Preparation and Photocatalytic Performance of Sodium Alginate/Polyacrylamide/Polypyrrole-TiO2 Nanocomposite Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polypyrrole-TiO2 Nanocomposite
2.3. Preparation of Sodium Alginate/Polyacrylamide/Polypyrrole-TiO2 Nanocomposite Hydrogel
2.4. Characterization
2.5. Swelling Performance Test of Composite Hydrogels
2.6. Mechanical Property Test of Composite Hydrogels
2.7. Photocatalytic Performance Test of Composite Hydrogels
3. Results and Discussion
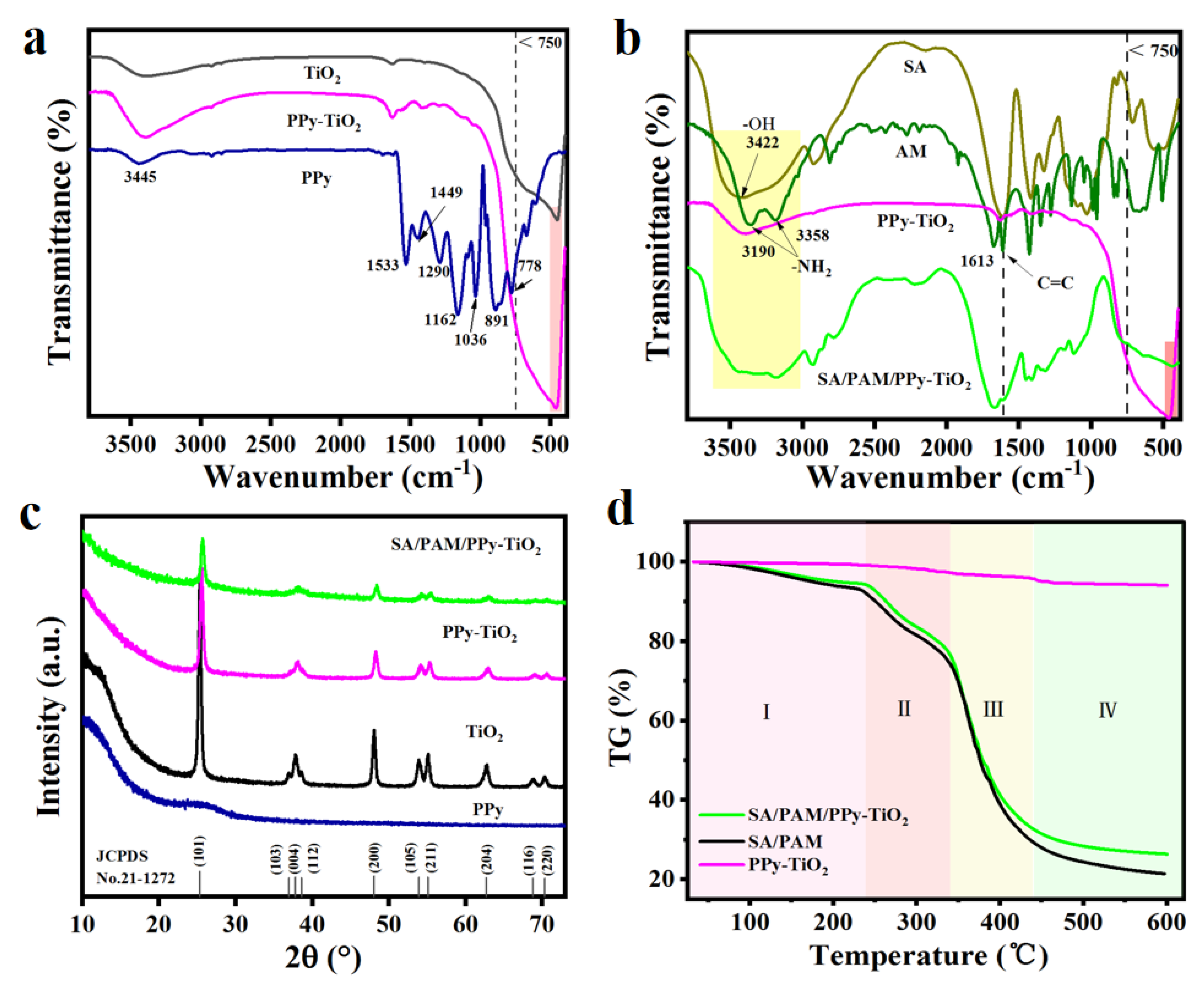
3.1. Structural Characterization Analysis of Materials
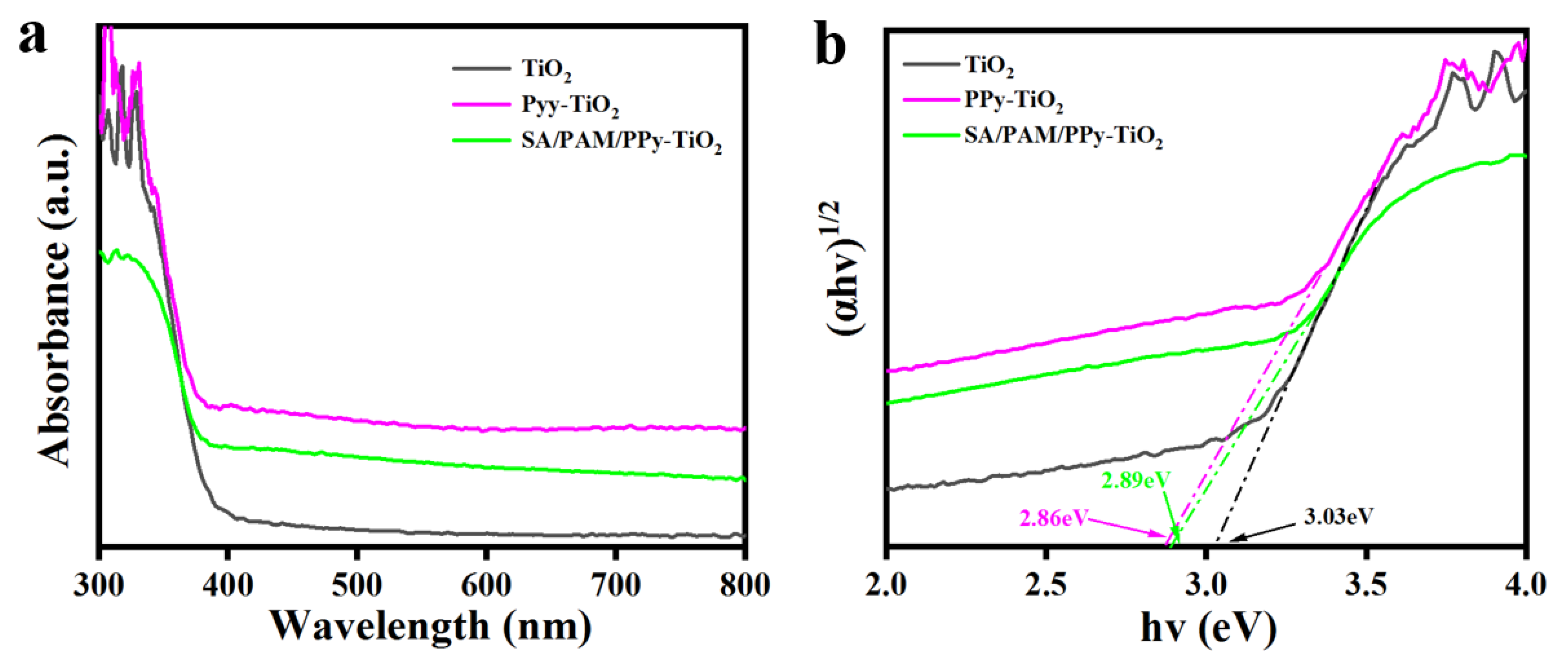
3.2. Analysis of Solid Ultraviolet–Visible Diffuse Reflection Spectra
3.3. Fluorescence Spectrum Analysis
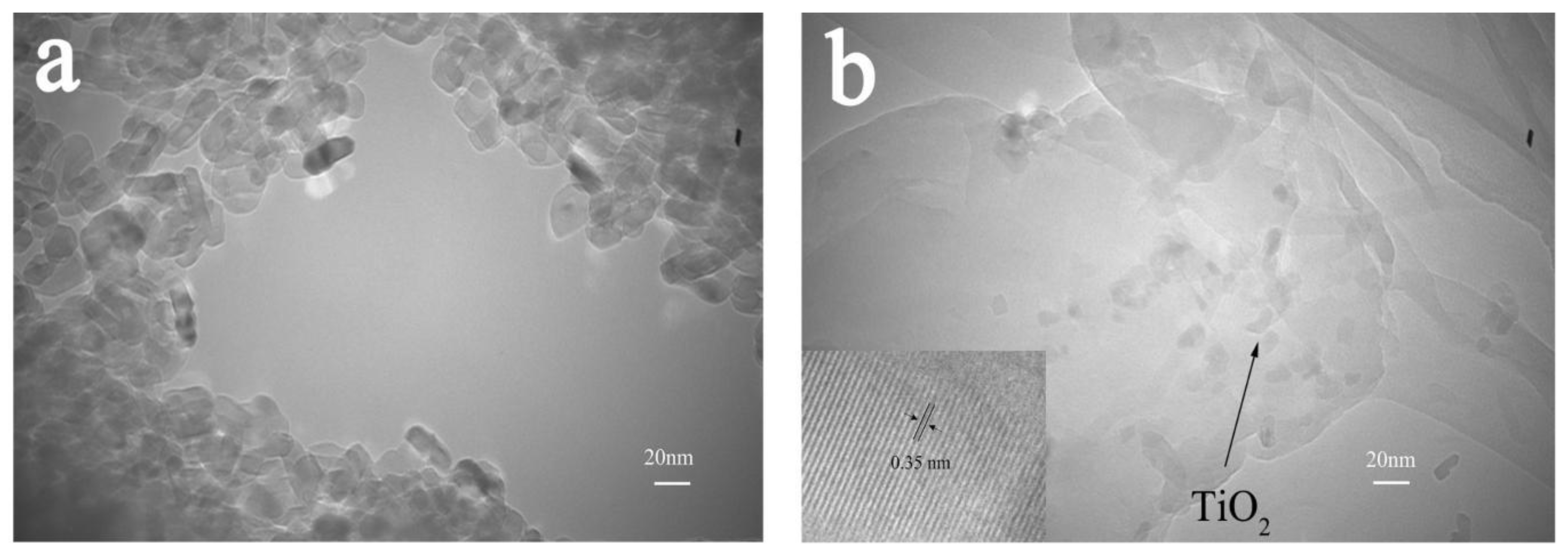
3.4. Analysis of the Microstructure of Materials
3.5. Swelling Performance Analysis of Composite Hydrogel
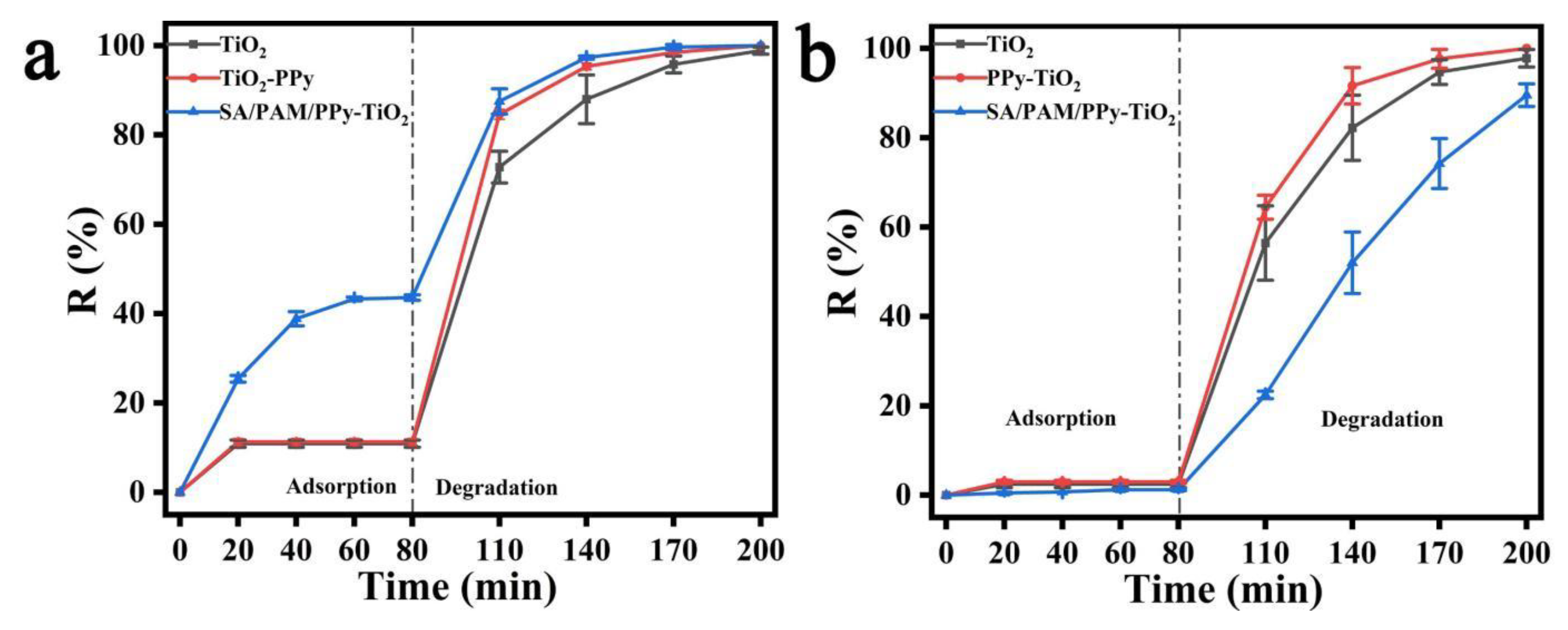
3.6. Analysis of Photocatalytic Properties of Composite Hydrogels towards Methyl Orange and Methylene Blue
3.7. Analysis of Photocatalytic Performance of Composite Hydrogels for Different Dyes
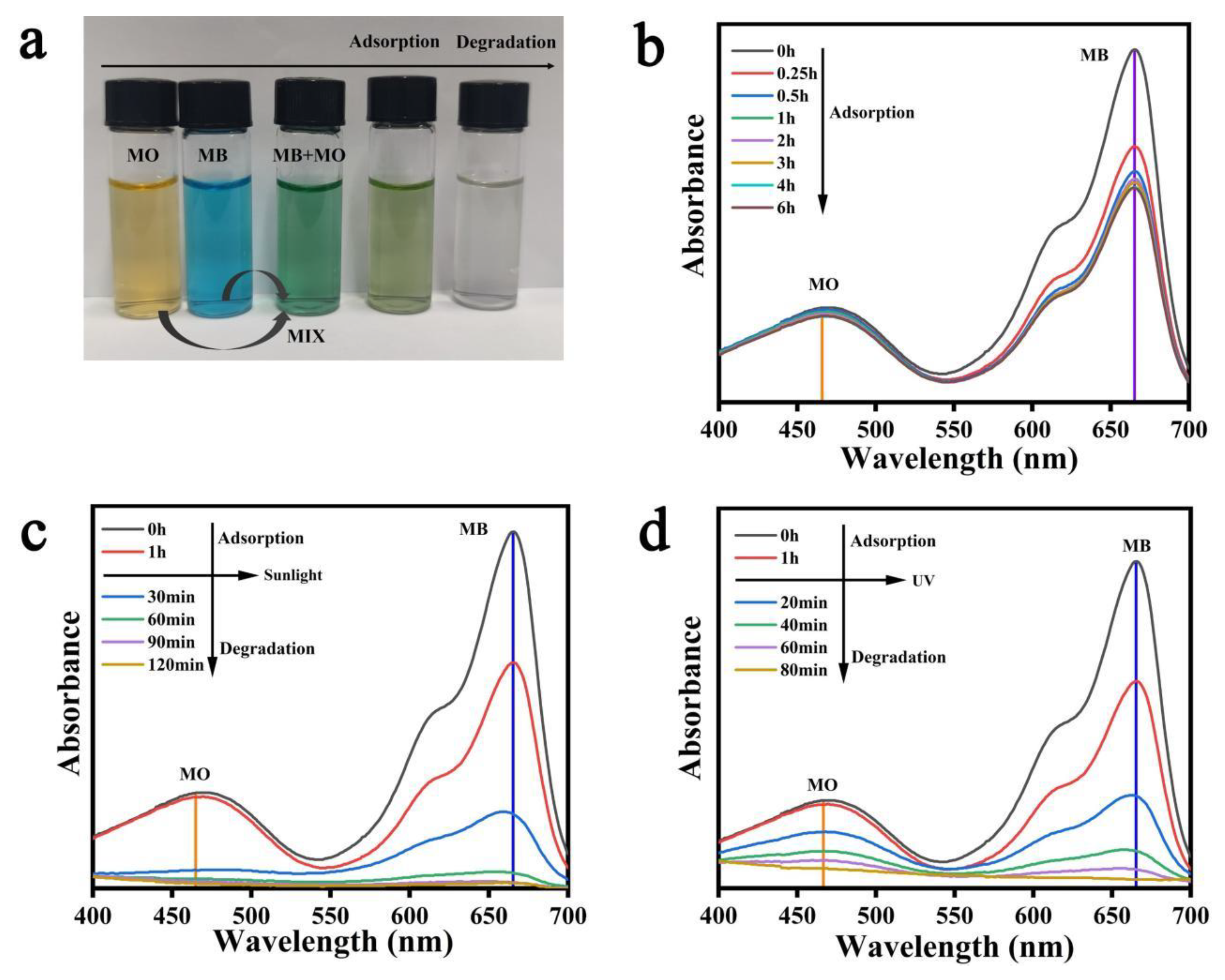
3.8. Analysis of Photocatalytic Performance of Composite Hydrogels for Mixed Dyes
3.9. Mechanical Properties and Macroscopic Morphology Analysis of Composite Hydrogels
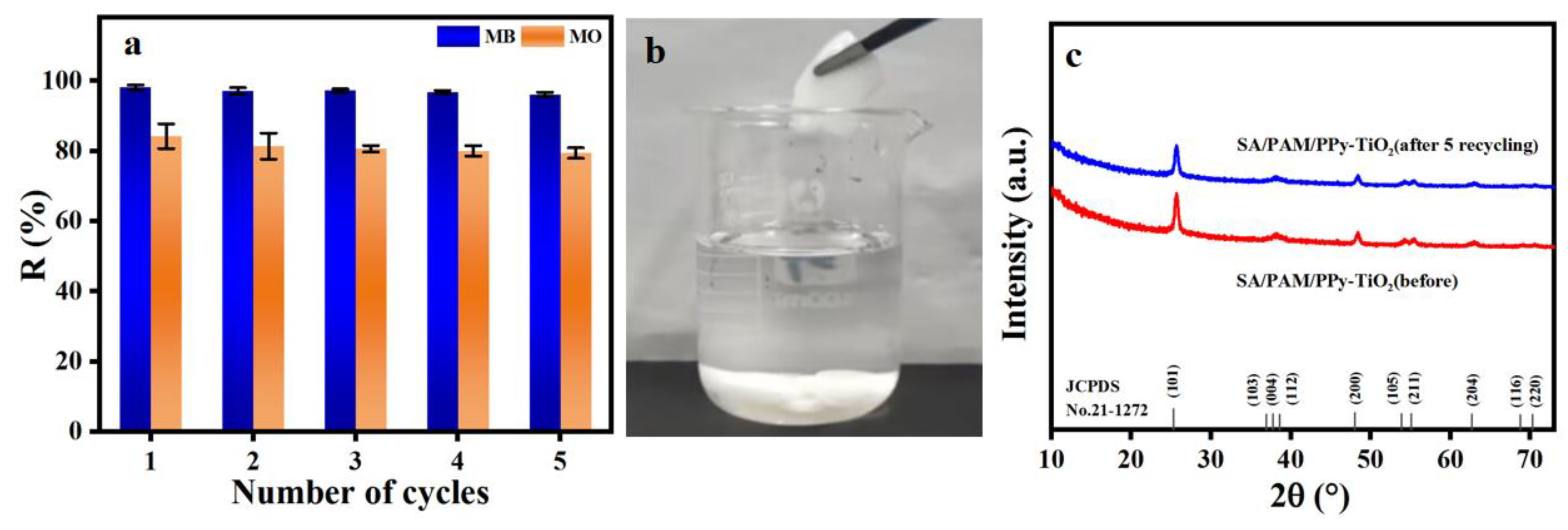
3.10. Recycle Performance Analysis of Composite Hydrogels
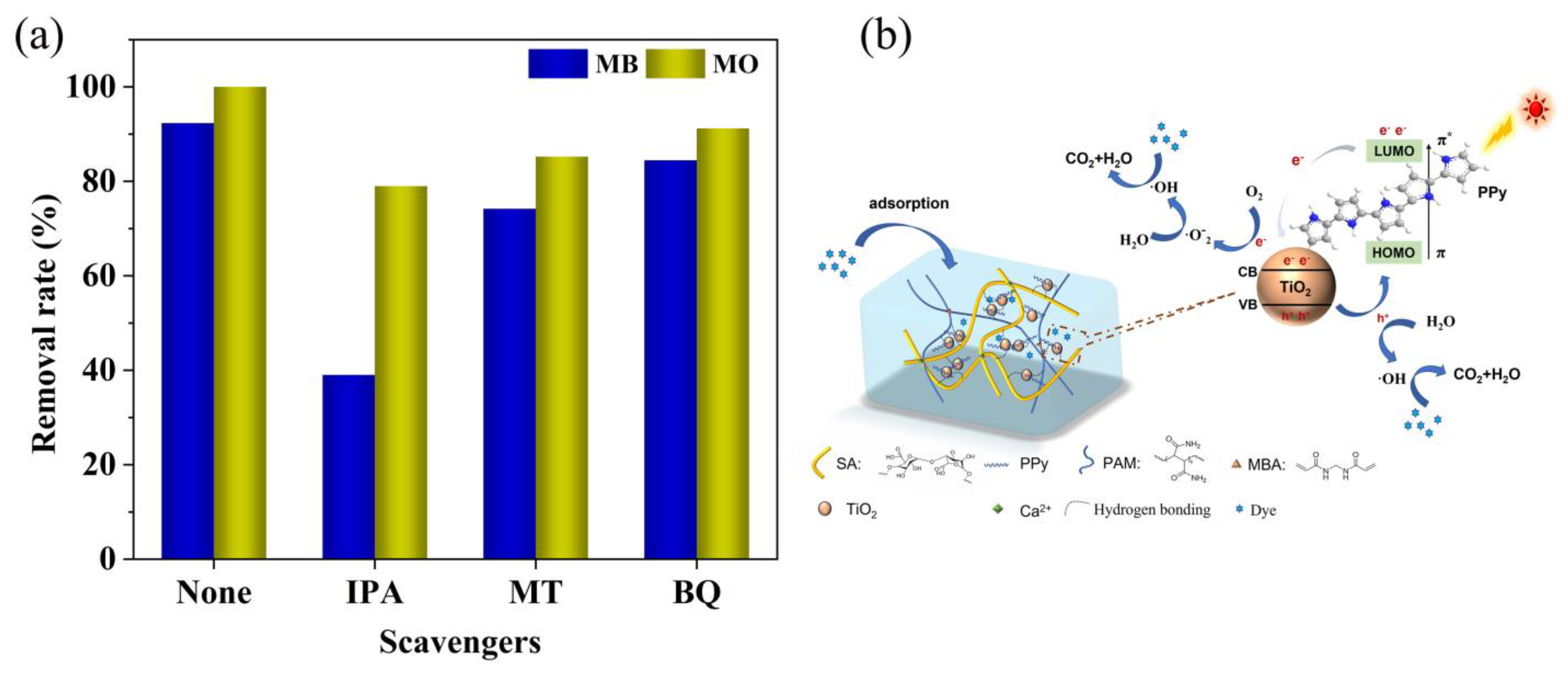
3.11. Analysis of Photocatalytic Mechanism of Composite Hydrogels
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive removal of dyes from wastewater using a metal-organic framework: A review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef] [PubMed]
