Recent Advances in Monocomponent Visible Light Photoinitiating Systems Based on Sulfonium Salts
Abstract
1. Introduction
2. Sulfonium Salts Activable under Visible Light
2.1. Advantages, Disadvantages and Limitations
2.2. 1,3,5-Triphenyl-2-Pyrazoline Derivatives
2.3. Phenothiazine Derivatives
2.4. Triphenylamine-Based Sulfonium Salts
2.5. Bopidy-Based Sulfonium Salts
2.6. Charge Transfer Complexes
2.7. Fluorene-Based Sulfonium Salts
2.8. Anthracene-Based Sulfonium Salts
2.9. Coumarin-Based Sulfonium Salts
3. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Allegrezza, M.L.; DeMartini, Z.M.; Kloster, A.J.; Digby, Z.A.; Konkolewicz, D. Visible and Sunlight Driven RAFT Photopolymerization Accelerated by Amines: Kinetics and Mechanism. Polym. Chem. 2016, 7, 6626–6636. [Google Scholar] [CrossRef]
- Sun, K.; Chen, H.; Zhang, Y.; Morlet-Savary, F.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. High-Performance Sunlight Induced Polymerization Using Novel Push-Pull Dyes with High Light Absorption Properties. Eur. Polym. J. 2021, 151, 110410. [Google Scholar] [CrossRef]
- Ciftci, M.; Tasdelen, M.A.; Yagci, Y. Sunlight Induced Atom Transfer Radical Polymerization by Using Dimanganese Decacarbonyl. Polym. Chem. 2014, 5, 600–606. [Google Scholar] [CrossRef]
- Decker, C.; Bendaikha, T. Interpenetrating Polymer Networks. II. Sunlight-Induced Polymerization of Multifunctional Acrylates. J. Appl. Polym. Sci. 1998, 70, 2269–2282. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lalevée, J.; Gigmes, D.; Fouassier, J.P. Green Chemistry: Sunlight-Induced Cationic Polymerization of Renewable Epoxy Monomers Under Air. Macromolecules 2010, 43, 1364–1370. [Google Scholar] [CrossRef]
- Wang, J.; Rivero, M.; Muñoz Bonilla, A.; Sanchez-Marcos, J.; Xue, W.; Chen, G.; Zhang, W.; Zhu, X. Natural RAFT Polymerization: Recyclable-Catalyst-Aided, Opened-to-Air, and Sunlight-Photolyzed RAFT Polymerizations. ACS Macro Lett. 2016, 5, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Gao, Y.; Jiang, S.; Sun, F. Naphthalimide Aryl Sulfide Derivative Norrish Type I Photoinitiators with Excellent Stability to Sunlight under Near-UV LED. Macromolecules 2019, 52, 1707–1717. [Google Scholar] [CrossRef]
- Bao, Y. Recent Trends in Advanced Photoinitiators for Vat Photopolymerization 3D Printing. Macromol. Rapid Commun. 2022, 43, 2200202. [Google Scholar] [CrossRef]
- Lalevée, J.; Fouassier, J.-P. Dyes and Chromophores in Polymer Science; ISTE Ltd.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; ISBN 978-1-84821-742-3. [Google Scholar]
- Fouassier, J.P.; Lalevée, J. Three-Component Photoinitiating Systems: Towards Innovative Tailor Made High Performance Combinations. RSC Adv. 2012, 2, 2621–2629. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Simon-Masseron, A.; Lalevée, J. Radical Photoinitiation with LEDs and Applications in the 3D Printing of Composites. Chem. Soc. Rev. 2021, 50, 3824–3841. [Google Scholar] [CrossRef]
- Lalevée, J.; Allonas, X.; Fouassier, J.P. Tris(Trimethylsilyl)Silane (TTMSS)-Derived Radical Reactivity toward Alkenes: A Combined Quantum Mechanical and Laser Flash Photolysis Study. J. Org. Chem. 2007, 72, 6434–6439. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Lalevée, J. Recent Applications of the (TMS)3SiH Radical-Based Reagent. Molecules 2012, 17, 527–555. [Google Scholar] [CrossRef] [PubMed]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Redox Two-Component Initiated Free Radical and Cationic Polymerizations: Concepts, Reactions and Applications. Prog. Polym. Sci. 2019, 94, 33–56. [Google Scholar] [CrossRef]
- Lalevée, J.; Mokbel, H.; Fouassier, J.-P. Recent Developments of Versatile Photoinitiating Systems for Cationic Ring Opening Polymerization Operating at Any Wavelengths and under Low Light Intensity Sources. Molecules 2015, 20, 7201–7221. [Google Scholar] [CrossRef]
- Jasinski, F.; Zetterlund, P.B.; Braun, A.M.; Chemtob, A. Photopolymerization in Dispersed Systems. Prog. Polym. Sci. 2018, 84, 47–88. [Google Scholar] [CrossRef]
- Noè, C.; Hakkarainen, M.; Sangermano, M. Cationic UV-Curing of Epoxidized Biobased Resins. Polymers 2021, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, C.; Zhang, R.; Liu, R.; Liu, J. Low Volume Shrinkage Photopolymerization System Using Hydrogen-Bond-Based Monomers. Prog. Org. Coat. 2019, 137, 105308. [Google Scholar] [CrossRef]
- Khudyakov, I.V.; Legg, J.C.; Purvis, M.B.; Overton, B.J. Kinetics of Photopolymerization of Acrylates with Functionality of 1–6. Ind. Eng. Chem. Res. 1999, 38, 3353–3359. [Google Scholar] [CrossRef]
- Dickens, S.H.; Stansbury, J.W.; Choi, K.M.; Floyd, C.J.E. Photopolymerization Kinetics of Methacrylate Dental Resins. Macromolecules 2003, 36, 6043–6053. [Google Scholar] [CrossRef]
- Maffezzoli, A.; Pietra, A.D.; Rengo, S.; Nicolais, L.; Valletta, G. Photopolymerization of Dental Composite Matrices. Biomaterials 1994, 15, 1221–1228. [Google Scholar] [CrossRef]
- Dikova, T.; Maximov, J.; Todorov, V.; Georgiev, G.; Panov, V. Optimization of Photopolymerization Process of Dental Composites. Processes 2021, 9, 779. [Google Scholar] [CrossRef]
- Andreu, A.; Su, P.-C.; Kim, J.-H.; Ng, C.S.; Kim, S.; Kim, I.; Lee, J.; Noh, J.; Subramanian, A.S.; Yoon, Y.-J. 4D Printing Materials for Vat Photopolymerization. Addit. Manuf. 2021, 44, 102024. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Zhang, Y.; Sun, K.; Liu, S.; Brunel, D.; Gigmes, D.; Graff, B.; Morlet-Savary, F.; Xiao, P.; et al. Photopolymerization and 3D/4D Applications Using Newly Developed Dyes: Search around the Natural Chalcone Scaffold in Photoinitiating Systems. Dyes Pigment. 2021, 188, 109213. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Tomal, W.; Kiliclar, H.C.; Fiedor, P.; Ortyl, J.; Yagci, Y. Visible Light Induced High Resolution and Swift 3D Printing System by Halogen Atom Transfer. Macromol. Rapid Commun. 2023, 44, 2200661. [Google Scholar] [CrossRef]
- Armstrong, B.K.; Kricker, A. The Epidemiology of UV Induced Skin Cancer. J. Photochem. Photobiol. B Biol. 2001, 63, 8–18. [Google Scholar] [CrossRef] [PubMed]
- de Gruijl, F.R. Skin Cancer and Solar UV Radiation. Eur. J. Cancer 1999, 35, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Winkel, A.; Eisenburger, M.; Menzel, H. Carboxylated Camphorquinone as Visible-Light Photoinitiator for Biomedical Application: Synthesis, Characterization, and Application. Arab. J. Chem. 2016, 9, 745–754. [Google Scholar] [CrossRef]
- Santini, A.; Gallegos, I.T.; Felix, C.M. Photoinitiators in Dentistry: A Review. Prim. Dent. J. 2013, 2, 30–33. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Visible Light Thiophene-Based Photoinitiators of Polymerization. Eur. Polym. J. 2022, 169, 111120. [Google Scholar] [CrossRef]
- Helmy, S.; Oh, S.; Leibfarth, F.A.; Hawker, C.J.; Read de Alaniz, J. Design and Synthesis of Donor–Acceptor Stenhouse Adducts: A Visible Light Photoswitch Derived from Furfural. J. Org. Chem. 2014, 79, 11316–11329. [Google Scholar] [CrossRef] [PubMed]
- Pigot, C.; Noirbent, G.; Brunel, D.; Dumur, F. Recent Advances on Push–Pull Organic Dyes as Visible Light Photoinitiators of Polymerization. Eur. Polym. J. 2020, 133, 109797. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Anthracene-Based Photoinitiators of Polymerization. Eur. Polym. J. 2022, 169, 111139. [Google Scholar] [CrossRef]
- Abdallah, M.; Le, H.; Hijazi, A.; Schmitt, M.; Graff, B.; Dumur, F.; Bui, T.-T.; Goubard, F.; Fouassier, J.-P.; Lalevée, J. Acridone Derivatives as High Performance Visible Light Photoinitiators for Cationic and Radical Photosensitive Resins for 3D Printing Technology and for Low Migration Photopolymer Property. Polymer 2018, 159, 47–58. [Google Scholar] [CrossRef]
- Bao, B.; You, J.; Li, D.; Zhan, H.; Zhang, L.; Li, M.; Wang, T. Double Benzylidene Ketones as Photoinitiators for Visible Light Photopolymerization. J. Photochem. Photobiol. A Chem. 2022, 429, 113938. [Google Scholar] [CrossRef]
- Fu, H.; Qiu, Y.; You, J.; Hao, T.; Fan, B.; Nie, J.; Wang, T. Photopolymerization of Acrylate Resin and Ceramic Suspensions with Benzylidene Ketones under Blue/Green LED. Polymer 2019, 184, 121841. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Wu, F.; Zhou, Y.; Huang, N.; Gu, Y.; Zou, Q.; Yang, W. Polyethylene Glycol-Functionalized Benzylidene Cyclopentanone Dyes for Two-Photon Excited Photodynamic Therapy. Org. Biomol. Chem. 2011, 9, 4168–4175. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liu, T.; Zou, Q.; Zhao, Y.; Wu, F. Cationic Benzylidene Cyclopentanone Photosensitizers for Selective Photodynamic Inactivation of Bacteria over Mammalian Cells. RSC Adv. 2015, 5, 56067–56074. [Google Scholar] [CrossRef]
- Xue, J.; Zhao, Y.; Wu, F.; Fang, D.-C. Effect of Bridging Position on the Two-Photon Polymerization Initiating Efficiencies of Novel Coumarin/Benzylidene Cyclopentanone Dyes. J. Phys. Chem. A 2010, 114, 5171–5179. [Google Scholar] [CrossRef]
- Egorov, A.E.; Kostyukov, A.A.; Shcherbakov, D.A.; Kolymagin, D.A.; Chubich, D.A.; Matital, R.P.; Arsenyev, M.V.; Burtsev, I.D.; Mestergazi, M.G.; Zhiganshina, E.R.; et al. Benzylidene Cyclopentanone Derivative Photoinitiator for Two-Photon Photopolymerization-Photochemistry and 3D Structures Fabrication for X-Ray Application. Polymers 2023, 15, 71. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Xiao, P.; Graff, B.; Morlet-Savary, F.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. New Chromone Based Photoinitiators for Polymerization Reactions under Visible Light. Polym. Chem. 2013, 4, 4234–4244. [Google Scholar] [CrossRef]
- You, J.; Fu, H.; Zhao, D.; Hu, T.; Nie, J.; Wang, T. Flavonol Dyes with Different Substituents in Photopolymerization. J. Photochem. Photobiol. A Chem. 2020, 386, 112097. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Garra, P.; Schmitt, M.; Toufaily, J.; Hamieh, T.; Graff, B.; Fouassier, J.P.; Dumur, F.; Lalevée, J. 3-Hydroxyflavone and N-Phenylglycine in High Performance Photoinitiating Systems for 3D Printing and Photocomposites Synthesis. Macromolecules 2018, 51, 4633–4641. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Frigoli, M.; Graff, B.; Morlet-Savary, F.; Wantz, G.; Bock, H.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Perylene Derivatives as Photoinitiators in Blue Light Sensitive Cationic or Radical Curable Films and Panchromatic Thiol-Ene Polymerizable Films. Eur. Polym. J. 2014, 53, 215–222. [Google Scholar] [CrossRef]
- Xiao, P.; Hong, W.; Li, Y.; Dumur, F.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Green Light Sensitive Diketopyrrolopyrrole Derivatives Used in Versatile Photoinitiating Systems for Photopolymerizations. Polym. Chem. 2014, 5, 2293–2300. [Google Scholar] [CrossRef]
- Xiao, P.; Hong, W.; Li, Y.; Dumur, F.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. Diketopyrrolopyrrole Dyes: Structure/Reactivity/Efficiency Relationship in Photoinitiating Systems upon Visible Lights. Polymer 2014, 55, 746–751. [Google Scholar] [CrossRef]
- Zivic, N.; Bouzrati-Zerrelli, M.; Villotte, S.; Morlet-Savary, F.; Dietlin, C.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. A Novel Naphthalimide Scaffold Based Iodonium Salt as a One-Component Photoacid/Photoinitiator for Cationic and Radical Polymerization under LED Exposure. Polym. Chem. 2016, 7, 5873–5879. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Naphthalimide Derivatives: Substituent Effects on the Photoinitiating Ability in Polymerizations under Near UV, Purple, White and Blue LEDs (385, 395, 405, 455, or 470 nm). Macromol. Chem. Phys. 2015, 216, 1782–1790. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Zhang, J.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Naphthalimide-Phthalimide Derivative Based Photoinitiating Systems for Polymerization Reactions under Blue Lights. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 665–674. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Fouassier, J.P.; Gigmes, D.; Lalevée, J. N-[2-(Dimethylamino)Ethyl]-1,8-Naphthalimide Derivatives as Photoinitiators under LEDs. Polym. Chem. 2018, 9, 994–1003. [Google Scholar] [CrossRef]
