Sustainable Polyhydroxyalkanoate Production from Food Waste via Bacillus mycoides ICRI89: Enhanced 3D Printing with Poly (Methyl Methacrylate) Blend
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Food Waste Collection
2.3. Pretreatment of Food Waste
2.3.1. Ultrasonic Pretreatment of FW
2.3.2. Ethanolic Organosolv Pretreatment of FW
2.4. FW Enzymatic Hydrolysis
2.5. PHB Production Using Hydrolysed FW with B. mycoides ICRI89
2.6. Preparation of PHB/PMMA Blends
2.7. Mechanical Properties of PHB/PMMA Blends
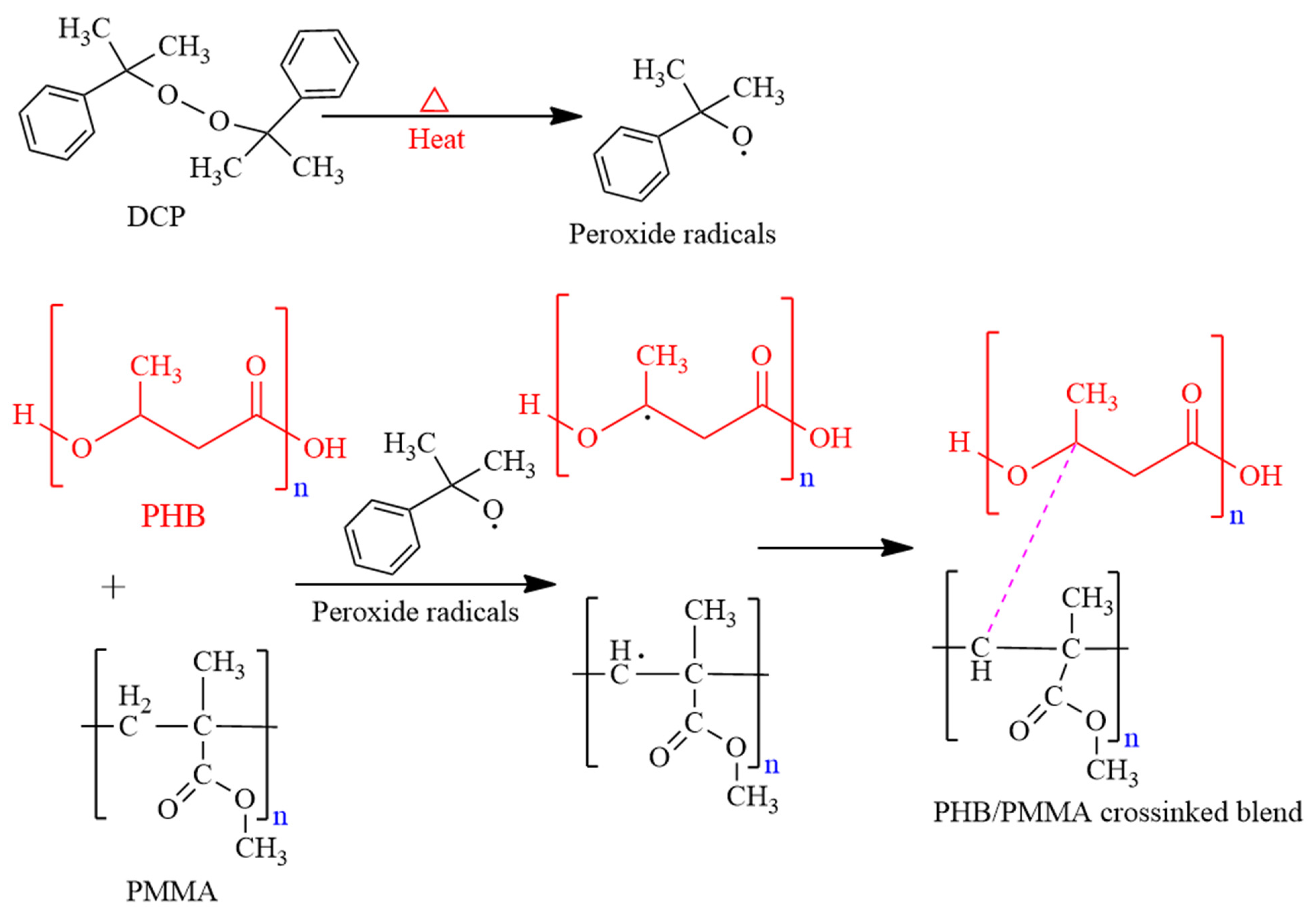
2.8. Dicumyl Peroxide (DCP) Crosslinking
2.9. Chemical Structure of the Generated PHB/PMMA Blend
FTIR
2.10. Thermal Characteristics of the Developed Crosslinked Polymeric Matrix
2.10.1. Thermogravimetric Analysis, TGA
2.10.2. Differential Scanning Calorimetry (DSC)
2.11. Degradation Studies of the DCP-PHB/PMMA Blend
2.11.1. Soil Degradation
2.11.2. Enzymatic Degradation
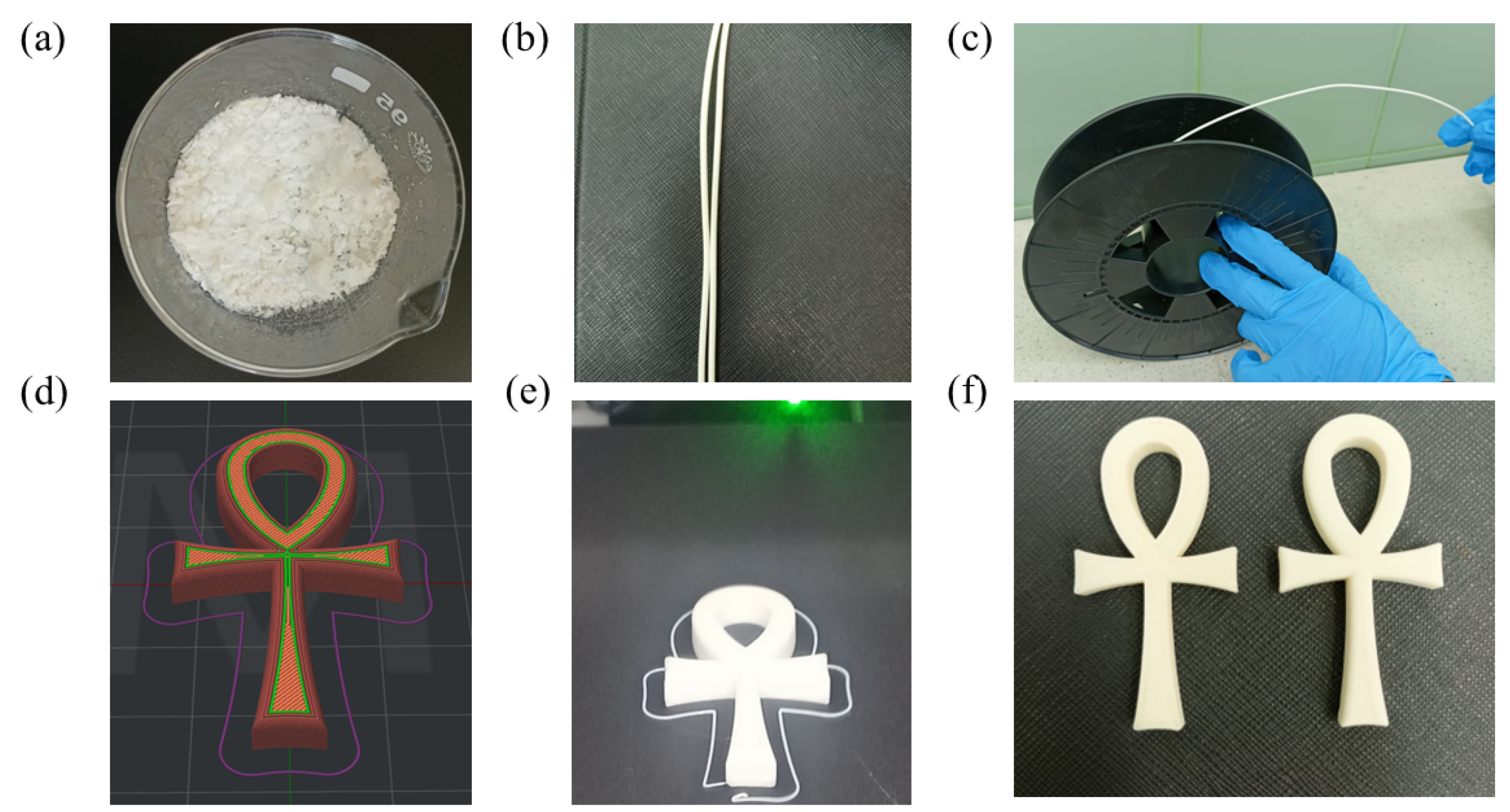
2.12. Filament Fabrication and SEM Analysis
2.13. Three-Dimensional Printing Models
2.14. Statistical Analysis
3. Results and Discussion
3.1. Glucose Liberation in Response to FW Hydrolysis