- El-Gaayda, J.; Titchou, F.E.; Oukhrib, R.; Yap, P.-S.; Liu, T.; Hamdani, M.; Ait Akbour, R. Natural flocculants for the treatment of wastewaters containing dyes or heavy metals: A state-of-the-art review. J. Environ. Chen. Eng. 2021, 9, 106060. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Hassan Shah, M.U.; Bhaskar Reddy, A.V.; Aminabhavi, T.M. Recent advances in the removal of dyes from wastewater using low-cost adsorbents. J. Environ. Manag. 2022, 321, 115981. [Google Scholar] [CrossRef] [PubMed]
- Solayman, H.M.; Hossen, M.A.; Aziz, A.A.; Yahya, N.Y.; Leong, K.H.; Sime, L.C.; Monir, M.U.; Zoh, K.-D. Performance evaluation of dye wastewater treatment technologies: A review. J. Environ. Chem. Eng. 2023, 11, 109610. [Google Scholar] [CrossRef]
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.-S.; Jeon, B.-H.; Park, Y.-K. A review on recent advances in the treatment of dye-polluted wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadizaniani, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Dang, Q.; Wang, L.; Liu, J.; Wang, D.; Chai, J.; Wu, M.; Tang, L. Recent progress of photoelectrocatalysis systems for wastewater treatment. J. Water Process Eng. 2023, 53, 103609. [Google Scholar] [CrossRef]
- Chowdhury, M.F.; Khandaker, S.; Sarker, F.; Islam, A.; Rahman, M.T.; Awual, M.R. Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: A review. J. Mol. Liq. 2020, 318, 114061. [Google Scholar] [CrossRef]
- Dlamini, M.C.; Maubane-Nkadimeng, M.S.; Moma, J.A. The use of TiO2/clay heterostructures in the photocatalytic remediation of water containing organic pollutants: A review. J. Environ. Chem. Eng. 2021, 9, 106546. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Wang, Y.; Selvakumar, K.; Oh, T.H.; Arunpandian, M.; Swaminathan, M. Boosting photocatalytic activity of single metal atom oxide anchored on TiO2 nanoparticles: An efficient catalyst for photodegradation of pharmaceutical pollutants. J. Alloys Compd. 