm−1, corresponding to the rotating vibration peak of –CH3, the out-of-plane oscillation peak of =CH–, and the asymmetric stretching vibration peak of –CH3, respectively. The concurrence of peaks for Ti3C2Tx MXene and NR in the FT-IR spectrum of Ti3C2Tx/NR indicates that these two components coexist stably in the obtained Ti3C2Tx/NR composites. Notably, the asymmetric stretching peak of –CH3 in the Ti3C2Tx/NR composites shifts from 2949 cm−1 to 2962 cm−1 compared to pure NR, implying the existence of an interaction between the methyl group on the NR molecular chain and the surface functional group of Ti3C2Tx. Actually, permanent dipole attraction and the presence of oxygen bonds can promote the surface hydration of rubber particles [45]. The above results confirm the formation of hydrogen bonds between the hydrated surface of NR and the polar groups (–OH and –F) of Ti3C2Tx. These hydrogen bonds enhance the interfacial interaction between Ti3C2Tx MXene sheets and the NR matrix, resulting in uniform dispersion of Ti3C2Tx MXene nanosheets in the NR matrix and holds promise for improving the mechanical and toxic chemical barrier properties of the Ti3C2Tx/NR composite material.
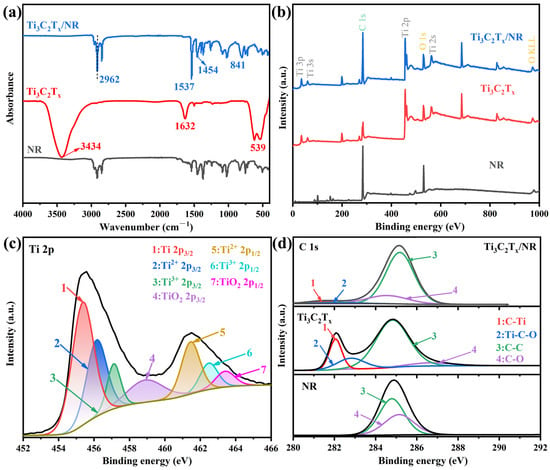
Figure 5.
(a) FITR spectra and (b) XPS survey scans of NR, Ti3C2Tx MXene, and Ti3C2Tx/NR. (c) XPS spectrum of Ti 2p in Ti3C2Tx/NR. (d) C 1s spectra of NR, Ti3C2Tx, and Ti3C2Tx/NR composites.
XPS analysis was conducted to further verify the interface between Ti3C2Tx MXene and NR. As shown in Figure 5b, both of the characteristic signals of Ti3C2Tx and NR appear in the XPS survey spectrum of Ti3C2Tx/NR. After hybridization, the atomic ratios of Ti/C and Ti/O decreases significantly (Ti3C2Tx/NR: Ti/C = 0.09 and Ti/O = 1.21; Ti3C2Tx: Ti/C = 0.96 and Ti/O = 1.45). In the Ti 2p spectrum of Ti3C2Tx/NR [Figure 5c], obvious C-Ti-Tx 2p3/2 and C-Ti-Tx 2p1/2 peaks indicate that Ti3C2Tx interacts with NR in the composite. Additionally, the C 1s spectrum of Ti3C2Tx/NR [Figure 5d] shows the characteristic peaks of C-Ti-Tx [46] from Ti3C2Tx MXene and intense C–C and C–O peaks from NR with polar groups and electronegative elements [45,47,48]. These polar groups and electronegative elements guarantee the steady distribution of rubber latex in the aqueous solution [45]. The action of permanent dipole attraction and oxygen bonds are able to promote the surface of the rubber particles to be hydrated. Hence, it is anticipated that the strong interaction between the Ti3C2Tx MXene nanosheets and NR originates from the hydrogen bonds generated between the –OH and =O groups on Ti3C2Tx MXene and the C–O groups on NR. For the composite, the C-Ti-Tx peak shifts to a lower binding energy compared with that of Ti3C2Tx [49], confirming the formation of hydrogen bonds in the composites. Overall, the interface constructed between the Ti3C2Tx MXene nanosheet filler and NR matrix forms a high-quality network in the nanocomposites, which optimizes the mechanical and barrier behaviours of MNx hybrids.