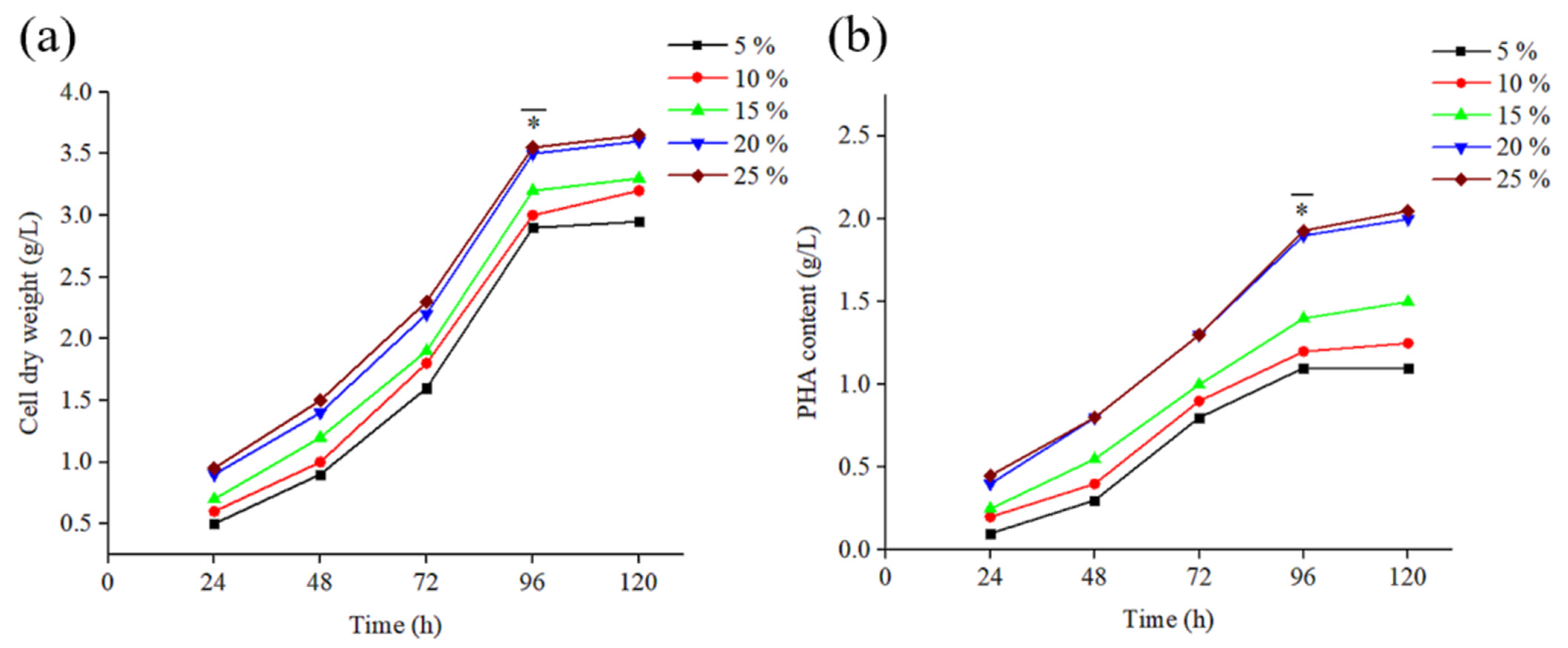
3.2. PHB Generation from Hydrolysed FW via B. mycoides ICRI89
3.3. Effect of Different Polymeric Ratios on PHB/PMMA Blends’ Mechanical Properties
3.4. DCP Crosslinked Blends
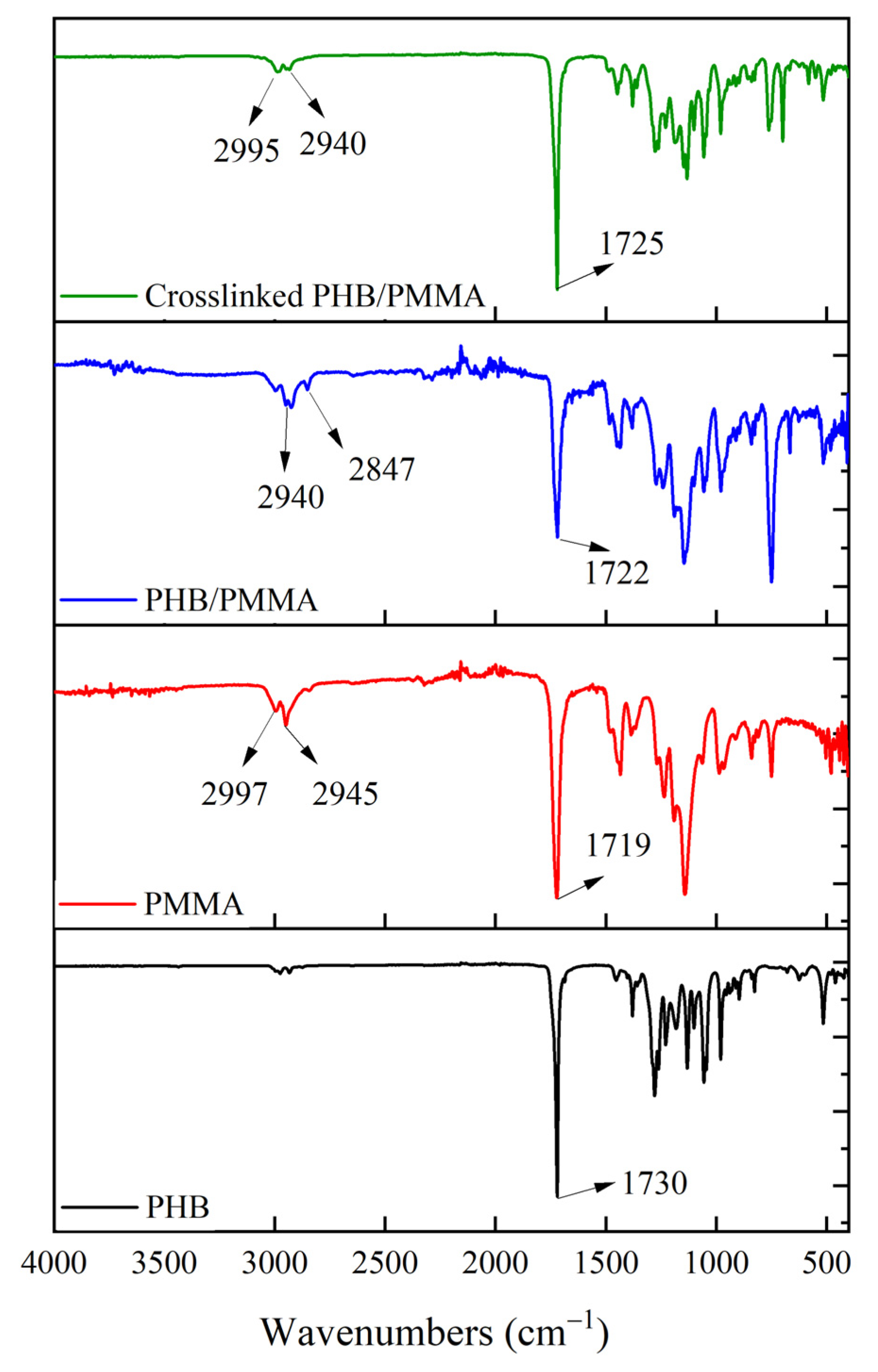
3.5. Chemical Characterisation of PHB/PMMA Film
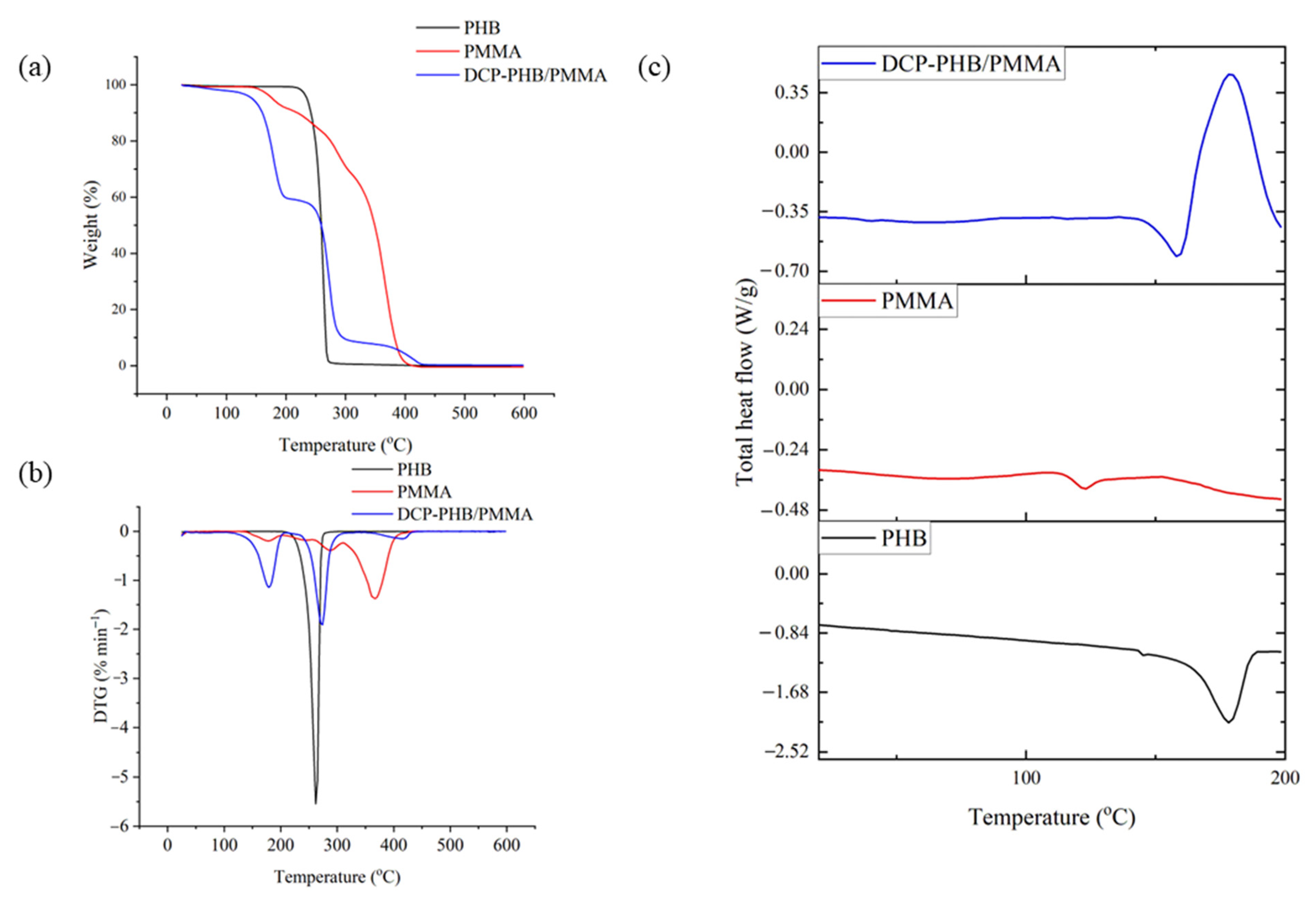
3.6. Thermal Analysis of the DCP-PHB/PMMA Blend as Compared to Native Polymers
3.7. Biodegradation Profiles of the Developed DCP-PHB/PMMA Blend
3.7.1. Soil Degradation
3.7.2. Enzymatic Degradation
3.8. Fabrication of the Polymeric Filament and 3D Printing Studies
4. Conclusions
5. Challenges and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, H.; Shin, G.; Kwak, H.; Hao, L.T.; Jegal, J.; Kim, H.J.; Jeon, H.; Park, J.; Oh, D.X. Review of polymer technologies for improving the recycling and upcycling efficiency of plastic waste. Chemosphere 2023, 320, 138089. [Google Scholar] [CrossRef]
- Bhatt, P.; Pathak, V.M.; Bagheri, A.R.; Bilal, M. Microplastic contaminants in the aqueous environment, fate, toxicity consequences, and remediation strategies. Environ. Res. 2021, 200, 111762. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- El-malek, F.A.; Steinbüchel, A. Post-Synthetic Enzymatic and Chemical Modifications for Novel Sustainable Polyesters. Front. Bioeng. Biotechnol. 2022, 9, 817023. [Google Scholar] [CrossRef] [PubMed]
- Rofeal, M.; El-Malek, F.A.; Qi, X. In vitro assessment of green polyhydroxybutyrate/chitosan blend loaded with kaempferol nanocrystals as a potential dressing for infected wounds. Nanotechnology 2021, 32, 375102. [Google Scholar] [CrossRef]
- Patil, P.B.; Sarkar, D.; Poddar, K.; Gu, J.-D.; Sarkar, A. Degradation profiling of in-vitro-produced polyhydroxyalkanoate synthesized by the soil bacterium Bacillus sp. PhNs9 under different microenvironments. Int. Biodeterior. Biodegrad. 2023, 181, 105615. [Google Scholar] [CrossRef]
- Trakunjae, C.; Boondaeng, A.; Apiwatanapiwat, W.; Kosugi, A.; Arai, T.; Sudesh, K.; Vaithanomsat, P. Enhanced polyhydroxybutyrate (PHB) production by newly isolated rare actinomycetes Rhodococcus sp. strain BSRT1-1 using response surface methodology. Sci. Rep. 2021, 11, 1896. [Google Scholar] [CrossRef]