2023, 950, 169821. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G. Trends in non-metal doping of anatase TiO2: B, C, N and F. Catal. Today 2013, 206, 12–18. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, B.; Zhao, Y.; Hou, M.; Xin, C.; Li, Q.; Yu, X. High-performance visible-light photocatalysis induced by dye-sensitized Ti3+-TiO2 microspheres. J. Phys. Chem. Solids 2023, 179, 111374. [Google Scholar] [CrossRef]
- Zhang, Q.; Rao, G.; Rogers, J.; Zhao, C.; Liu, L.; Li, Y. Novel anti-fouling Fe2O3/TiO2 nanowire membranes for humic acid removal from water. Chem. Eng. J. 2015, 271, 180–187. [Google Scholar] [CrossRef]
- Ahmad, N.; Anae, J.; Khan, M.Z.; Sabir, S.; Yang, X.J.; Thakur, V.K.; Campo, P.; Coulon, F. Visible light-conducting polymer nanocomposites as efficient photocatalysts for the treatment of organic pollutants in wastewater. J. Environ. Manag. 2021, 295, 113362. [Google Scholar] [CrossRef]
- Kumar, R.; Travas-Sejdic, J.; Padhye, L.P. Conducting polymers-based photocatalysis for treatment of organic contaminants in water. Chem. Eng. J. Adv. 2020, 4, 100047. [Google Scholar] [CrossRef]
- Kasisomayajula, S.; Jadhav, N.; Gelling, V.J. Conductive polypyrrole and acrylate nanocomposite coatings: Mechanistic study on simultaneous photopolymerization. Prog. Org. Coat. 2016, 101, 440–454. [Google Scholar] [CrossRef]
- Punnakkal, V.S.; Anila, E.I. Polypyrrole/ silver/graphene ternary nanocomposite synthesis and study on photocatalytic property in degrading Congo red dye under visible light. Surf. Interfaces 2023, 42, 103342. [Google Scholar] [CrossRef]