- Noirbent, G.; Dumur, F. Recent Advances on Naphthalic Anhydrides and 1,8-Naphthalimide-Based Photoinitiators of Polymerization. Eur. Polym. J. 2020, 132, 109702. [Google Scholar] [CrossRef]
- Zivic, N.; Zhang, J.; Bardelang, D.; Dumur, F.; Xiao, P.; Jet, T.; Versace, D.-L.; Dietlin, C.; Morlet-Savary, F.; Graff, B.; et al. Novel Naphthalimide–Amine Based Photoinitiators Operating under Violet and Blue LEDs and Usable for Various Polymerization Reactions and Synthesis of Hydrogels. Polym. Chem. 2015, 7, 418–429. [Google Scholar] [CrossRef]
- Liu, S.; Giacoletto, N.; Graff, B.; Morlet-Savary, F.; Nechab, M.; Xiao, P.; Dumur, F.; Lalevée, J. N-Naphthalimide Ester Derivatives as Type Ⅰ Photoinitiators for LED Photopolymerization. Mater. Today Chem. 2022, 26, 101137. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Ali, S.; Akram, M.Y.; Nie, J.; Zhu, X. The Effect of Polyethylene Glycoldiacrylate Complexation on Type II Photoinitiator and Promotion for Visible Light Initiation System. J. Photochem. Photobiol. A Chem. 2019, 384, 112037. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Li, Y.; Li, R.; Nie, J.; Zhu, X. In Situ Monitoring of Photopolymerization by Photoinitiator with Luminescence Characteristics. J. Photochem. Photobiol. A Chem. 2020, 389, 112225. [Google Scholar] [CrossRef]
- Li, J.; Hao, Y.; Zhong, M.; Tang, L.; Nie, J.; Zhu, X. Synthesis of Furan Derivative as LED Light Photoinitiator: One-Pot, Low Usage, Photobleaching for Light Color 3D Printing. Dyes Pigment. 2019, 165, 467–473. [Google Scholar] [CrossRef]
- Xu, Y.; Noirbent, G.; Brunel, D.; Ding, Z.; Gigmes, D.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Novel Ketone Derivative-Based Photoinitiating Systems for Free Radical Polymerization under Mild Conditions and 3D Printing. Polym. Chem. 2020, 11, 5767–5777. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Sun, K.; Brunel, D.; Gigmes, D.; Morlet-Savary, F.; Zhang, Y.; Liu, S.; Xiao, P.; Dumur, F.; et al. Photoinitiators Derived from Natural Product Scaffolds: Monochalcones in Three-Component Photoinitiating Systems and Their Applications in 3D Printing. Polym. Chem. 2020, 11, 4647–4659. [Google Scholar] [CrossRef]
- Chen, H.; Noirbent, G.; Liu, S.; Brunel, D.; Graff, B.; Gigmes, D.; Zhang, Y.; Sun, K.; Morlet-Savary, F.; Xiao, P.; et al. Bis-Chalcone Derivatives Derived from Natural Products as near-UV/Visible Light Sensitive Photoinitiators for 3D/4D Printing. Mater. Chem. Front. 2021, 5, 901–916. [Google Scholar] [CrossRef]
- Sun, K.; Xu, Y.; Dumur, F.; Morlet-Savary, F.; Chen, H.; Dietlin, C.; Graff, B.; Lalevée, J.; Xiao, P. In Silico Rational Design by Molecular Modeling of New Ketones as Photoinitiators in Three-Component Photoinitiating Systems: Application in 3D Printing. Polym. Chem. 2020, 11, 2230–2242. [Google Scholar] [CrossRef]
- Yen, S.-C.; Ni, J.-S.; Chen, Y.-C. Triphenylamine-Functionalized Chalcones as One-Component Type II Visible-Light-Absorbing Photoinitiators for Free Radical Photopolymerization. Eur. Polym. J. 2023, 187, 111885. [Google Scholar] [CrossRef]
- Gao, Y.; Qu, J. New Long-Wavelength D–π-A–π-D Chalcone Photoinitiator for Visible Light Polymerization with Photobleaching and Biocompatibility Properties. Polym. Chem. 2023, 14, 952–962. [Google Scholar] [CrossRef]
- Deng, L.; Qu, J. Synthesis and Properties of Novel Bis-Chalcone-Based Photoinitiators for LED Polymerization with Photobleaching and Low Migration. Prog. Org. Coat. 2023, 174, 107240. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Garra, P.; Sallenave, X.; Dumur, F.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. π-Conjugated Dithienophosphole Derivatives as High Performance Photoinitiators for 3D Printing Resins. Macromolecules 2018, 51, 1811–1821. [Google Scholar] [CrossRef]
- Topa-Skwarczyńska, M.; Galek, M.; Jankowska, M.; Morlet-Savary, F.; Graff, B.; Lalevée, J.; Popielarz, R.; Ortyl, J. Development of the First Panchromatic BODIPY-Based One-Component Iodonium Salts for Initiating the Photopolymerization Processes. Polym. Chem. 2021, 12, 6873–6893. [Google Scholar] [CrossRef]
- Karaca, N.; Ocal, N.; Arsu, N.; Jockusch, S. Thioxanthone-Benzothiophenes as Photoinitiator for Free Radical Polymerization. J. Photochem. Photobiol. A Chem. 2016, 331, 22–28. [Google Scholar] [CrossRef]
- Balta, D.K.; Cetiner, N.; Temel, G.; Turgut, Z.; Arsu, N. An Annelated Thioxanthone as a New Type II Initiator. J. Photochem. Photobiol. A Chem. 2008, 199, 316–321. [Google Scholar] [CrossRef]
- Balta, D.K.; Temel, G.; Goksu, G.; Ocal, N.; Arsu, N. Thioxanthone–Diphenyl Anthracene: Visible Light Photoinitiator. Macromolecules 2012, 45, 119–125. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Aydogan, C.; Yagci, Y. Shining a Light on an Adaptable Photoinitiator: Advances in Photopolymerizations Initiated by Thioxanthones. Polym. Chem. 2015, 6, 6595–6615. [Google Scholar] [CrossRef]
- Eren, T.N.; Yasar, N.; Aviyente, V.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Lalevee, J.; Avci, D. Photophysical and Photochemical Studies of Novel Thioxanthone-Functionalized Methacrylates through LED Excitation. Macromol. Chem. Phys. 2016, 217, 1501–1512. [Google Scholar] [CrossRef]
- Qiu, J.; Wei, J. Thioxanthone Photoinitiator Containing Polymerizable N-Aromatic Maleimide for Photopolymerization. J. Polym. Res. 2014, 21, 559. [Google Scholar] [CrossRef]
- Tar, H.; Sevinc Esen, D.; Aydin, M.; Ley, C.; Arsu, N.; Allonas, X. Panchromatic Type II Photoinitiator for Free Radical Polymerization Based on Thioxanthone Derivative. Macromolecules 2013, 46, 3266–3272. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Xiong, Y.; Yang, J.; Tang, H. Thioxanthone Based One-Component Polymerizable Visible Light Photoinitiator for Free Radical Polymerization. RSC Adv. 2016, 6, 66098–66107. [Google Scholar] [CrossRef]
- Wu, Q.; Tang, K.; Xiong, Y.; Wang, X.; Yang, J.; Tang, H. High-Performance and Low Migration One-Component Thioxanthone Visible Light Photoinitiators. Macromol. Chem. Phys. 2017, 218, 1600484. [Google Scholar] [CrossRef]
- Wu, X.; Jin, M.; Malval, J.-P.; Wan, D.; Pu, H. Visible Light-Emitting Diode-Sensitive Thioxanthone Derivatives Used in Versatile Photoinitiating Systems for Photopolymerizations. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 4037–4045. [Google Scholar] [CrossRef]
- Esen, D.S.; Karasu, F.; Arsu, N. The Investigation of Photoinitiated Polymerization of Multifunctional Acrylates with TX-BT by Photo-DSC and RT-FTIR. Prog. Org. Coat. 2011, 70, 102–107. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Fries, C.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P. New Thioxanthone and Xanthone Photoinitiators Based on Silyl Radical Chemistry. Polym. Chem. 2011, 2, 1077–1084. [Google Scholar] [CrossRef]
- Gencoglu, T.; Eren, T.N.; Lalevée, J.; Avci, D. A Water Soluble, Low Migration, and Visible Light Photoinitiator by Thioxanthone-Functionalization of Poly(Ethylene Glycol)-Containing Poly(β-Amino Ester). Macromol. Chem. Phys. 2022, 223, 2100450. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Liu, D.; Peng, C.; Wang, J.; Dong, X. New Functionalized Thioxanthone Derivatives as Type I Photoinitiators for Polymerization under UV-Vis LEDs. New J. Chem. 2023, 47, 5330–5337. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Zhang, Y.; Sun, K.; Xu, Y.; Morlet-Savary, F.; Graff, B.; Noirbent, G.; Pigot, C.; Brunel, D.; et al. Monocomponent Photoinitiators Based on Benzophenone-Carbazole Structure for LED Photoinitiating Systems and Application on 3D Printing. Polymers 2020, 12, 1394. [Google Scholar] [CrossRef]
- Liu, S.; Brunel, D.; Sun, K.; Zhang, Y.; Chen, H.; Xiao, P.; Dumur, F.; Lalevée, J. Novel Photoinitiators Based on Benzophenone-Triphenylamine Hybrid Structure for LED Photopolymerization. Macromol. Rapid Commun. 2020, 41, 2000460. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Brunel, D.; Sun, K.; Xu, Y.; Morlet-Savary, F.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. A Monocomponent Bifunctional Benzophenone–Carbazole Type II Photoinitiator for LED Photoinitiating Systems. Polym. Chem. 2020, 11, 3551–3556. [Google Scholar] [CrossRef]
- Lin, J.-T.; Lalevee, J. Efficacy Modeling of New Multi-Functional Benzophenone-Based System for Free-Radical/Cationic Hybrid Photopolymerization Using 405 nm LED. J. Polym. Res. 2021, 29, 100. [Google Scholar] [CrossRef]
- Launay, V.; Wolf, R.; Dumur, F.; Lalevée, J. Photothermal Activation in the near Infrared Range for 4-Dimensional Printing Using Relevant Organic Dyes. Addit. Manuf. 2022, 58, 103031. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, T.; Yang, T.; Wei, H.; Yang, H.; Li, G.; Zhao, M.; Liu, S.; Huang, W.; Zhao, Q. Utilizing Intramolecular Photoinduced Electron Transfer to Enhance Photothermal Tumor Treatment of Aza-BODIPY-Based Near-Infrared Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 16299–16307. [Google Scholar] [CrossRef] [PubMed]
- Skotnicka, A.; Kabatc, J. New BODIPY Dyes Based on Benzoxazole as Photosensitizers in Radical Polymerization of Acrylate Monomers. Materials 2022, 15, 662. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Chung, K.-Y.; Stafford, A.; Kiker, M.; Kafle, K.; Page, Z.A. Boron Dipyrromethene (BODIPY) in Polymer Chemistry. Polym. Chem. 2021, 12, 327–348. [Google Scholar] [CrossRef]
- Telitel, S.; Blanchard, N.; Schweizer, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. BODIPY Derivatives and Boranil as New Photoinitiating Systems of Cationic Polymerization Exhibiting a Tunable Absorption in the 400–600 nm Spectral Range. Polymer 2013, 54, 2071–2076. [Google Scholar] [CrossRef]
- Telitel, S.; Lalevée, J.; Blanchard, N.; Kavalli, T.; Tehfe, M.-A.; Schweizer, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P. Photopolymerization of Cationic Monomers and Acrylate/Divinylether Blends under Visible Light Using Pyrromethene Dyes. Macromolecules 2012, 45, 6864–6868. [Google Scholar] [CrossRef]
- Bonardi, A.; Bonardi, F.; Noirbent, G.; Dumur, F.; Dietlin, C.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Different NIR Dye Scaffolds for Polymerization Reactions under NIR Light. Polym. Chem. 2019, 10, 6505–6514. [Google Scholar] [CrossRef]
- Zhang, J.; Lalevée, J.; Zhao, J.; Graff, B.; Stenzel, M.H.; Xiao, P. Dihydroxyanthraquinone Derivatives: Natural Dyes as Blue-Light-Sensitive Versatile Photoinitiators of Photopolymerization. Polym. Chem. 2016, 7, 7316–7324. [Google Scholar] [CrossRef]
- Zhao, J.; Lalevée, J.; Lu, H.; MacQueen, R.; Kable, S.H.; Schmidt, T.W.; Stenzel, M.H.; Xiao, P. A New Role of Curcumin: As a Multicolor Photoinitiator for Polymer Fabrication under Household UV to Red LED Bulbs. Polym. Chem. 2015, 6, 5053–5061. [Google Scholar] [CrossRef]
- Crivello, J.V.; Bulut, U. Curcumin: A Naturally Occurring Long-Wavelength Photosensitizer for Diaryliodonium Salts. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5217–5231. [Google Scholar] [CrossRef]