3.5. Bound Rubber Contents and Crosslinking Densities of MNx Composites
Table 1 displays the contents of bound rubber and CDs of MNx. The rubber combined with the filler prior to crosslinking is defined as bound rubber and mainly results from interfacial interactions between the fillers and rubber molecules. Compared with pure NR, the combination of NR with Ti3C2Tx enhances the content of bound rubber significantly, confirming the existence of strong interfacial interactions between Ti3C2Tx and adjacent NR molecules. This observation is consistent with the rheological tests depicted in Figure 4b. The enhanced CD after the combination of NR with Ti3C2Tx benefits from two factors: (1) the optimized crosslinking network induced by intense interactions, such as hydrogen bonds between the Ti3C2Tx and NR molecules; (2) the general sulphur crosslinking of the network. The enhanced CD of MNx composites benefits from the reduction in the distance among NR molecules, thereby optimizing the barrier behaviour [50].

Table 1.
Bound rubber content and CD of NR composites.
3.6. Barrier Properties of MNx Composites
It remains a challenge to achieve excellent chemical protection properties of polymer composites with low content of Ti3C2Tx MXene. Figure 6a–c show the BTT of various toxic chemicals to MNx. The BTT of MNx to toluene, dichloromethane, and concentrated sulfuric acid gradually increased with an increase in x until x = 1, whereas the BTT was enhanced by 140%, 178.6%, and 92.5%. The delay in the penetration process of the MNx composites is attributed to two factors, as shown in Figure 6d. First, the free volume of NR molecular chains shrinks significantly because of the intricate 3D network of Ti3C2Tx MXene sheet filler and strong interfacial interaction between the Ti3C2Tx and NR molecules. Second, the 2D structure of Ti3C2Tx MXene nanosheets extends the diffusion pathway of harmful chemicals in the NR matrix. Composites with negligible amounts of Ti3C2Tx MXene have a weak blocking impact on rubber chain slip and interface interactions, whereas excessive Ti3C2Tx MXene accumulates in the composites, decreasing its blocking impact and increasing flaws in the composite. To sum up, the eye-catching chemical protection property was achieved in MNx composites with merely 1 wt% Ti3C2Tx MXene. As shown in Table S4, the chemical protection properties of this work (the BTT of MN1 to toluene and dichloromethane) significantly improved, compared with those of the published materials, especially for the innovative precise characterization of the corrosion resistance of concentrated sulfuric acid [51,52].
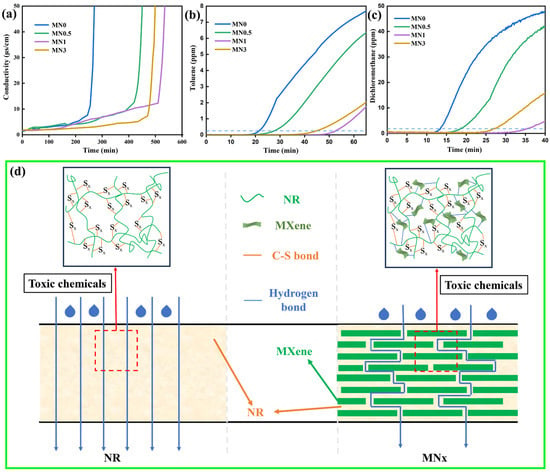
Figure 6.
Permeability of MNx composites (x = 0, 0.5, 1, and 3) for (a) concentrated H2SO4, (b) toluene, and (c) dichloromethane. (d) Schematic illustration for the diffusion of the toxic chemicals in NR and MNx.
3.7. Mechanical properties of MNx composites
Excellent stretchability, abrasion resistance, and flexural flexibility guarantee the proper functioning of the CPC in practical applications. The evolutions of the tensile strength and elongation at the break of the MNx system are shown in Figure 7a, and other mechanical properties are exhibited in Table S3. After the combination of Ti3C2Tx with NR, the tensile strength, elongation at break, and modulus at 100% and 300% strain of the MNx (x = 0.5~3) significantly improved in comparison with bare NR, especially for MN1 (increased by 7.847 MPa, 194% and 12 HA). It is the strong interfacial interaction that efficiently accelerated the movement of stress from the NR to the 2D Ti3C2Tx MXene nanosheets. However, the incorporation of excessive Ti3C2Tx MXene weakened the mechanical properties of MNx, mainly due to the increase in defects as well as a decrease in hydrogen bonds resulting from the aggregation of Ti3C2Tx in the NR matrix.