- Faisal, M.; Ahmed, J.; Algethami, J.S.; Jalalah, M.; Alsareii, S.A.; Alsaiari, M.; Harraz, F.A. Visible-light responsive Au nanoparticle-decorated polypyrrole-carbon black/SnO2 ternary nanocomposite for ultrafast removal of insecticide imidacloprid and methylene blue. J. Ind. Eng. Chem. 2023, 121, 287–298. [Google Scholar] [CrossRef]
- Yuan, X.; Kobylanski, M.P.; Cui, Z.; Li, J.; Beaunier, P.; Dragoe, D.; Colbeau-Justin, C.; Zaleska-Medynska, A.; Remita, H. Highly active composite TiO2-polypyrrole nanostructures for water and air depollution under visible light irradiation. J. Environ. Chem. Eng. 2020, 8, 104178. [Google Scholar] [CrossRef]
- Amer, W.A.; Al-Saida, B.; Ayad, M.M. Rational design of a polypyrrole-based competent bifunctional magnetic nanocatalyst. RSC Adv. 2019, 9, 18245–18255. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, J.; He, G.; Jiang, G.; Tan, Y.; Xue, B. Macroporous polypyrrole-TiO2 composites with improved photoactivity and electrochemical sensitivity. J. Colloid Interface Sci. 2013, 411, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Singh Jassal, P.; Kaur, D.; Prasad, R.; Singh, J. Green synthesis of titanium dioxide nanoparticles: Development and applications. J. Agric. Food Chem. 2022, 10, 100361. [Google Scholar] [CrossRef]
- Bai, W.; Tian, X.; Yao, R.; Chen, Y.; Lin, H.; Zheng, J.; Xu, Y.; Lin, J. Preparation of nano-TiO2@polyfluorene composite particles for the photocatalytic degradation of organic pollutants under sunlight. Sol. Energy 2020, 196, 616–624. [Google Scholar] [CrossRef]
- Aslam, M.; Abdullah, A.Z.; Rafatullah, M. Recent development in the green synthesis of titanium dioxide nanoparticles using plant-based biomolecules for environmental and antimicrobial applications. J. Ind. Eng. Chem. 2021, 98, 1–16. [Google Scholar] [CrossRef]