- Kiselev, E.G.; Demidenko, A.V.; Zhila, N.O.; Shishatskaya, E.I.; Volova, T.G. Sugar Beet Molasses as a Potential C-Substrate for PHA Production by Cupriavidus necator. Bioengineering 2022, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Kanavaki, I.; Drakonaki, A.; Geladas, E.D.; Spyros, A.; Xie, H.; Tsiotis, G. Polyhydroxyalkanoate (PHA) Production in Pseudomonas sp. phDV1 Strain Grown on Phenol as Carbon Sources. Microorganisms 2021, 9, 1636. [Google Scholar] [CrossRef]
- Abd El-Malek, F.; Rofeal, M.; Zabed, H.M.; Nizami, A.-S.; Rehan, M.; Qi, X. Microorganism-mediated algal biomass processing for clean products manufacturing: Current status, challenges and future outlook. Fuel 2022, 311, 122612. [Google Scholar] [CrossRef]
- Abdelmalek, F.; Steinbüchel, A.; Rofeal, M. The Hyperproduction of Polyhydroxybutyrate Using Bacillus mycoides ICRI89 through Enzymatic Hydrolysis of Affordable Cardboard. Polymers 2022, 14, 2810. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Thakor, A.; Mekonnen, T.H.; Charles, T.C.; Lee, H.-S. Production of polyhydroxyalkanoate (PHA) copolymer from food waste using mixed culture for carboxylate production and Pseudomonas putida for PHA synthesis. J. Environ. Manag. 