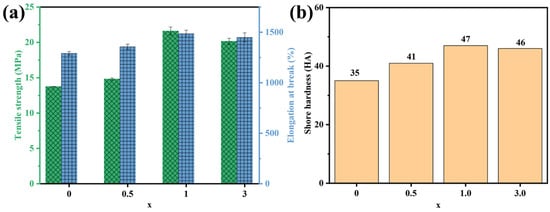
Figure 7.
Mechanical properties of MNx system (x = 0~3): (a) tensile strength, elongation at break, and (b) shore hardness as a function of x curves.
4. Conclusions
In summary, an MNx composite was successfully synthesized via a combination of latex mixing, emulsion flocculation, and flat-plate vulcanisation technologies. Due to the strong interfacial interactions between the Ti3C2Tx MXene filler and NR matrix, a high-quality 3D network forms in the nanocomposites and it highly optimizes the toxic chemical barrier and mechanical behaviours of NR. The nanocomposite with 1 wt% Ti3C2Tx MXene nanosheets achieves a substantial enhancement of BTT to toluene, dichloromethane, and concentrated sulfuric acid when compared with those of pure NR (increased by 140%, 178.6%, and 92.5%, respectively). Furthermore, the tensile strength, elongation at break, and shore hardness of MN1 were increased by 7.847 MPa, 194%, and 12 HA, respectively, compared with MN0. This study not only implies Ti3C2Tx MXene/NR as a potential CPC material but also provides a universal strategy to design multifunctional MXene/rubber composites for strain sensors and other applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym15214260/s1, Figure S1: SEM images of Ti3AlC2 MAX with loose accordion-like structure; Table S1: Composition ratio design of Ti3C2Tx MXene/NR; Table S2: Test Prescription; Table S3: Mechanical properties of NR composites; Table S4: The chemical protection properties of rubber and rubber composites.
Author Contributions
Conceptualization, Q.C., M.Z. and X.Z.; methodology, Q.C., M.Z. and H.L.; validation, Q.C. and M.Z.; formal analysis, Q.C., X.Z. and H.L.; investigation, Q.C.; resources, Q.C. and X.Z.; data curation, Q.C.; writing—original draft preparation, Q.C. and M.Z.; writing—review and editing, Q.C., M.Z. and X.Z.; project administration, X.L., C.Z. and G.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (project no. 22075319), the State Key Laboratory of NBC Protection for Civilian Foundation of China (SKLNBC202103).
Data Availability Statement
All information is available within the article.
Acknowledgments
This work was sponsored by the National Natural Science Foundation of China (project no. 22075319), the State Key Laboratory of NBC Protection for Civilian Foundation of China (SKLNBC202103). The authors also thank the teachers and students of the research group for their full support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, R.; Xu, K.; Xu, Y.; Wu, Y. Study on prediction model of hazardous chemical accidents. J. Loss Prev. Process Ind. 2020, 66, 104183. [Google Scholar] [CrossRef]
- Wang, B.; Li, D.; Wu, C. Characteristics of hazardous chemical accidents during hot season in China from 1989 to 2019: A statistical investigation. Saf. Sci. 2020, 129, 104788. [Google Scholar] [CrossRef]
- Mercey, G.; Verdelet, T.; Renou, J.; Kliachyna, M.; Baati, R.; Nachon, F.; Jean, L.; Renard, P.-Y. Reactivators of Acetylcholinesterase Inhibited by Organophosphorus Nerve Agents. Accounts Chem. Res. 2012, 45, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Rosman, Y.; Eisenkraft, A.; Milk, N.; Shiyovich, A.; Ophir, N.; Shrot, S.; Kreiss, Y.; Kassirer, M. Lessons Learned From the Syrian Sarin Attack: Evaluation of a Clinical Syndrome Through Social Media. Ann. Intern. Med. 2014, 160, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, M.; Heo, J.; Jung, S.; Ka, D.; Jung, H.; Lee, S.; Jin, Y.; Hong, J. Effect of surface charge density of graphene oxide on chemical warfare agent simulants blocking. Appl. Surf. Sci. 2021, 579, 152225. [Google Scholar] [CrossRef]