- Han, W.; Fu, H.; Xue, T.; Liu, T.; Wang, Y.; Wang, T. Facilely Prepared Blue-Green Light Sensitive Curcuminoids with Excellent Bleaching Properties as High Performance Photosensitizers in Cationic and Free Radical Photopolymerization. Polym. Chem. 2018, 9, 1787–1798. [Google Scholar] [CrossRef]
- Mishra, A.; Daswal, S. Curcumin, A Novel Natural Photoinitiator for the Copolymerization of Styrene and Methylmethacrylate. Null 2005, 42, 1667–1678. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Furan-Based Visible Light Photoinitiators of Polymerization. Catalysts 2023, 13, 493. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Faury, T.; Graff, B.; Tehfe, M.-A.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. New Core-Pyrene π Structure Organophotocatalysts Usable as Highly Efficient Photoinitiators. Beilstein J. Org. Chem. 2013, 9, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Nakano, H.; Igarashi, T.; Sakurai, T. Nonsalt 1-(Arylmethyloxy)Pyrene Photoinitiators Capable of Initiating Cationic Polymerization. J. Appl. Polym. Sci. 2014, 131, 40510. [Google Scholar] [CrossRef]
- Mishra, A.; Daswal, S. 1-(Bromoacetyl)Pyrene, a Novel Photoinitiator for the Copolymerization of Styrene and Methylmethacrylate. Radiat. Phys. Chem. 2006, 75, 1093–1100. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Pyrene-Based Photoinitiators of Polymerization. Eur. Polym. J. 2020, 126, 109564. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Kumbaraci, V.; Jockusch, S.; Turro, N.J.; Talinli, N.; Yagci, Y. Photoacid Generation by Stepwise Two-Photon Absorption: Photoinitiated Cationic Polymerization of Cyclohexene Oxide by Using Benzodioxinone in the Presence of Iodonium Salt. Macromolecules 2008, 41, 295–297. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Diaryliodonium Salts. A New Class of Photoinitiators for Cationic Polymerization. Macromolecules 1977, 10, 1307–1315. [Google Scholar] [CrossRef]
- He, Y.; Zhou, W.; Wu, F.; Li, M.; Wang, E. Photoreaction and Photopolymerization Studies on Squaraine Dyes/Iodonium Salts Combination. J. Photochem. Photobiol. A Chem. 2004, 162, 463–471. [Google Scholar] [CrossRef]
- Jun, L.I.; Miaozhen, L.I.; Huaihai, S.; Yongyuan, Y.; Erjian, W. Photopolymerization Initiated by Dimethylaminochalcone/Diphenyliodonium Salt Combination System Sensitive to Visible Light. Chin. J. Polym. Sci. 1993, 11, 163–170. [Google Scholar]
- Petko, F.; Galek, M.; Hola, E.; Topa-Skwarczyńska, M.; Tomal, W.; Jankowska, M.; Pilch, M.; Popielarz, R.; Graff, B.; Morlet-Savary, F.; et al. Symmetric Iodonium Salts Based on Benzylidene as One-Component Photoinitiators for Applications in 3D Printing. Chem. Mater. 2022, 34, 10077–10092. [Google Scholar] [CrossRef]
- Petko, F.; Galek, M.; Hola, E.; Popielarz, R.; Ortyl, J. One-Component Cationic Photoinitiators from Tunable Benzylidene Scaffolds for 3D Printing Applications. Macromolecules 2021, 54, 7070–7087. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. The Carbazole-Bound Ferrocenium Salt as a Specific Cationic Photoinitiator upon near-UV and Visible LEDs (365–405 nm). Polym. Bull. 2016, 73, 493–507. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Garra, P.; Dumur, F.; Bui, T.-T.; Goubard, F.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; et al. Novel Carbazole Skeleton-Based Photoinitiators for LED Polymerization and LED Projector 3D Printing. Molecules 2017, 22, 2143. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Carbazole-Based Photoinitiators of Polymerization. Eur. Polym. J. 2020, 125, 109503. [Google Scholar] [CrossRef]
- Xu, C.; Gong, S.; Wu, X.; Wu, Y.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. High-Efficient Carbazole-Based Photo-Bleachable Dyes as Free Radical Initiators for Visible Light Polymerization. Dyes Pigment. 2022, 198, 110039. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Carbazole-Based Oxime Esters as Photoinitiators of Polymerization. Eur. Polym. J. 2022, 175, 111330. [Google Scholar] [CrossRef]
- Liao, W.; Liao, Q.; Xiong, Y.; Li, Z.; Tang, H. Design, Synthesis and Properties of Carbazole-Indenedione Based Photobleachable Photoinitiators for Photopolymerization. J. Photochem. Photobiol. A Chem. 2023, 435, 114297. [Google Scholar] [CrossRef]
- Bin, F.-C.; Guo, M.; Li, T.; Zheng, Y.-C.; Dong, X.-Z.; Liu, J.; Jin, F.; Zheng, M.-L. Carbazole-Based Anion Ionic Water-Soluble Two-Photon Initiator for Achieving 3D Hydrogel Structures. Adv. Funct. Mater. 2023, 33, 2300293. [Google Scholar] [CrossRef]
- Corakci, B.; Hacioglu, S.O.; Toppare, L.; Bulut, U. Long Wavelength Photosensitizers in Photoinitiated Cationic Polymerization: The Effect of Quinoxaline Derivatives on Photopolymerization. Polymer 2013, 54, 3182–3187. [Google Scholar] [CrossRef]
- Pyszka, I.; Skowroński, Ł.; Jędrzejewska, B. Study on New Dental Materials Containing Quinoxaline-Based Photoinitiators in Terms of Exothermicity of the Photopolymerization Process. Int. J. Mol. Sci. 2023, 24, 2752. [Google Scholar] [CrossRef] [PubMed]
- Pyszka, I.; Jędrzejewska, B. Photoinitiation Abilities of Indeno- and Indoloquinoxaline Derivatives and Mechanical Properties of Dental Fillings Based on Multifunctional Acrylic Monomers and Glass Ionomer. Polymer 2023, 266, 125625. [Google Scholar] [CrossRef]
- Arsu, N.; Aydın, M. Photoinduced Free Radical Polymerization Initiated with Quinoxalines. Angew. Makromol. Chem. 1999, 270, 1–4. [Google Scholar] [CrossRef]
- Aydın, M.; Arsu, N. Photoinitiated Free Radical Polymerization of Methylmethacrylate by Using of Quinoxalines in the Presence of Aldehydes. Prog. Org. Coat. 2006, 56, 338–342. [Google Scholar] [CrossRef]
- Bulut, U.; Gunbas, G.E.; Toppare, L. A Quinoxaline Derivative as a Long Wavelength Photosensitizer for Diaryliodonium Salts. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 209–213. [Google Scholar] [CrossRef]
- Bulut, U.; Kolay, M.; Tarkuc, S.; Udum, Y.A.; Toppare, L. Quinoxaline Derivatives as Long Wavelength Photosensitizers in Photoinitiated Cationic Polymerization of Diaryliodonium Salts. Prog. Org. Coat. 2012, 73, 215–218. [Google Scholar] [CrossRef]
- Cao, X.; Jin, F.; Li, Y.-F.; Chen, W.-Q.; Duan, X.-M.; Yang, L.-M. Triphenylamine-Modified Quinoxaline Derivatives as Two-Photon Photoinitiators. New J. Chem. 2009, 33, 1578–1582. [Google Scholar] [CrossRef]
- Karaca Balta, D.; Keskin, S.; Karasu, F.; Arsu, N. Quinoxaline Derivatives as Photoinitiators in UV-Cured Coatings. Prog. Org. Coat. 2007, 60, 207–210. [Google Scholar] [CrossRef]
- Kucybała, Z.; Pyszka, I.; Pączkowski, J. Development of New Dyeing Photoinitiators for Free Radical Polymerization Based on the 1H-Pyrazolo[3,4-b]Quinoxaline Skeleton. Part 2. J. Chem. Soc. Perkin Trans. 2 2000, 7, 1559–1567. [Google Scholar] [CrossRef]
- Podsiadły, R.; Szymczak, A.M.; Podemska, K. The Synthesis of Novel, Visible-Wavelength, Oxidizable Polymerization Sensitizers Based on the 8-Halogeno-5,12-Dihydroquinoxalino[2,3-b]Quinoxaline Skeleton. Dyes Pigment. 2009, 82, 365–371. [Google Scholar] [CrossRef]
- Yao, J.-Y.; Hou, H.-H.; Ma, X.-D.; Xu, H.-J.; Shi, Z.-X.; Yin, J.; Jiang, X.-S. Combining Photo-Cleavable and Hydrogen-Abstracting Groups in Quinoxaline with Thioether Bond as Hybrid Photoinitiator. Chin. Chem. Lett. 2017, 28, 6–12. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, X.; Yin, J. Study of Methoxyphenylquinoxalines (MOPQs) as Photoinitiators in the Negative Photo-Resist. Prog. Org. Coat. 2010, 67, 225–232. [Google Scholar] [CrossRef]
- Ercan, B.T.; Gultekin, S.S.; Yesil, T.; Dincalp, H.; Koyuncu, S.; Yagci, Y.; Zafer, C. Highly Conjugated Isoindigo and Quinoxaline Dyes as Sunlight Photosensitizers for Onium Salt-Photoinitiated Cationic Polymerization of Epoxy Resins. Polym. Int. 2022, 71, 867–873. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Glyoxylates and Related Structures as Photoinitiators of Polymerization. Macromol 2023, 3, 149–174. [Google Scholar] [CrossRef]
- Mousawi, A.A.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Goubard, F.; Bui, T.-T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; et al. Azahelicenes as Visible Light Photoinitiators for Cationic and Radical Polymerization: Preparation of Photoluminescent Polymers and Use in High Performance LED Projector 3D Printing Resins. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1189–1199. [Google Scholar] [CrossRef]
- Noirbent, G.; Xu, Y.; Bonardi, A.-H.; Duval, S.; Gigmes, D.; Lalevée, J.; Dumur, F. New Donor-Acceptor Stenhouse Adducts as Visible and Near Infrared Light Polymerization Photoinitiators. Molecules 2020, 25, 2317. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Naphthoquinone-Based Photoinitiators of Polymerization. Eur. Polym. J. 2023, 193, 112120. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Gigmes, D.; Al Mousawi, A.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Lalevée, J. Copper (Photo)Redox Catalyst for Radical Photopolymerization in Shadowed Areas and Access to Thick and Filled Samples. Macromolecules 2017, 50, 3761–3771. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Lalevée, J. A New Highly Efficient Amine-Free and Peroxide-Free Redox System for Free Radical Polymerization under Air with Possible Light Activation. Macromolecules 2016, 49, 6296–6309. [Google Scholar] [CrossRef]
- Lin, J.-T.; Lalevee, J.; Cheng, D.-C. Efficacy Analysis of New Copper Complex for Visible Light (455, 530 nm) Radical/Cationic Photopolymerization: The Synergic Effects and Catalytic Cycle. PLoS ONE 2022, 17, e0270679. [Google Scholar] [CrossRef] [PubMed]
- Tehfe, M.-A.; Dumur, F.; Telitel, S.; Gigmes, D.; Contal, E.; Bertin, D.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Zinc-Based Metal Complexes as New Photocatalysts in Polymerization Initiating Systems. Eur. Polym. J. 2013, 49, 1040–1049. [Google Scholar] [CrossRef]
- Nzulu, F.; Telitel, S.; Stoffelbach, F.; Graff, B.; Morlet-Savary, F.; Lalevée, J.; Fensterbank, L.; Goddard, J.-P.; Ollivier, C. A Dinuclear Gold(i) Complex as a Novel Photoredox Catalyst for Light-Induced Atom Transfer Radical Polymerization. Polym. Chem. 2015, 6, 4605–4611. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lepeltier, M.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Structural Effects in the Iridium Complex Series: Photoredox Catalysis and Photoinitiation of Polymerization Reactions under Visible Lights. Macromol. Chem. Phys. 2017, 218, 1700192. [Google Scholar] [CrossRef]
- Dumur, F.; Bertin, D.; Gigmes, D. Iridium (III) Complexes as Promising Emitters for Solid–State Light–Emitting Electrochemical Cells (LECs). Int. J. Nanotechnol. 2012, 9, 377–395. [Google Scholar] [CrossRef]
- Dumur, F.; Nasr, G.; Wantz, G.; Mayer, C.R.; Dumas, E.; Guerlin, A.; Miomandre, F.; Clavier, G.; Bertin, D.; Gigmes, D. Cationic Iridium Complex for the Design of Soft Salt-Based Phosphorescent OLEDs and Color-Tunable Light-Emitting Electrochemical Cells. Org. Electron. 2011, 12, 1683–1694. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Morlet-Savary, F.; Yamashita, K.; Imahashi, S. The Role of the Dye/Iron Arene Complex/Amine System as a Photoinitiator for Photopolymerization Reactions. Polymer 1997, 38, 1415–1421. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Campolo, D.; Poly, J.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Iron Complexes as Potential Photocatalysts for Controlled Radical Photopolymerizations: A Tool for Modifications and Patterning of Surfaces. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 702–713. [Google Scholar] [CrossRef]