- Ali, H.M.; Arabpour Roghabadi, F.; Ahmadi, V. Solid-supported photocatalysts for wastewater treatment: Supports contribution in the photocatalysis process. Sol. Energy 2023, 255, 99–125. [Google Scholar] [CrossRef]
- Yang, D. Recent Advances in Hydrogels. Chem. Mater. 2022, 34, 1987–1989. [Google Scholar] [CrossRef]
- Mehta, P.; Sharma, M.; Devi, M. Hydrogels: An overview of its classifications, properties, and applications. J. Mech. Behav. Biomed. 2023, 147, 106145. [Google Scholar] [CrossRef]
- Zhao, S.; Hou, C.; Shao, L.; An, W.; Cui, W. Adsorption and in-situ photocatalytic synergy degradation of 2,4-dichlorophenol by three-dimensional graphene hydrogel modified with highly dispersed TiO2 nanoparticles. Appl. Surf. Sci. 2022, 590, 153088. [Google Scholar] [CrossRef]
- Kangwansupamonkon, W.; Klaikaew, N.; Kiatkamjornwong, S. Green synthesis of titanium dioxide/acrylamide-based hydrogel composite, self degradation and environmental applications. Eur. Polym. J. 2018, 107, 118–131. [Google Scholar] [CrossRef]
- Heidarpour, H.; Golizadeh, M.; Padervand, M.; Karimi, A.; Vossoughi, M.; Tavakoli, M.H. In-situ formation and entrapment of Ag/AgCl photocatalyst inside cross-linked carboxymethyl cellulose beads: A novel photoactive hydrogel for visible-light-induced photocatalysis. J. Photochem. Photobiol. A Chem. 2020, 398, 112559. [Google Scholar] [CrossRef]
- Liao, W.; Zhao, M.; Rong, H.; Jiang, P.; Liao, Q.; Zhang, C.; Chen, Y. Photocatalyst immobilized by hydrogel, efficient degradation and self regeneration: A review. Mat. Sci. Semicon. Proc. 2022, 150, 106929. [Google Scholar] [CrossRef]
- Wu, T.; Sawut, A.; Simayi, R.; Gong, X.; Zhang, X.; Jiang, M.; Zhu, Z. Green synthesis and environmental applications of alginate/polyacrylamide/titanium dioxide composite hydrogel. J. Appl. Polym. Sci. 2023, 140, e54394. [Google Scholar] [CrossRef]