- Wisniewski, A.; Pirszel, J. Protection of armoured vehicles against chemical, biological and radiological contamination. Def. Technol. 2020, 17, 384–392. [Google Scholar] [CrossRef]
- Mao, N. 3-High performance textiles for protective clothing. In High Performance Textiles and Their Applications; Lawrence, C.A., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 91–143. [Google Scholar]
- Bensel, C.K. The effects of various thicknesses of chemical protective gloves on manual dexterity. Ergonomics 1993, 36, 687–696. [Google Scholar] [CrossRef]
- Kincl, L.D.; Bhattacharya, A.; Succop, P.A.; Clark, C.S. Postural Sway Measurements: A Potential Safety Monitoring Technique for Workers Wearing Personal Protective Equipment. Appl. Occup. Environ. Hyg. 2002, 17, 256–266. [Google Scholar] [CrossRef]
- Rothe, M.J. Hand eczema. 2nd edition. J. Am. Acad. Dermatol. 2002, 46, 472. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.R.; Wang, L.; Shaid, A.; A Shanks, R.; Ding, J. Advances and applications of chemical protective clothing system. J. Ind. Text. 2018, 49, 97–138. [Google Scholar] [CrossRef]
- Zhang, N.; Pang, Y.; Li, Z.; Yang, C.; Zong, L.; Yang, H.; Wu, H.; Duan, Y.; Zhang, J. Rubber-like and biodegradable poly (vinyl alcohol) composites with triple networks for high-efficiency solvent barrier. Compos. Sci. Technol. 2023, 231, 109801. [Google Scholar] [CrossRef]
- Jose, S.; Thomas, S.; Jibin, K.; Sisanth, K.; Kadam, V.; Shakyawar, D. Surface modification of wool fabric using sodium lignosulfonate and subsequent improvement in the interfacial adhesion of natural rubber latex in the wool/rubber composites. Ind. Crop. Prod. 2022, 177, 114489. [Google Scholar] [CrossRef]
- Kim, K.; Jung, H.; Cho, K.M. ZIF-8/Graphene Oxide Hybrid Membranes as Breathable and Protective Barriers against Chemical Warfare Agents. ACS Appl. Mater. Interfaces 2023, 15, 41755–41762. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Barrera, C.S.; Tardiff, J.L.; Gil, A.; Cornish, K. Liquid guayule natural rubber, a renewable and crosslinkable processing aid in natural and synthetic rubber compounds. J. Clean. Prod. 2020, 276, 122933. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, T.; Qiao, Y.; Liu, H.; Zhang, C.; Zhang, X.; Sun, Y. Effects of microstructures of liquid polyisoprene on the properties of styrene–butadiene rubber/butadiene rubber compounds. Polym. Int. 2023, 72, 798–803. [Google Scholar] [CrossRef]
- Jayathilaka, L.P.I.; Ariyadasa, T.U.; Egodage, S.M. Development of biodegradable natural rubber latex composites by employing corn derivative bio-fillers. J. Appl. Polym. Sci. 2020, 137, 49205. [Google Scholar] [CrossRef]
- George, N.; Varghese, G.A.; Joseph, R. Improved mechanical and barrier properties of Natural rubber-Multiwalled carbon nanotube composites with segregated network structure. Mater. Today Proc. 2019, 9, 13–20. [Google Scholar] [CrossRef]
- Nouraei, M.; Liaghat, G.; Ahmadi, H.; Bahramian, A.R.; Taherzadeh-Fard, A.; Vahid, S. High strain-rate and quasi-static mechanical characteristics of the natural rubber-based elastomer nanocomposite reinforced with alumina nanoparticles. J. Reinf. Plast. Compos. 2022, 42, 871–892. [Google Scholar] [CrossRef]
- Wongwat, S.; Yoksan, R.; Hedenqvist, M.S. Bio-based thermoplastic natural rubber based on poly(lactic acid)/thermoplastic starch/calcium carbonate nanocomposites. Int. J. Biol. Macromol. 2022, 208, 973–982. [Google Scholar] [CrossRef]
- Li, Q.; Zhong, B.; Zhang, W.; Jia, Z.; Jia, D.; Qin, S.; Wang, J.; Razal, J.M.; Wang, X. Ti3C2 MXene as a new nanofiller for robust and conductive elastomer composites. Nanoscale 2019, 11, 14712–14719. [Google Scholar] [CrossRef]