- Bonardi, A.H.; Dumur, F.; Grant, T.M.; Noirbent, G.; Gigmes, D.; Lessard, B.H.; Fouassier, J.-P.; Lalevée, J. High Performance Near-Infrared (NIR) Photoinitiating Systems Operating under Low Light Intensity and in the Presence of Oxygen. Macromolecules 2018, 51, 1314–1324. [Google Scholar] [CrossRef]
- Bonardi, A.-H.; Bonardi, F.; Noirbent, G.; Dumur, F.; Gigmes, D.; Dietlin, C.; Lalevée, J. Free-Radical Polymerization upon near-Infrared Light Irradiation, Merging Photochemical and Photothermal Initiating Methods. J. Polym. Sci. 2020, 58, 300–308. [Google Scholar] [CrossRef]
- Lago, M.A.; de Quirós, A.R.-B.; Sendón, R.; Bustos, J.; Nieto, M.T.; Paseiro, P. Photoinitiators: A Food Safety Review. Food Addit. Contam. Part A 2015, 32, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, F.; Hijazi, A.; Schmitt, M.; Dumur, F.; Lalevée, J. A Review on Recently Proposed Oxime Ester Photoinitiators. Eur. Polym. J. 2023, 188, 111901. [Google Scholar] [CrossRef]
- Sun, K.; Xiao, P.; Dumur, F.; Lalevée, J. Organic Dye-Based Photoinitiating Systems for Visible-Light-Induced Photopolymerization. J. Polym. Sci. 2021, 59, 1338–1389. [Google Scholar] [CrossRef]
- Mokbel, H.; Toufaily, J.; Hamieh, T.; Dumur, F.; Campolo, D.; Gigmes, D.; Fouassier, J.P.; Ortyl, J.; Lalevée, J. Specific Cationic Photoinitiators for near UV and Visible LEDs: Iodonium versus Ferrocenium Structures. J. Appl. Polym. Sci. 2015, 132, 42759. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Diaryliodonium-Based Monocomponent Photoinitiating Systems. Eur. Polym. J. 2023, 195, 112193. [Google Scholar] [CrossRef]
- Boeira, P.O.; Meereis, C.T.W.; Suárez, C.E.C.; de Almeida, S.M.; Piva, E.; da Silveira Lima, G. Coumarin-Based Iodonium Hexafluoroantimonate as an Alternative Photoinitiator for Experimental Dental Adhesives Resin. Appl. Adhes. Sci. 2017, 5, 2. [Google Scholar] [CrossRef][Green Version]
- Gómez, M.L.; Previtali, C.M.; Montejano, H.A. Two- and Three-Component Visible Light Photoinitiating Systems for Radical Polymerization Based on Onium Salts: An Overview of Mechanistic and Laser Flash Photolysis Studies. Int. J. Photoenergy 2012, 2012, 260728. [Google Scholar] [CrossRef]
- Prabhakaran, P.; Lee, K.-S. Photo-Polymerization. In Functional Polymers; Jafar Mazumder, M.A., Sheardown, H., Al-Ahmed, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–52. ISBN 978-3-319-95987-0. [Google Scholar]
- Crivello, J.V. The Discovery and Development of Onium Salt Cationic Photoinitiators. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 4241–4254. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Photoinitiated Cationic Polymerization with Triarylsulfonium Salts. J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 977–999. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. A New Preparation of Triarylsulfonium and -Selenonium Salts via the Copper(II)-Catalyzed Arylation of Sulfides and Selenides with Diaryliodonium Salts. J. Org. Chem. 1978, 43, 3055–3058. [Google Scholar] [CrossRef]
- Sangermano, M.; Roppolo, I.; Chiappone, A. New Horizons in Cationic Photopolymerization. Polymers 2018, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Borjigin, T.; Schmitt, M.; Morlet-Savary, F.; Xiao, P.; Lalevée, J. High-Performance Photoinitiating Systems for LED-Induced Photopolymerization. Polymers 2023, 15, 342. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.J.; Rapenne, G.; Nakashima, T.; Kawai, T. Recent Progress in Development of Photoacid Generators. J. Photochem. Photobiol. C Photochem. Rev. 2018, 34, 41–51. [Google Scholar] [CrossRef]
- Gachet, B.; Lecompère, M.; Croutxé-Barghorn, C.; Burr, D.; L’Hostis, G.; Allonas, X. Highly Reactive Photothermal Initiating System Based on Sulfonium Salts for the Photoinduced Thermal Frontal Cationic Polymerization of Epoxides: A Way to Create Carbon-Fiber Reinforced Polymers. RSC Adv. 2020, 10, 41915–41920. [Google Scholar] [CrossRef] [PubMed]
- Klikovits, N.; Knaack, P.; Bomze, D.; Krossing, I.; Liska, R. Novel Photoacid Generators for Cationic Photopolymerization. Polym. Chem. 2017, 8, 4414–4421. [Google Scholar] [CrossRef]
- Niu, X.-Z.; Pepel, R.D.; Paniego, R.; Field, J.A.; Chorover, J.; Abrell, L.; Sáez, A.E.; Sierra-Alvarez, R. Photochemical Fate of Sulfonium Photoacid Generator Cations under Photolithography Relevant UV Irradiation. J. Photochem. Photobiol. A Chem. 2021, 416, 113324. [Google Scholar] [CrossRef]
- Taschner, R.; Koch, T.; Wolff, R.; Stampfl, J.; Liska, R.; Knaack, P. Evaluation of Sulfonium Borate Initiators for Cationic Photopolymerization and Their Application in Hot Lithography. ACS Appl. Polym. Mater. 2023, 5, 3023–3033. [Google Scholar] [CrossRef]
- Peter Pappas, S.; Pappas, B.C.; Gatechair, L.R.; Jilek, J.H.; Schnabel, W. Photoinitiation of Cationic Polymerization. IV. Direct and Sensitized Photolysis of Aryl Iodonium and Sulfonium Salts. Polym. Photochem. 1984, 5, 1–22. [Google Scholar] [CrossRef]
- Crivello, J.V. Cationic Polymerization—Iodonium and Sulfonium Salt Photoinitiators. In Proceedings of the Initiators—Poly-Reactions—Optical Activity; Springer: Berlin/Heidelberg, Germany, 1984; pp. 1–48. [Google Scholar]
- Crivello, J.V.; Lee, J.L. Photosensitized Cationic Polymerizations Using Dialkylphenacylsulfonium and Dialkyl(4-Hydroxyphenyl)Sulfonium Salt Photoinitiators. Macromolecules 1981, 14, 1141–1147. [Google Scholar] [CrossRef]
- Saeva, F.D.; Breslin, D.T.; Luss, H.R. Intramolecular Photoinduced Rearrangements via Electron-Transfer-Induced, Concerted Bond Cleavage and Cation Radical/Radical Coupling. J. Am. Chem. Soc. 1991, 113, 5333–5337. [Google Scholar] [CrossRef]
- Jin, M.; Wu, X.; Malval, J.P.; Wan, D.; Pu, H. Dual Roles for Promoting Monomers to Polymers: A Conjugated Sulfonium Salt Photoacid Generator as Photoinitiator and Photosensitizer in Cationic Photopolymerization. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2722–2730. [Google Scholar] [CrossRef]
- Denizligil, S.; Yagci, Y.; McArdle, C. Photochemically and Thermally Induced Radical Promoted Cationic Polymerization Using an Allylic Sulfonium Salt. Polymer 1995, 36, 3093–3098. [Google Scholar] [CrossRef]
- Denizligil, S.; Resul, R.; Yagci, Y.; Mc Ardle, C.; Fouassier, J.-P. Photosensitized Cationic Polymerization Using Allyl Sulfonium Salt. Macromol. Chem. Phys. 1996, 197, 1233–1240. [Google Scholar] [CrossRef]
- Wang, D.; Kaya, K.; Garra, P.; Fouassier, J.-P.; Graff, B.; Yagci, Y.; Lalevée, J. Sulfonium Salt Based Charge Transfer Complexes as Dual Thermal and Photochemical Polymerization Initiators for Composites and 3D Printing. Polym. Chem. 2019, 10, 4690–4698. [Google Scholar] [CrossRef]
- Hamazu, F.; Akashi, S.; Koizumi, T.; Takata, T.; Endo, T. Novel Benzyl Sulfonium Salt Having an Aromatic Group on Sulfur Atom as a Latent Thermal Initiator. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 1675–1680. [Google Scholar] [CrossRef]
- Pappas, S.P. Kinetic Parameter Considerations for Maximizing Stability and Minimizing Cure Temperature of Thermo-Setting Coatings: Sulfonium Salts as Latent Thermal Initiators for Cationic Polymerization. J. Coat. Technol. 1981, 53, 43–51. [Google Scholar]
- Endo, T.; Arita, H. Polymerization of Spiroorthocarbonates by Sulfonium Salts, as Latent Thermal Catalysts. Makromol. Chem. Rapid Commun. 1985, 6, 137–139. [Google Scholar] [CrossRef]
- Toba, Y.; Yasuike, M.; Usui, Y. Visible laser polymerizations with the sulfonium borate styryl dyes as new photoinitiator systems. J. Photosci. Int. J. Off. Organ Korean Soc. Photosci. 1998, 5, 63–67. [Google Scholar]
- Kaya, K.; Kreutzer, J.; Yagci, Y. Diphenylphenacyl Sulfonium Salt as Dual Photoinitiator for Free Radical and Cationic Polymerizations. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 451–457. [Google Scholar] [CrossRef]
- Saeva, F.D.; Garcia, E.; Martic, P.A. Comparative Photochemical Behavior of Some Anthracenyl and Naphthacenyl Sulfonium Salt Derivatives. J. Photochem. Photobiol. A Chem. 1995, 86, 149–154. [Google Scholar] [CrossRef]
- Saeva, F.D.; Breslin, D.T.; Martic, P.A. The Effect of the Nature of the Lowest Unoccupied Molecular Orbital (LUMO) of Some Arylmethylsulfonium Salt Derivatives on Photochemical and Redox Behavior. J. Am. Chem. Soc. 1989, 111, 1328–1330. [Google Scholar] [CrossRef]
- Zhou, W.; Kuebler, S.M.; Carrig, D.; Perry, J.W.; Marder, S.R. Efficient Photoacids Based upon Triarylamine Dialkylsulfonium Salts. J. Am. Chem. Soc. 2002, 124, 1897–1901. [Google Scholar] [CrossRef] [PubMed]
- Zivic, N.; Kuroishi, P.K.; Dumur, F.; Gigmes, D.; Dove, A.P.; Sardon, H. Recent Advances and Challenges in the Design of Organic Photoacid and Photobase Generators for Polymerizations. Angew. Chem. Int. Ed. 2019, 58, 10410–10422. [Google Scholar] [CrossRef] [PubMed]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced Electron Transfer Reactions for Macromolecular Syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef]
- Ichimura, K.; Horie, S.; Nagano, S. Solid-State Photoelectron Transfer in Powdery Nanocomposites Comprised of a Sensitiser, Photoacid Generators and Silica Nanoparticles. Phys. Chem. Chem. Phys. 2011, 13, 5974–5980. [Google Scholar] [CrossRef]
- Chen, S.; Cao, C.; Shen, X.; Qiu, Y.; Kuang, C.; Wan, D.; Jin, M. One/Two-Photon Sensitive Sulfonium Salt Photoinitiators Based on 1,3,5-Triphenyl-2-Pyrazoline. Eur. Polym. J. 2021, 153, 110525. [Google Scholar] [CrossRef]
- Mysliwiec, J.; Szukalski, A.; Sznitko, L.; Miniewicz, A.; Haupa, K.; Zygadlo, K.; Matczyszyn, K.; Olesiak-Banska, J.; Samoc, M. Synthesis, Optical and Nonlinear Optical Properties of New Pyrazoline Derivatives. Dyes Pigment. 2014, 102, 63–70. [Google Scholar] [CrossRef]
- Mori, T.; Saomoto, H.; Machitani, K.; Inoue, K.; Aoki, Y.; Koshitani, T.; Koumura, N.; Murakami, T.N. Influence of the Non-Conjugated 5-Position Substituent of 1,3,5-Triaryl-2-Pyrazoline-Based Photosensitizers on the Photophysical Properties and Performance of a Dye-Sensitized Solar Cell. RSC Adv. 2016, 6, 13964–13970. [Google Scholar] [CrossRef]
- Jin, M.; Hong, H.; Xie, J.; Malval, J.-P.; Spangenberg, A.; Soppera, O.; Wan, D.; Pu, H.; Versace, D.-L.; Leclerc, T.; et al. π-Conjugated Sulfonium-Based Photoacid Generators: An Integrated Molecular Approach for Efficient One and Two-Photon Polymerization. Polym. Chem. 2014, 5, 4747–4755. [Google Scholar] [CrossRef]
- Rehm, D.; Weller, A. Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
- Suppan, P.; Vauthey, E. The Energy Balance of Photoinduced Electron Transfer Reactions. J. Photochem. Photobiol. A Chem. 1989, 49, 239–248. [Google Scholar] [CrossRef]
- Verma, M.; Chaudhry, A.F.; Fahrni, C.J. Predicting the Photoinduced Electron Transfer Thermodynamics in Polyfluorinated 1,3,5-Triarylpyrazolines Based on Multiple Linear Free Energy Relationships. Org. Biomol. Chem. 2009, 7, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Farid, S.; Dinnocenzo, J.P.; Merkel, P.B.; Young, R.H.; Shukla, D.; Guirado, G. Reexamination of the Rehm–Weller Data Set Reveals Electron Transfer Quenching That Follows a Sandros–Boltzmann Dependence on Free Energy. J. Am. Chem. Soc. 2011, 133, 11580–11587. [Google Scholar] [CrossRef] [PubMed]
- Taherpour, A.A.; Talebi-Haftadori, Z. Free Energies, Kinetics, and Photoelectron-Transfer Properties, and Theoretical and Quantitative Structural Relationship Studies of [SWCNT(5,5)-Armchair-CnH20][R] (R = H2-CmPd(Dppf), H2-CmPd(Dppr), and H2-CmPd(Dppcym)2, n = 20 to 300 and m = 60 and 70) Nanostructure Complexes. Int. Nano Lett. 2013, 3, 22. [Google Scholar] [CrossRef]