- Sawut, A.; Wu, T.; Simayi, R.; Wu, T.; Gong, X.; Wang, Z. Reduced graphene oxide-TiO2/sodium alginate/polyacrylamide composite hydrogel for enhanced adsorption-photocatalytic degradation of methylene blue. Colloid Surf. A 2023, 678, 132531. [Google Scholar] [CrossRef]
- Kratofil Krehula, L.; Stjepanović, J.; Perlog, M.; Krehula, S.; Gilja, V.; Travas-Sejdic, J.; Hrnjak-Murgić, Z. Conducting polymer polypyrrole and titanium dioxide nanocomposites for photocatalysis of RR45 dye under visible light. Polym. Bull. 2019, 76, 1697–1715. [Google Scholar] [CrossRef]
- Saarai, A.; Kasparkova, V.; Sedlacek, T.; Saha, P. On the development and characterisation of crosslinked sodium alginate/gelatine hydrogels. J. Mech. Behav. Biomed. 2013, 18, 152–166. [Google Scholar] [CrossRef]
- Landi, S. Comment on “Photocatalytic degradation of RhB from an aqueous solution using Ag3PO4/N-TiO2 heterostructure” and “Evaluation of the effect of dose change of Fe3O4 nanoparticles on electrochemical biosensor compatibility using hydrogels as an experimental living organism model”. J. Mol. Liq. 2021, 338, 116635. [Google Scholar] [CrossRef]
- Adjimi, S.; Roux, J.C.; Sergent, N.; Delpech, F.; Thivel, P.X.; Pera-Titus, M. Photocatalytic oxidation of ethanol using paper-based nano-TiO2 immobilized on porous silica: A modelling study. Chem. Eng. J. 2014, 251, 381–391. [Google Scholar] [CrossRef]
- Cheng, G.; Akhtar, M.S.; Yang, O.B.; Stadler, F.J. Novel preparation of anatase TiO2@reduced graphene oxide hybrids for high-performance dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2013, 5, 6635–6642. [Google Scholar] [CrossRef]
- Nawaz, M.; Khan, A.A.; Hussain, A.; Jang, J.; Jung, H.Y.; Lee, D.S. Reduced graphene oxide-TiO2/sodium alginate 3-dimensional structure aerogel for enhanced photocatalytic degradation of ibuprofen and sulfamethoxazole. Chemosphere 2020, 261, 127702. [Google Scholar] [CrossRef] [PubMed]