- Naguib, M. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2016, 2, 1502–1506. [Google Scholar] [CrossRef]
- Peng, W.; Han, J.; Lu, Y.-R.; Luo, M.; Chan, T.-S.; Peng, M.; Tan, Y. A General Strategy for Engineering Single-Metal Sites on 3D Porous N, P Co-Doped Ti3C2TX MXene. ACS Nano 2022, 16, 4116–4125. [Google Scholar] [CrossRef]
- Xue, R.; Wang, C.-X.; Zhao, Z.-G.; Chen, Y.-H.; Yang, J.; Feng, C.-P. Flexible Silica/MXene/Natural rubber film strain sensors with island chain structure for Healthcare monitoring. J. Colloid Interface Sci. 2023, 650, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Su, J.; Lin, J.; Huang, J.; Weng, M.; Min, Y. Enhancing the light-thermal absorption and conversion capacity of diatom-based biomass/polyethylene glycol composites phase change material by introducing MXene. J. Energy Storage 2023, 72, 108253. [Google Scholar] [CrossRef]
- Han, R.; Xie, Y.; Ma, X. Crosslinked P84 copolyimide/MXene mixed matrix membrane with excellent solvent resistance and permselectivity. Chin. J. Chem. Eng. 2018, 27, 877–883. [Google Scholar] [CrossRef]
- Aakyiir, M.; Oh, J.-A.; Araby, S.; Zheng, Q.; Naeem, M.; Ma, J.; Adu, P.; Zhang, L.; Mai, Y.-W. Combining hydrophilic MXene nanosheets and hydrophobic carbon nanotubes for mechanically resilient and electrically conductive elastomer nanocomposites. Compos. Sci. Technol. 2021, 214, 108997. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Baumann, W.; Ismeier, M. Grundlagen zu Kautschuk. In Kautschuk und Gummi: Daten und Fakten zum Umweltschutz Band ½; Baumann, W., Ismeier, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 28–51. [Google Scholar]
- Ng, V.M.H.; Huang, H.; Zhou, K.; Lee, P.S.; Que, W.; Xu, J.Z.; Kong, L.B. Recent progress in layered transition metal carbides and/or nitrides (MXenes) and their composites: Synthesis and applications. J. Mater. Chem. A 2016, 5, 3039–3068. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2T00000000 MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, L.; Wen, S.; Liu, L. Graphene oxide-supported zinc oxide nanoparticles for chloroprene rubber with improved crosslinking network and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105492. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, X.; Wang, J.; Wen, Y.; Jia, H.; Ji, Q.; Ding, L. Ionic liquid functionalized graphene oxide for enhancement of styrene-butadiene rubber nanocomposites. Polym. Adv. Technol. 2017, 28, 293–302. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, H.; Wu, S.; Tang, Z.; Guo, B.; Zhang, L. A green method for preparing conductive elastomer composites with interconnected graphene network via Pickering emulsion templating. Chem. Eng. J. 2018, 342, 112–119. [Google Scholar] [CrossRef]
- Punetha, V.D.; Rana, S.; Yoo, H.J.; Chaurasia, A.; McLeskey, J.T., Jr.; Ramasamy, M.S.; Sahoo, N.G.; Cho, J.W. Functionalization of carbon nanomaterials for advanced polymer nanocomposites: A comparison study between CNT and graphene. Prog. Polym. Sci. 2017, 67, 1–47. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, L.; Feng, W.; Guo, B.; Liu, F.; Jia, D. Rational Design of Graphene Surface Chemistry for High-Performance Rubber/Graphene Composites. Macromolecules 2014, 47, 8663–8673. [Google Scholar] [CrossRef]
- Rao, J.M. Pochan, Mechanics of Polymer−Clay Nanocomposites. Macromolecules 2007, 40, 290–296. [Google Scholar] [CrossRef]
- Angellier, H.; Molina-Boisseau, S.; Dufresne, A. Mechanical Properties of Waxy Maize Starch Nanocrystal Reinforced Natural Rubber. Macromolecules 2005, 38, 9161–9170. [Google Scholar] [CrossRef]