- Abdallah, M.; Bui, T.-T.; Goubard, F.; Theodosopoulou, D.; Dumur, F.; Hijazi, A.; Fouassier, J.-P.; Lalevée, J. Phenothiazine Derivatives as Photoredox Catalysts for Cationic and Radical Photosensitive Resins for 3D Printing Technology and Photocomposite Synthesis. Polym. Chem. 2019, 10, 6145–6156. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Visible Light Phenothiazine-Based Photoinitiators of Polymerization. Eur. Polym. J. 2022, 165, 110999. [Google Scholar] [CrossRef]
- Zhang, Y.; Morlet-Savary, F.; Schmitt, M.; Graff, B.; Rico, A.; Ibrahim-Ouali, M.; Dumur, F.; Lalevée, J. Photoinitiation Behavior of Phenothiazines Containing Two Oxime Ester Functionalities (OXEs) in Free Radical Photopolymerization and 3D Printing Application. Dyes Pigment. 2023, 215, 111202. [Google Scholar] [CrossRef]
- Deng, L.; Tang, L.; Qu, J. Novel Chalcone-Based Phenothiazine Derivative Photoinitiators for Visible Light Induced Photopolymerization with Photobleaching and Good Biocompatibility. Prog. Org. Coat. 2022, 167, 106859. [Google Scholar] [CrossRef]
- Gomurashvili, Z.; Crivello, J.V. Phenothiazine Photosensitizers for Onium Salt Photoinitiated Cationic Polymerization. In Photoinitiated Polymerization; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2003; Volume 847, pp. 231–241. ISBN 978-0-8412-3813-8. [Google Scholar]
- Gomurashvili, Z.; Crivello, J.V. Phenothiazine Photosensitizers for Onium Salt Photoinitiated Cationic Polymerization. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 1187–1197. [Google Scholar] [CrossRef]
- Li, M.; Bao, B.; You, J.; Du, Y.; Li, D.; Zhan, H.; Zhang, L.; Wang, T. Phenothiazine Derivatives Based on Double Benzylidene Ketone Structure: Application to Red Light Photopolymerization. Prog. Org. Coat. 2022, 173, 107217. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.N.; Al-Ghamdi, H.A.; El-Shishtawy, R.M.; Asiri, A.M. Advances in Phenothiazine and Phenoxazine-Based Electron Donors for Organic Dye-Sensitized Solar Cells. Dyes Pigment. 2021, 194, 109638. [Google Scholar] [CrossRef]
- Gangadhar, P.S.; Reddy, G.; Prasanthkumar, S.; Giribabu, L. Phenothiazine Functional Materials for Organic Optoelectronic Applications. Phys. Chem. Chem. Phys. 2021, 23, 14969–14996. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-S.; Meier, H.; Cao, D. Phenothiazine-Based Dyes for Efficient Dye-Sensitized Solar Cells. J. Mater. Chem. C 2016, 4, 2404–2426. [Google Scholar] [CrossRef]
- Buene, A.F.; Almenningen, D.M. Phenothiazine and Phenoxazine Sensitizers for Dye-Sensitized Solar Cells—An Investigative Review of Two Complete Dye Classes. J. Mater. Chem. C 2021, 9, 11974–11994. [Google Scholar] [CrossRef]
- Mao, L.; Wu, Y.; Jiang, J.; Guo, X.; Heng, P.; Wang, L.; Zhang, J. Rational Design of Phenothiazine-Based Organic Dyes for Dye-Sensitized Solar Cells: The Influence of π-Spacers and Intermolecular Aggregation on Their Photovoltaic Performances. J. Phys. Chem. C 2020, 124, 9233–9242. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P. Prediction of Power Conversion Efficiency of Phenothiazine-Based Dye-Sensitized Solar Cells Using Monte Carlo Method with Index of Ideality of Correlation. SAR QSAR Environ. Res. 2021, 32, 817–834. [Google Scholar] [CrossRef]
- Buene, A.F.; Christensen, M.; Hoff, B.H. Effect of Auxiliary Donors on 3,8-Phenothiazine Dyes for Dye-Sensitized Solar Cells. Molecules 2019, 24, 4485. [Google Scholar] [CrossRef]
- Slodek, A.; Zych, D.; Szafraniec-Gorol, G.; Gnida, P.; Vasylieva, M.; Schab-Balcerzak, E. Investigations of New Phenothiazine-Based Compounds for Dye-Sensitized Solar Cells with Theoretical Insight. Materials 2020, 13, 2292. [Google Scholar] [CrossRef] [PubMed]
- TamilSelvan, S.; Prakasam, A.; Venkatesh, G.; Kamal, C.; Sheena Mary, Y.; Parveen Banu, S.; Vennila, P.; Shyma Mary, Y. Synthesis, Spectral Characterizations, Molecular Geometries and Electronic Properties of Phenothiazine Based Organic Dyes for Dye-Sensitized Solar Cells. Z. Phys. Chem. 2021, 235, 1355–1380. [Google Scholar] [CrossRef]
- Pan, B.; Zhu, Y.-Z.; Qiu, C.; Wang, B.; Zheng, J.-Y. Synthesis of Phenothiazine Dyes Featuring Benzothiadiazole Unit for Efficient Dye-Sensitized Solar Cells. Acta Chim. Sin. 2018, 76, 215. [Google Scholar] [CrossRef]
- Nagane, S.; Ichake, A.; Agrawal, I.; Sadhanala, A.; Friend, R.H.; Ogale, S.; Wadgaonkar, P.P. Phenothiazine-Based D–A–π–A Dyes for Highly Efficient Dye-Sensitized Solar Cells: Effect of Internal Acceptor and Non-Conjugated π-Spacer on Device Performance. ChemPlusChem 2017, 82, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Kafafy, H.; Wu, H.; Peng, M.; Hu, H.; Yan, K.; El-Shishtawy, R.M.; Zou, D. Steric and Solvent Effect in Dye-Sensitized Solar Cells Utilizing Phenothiazine-Based Dyes. Int. J. Photoenergy 2014, 2014, 548914. [Google Scholar] [CrossRef]
- Chiu, K.Y.; Tseng, Z.-L.; Chen, L.-C.; Shaw, H.; Chen, C.-J.; Chen, Y.-C.; Yang, S.-H.; Wu, W.-T.; Su, Y.O. D-π-A Type Phenothiazine Organic Dyes for Dye-Sensitized TiO2 Solar Cells. Sci. Adv. Mater. 2018, 10, 801–807. [Google Scholar] [CrossRef]
- Salunke, J.K.; Wong, F.L.; Feron, K.; Manzhos, S.; Lo, M.F.; Shinde, D.; Patil, A.; Lee, C.S.; Roy, V.A.L.; Sonar, P.; et al. Phenothiazine and Carbazole Substituted Pyrene Based Electroluminescent Organic Semiconductors for OLED Devices. J. Mater. Chem. C 2016, 4, 1009–1018. [Google Scholar] [CrossRef]
- He, J.; Xu, Y.; Luo, S.; Miao, J.; Cao, X.; Zou, Y. Phenoxazine and Phenothiazine Embedded Multi-Resonance Emitters for Highly Efficient Pure-Red OLEDs with Improved Color Purity. Chem. Eng. J. 2023, 471, 144565. [Google Scholar] [CrossRef]
- Khan, F.; Mahmoudi, M.; Volyniuk, D.; Grazulevicius, J.V.; Misra, R. Stimuli-Responsive Phenothiazine-S,S-Dioxide-Based Nondoped OLEDs with Color-Changeable Electroluminescence. J. Phys. Chem. C 2022, 126, 15573–15586. [Google Scholar] [CrossRef]
- Lade, J.J.; Chiou, S.-S.; Tseng, Y.-H.; Chiu, M.-H.; Lin, Y.-F.; Chang, C.-H.; Chetti, P.; Chaskar, A.C. Design and Synthesis of Novel Phenothiazine-Benzothiadiazine-1,1-Dioxide Hybrid Organic Material for OLED Applications. ChemistrySelect 2021, 6, 11029–11038. [Google Scholar] [CrossRef]
- Kim, B.; Lee, J.; Park, Y.; Lee, C.; Park, J.W. Highly Efficient New Hole Injection Materials for Organic Light Emitting Diodes Base on Phenothiazine Derivatives. J. Nanosci. Nanotechnol. 2014, 14, 6404–6408. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Chen, Z.; Liu, Y.; Cao, H.; Xu, S.; Cao, S.; Lan, Z.; Wang, Z.; Gong, Q. Orange and Red Emitting OLEDs Based on Phenothiazine Polymers. J. Phys. D Appl. Phys. 2006, 39, 2680. [Google Scholar] [CrossRef]
- Aizawa, N.; Tsou, C.-J.; Park, I.S.; Yasuda, T. Aggregation-Induced Delayed Fluorescence from Phenothiazine-Containing Donor–Acceptor Molecules for High-Efficiency Non-Doped Organic Light-Emitting Diodes. Polym. J. 2017, 49, 197–202. [Google Scholar] [CrossRef]
- Baraket, F.; Pedras, B.; Torres, É.; Brites, M.J.; Dammak, M.; Berberan-Santos, M.N. Novel Phenoxazine-Benzonitrile and Phenothiazine-Benzonitrile Donor-Acceptor Molecules with Thermally Activated Delayed Fluorescence (TADF). Dyes Pigment. 2020, 175, 108114. [Google Scholar] [CrossRef]
- May, L.; Müller, T.J.J. Dithieno[1,4]Thiazines and Bis[1]Benzothieno[1,4]Thiazines—Organometallic Synthesis and Functionalization of Electron Density Enriched Congeners of Phenothiazine. Molecules 2020, 25, 2180. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.-M.; Ou, Q.; Zhang, Q.; Zhang, J.-K.; Dai, G.-L.; Zhao, X.; Wei, H.-X. Two Novel Blue Phosphorescent Host Materials Containing Phenothiazine-5,5-Dioxide Structure Derivatives. Beilstein J. Org. Chem. 2018, 14, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, B.; Lee, C.; Lee, J.; Lee, J.-H.; Park, J. High Efficiency New Hole Injection Materials for Organic Light Emitting Diodes Based on Dimeric Phenothiazine and Phenoxazine Moiety Derivatives. J. Nanosci. Nanotechnol. 2012, 12, 4356–4360. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, S.; Deng, Z.; Islam, A.; Wu, S.; He, J.; Ni, S.; Dang, L.; Li, M.-D. Uncovering the Substituted-Position Effect on Excited-State Evolution of Benzophenone-Phenothiazine Dyads. J. Chem. Phys. 2023, 159, 144502. [Google Scholar] [CrossRef]
- Can, M.; Ali Havare, K. OLED Application of Pi-Conjugated Phenylimino Carboxylic Acid Organic Semiconductor Material. Eur. Phys. J. Appl. Phys. 2022, 97, 33. [Google Scholar] [CrossRef]
- Truong, N.T.T.; Nguyen, L.T.; Mai, H.L.T.; Doan, B.K.; Tran, D.H.; Truong, K.T.; Nguyen, V.Q.; Nguyen, L.-T.T.; Hoang, M.H.; Van Pham, T.; et al. Phenothiazine Derivatives, Diketopyrrolopyrrole-Based Conjugated Polymers: Synthesis, Optical and Organic Field Effect Transistor Properties. J. Polym. Res. 2020, 27, 223. [Google Scholar] [CrossRef]
- Al-Busaidi, I.J.; Haque, A.; Al Rasbi, N.K.; Khan, M.S. Phenothiazine-Based Derivatives for Optoelectronic Applications: A Review. Synth. Metals 2019, 257, 116189. [Google Scholar] [CrossRef]
- Lee, M.; Park, J.E.; Park, C.; Choi, H.C. Synthesis of a P-Type Semiconducting Phenothiazine Exfoliatable Layered Crystal. Langmuir 2013, 29, 9967–9971. [Google Scholar] [CrossRef] [PubMed]
- May, L.; Müller, T.J.J. Electron-Rich Phenothiazine Congeners and Beyond: Synthesis and Electronic Properties of Isomeric Dithieno[1,4]Thiazines. Chem. A Eur. J. 2020, 26, 12111–12118. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fan, S.; Huang, R.; Kawamoto, T.; Mori, T. Transistor Properties of Charge-Transfer Complexes─Combined Requirements from Energy Levels and Orbital Symmetry. J. Phys. Chem. C 2023, 127, 5125–5133. [Google Scholar] [CrossRef]
- Hwang, D.-H.; Kim, S.-K.; Park, M.-J.; Lee, J.-H.; Koo, B.-W.; Kang, I.-N.; Kim, S.-H.; Zyung, T. Conjugated Polymers Based on Phenothiazine and Fluorene in Light-Emitting Diodes and Field Effect Transistors. Chem. Mater. 2004, 16, 1298–1303. [Google Scholar] [CrossRef]
- Hu, Y.; Shang, Z.; Wang, J.; Hong, M.; Zhang, R.; Meng, Q.; Zhang, Z. A Phenothiazine-Based Turn-on Fluorescent Probe for the Selective Detection of Hydrogen Sulfide in Food, Live Cells and Animals. Analyst 2021, 146, 7528–7536. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Li, D.; Le, Y.; Dong, P.; Liu, L. Phenothiazine-Based Fluorescent Probe for Fluoride Ions and Its Applications in Rapid Detection of Endemic Disease. Dyes Pigment. 2022, 201, 110200. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Li, X.; Nie, S.; Liu, C.; Zhang, Y.; Guo, J. A Fluorescent Probe Based on Phenothiazine for Detection of ClO− with Naked-Eye Color Change Properties. Anal. Biochem. 2023, 670, 115131. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Xiao, M.; Ai, Y.; Liu, G.; Ding, H.; Pu, S. A “Turn-on” Fluorescent Probe Based on Phenothiazine for Selectively Recognizing ClO− and Its Practical Applications. J. Fluoresc. 2023. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, P.; Li, Y.; Sun, J.; Cui, H.; Zhang, H.; Chen, S. A Phenothiazine-HPQ Based Fluorescent Probe with a Large Stokes Shift for Sensing Biothiols in Living Systems. Molecules 2021, 26, 2337. [Google Scholar] [CrossRef]
- Christian, S.T. The Interaction of Phenothiazine Derivatives with Synaptosomal Membranes: The Use of Fluorescent Probes as an Indicator of Drug-Induced Conformational Perturbations. Int. J. Neurosci. 1973, 6, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Zhang, L.; Li, X.; Nie, S.; Liu, C. A Near-Infrared Fluorescent Probe Based on Phenothiazine for Rapid Detecting of CN− and ClO−. Opt. Mater. 2022, 133, 112959. [Google Scholar] [CrossRef]