2023, 336, 117650. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.; Hathi, Z.; Haque, M.A.; Kumar, S.; Kumar, A.; Singh, E.; Lin, C.S.K. Effect of levulinic acid on production of polyhydroxyalkanoates from food waste by Haloferax mediterranei. Environ. Res. 2022, 214, 114001. [Google Scholar] [CrossRef]
- Wu, M.; Gong, X.; Liu, X.; Tu, W.; Yu, P.; Zou, Y.; Wang, H. Comprehensive Techno-environmental Evaluation of a Pilot-Scale PHA Production from Food Waste in China. Environ. Sci. Technol. 2023, 57, 1467–1478. [Google Scholar] [CrossRef]
- Wellenreuther, C.; Wolf, A.; Zander, N. Cost competitiveness of sustainable bioplastic feedstocks—A Monte Carlo analysis for polylactic acid. Clean. Eng. Technol. 2022, 6, 100411. [Google Scholar] [CrossRef]
- Kuna, E.; Behling, R.; Valange, S.; Chatel, G.; Colmenares, J.C. Sonocatalysis: A Potential Sustainable Pathway for the Valorization of Lignocellulosic Biomass and Derivatives. Top. Curr. Chem. 2017, 375, 41. [Google Scholar] [CrossRef]
- Pau, S.; Tan, L.C.; Arriaga Garcia, S.L.; Lens, P.N. Effect of thermal and ultrasonic pretreatment on lactic acid fermentation of food waste. Waste Manag. Res. 2023, 41, 566–574. [Google Scholar] [CrossRef]