- Sangareswari, M.; Sundaram, M.M. Development of efficiency improved polymer-modified TiO2 for the photocatalytic degradation of an organic dye from wastewater environment. Appl. Water Sci. 2015, 7, 1781–1790. [Google Scholar] [CrossRef]
- Sun, L.; Shi, Y.; Li, B.; Li, X.; Wang, Y. Preparation and characterization of polypyrrole/TiO2 nanocomposites by reverse microemulsion polymerization and its photocatalytic activity for the degradation of methyl orange under natural light. Polym. Compos. 2013, 34, 1076–1080. [Google Scholar] [CrossRef]
- Deng, F.; Li, Y.; Luo, X.; Yang, L.; Tu, X. Preparation of conductive polypyrrole/TiO2 nanocomposite via surface molecular imprinting technique and its photocatalytic activity under simulated solar light irradiation. Colloids Surf. A 2011, 395, 183–189. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.J. Three Isomeric Zn(II)-Sn(IV)-Zn(II) Porphyrin-Triad-Based Supramolecular Nanoarchitectures for the Morphology-Dependent Photocatalytic Degradation of Methyl Orange. ACS Omega 2022, 7, 9775–9784. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Jo, H.J.; Kim, H.J. Coordination framework materials fabricated by the self-assembly of Sn(iv) porphyrins with Ag(i) ions for the photocatalytic degradation of organic dyes in wastewater. Inorg. Chem. Front. 2022, 9, 1270–1280. [Google Scholar] [CrossRef]




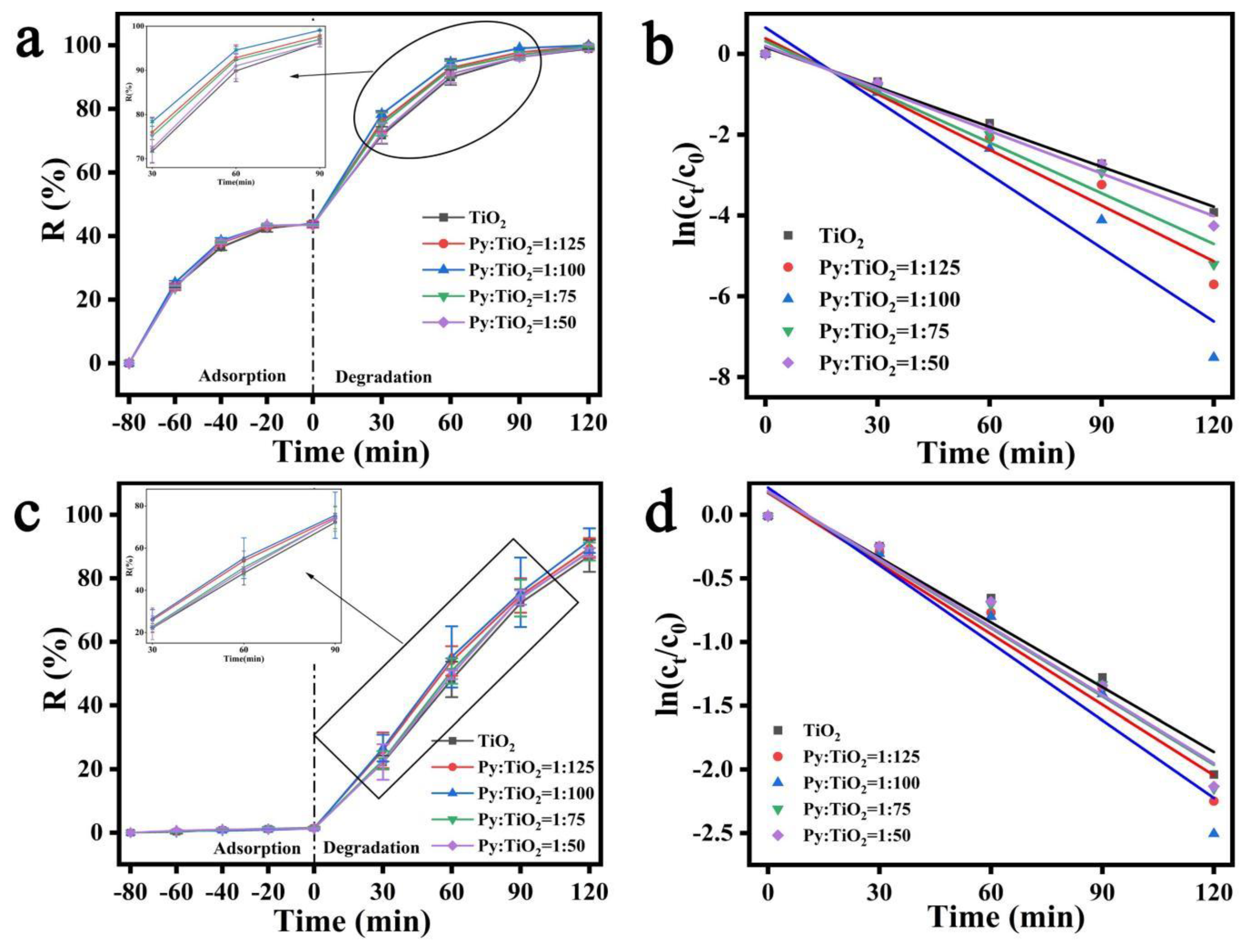




| Factor | K | R2 | |
|---|---|---|---|
| Py:TiO2 | 0 | 0.03292 | 0.99165 |
| 1:125 | 0.04598 | 0.9569 | |
| 1:100 | 0.06062 | 0.93871 | |
| 1:75 | 0.04181 | 0.95925 | |
| 1:50 | 0.03509 | 0.98427 | |
| pH | 3 | 0.01203 | 0.98872 |
| 5 | 0.02036 | 0.99164 | |
| 7 | 0.05759 | 0.99693 | |