- Al-Zahrani, F.A.M.; El-Shishtawy, R.M.; Asiri, A.M.; Al-Soliemy, A.M.; Mellah, K.A.; Ahmed, N.S.E.; Jedidi, A. A New Phenothiazine-Based Selective Visual and Fluorescent Sensor for Cyanide. BMC Chem. 2020, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yue, X.; Li, W.; Hao, Y.; Zhang, L.; Zhu, L.; Sheng, J.; Song, X. A Phenothiazine Coumarin-Based Red Emitting Fluorescent Probe for Nanomolar Detection of Thiophenol with a Large Stokes Shift. Sens. Actuators B Chem. 2017, 245, 702–710. [Google Scholar] [CrossRef]
- Rout, Y.; Montanari, C.; Pasciucco, E.; Misra, R.; Carlotti, B. Tuning the Fluorescence and the Intramolecular Charge Transfer of Phenothiazine Dipolar and Quadrupolar Derivatives by Oxygen Functionalization. J. Am. Chem. Soc. 2021, 143, 9933–9943. [Google Scholar] [CrossRef] [PubMed]
- Son, M.S.; Chang, S.H. Synthesis and Fluorescent Properties of New Rhodamine B Containing Phenothiazine Moiety. J. Environ. Sci. Int. 2015, 24, 955–960. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, D.; Zhang, Y.; Zhou, Y.; Yagci, Y.; Liu, R. Phenacyl Phenothiazinium Salt as a New Broad-Wavelength-Absorbing Photoinitiator for Cationic and Free Radical Polymerizations. Angew. Chem. Int. Ed. 2021, 60, 16917–16921. [Google Scholar] [CrossRef] [PubMed]
- Jockusch, S.; Yagci, Y. The Active Role of Excited States of Phenothiazines in Photoinduced Metal Free Atom Transfer Radical Polymerization: Singlet or Triplet Excited States? Polym. Chem. 2016, 7, 6039–6043. [Google Scholar] [CrossRef]
- Ladika, D.; Noirbent, G.; Dumur, F.; Gigmes, D.; Mourka, A.; Barmparis, G.D.; Farsari, M.; Gray, D. Synthesis and Application of Triphenylamine-Based Aldehydes as Photo-Initiators for Multi-Photon Lithography. Appl. Phys. A 2022, 128, 745. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Visible Light Triphenylamine-Based Photoinitiators of Polymerization. Eur. Polym. J. 2022, 166, 111036. [Google Scholar] [CrossRef]
- Mahmood, A. Triphenylamine Based Dyes for Dye Sensitized Solar Cells: A Review. Solar Energy 2016, 123, 127–144. [Google Scholar] [CrossRef]
- Holliman, P.J.; Mohsen, M.; Connell, A.; Kershaw, C.P.; Meza-Rojas, D.; Jones, E.W.; Geatches, D.; Sen, K.; Hsiao, Y.-W. Double Linker Triphenylamine Dyes for Dye-Sensitized Solar Cells. Energies 2020, 13, 4637. [Google Scholar] [CrossRef]
- Mahmoud, S.E.; Fadda, A.A.; Abdel-Latif, E.; Elmorsy, M.R. Synthesis of Novel Triphenylamine-Based Organic Dyes with Dual Anchors for Efficient Dye-Sensitized Solar Cells. Nanoscale Res. Lett. 2022, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Pakravesh, F.; Izadyar, M.; Arkan, F. Molecular Engineering of Triphenylamine-Based Metal-Free Organic Dyes for Dye-Sensitized Solar Cells. Int. J. Quantum Chem. 2021, 121, e26620. [Google Scholar] [CrossRef]
- Zhou, W.; Kuebler, S.M.; Braun, K.L.; Yu, T.; Cammack, J.K.; Ober, C.K.; Perry, J.W.; Marder, S.R. An Efficient Two-Photon-Generated Photoacid Applied to Positive-Tone 3D Microfabrication. Science 2002, 296, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Kuebler, S.M.; Braun, K.L.; Zhou, W.; Cammack, J.K.; Yu, T.; Ober, C.K.; Marder, S.R.; Perry, J.W. Design and Application of High-Sensitivity Two-Photon Initiators for Three-Dimensional Microfabrication. J. Photochem. Photobiol. A Chem. 2003, 158, 163–170. [Google Scholar] [CrossRef]
- Yu, T.; Ober, C.K.; Kuebler, S.M.; Zhou, W.; Marder, S.R.; Perry, J.W. Chemically Amplified Positive Resists for Two-Photon Three-Dimensional Microfabrication. Adv. Mater. 2003, 15, 517–521. [Google Scholar] [CrossRef]
- Pohlers, G.; Scaiano, J.C.; Sinta, R. A Novel Photometric Method for the Determination of Photoacid Generation Efficiencies Using Benzothiazole and Xanthene Dyes as Acid Sensors. Chem. Mater. 1997, 9, 3222–3230. [Google Scholar] [CrossRef]
- Gu, H.; Ren, K.; Grinevich, O.; Malpert, J.H.; Neckers, D.C. Characterization of Iodonium Salts Differing in the Anion. J. Org. Chem. 2001, 66, 4161–4164. [Google Scholar] [CrossRef]
- Park, J.; Kihara, N.; Ikeda, T.; Endo, T. Photo-Induced Cationic Ring-Opening Polymerization of 2-Alkenyl-4-Methylene-1,3-Dioxolanes by Benzylsulfonium Salt. Macromolecules 1997, 30, 3414–3416. [Google Scholar] [CrossRef]
- Yanez, C.O.; Andrade, C.D.; Belfield, K.D. Characterization of Novel Sulfonium Photoacid Generators and Their Microwave-Assisted Synthesis. Chem. Commun. 2009, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Yanez, C.O.; Andrade, C.D.; Yao, S.; Luchita, G.; Bondar, M.V.; Belfield, K.D. Photosensitive Polymeric Materials for Two-Photon 3D WORM Optical Data Storage Systems. ACS Appl. Mater. Interfaces 2009, 1, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Steidl, L.; Jhaveri, S.J.; Ayothi, R.; Sha, J.; McMullen, J.D.; Ng, S.Y.C.; Zipfel, W.R.; Zentel, R.; Ober, C.K. Non-Ionic Photo-Acid Generators for Applications in Two-Photon Lithography. J. Mater. Chem. 2009, 19, 505–513. [Google Scholar] [CrossRef]
- Jin, M.; Xu, H.; Hong, H.; Malval, J.-P.; Zhang, Y.; Ren, A.; Wan, D.; Pu, H. Design of D–π–A Type Photoacid Generators for High Efficiency Excitation at 405 nm and 800 nm. Chem. Commun. 2013, 49, 8480–8482. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Malval, J.-P.; Jin, M.; Spangenberg, A.; Wan, D.; Pu, H.; Vergote, T.; Morlet-Savary, F.; Chaumeil, H.; Baldeck, P.; et al. Enhancement of Acid Photogeneration Through a Para-to-Meta Substitution Strategy in a Sulfonium-Based Alkoxystilbene Designed for Two-Photon Polymerization. Chem. Mater. 2012, 24, 237–244. [Google Scholar] [CrossRef]
- Wu, X.; Jin, M.; Xie, J.; Malval, J.P.; Wan, D. One/Two-Photon Cationic Polymerization in Visible and near Infrared Ranges Using Two-Branched Sulfonium Salts as Efficient Photoacid Generators. Dyes Pigment. 2016, 133, 363–371. [Google Scholar] [CrossRef]
- Jin, M.; Wu, X.; Xie, J.; Malval, J.P.; Wan, D. One/Two-Photon-Sensitive Photoacid Generators Based on Benzene Oligomer-Containing D–π–A-Type Aryl Dialkylsulfonium Salts. RSC Adv. 2015, 5, 55340–55347. [Google Scholar] [CrossRef]
- Wua, X.; Jin, M.; Xie, J.; Wan, D.; Malval, J.P. Effects of Conjugated Systems on UV-Visible Light-Sensitive D-π-A Type Sulfonium Salt Photoacid Generators. Chin. J. Polym. Sci. 2016, 34, 1456–1468. [Google Scholar] [CrossRef]
- Wang, C.; Meng, X.; Li, Z.; Li, M.; Jin, M.; Liu, R.; Yagci, Y. Chemiluminescence Induced Cationic Photopolymerization Using Sulfonium Salt. ACS Macro Lett. 2020, 9, 471–475. [Google Scholar] [CrossRef]
- Meng, X.; Li, L.; Huang, Y.; Deng, X.; Liu, X.; Li, Z. Upconversion Nanoparticle-Assisted Cationic and Radical/Cationic Hybrid Photopolymerization Using Sulfonium Salts. Polym. Chem. 2021, 12, 7005–7009. [Google Scholar] [CrossRef]
- Méndez-Ramos, J.; Ruiz-Morales, J.C.; Acosta-Mora, P.; Khaidukov, N.M. Infrared-Light Induced Curing of Photosensitive Resins through Photon up-Conversion for Novel Cost-Effective Luminescent 3D-Printing Technology. J. Mater. Chem. C 2016, 4, 801–806. [Google Scholar] [CrossRef]
- Hu, P.; Xu, H.; Pan, Y.; Sang, X.; Liu, R. Upconversion Particle-Assisted NIR Polymerization Enables Microdomain Gradient Photopolymerization at Inter-Particulate Length Scale. Nat. Commun. 2023, 14, 3653. [Google Scholar] [CrossRef] [PubMed]
- Rocheva, V.V.; Koroleva, A.V.; Savelyev, A.G.; Khaydukov, K.V.; Generalova, A.N.; Nechaev, A.V.; Guller, A.E.; Semchishen, V.A.; Chichkov, B.N.; Khaydukov, E.V. High-Resolution 3D Photopolymerization Assisted by Upconversion Nanoparticles for Rapid Prototyping Applications. Sci. Rep. 2018, 8, 3663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Boniface, A.; Parashar, V.K.; Gijs, M.A.M.; Moser, C. Multi-Photon Polymerization Using Upconversion Nanoparticles for Tunable Feature-Size Printing. Nanophotonics 2023, 12, 1527–1536. [Google Scholar] [CrossRef]
- Zhakeyev, A.; Abdulrhman, M.; Zhang, Y.; Li, F.; Chen, G.; Marques-Hueso, J. Upconversion 3D Printing Enables Single-Immersion Multi-Material Stereolithography. Appl. Mater. Today 2023, 32, 101854. [Google Scholar] [CrossRef]
- Hermes, P.; Hermsen, A.; Jäger, M.; Gutmann, J.S.; Strehmel, V.; Strehmel, B. Challenges and Limits of Upconversion Nanoparticles for Cationic Photopolymerization with UV Initiators Excited at 980 nm. Polym. Chem. 2022, 13, 4879–4886. [Google Scholar] [CrossRef]
- Limberg, D.K.; Kang, J.-H.; Hayward, R.C. Triplet–Triplet Annihilation Photopolymerization for High-Resolution 3D Printing. J. Am. Chem. Soc. 2022, 144, 5226–5232. [Google Scholar] [CrossRef] [PubMed]
- Tehfe, M.A.; Louradour, F.; Lalevée, J.; Fouassier, J.-P. Photopolymerization Reactions: On the Way to a Green and Sustainable Chemistry. Appl. Sci. 2013, 3, 490–514. [Google Scholar] [CrossRef]
- Hola, E.; Morlet-Savary, F.; Lalevée, J.; Ortyl, J. Photoinitiator or Photosensitizer? Dual Behaviour of m-Terphenyls in Photopolymerization Processes. Eur. Polym. J. 2023, 189, 111971. [Google Scholar] [CrossRef]
- Samoilenko, T.F.; Iarova, N.V.; Ostapiuk, S.M.; Tkalich, M.H.; Vorontsova, L.O.; Klymchuk, D.O.; Brovko, O.O. Influence of N-Vynilcarbazole on the Photopolymerization Process and Properties of Epoxy-Acrylate Interpenetrating Polymer Networks. e-Polymers 2016, 16, 429–435. [Google Scholar] [CrossRef][Green Version]
- Gallastegui, A.; Dominguez-Alfaro, A.; Lezama, L.; Alegret, N.; Prato, M.; Gómez, M.L.; Mecerreyes, D. Fast Visible-Light Photopolymerization in the Presence of Multiwalled Carbon Nanotubes: Toward 3D Printing Conducting Nanocomposites. ACS Macro Lett. 2022, 11, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Lalevée, J.; Zhao, J.; Stenzel, M.H. N-Vinylcarbazole as Versatile Photoinaddimer of Photopolymerization under Household UV LED Bulb (392 nm). Macromol. Rapid Commun. 2015, 36, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, J.; Dirani, A.; El-Roz, M.; Allonas, X.; Fouassier, J.P. Germanes as Efficient Coinitiators in Radical and Cationic Photopolymerizations. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3042–3047. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Lalevée, J. Photochemical Production of Interpenetrating Polymer Networks; Simultaneous Initiation of Radical and Cationic Polymerization Reactions. Polymers 2014, 6, 2588–2610. [Google Scholar] [CrossRef]
- Li, Y.; Shaukat, U.; Schlögl, S.; Xue, T.; Li, J.; Nie, J.; Zhu, X. A Pyrrole–Carbazole Photoinitiator for Radical and Cationic Visible Light LED Photopolymerization. Eur. Polym. J. 2023, 182, 111700. [Google Scholar] [CrossRef]
- Motiekaityte, G.; Navaruckiene, A.; Raudoniene, V.; Bridziuviene, D.; Jaras, J.; Kantminiene, K.; Ostrauskaite, J. Antimicrobial Dual-Cured Photopolymers of Vanillin Alcohol Diglycidyl Ether and Glycerol Dimethacrylate. J. Appl. Polym. Sci. 2023, 140, e53289. [Google Scholar] [CrossRef]
- Urano, T.; Ohno-Okumura, E.; Sakamoto, K.; Suzuki, S.; Yamaoka, T. Effect of Ethyl Group in Sensitization Mechanisms of Pyrromethene Dyes in Photopolymer Coating Layer. J. Photopolym. Sci. Technol. 2000, 13, 673–677. [Google Scholar] [CrossRef][Green Version]
- Sambath, K.; Wan, Z.; Wang, Q.; Chen, H.; Zhang, Y. BODIPY-Based Photoacid Generators for Light-Induced Cationic Polymerization. Org. Lett. 2020, 22, 1208–1212. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Chen, Y.; Pi, J.; Liu, R.; Zhu, Y. Recent Advances and Challenges in Long Wavelength Sensitive Cationic Photoinitiating Systems. Polymers 2023, 15, 2524. [Google Scholar] [CrossRef]
- Wang, X.; Saeva, F.D.; Kampmeier, J.A. Photosensitized Reduction of Sulfonium Salts: Evidence for Nondissociative Electron Transfer. J. Am. Chem. Soc. 1999, 121, 4364–4368. [Google Scholar] [CrossRef]