- Das, A.; Kasaliwal, G.R.; Jurk, R.; Boldt, R.; Fischer, D.; Stöckelhuber, K.W.; Heinrich, G. Rubber composites based on graphene nanoplatelets, expanded graphite, carbon nanotubes and their combination: A comparative study. Compos. Sci. Technol. 2012, 72, 1961–1967. [Google Scholar] [CrossRef]
- Sorokin, V.V.; Ecker, E.; Stepanov, G.V.; Shamonin, M.; Monkman, G.J.; Kramarenko, E.Y.; Khokhlov, A.R. Experimental study of the magnetic field enhanced Payne effect in magnetorheological elastomers. Soft Matter 2014, 10, 8765–8776. [Google Scholar] [CrossRef]
- Gan, S.; Wu, Z.L.; Xu, H.; Song, Y.; Zheng, Q. Viscoelastic Behaviors of Carbon Black Gel Extracted from Highly Filled Natural Rubber Compounds: Insights into the Payne Effect. Macromolecules 2016, 49, 1454–1463. [Google Scholar] [CrossRef]
- Luo, J.-Q.; Zhao, S.; Zhang, H.-B.; Deng, Z.; Li, L.; Yu, Z.-Z. Flexible, stretchable and electrically conductive MXene/natural rubber nanocomposite films for efficient electromagnetic interference shielding. Compos. Sci. Technol. 2019, 182, 107754. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, H.; Liu, J.; Xie, X.; Yang, R.; Li, Y.; Hong, S.; Yu, Z. Highly Conductive Transition Metal Carbide/Carbonitride(MXene)@polystyrene Nanocomposites Fabricated by Electrostatic Assembly for Highly Efficient Electromagnetic Interference Shielding. Adv. Funct. Mater. 2017, 27, 1702807. [Google Scholar] [CrossRef]
- Boota, M.; Anasori, B.; Voigt, C.; Zhao, M.-Q.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive Electrodes Produced by Oxidant-Free Polymerization of Pyrrole between the Layers of 2D Titanium Carbide (MXene). Adv. Mater. 2015, 28, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.-L.; D’auzac, J.; Prevôt, J.-C. The composition of natural latex fromHevea brasiliensis. Clin. Rev. Allergy 1993, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Halim, J.; Cook, K.M.; Naguib, M.; Eklund, P.; Gogotsi, Y.; Rosen, J.; Barsoum, M.W. X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Appl. Surf. Sci. 2016, 362, 406–417. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, H.-B.; Luo, J.-Q.; Wang, Q.-W.; Xu, B.; Hong, S.; Yu, Z.-Z. Highly Electrically Conductive Three-Dimensional Ti3C2Tx MXene/Reduced Graphene Oxide Hybrid Aerogels with Excellent Electromagnetic Interference Shielding Performances. ACS Nano 2018, 12, 11193–11202. [Google Scholar] [CrossRef]
- Yan, N.; Buonocore, G.; Lavorgna, M.; Kaciulis, S.; Balijepalli, S.K.; Zhan, Y.; Xia, H.; Ambrosio, L. The role of reduced graphene oxide on chemical, mechanical and barrier properties of natural rubber composites. Compos. Sci. Technol. 2014, 102, 74–81. [Google Scholar] [CrossRef]
- Bokobza, L. Natural Rubber Nanocomposites: A Review. Nanomaterials 2019, 9, 12. [Google Scholar] [CrossRef]
- Zheng, L.; Jerrams, S.; Xu, Z.; Zhang, L.; Liu, L.; Wen, S. Enhanced gas barrier properties of graphene oxide/rubber composites with strong interfaces constructed by graphene oxide and sulfur. Chem. Eng. J. 2019, 383, 123100. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, D.; Xu, Z.; Zhang, L.; Liu, L.; Wen, S. High barrier properties against sulfur mustard of graphene oxide/butyl rubber composites. Compos. Sci. Technol. 2018, 170, 141–147. [Google Scholar] [CrossRef]
- De Kee, D.; Fong, C.F.C.M.; Pintauro, P.; Hinestroza, J.; Yuan, G.; Burczyk, A. Effect of temperature and elongation on the liquid diffusion and permeation characteristics of natural rubber, nitrile rubber, and bromobutyl rubber. J. Appl. Polym. Sci. 2000, 78, 1250–1255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).