| 9 | 0.03767 | 0.99685 | |
| 11 | 0.03331 | 0.99392 | |
| Factor | K | R2 | |
|---|---|---|---|
| Py:TiO2 | 0 | 0.01696 | 0.95721 |
| 1:125 | 0.0185 | 0.96055 | |
| 1:100 | 0.02032 | 0.94372 | |
| 1:75 | 0.01787 | 0.95833 | |
| 1:50 | 0.01782 | 0.95735 | |
| pH | 3 | 0.02916 | 0.9256 |
| 5 | 0.01837 | 0.95797 | |
| 7 | 0.01672 | 0.96463 | |
| 9 | 0.00577 | 0.99896 | |
| 11 | 0.00529 | 0.99841 | |
| No. | Materials | Irradiation Time—Illumination Source | MO/MB Degradation Efficiency | References |
|---|---|---|---|---|
| 1 | PPy-TiO2 | 90 min—Sunlight | 93% MB | [42] |
| 2 | PPy-TiO2 | 180 min—Sunlight | 80% MO | [43] |
| 3 | PPy-TiO2 | 120 min—Sunlight | 83% MO | [44] |
| 4 | SA/PAM/PPy-TiO2 | 120 min—Sunlight | 100% MB/91.85% MO | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawut, A.; Wu, T.; Simayi, R.; Jiao, X.; Feng, Y. Preparation and Photocatalytic Performance of Sodium Alginate/Polyacrylamide/Polypyrrole-TiO2 Nanocomposite Hydrogels. Polymers 2023, 15, 4174. https://doi.org/10.3390/polym15204174
Sawut A, Wu T, Simayi R, Jiao X, Feng Y. Preparation and Photocatalytic Performance of Sodium Alginate/Polyacrylamide/Polypyrrole-TiO2 Nanocomposite Hydrogels. Polymers. 2023; 15(20):4174. https://doi.org/10.3390/polym15204174
Chicago/Turabian StyleSawut, Amatjan, Tongmeng Wu, Rena Simayi, Xueying Jiao, and Yurou Feng. 2023. "Preparation and Photocatalytic Performance of Sodium Alginate/Polyacrylamide/Polypyrrole-TiO2 Nanocomposite Hydrogels" Polymers 15, no. 20: 4174. https://doi.org/10.3390/polym15204174
APA StyleSawut, A., Wu, T., Simayi, R., Jiao, X., & Feng, Y. (2023). Preparation and Photocatalytic Performance of Sodium Alginate/Polyacrylamide/Polypyrrole-TiO2 Nanocomposite Hydrogels. Polymers, 15(20), 4174. https://doi.org/10.3390/polym15204174