- Garra, P.; Fouassier, J.P.; Lakhdar, S.; Yagci, Y.; Lalevée, J. Visible Light Photoinitiating Systems by Charge Transfer Complexes: Photochemistry without Dyes. Prog. Polym. Sci. 2020, 107, 101277. [Google Scholar] [CrossRef]
- Garra, P.; Graff, B.; Morlet-Savary, F.; Dietlin, C.; Becht, J.-M.; Fouassier, J.-P.; Lalevée, J. Charge Transfer Complexes as Pan-Scaled Photoinitiating Systems: From 50 μm 3D Printed Polymers at 405 nm to Extremely Deep Photopolymerization (31 cm). Macromolecules 2018, 51, 57–70. [Google Scholar] [CrossRef]
- Kaya, K.; Kreutzer, J.; Yagci, Y. A Charge-Transfer Complex of Thioxanthonephenacyl Sulfonium Salt as a Visible-Light Photoinitiator for Free Radical and Cationic Polymerizations. ChemPhotoChem 2019, 3, 1187–1192. [Google Scholar] [CrossRef]
- Wu, X.; Malval, J.; Wan, D.; Jin, M. D-π-A-Type Aryl Dialkylsulfonium Salts as One-Component Versatile Photoinitiators under UV/Visible LEDs Irradiation. Dyes Pigment. 2016, 132, 128–135. [Google Scholar] [CrossRef]
- Jeon, N.J.; Na, H.; Jung, E.H.; Yang, T.-Y.; Lee, Y.G.; Kim, G.; Shin, H.-W.; Il Seok, S.; Lee, J.; Seo, J. A Fluorene-Terminated Hole-Transporting Material for Highly Efficient and Stable Perovskite Solar Cells. Nat. Energy 2018, 3, 682–689. [Google Scholar] [CrossRef]
- Suman; Bagui, A.; Datt, R.; Gupta, V.; Singh, S.P. A Simple Fluorene Core-Based Non-Fullerene Acceptor for High Performance Organic Solar Cells. Chem. Commun. 2017, 53, 12790–12793. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Dong, Y.; Su, Y.-J.; Yu, R.; Gao, H.; Gong, Y.; Lee, Z.-Y.; Yang, C.; Hsu, C.-S.; Tan, Z. Morphological Stabilization in Organic Solar Cells via a Fluorene-Based Crosslinker for Enhanced Efficiency and Thermal Stability. ACS Appl. Mater. Interfaces 2022, 14, 1187–1194. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, J.; Xu, B.; Liu, P.; Cheng, M.; Kloo, L.; Johansson, E.M.J.; Sveinbjörnsson, K.; Aitola, K.; Boschloo, G.; et al. Facile Synthesis of Fluorene-Based Hole Transport Materials for Highly Efficient Perovskite Solar Cells and Solid-State Dye-Sensitized Solar Cells. Nano Energy 2016, 26, 108–113. [Google Scholar] [CrossRef]
- Suman; Kovvuri, J.; Islavath, N. Molecular Modifications in Fluorene Core for Efficient Organic Photovoltaic Cells. J. Photochem. Photobiol. A Chem. 2024, 446, 115162. [Google Scholar] [CrossRef]
- Nagarjuna, P.; Chaturvedi, N.; Bagui, A.; Runjhun, R.; Garg, A.; Singh, S.P. Solution-Processed Organic Solar Cells Using New Electron Acceptor Derived from Naphthalene and Fluorene Unit. ChemistrySelect 2017, 2, 7913–7917. [Google Scholar] [CrossRef]
- Kumar, D.; Justin Thomas, K.R.; Lee, C.-P.; Ho, K.-C. Organic Dyes Containing Fluorene Decorated with Imidazole Units for Dye-Sensitized Solar Cells. J. Org. Chem. 2014, 79, 3159–3172. [Google Scholar] [CrossRef]
- Kacimi, R.; Abram, T.; Bourass, M.; Bejjit, L.; Alimi, K.; Bouachrine, M. Molecular Design of D–A–D Conjugated Molecules Based on Fluorene for Organic Solar Cells. Opt. Quantum Electron. 2019, 51, 76. [Google Scholar] [CrossRef]
- Gayathri, R.D.; Gokulnath, T.; Park, H.-Y.; Xie, Z.; Jin, S.-H.; Han, S.C.; Lee, J.W. Facile and Stable Fluorene Based Organic Hole Transporting Materials for Efficient Perovskite Solar Cells. Macromole. Res. 2022, 30, 745–750. [Google Scholar] [CrossRef]
- Justin Thomas, K.R.; Baheti, A. Fluorene Based Organic Dyes for Dye Sensitised Solar Cells: Structure–Property Relationships. Mater. Technol. 2013, 28, 71–87. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Huang, S.; Peng, X. Fluorene Dimers as the Cathode Interlayers in Organic Solar Cells. Synth. Metals 2019, 253, 110–115. [Google Scholar] [CrossRef]
- Kim, K.-S.; Jeon, Y.-M.; Kim, J.-W.; Lee, C.-W.; Gong, M.-S. Blue Light-Emitting OLED Using New Spiro[Fluorene-7,9′-Benzofluorene] Host and Dopant Materials. Org. Electron. 2008, 9, 797–804. [Google Scholar] [CrossRef]
- Li, G.; Zheng, J.; Klimes, K.; Zhu, Z.-Q.; Wu, J.; Zhu, H.; Li, J. Novel Carbazole/Fluorene-Based Host Material for Stable and Efficient Phosphorescent OLEDs. ACS Appl. Mater. Interfaces 2019, 11, 40320–40331. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, N.; Xiao, Y.; Wang, S.; Li, X. Novel Carbazolyl-Substituted Spiro[Acridine-9,9′-Fluorene] Derivatives as Deep-Blue Emitting Materials for OLED Applications. Dyes Pigment. 2018, 154, 30–37. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, W.-H.; Huang, Y.-S.; Wu, C.-H.; Gnanasekaran, P.; Chang, Y.-M.; Teng, S.-W.; Lu, C.-W.; Chang, C.-H.; Chang, Y.J. Highly Efficient (EQE > 27%) Yellow OLEDs Using Spiro[Fluorene-9,9′-Phenanthren-10′-One]-Carbazole-Based Donor–Acceptor–Donor Host Materials. J. Mater. Chem. C 2023, 11, 3101–3111. [Google Scholar] [CrossRef]
- Lozano-Hernández, L.-A.; Cameron, J.; Riggs, C.J.; Reineke, S.; Skabara, P.J. A Study on Varying the Number of Fluorene Units in 2, 1, 3-Benzothiadiazole-Containing Oligomers and the Effect on OLED Performance and Stability. ChemPhotoChem 2023, 7, e202200256. [Google Scholar] [CrossRef]
- Lee, J.; Kim, B.; Park, Y.; Kim, S.; Park, J. Fluorine Effects in New Indenofluorenedione Derivatives for Electron Transporting Layer in OLED Devices. J. Nanosci. Nanotechnol. 2014, 14, 6431–6434. [Google Scholar] [CrossRef] [PubMed]
- Braveenth, R.; Jung, H.; Kim, K.; Kim, B.M.; Bae, I.-J.; Kim, M.; Chai, K.Y. Fluorene–Triphenylamine-Based Bipolar Materials: Fluorescent Emitter and Host for Yellow Phosphorescent OLEDs. Appl. Sci. 2020, 10, 519. [Google Scholar] [CrossRef]
- Kim, D.; Reddy, M.R.; Cho, K.; Ho, D.; Kim, C.; Seo, S. Solution-Processable Fluorene Derivative for Organic Thin-Film Transistors. Org. Electron. 2020, 76, 105464. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lai, Y.-Y.; Cao, F.-Y.; Hsu, J.-Y.; Lin, Z.-L.; Jeng, U.-S.; Su, C.-J.; Cheng, Y.-J. Synthesis, Molecular and Photovoltaic/Transistor Properties of Heptacyclic Ladder-Type Di(Thienobenzo)Fluorene-Based Copolymers. J. Mater. Chem. C 2016, 4, 11427–11435. [Google Scholar] [CrossRef]
- Dong, L.; Sun, H.-S.; Wang, J.-T.; Lee, W.-Y.; Chen, W.-C. Fluorene Based Donor-Acceptor Polymer Electrets for Nonvolatile Organic Transistor Memory Device Applications. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 602–614. [Google Scholar] [CrossRef]
- Cho, K.; Reddy, M.R.; Kim, D.; Ho, D.; Yun, C.; Seo, S.; Kim, C. Synthesis and Characterization of Fluorene Derivatives as Organic Semiconductors for Organic Field-Effect Transistor. Mol. Cryst. Liquid Cryst. 2019, 690, 56–66. [Google Scholar] [CrossRef]
- Koshiba, Y.; Kato, T.; Misaki, M.; Ishida, K.; Torii, M.; Kato, T.; Tsutsui, K.; Tanigaki, N.; Yase, K.; Ueda, Y. Fabrication of One-Dimensionally Oriented Fluorene–Thiophene Copolymer Thin Films and Anisotropic Transistor Characteristics. Jpn. J. Appl. Phys. 2010, 49, 01AE13. [Google Scholar] [CrossRef]
- Kim, Z.-S.; Lim, S.C.; Kim, S.H.; Yang, Y.S.; Hwang, D.-H. Biotin-Functionalized Semiconducting Polymer in an Organic Field Effect Transistor and Application as a Biosensor. Sensors 2012, 12, 11238–11248. [Google Scholar] [CrossRef]
- Noh, Y.-Y.; Kim, D.-Y.; Yoshida, Y.; Yase, K.; Jung, B.-J.; Lim, E.; Shim, H.-K.; Azumi, R. Keto Defect Sites in Fluorene-Based Organic Field-Effect Transistors: The Origin of Rapid Degradation on the Performance of the Device. J. Appl. Phys. 2005, 97, 104504. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Chen, C.-W.; Chueh, C.-C.; Yang, C.-C.; Chen, W.-C. Synthesis of New Fluorene-Indolocarbazole Alternating Copolymers for Light-Emitting Diodes and Field Effect Transistors. Polym. J. 2008, 40, 249–255. [Google Scholar] [CrossRef]
- Heeney, M.; Bailey, C.; Giles, M.; Shkunov, M.; Sparrowe, D.; Tierney, S.; Zhang, W.; McCulloch, I. Alkylidene Fluorene Liquid Crystalline Semiconducting Polymers for Organic Field Effect Transistor Devices. Macromolecules 2004, 37, 5250–5256. [Google Scholar] [CrossRef]
- Schafer Hales, K.J.; Belfield, K.D.; Yao, S.; Frederiksen, P.K.; Hales, J.M.; Kolattukudy, P.E. Fluorene-Based Fluorescent Probes with High Two-Photon Action Cross-Sections for Biological Multiphoton Imaging Applications. J. Biomed. Opt. 2005, 10, 051402. [Google Scholar] [CrossRef] [PubMed]
- Shaya, J.; Corridon, P.R.; Al-Omari, B.; Aoudi, A.; Shunnar, A.; Mohideen, M.I.H.; Qurashi, A.; Michel, B.Y.; Burger, A. Design, Photophysical Properties, and Applications of Fluorene-Based Fluorophores in Two-Photon Fluorescence Bioimaging: A Review. J. Photochem. Photobiol. C Photochem. Rev. 2022, 52, 100529. [Google Scholar] [CrossRef]
- Yang, T.; Zuo, Y.; Zhang, Y.; Gou, Z.; Wang, X.; Lin, W. Novel Fluorene-Based Fluorescent Probe with Excellent Stability for Selective Detection of SCN− and Its Applications in Paper-Based Sensing and Bioimaging. J. Mater. Chem. B 2019, 7, 4649–4654. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, X.; Ren, H.; Lu, M.; Cui, X.; Wang, L.; Wang, H.; Tang, Q. Fluorene-Based Polymers of Intrinsic Microporosity as Fluorescent Probes for Metal Ions. React. Funct. Polym. 2022, 181, 105431. [Google Scholar] [CrossRef]
- Xochitiotzi-Flores, E.; Jiménez-Sánchez, A.; García-Ortega, H.; Sánchez-Puig, N.; Romero-Ávila, M.; Santillan, R.; Farfán, N. Optical Properties of Two Fluorene Derived BODIPY Molecular Rotors as Fluorescent Ratiometric Viscosity Probes. New J. Chem. 2016, 40, 4500–4512. [Google Scholar] [CrossRef]
- Morales, A.R.; Schafer-Hales, K.J.; Marcus, A.I.; Belfield, K.D. Amine-Reactive Fluorene Probes: Synthesis, Optical Characterization, Bioconjugation, and Two-Photon Fluorescence Imaging. Bioconjug. Chem. 2008, 19, 2559–2567. [Google Scholar] [CrossRef][Green Version]
- Lala, A.K.; Koppaka, V. Transverse Location of New Fluorene Based Depth Dependent Fluorescent Probes in Membranes—Quenching Studies with 9,10-Dibromostearic Acid. Photochem. Photobiol. 1989, 49, 763–767. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Mohadjerani, M.; Pooryousef, M. A New Selective Fluorene-Based Fluorescent Internal Charge Transfer (ICT) Sensor for Sugar Alcohols in Aqueous Solution. Anal. Bioanal. Chem. 2016, 408, 1901–1908. [Google Scholar] [CrossRef]
- Yilmaz, G.; Aydogan, B.; Temel, G.; Arsu, N.; Moszner, N.; Yagci, Y. Thioxanthone−Fluorenes as Visible Light Photoinitiators for Free Radical Polymerization. Macromolecules 2010, 43, 4520–4526. [Google Scholar] [CrossRef]
- Peter Pappas, S.; Tilley, M.G.; Pappas, B.C. Anthracene-Bound Sulfonium Salts: Highly Efficient Photoinitiators for Cationic Polymerization: A New Synthesis of Sulfonium Salts Which Avoids the Use of Silver Salts. J. Photochem. Photobiol. A Chem. 2003, 159, 161–171. [Google Scholar] [CrossRef]
- Balta, D.K.; Arsu, N.; Yagci, Y.; Jockusch, S.; Turro, N.J. Thioxanthone−Anthracene: A New Photoinitiator for Free Radical Polymerization in the Presence of Oxygen. Macromolecules 2007, 40, 4138–4141. [Google Scholar] [CrossRef]
- Balta, D.K.; Arsu, N.; Yagci, Y.; Sundaresan, A.K.; Jockusch, S.; Turro, N.J. Mechanism of Photoinitiated Free Radical Polymerization by Thioxanthone−Anthracene in the Presence of Air. Macromolecules 2011, 44, 2531–2535. [Google Scholar] [CrossRef]
- Balta, D.K.; Arsu, N. Photodimerization of Thioxanthone–Anthracene: Formation of Monochromophoric Type II Initiator. Des. Monomers Polym. 2016, 19, 222–226. [Google Scholar] [CrossRef]
- Balta, D.K.; Arsu, N. Thioxanthone-Ethyl Anthracene. J. Photochem. Photobiol. A Chem. 2013, 257, 54–59. [Google Scholar] [CrossRef]
- Crivello, J.V.; Jang, M. Anthracene Electron-Transfer Photosensitizers for Onium Salt Induced Cationic Photopolymerizations. J. Photochem. Photobiol. A Chem. 2003, 159, 173–188. [Google Scholar] [CrossRef]
- Toba, Y. Anthracene-Sensitized Polymerization of Vinyl Ethers by Onium Tetrakis(Pentafluorophenyl)Borate Initiators. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 982–987. [Google Scholar] [CrossRef]
- Cho, J.-D.; Kim, H.-K.; Kim, Y.-S.; Hong, J.-W. Dual Curing of Cationic UV-Curable Clear and Pigmented Coating Systems Photosensitized by Thioxanthone and Anthracene. Polym. Test. 2003, 22, 633–645. [Google Scholar] [CrossRef]
- Cho, J.-D.; Kim, E.-O.; Kim, H.-K.; Hong, J.-W. An Investigation of the Surface Properties and Curing Behavior of Photocurable Cationic Films Photosensitized by Anthracene. Polym. Test. 2002, 21, 781–791. [Google Scholar] [CrossRef]
- Iwaki, J.; Suzuki, S.; Park, C.; Miyagawa, N.; Takahara, S.; Yamaoka, T. Sensitization Mechanism of the Anthracene Derivatives/Photoacid Generator Photoinitiating System. J. Photopolym. Sci. Technol. 2004, 17, 123–124. [Google Scholar] [CrossRef][Green Version]
- Pappas, S.P.; Pappas, B.C.; Gatechair, L.R.; Schnabel, W. Photoinitation of Cationic Polymerization. II. Laser Flash Photolysis of Diphenyliodonium Salts. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 69–76. [Google Scholar] [CrossRef]
- DeVoe, R.J.; Sahyun, M.R.V.; Schmidt, E.; Serpone, N.; Sharma, D.K. Electron Transfer Sensitized Photolysis of ’onium Salts. Can. J. Chem. 1988, 66, 319–324. [Google Scholar] [CrossRef]
- Runemark, A.; Sundén, H. Overcoming Back Electron Transfer in the Electron Donor–Acceptor Complex-Mediated Visible Light-Driven Generation of α-Aminoalkyl Radicals from Secondary Anilines. J. Org. Chem. 2023, 88, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Pączkowski, J.; Pietrzak, M.; Paczkowska, B. Does the Back Electron Transfer Affect the Rate of Photoinduced Free Radical Polymerization? Polimery 2022, 48, 765–773. [Google Scholar] [CrossRef]
- Christmann, J.; Allonas, X.; Ley, C.; Ibrahim, A.; Croutxé-Barghorn, C. Triazine-Based Type-II Photoinitiating System for Free Radical Photopolymerization: Mechanism, Efficiency, and Modeling. Macromol. Chem. Phys. 2017, 218, 1600597. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Gnonlonfin, G.J.B.; Sanni, A.; Brimer, L. Review Scopoletin—A Coumarin Phytoalexin with Medicinal Properties. Null 2012, 31, 47–56. [Google Scholar] [CrossRef]
- Lončarić, M.; Gašo-Sokač, D.; Jokić, S.; Molnar, M. Recent Advances in the Synthesis of Coumarin Derivatives from Different Starting Materials. Biomolecules 2020, 10, 151. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Q.; Gao, P.; Chi, B.; Nie, J.; He, Y. Photopolymerization of Coumarin-Containing Reversible Photoresponsive Materials Based on Wavelength Selectivity. Ind. Eng. Chem. Res. 2019, 58, 2970–2975. [Google Scholar] [CrossRef]
- Li, Z.; Zou, X.; Zhu, G.; Liu, X.; Liu, R. Coumarin-Based Oxime Esters: Photobleachable and Versatile Unimolecular Initiators for Acrylate and Thiol-Based Click Photopolymerization under Visible Light-Emitting Diode Light Irradiation. ACS Appl. Mater. Interfaces 2018, 10, 16113–16123. [Google Scholar] [CrossRef]
- Rahal, M.; Mokbel, H.; Graff, B.; Toufaily, J.; Hamieh, T.; Dumur, F.; Lalevée, J. Mono vs. Difunctional Coumarin as Photoinitiators in Photocomposite Synthesis and 3D Printing. Catalysts 2020, 10, 1202. [Google Scholar] [CrossRef]
- Rajeshirke, M.; Sreenath, M.C.; Chitrambalam, S.; Joe, I.H.; Sekar, N. Enhancement of NLO Properties in OBO Fluorophores Derived from Carbazole–Coumarin Chalcones Containing Carboxylic Acid at the N-Alykl Terminal End. J. Phys. Chem. C 2018, 122, 14313–14325. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Coumarin-Based Photoinitiators of Polymerization. Eur. Polym. J. 2022, 163, 110962. [Google Scholar] [CrossRef]
- Liu, Z.; Dumur, F. Recent Advances on Visible Light Coumarin-Based Oxime Esters as Initiators of Polymerization. Eur. Polym. J. 2022, 177, 111449. [Google Scholar] [CrossRef]
- Camposeo, A.; Arkadii, A.; Romano, L.; D’Elia, F.; Fabbri, F.; Zussman, E.; Pisignano, D. Impact of Size Effects on Photopolymerization and Its Optical Monitoring In-Situ. Addit. Manuf. 2022, 58, 103020. [Google Scholar] [CrossRef]
- Breloy, L.; Brezová, V.; Barbieriková, Z.; Ito, Y.; Akimoto, J.; Chiappone, A.; Abbad-Andaloussi, S.; Malval, J.-P.; Versace, D.-L. Methacrylated Quinizarin Derivatives for Visible-Light Mediated Photopolymerization: Promising Applications in 3D-Printing Biosourced Materials under LED@405 nm. ACS Appl. Polym. Mater. 2022, 4, 210–228. [Google Scholar] [CrossRef]
- Chao, P.; Gu, R.; Ma, X.; Wang, T.; Zhao, Y. Thiophene-Substituted Phenothiazine-Based Photosensitisers for Radical and Cationic Photopolymerization Reactions under Visible Laser Beams (405 and 455 nm). Polym. Chem. 2016, 7, 5147–5156. [Google Scholar] [CrossRef]
- Sun, K.; Peng, X.; Gan, Z.; Chen, W.; Li, X.; Gong, T.; Xiao, P. 3D Printing/Vat Photopolymerization of Photopolymers Activated by Novel Organic Dyes as Photoinitiators. Catalysts 2022, 12, 1272. [Google Scholar] [CrossRef]
- Shah, M.; Ullah, A.; Azher, K.; Rehman, A.U.; Juan, W.; Aktürk, N.; Tüfekci, C.S.; Salamci, M.U. Vat Photopolymerization-Based 3D Printing of Polymer Nanocomposites: Current Trends and Applications. RSC Adv. 2023, 13, 1456–1496. [Google Scholar] [CrossRef]
- Pagac, M.; Hajnys, J.; Ma, Q.-P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A Review of Vat Photopolymerization Technology: Materials, Applications, Challenges, and Future Trends of 3D Printing. Polymers 2021, 13, 598. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, L.; Zhang, Y.; Ou, Y.; Zhang, J.; Yagci, Y.; Liu, R. Broad Wavelength Sensitive Coumarin Sulfonium Salts as Photoinitiators for Cationic, Free Radical and Hybrid Photopolymerizations. Prog. Org. Coat. 2023, 174, 107272. [Google Scholar] [CrossRef]
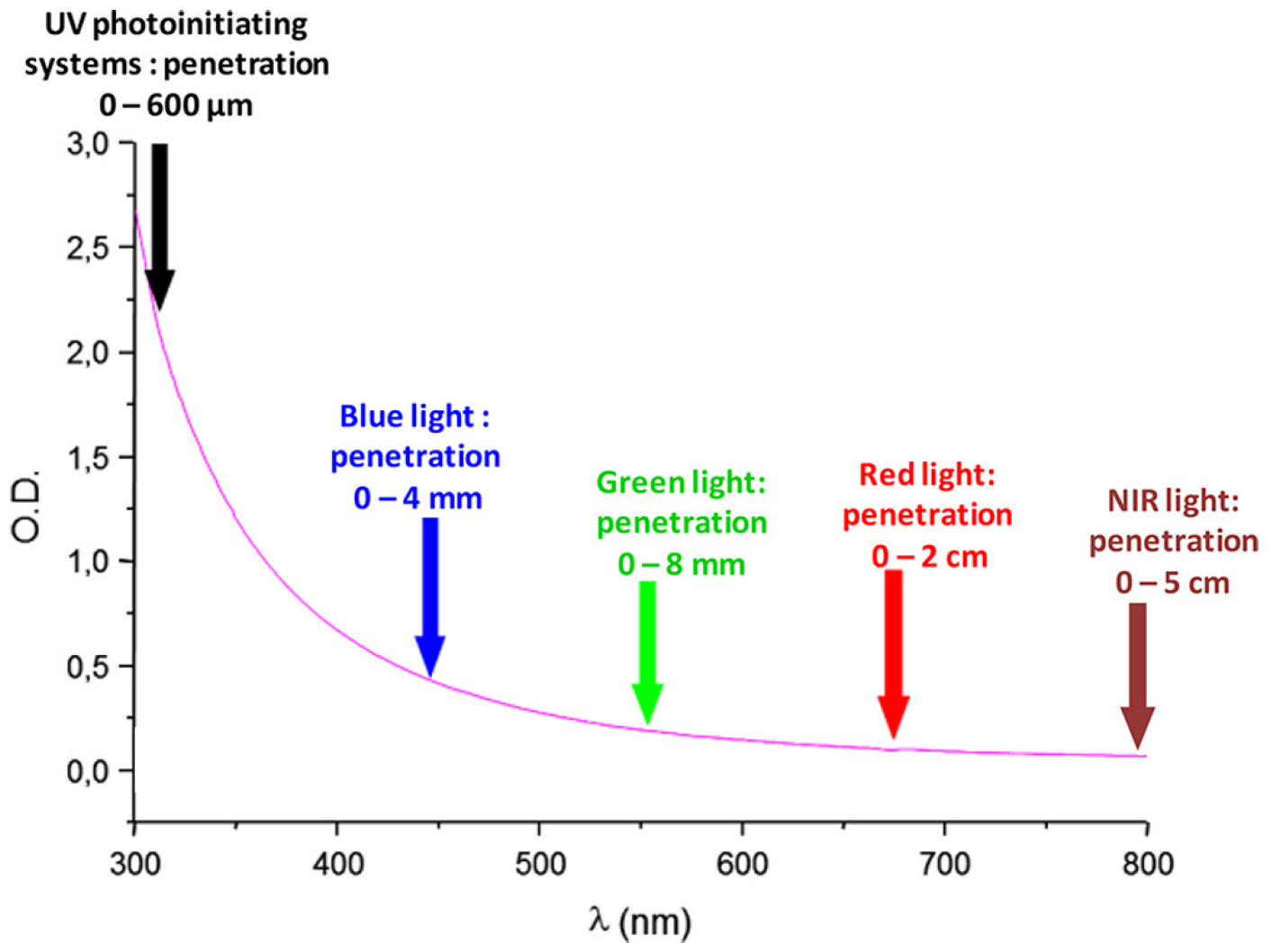














































| Parameters | Monocomponent Sulfonium Salts | Other Sulfonium Salts |
|---|---|---|
| Cost/synthesis | Compounds are often prepared in multistep syntheses rendering these compounds expensive. | Numerous sulfonium salts are commercially available and can be purchased at low cost. |
| Environmental impact | Most synthetic dyes are often prepared in an organic solvent. Purification by column chromatography is also required. | The same comments can be performed concerning benchmark sulfonium salts. |
| Photochemical stability | Chromophore-substituted sulfonium salts are stable compounds. Therefore, their photochemical stability is good. | Sulfonium salts are highly stable, notably when comprising aromatic rings. |
| Absorption range | Chromophores bounded to sulfonium salts exhibit a broad absorption and their absorption spectra can be finely tuned from the near-UV visible range up to the near-infrared range | Benchmark sulfonium salts exhibit a UV-centered absorption, with no absorption in the visible range. |
| Photoinitiating ability | Chromophores are ideal candidates for photoinitiation in the visible range and even in the near-infrared region. Ability of chromophores to release or accept electrons is well-documented in the literature. | Good photoinitiating ability of sulfonium salts in the UV range |
| Availability | Availability can be restricted, especially when multistep syntheses are used. Expensive compounds, which can limit their scope of applications. | Easy availability. Benchmark photoinitiators can be purchased in the Kg scale at low cost. Numerous applications of benchmark sulfonium salts in industry. |
| 365 nm | 385 nm | 395 nm | 405 nm | 425 nm | |
|---|---|---|---|---|---|
| PI-CF3 | 0.60 | 0.56 | 0.61 | 0.59 | 0.31 |
| PI-H | 0.62 | 0.59 | 0.62 | 0.63 | 0.26 |
| PI-EtO | 0.65 | 0.61 | 0.64 | 0.46 | 0.20 |
| 365 nm | 385 nm | 395 nm | 405 nm | 425 nm | |
|---|---|---|---|---|---|
| PI-CF3 | 49.15 | 50.63 | 52.66 | 55.18 | 51.87 |
| PI-H | 52.84 | 54.06 | 56.37 | 59.74 | 40.25 |
| PI-EtO | 55.06 | 56.47 | 58.82 | 61.97 | 49.57 |
| PI-6992M | 59.31 | 43.63 | - | - | - |
| 365 nm | 385 nm | 395 nm | 405 nm | |
|---|---|---|---|---|
| PI-CF3 | 43.1 | 44.9 | 41.3 | 35.3 |
| PI-H | 50.9 | 48.9 | 45.5 | 42.4 |
| OXE-02 | 65.6 | 61.5 | 44.4 | 27.0 |
| Initiator | λ = 710 nm |
|---|---|
| BSB-S2 | 2.4 |
| CD1012/ITX | 44 |
| CD1012 | 212 |
| TPS | >317 a |
| DPI-DMAS | >317 a |
| Initiator | λmax (nm) | εmax (M−1·cm−1) | ϕH+ a | δmax (GM) |
|---|---|---|---|---|
| PAG1 | 346 | 36,100 | 0.10 | 73 (760 nm) |
| PAG2 | 324 | 26,000 | 0.24 | 68 (710 nm) |
| PAG3 | 395 | 34,300 | 0.05 | 643 (870 nm) |
| PAG4 | 381 | 23,700 | 0.44 | 650 (800 nm) |
| PAG5 | 400 | 34,400 | 0.31 | 680 (880 nm) |
| PAG6 | 380 | 25,200 | 0.50 | 648 (800 nm) |
| Compound | λabs (nm) | εmax (M−1·cm−1) | ϕH+ a | ϕH+ b | δ780 nm (GM) | ϕH+ |
|---|---|---|---|---|---|---|
| Pre-Para | 394 | 53,200 | - | - | - | - |
| Mono-Para | 404 | 40,600 | 0.002 | 0.02 | 528 | 1.0 |
| Bi-Para | 413 | 37,000 | 0.004 | 0.04 | 816 | 3.2 |
| Pre-Meta | 385 | 37,000 | - | - | - | - |
| Mono-Meta | 392 | 32,000 | 0.20 | 0.41 | 234 | 46.8 |
| Bi-Meta | 399 | 30,800 | 0.40 | 0.49 | 745 | 298 |
| Monomer | Photoinitiator | Intensity (mW/cm2) | Conversion (%) |
|---|---|---|---|
| DVE-3 | Flu-MS | 2 | 98 |
| DVE-3 | Flu-PS | 2 | 99 |
| CHO | Flu-MS | 2 | 84.5 |
| CHO | Flu-PS | 2 | 79 |
| EPOX | Flu-MS | 4 | 70 |
| EPOX | Flu-PS | 4 | 59 |
| TMPTA | Flu-MS | 4 | 58 |
| TMPTA | Flu-PS | 4 | 61 |
| EPOX/TMPTA | Flu-MS | 2 | 39/71 |
| EPOX/TMPTA | Flu-PS | 2 | 43/71 |
| trithiol/TMPTA | Flu-MS | 2 | 50/82 |
| trithiol/TMPTA | Flu-PS | 2 | 47/85 |
| Monomers | PIs | Light Source | Intensity (mW/cm2) | Conversion (%) |
|---|---|---|---|---|
| EPOX | Flu-MS | 385 nm | 4 | 65.6 |
| EPOX | Flu-MS | 405 nm | 4 | 73.0 |
| EPOX | Flu-MS | 425 nm | 4 | 63.2 |
| EPOX | Flu-PS | 385 nm | 4 | 63.3 |
| EPOX | Flu-PS | 405 nm | 4 | 68.6 |
| EPOX | Flu-PS | 425 nm | 4 | 64.3 |
| CHO | Flu-MS | 385 nm | 2 | 81.1 |
| CHO | Flu-MS | 405 nm | 2 | 64.3 |
| CHO | Flu-MS | 450 nm | 16 | 65.0 |
| CHO | Flu-PS | 385 nm | 2 | 76.3 |
| CHO | Flu-PS | 405 nm | 2 | 76.9 |
| CHO | Flu-PS | 450 nm | 16 | 85.4 |
| TMPTA | Flu-MS | 385 nm | 4 | 48.5 |
| TMPTA | Flu-MS | 405 nm | 4 | 42.8 |
| TMPTA | Flu-PS | 385 nm | 4 | 60.8 |
| TMPTA | Flu-PS | 405 nm | 4 | 56.2 |
| EPOX/TMPTA | Flu-MS | 365 nm | 2 | 22.8/5.3 |
| EPOX/TMPTA | Flu-PS | 365 nm | 2 | 13.1/13.3 |
| 365 nm | p-H-Me CSS | p-H-Bu CSS | p-H-Hep CSS | p-OMe-Me CSS | p-OMe-Bu CSS | p-OMe-Hep CSS | |
|---|---|---|---|---|---|---|---|
| EPOX | 18 | 64 | 37 | 18 | 55 | 32 | 55 (201s) |
| TMPTA | 44 | 69 | 54 | 48 | 62 | 54 | 68 (ITX) |
| 405 nm | p-H-Me CSS | p-H-Bu CSS | p-H-Hep CSS | p-OMe-Me CSS | p-OMe-Bu CSS | p-OMe-Hep CSS | 201s |
| EPOX | 12 | 53 | 44 | 15 | 69 | 45 | 12 (201s) |
| TMPTA | 40 | 58 | 43 | 39 | 60 | 52 | 65 (ITX) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumur, F. Recent Advances in Monocomponent Visible Light Photoinitiating Systems Based on Sulfonium Salts. Polymers 2023, 15, 4202. https://doi.org/10.3390/polym15214202
Dumur F. Recent Advances in Monocomponent Visible Light Photoinitiating Systems Based on Sulfonium Salts. Polymers. 2023; 15(21):4202. https://doi.org/10.3390/polym15214202
Chicago/Turabian StyleDumur, Frédéric. 2023. "Recent Advances in Monocomponent Visible Light Photoinitiating Systems Based on Sulfonium Salts" Polymers 15, no. 21: 4202. https://doi.org/10.3390/polym15214202
APA StyleDumur, F. (2023). Recent Advances in Monocomponent Visible Light Photoinitiating Systems Based on Sulfonium Salts. Polymers, 15(21), 4202. https://doi.org/10.3390/polym15214202