- Taheri, M.E.; Salimi, E.; Saragas, K.; Novakovic, J.; Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Effect of pretreatment techniques on enzymatic hydrolysis of food waste. Biomass Convers. Biorefin. 2021, 11, 219–226. [Google Scholar] [CrossRef]
- Premphet, P.; Leksakul, K.; Boonyawan, D.; Vichiansan, N. Process parameters optimization and mechanical properties of 3D PLA/HA printing scaffold. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Vigil Fuentes, M.A.; Thakur, S.; Wu, F.; Misra, M.; Gregori, S.; Mohanty, A.K. Study on the 3D printability of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(lactic acid) blends with chain extender using fused filament fabrication. Sci. Rep. 2020, 10, 11804. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalil, S.; Abid, S. Recent progress in development and chemical modification of poly(hydroxybutyrate)-based blends for potential medical applications. Int. J. Biol. Macromol. 2020, 160, 77–100. [Google Scholar] [CrossRef]
- Wang, Y.; Duo, T.; Xu, X.; Xiao, Z.; Xu, A.; Liu, R.; Jiang, C.; Lu, J. Eco-Friendly High-Performance Poly(methyl methacrylate) Film Reinforced with Methylcellulose. ACS Omega 2020, 5, 24256–24261. [Google Scholar] [CrossRef]
- Özsin, G. Assessing thermal behaviours of cellulose and poly(methyl methacrylate) during co-pyrolysis based on an unified thermoanalytical study. Bioresour. Technol. 2020, 300, 122700. [Google Scholar] [CrossRef]
- Anakabe, J.; Orue, A.; Zaldua Huici, A.M.; Eceiza, A.; Arbelaiz, A. Properties of PLA/PMMA blends with high polylactide content prepared by reactive mixing in presence of poly(styrene-co-glycidyl methacrylate) copolymer. J. Appl. Polym. Sci. 2018, 135, 46825. [Google Scholar] [CrossRef]
- Formela, K.; Zedler, Ł.; Hejna, A.; Tercjak, A. Reactive extrusion of bio-based polymer blends and composites-Current trends and future developments. Express Polym. Lett. 2018, 12, 24–57. [Google Scholar] [CrossRef]
- Ma, P.; Cai, X.; Zhang, Y.; Wang, S.; Dong, W.; Chen, M.; Lemstra, P.J. In-situ compatibilization of poly(lactic acid) and poly(butylene adipate-co-terephthalate) blends by using dicumyl peroxide as a free-radical initiator. Polym. Degrad. Stab. 2014, 102, 145–151. [Google Scholar] [CrossRef]
- McCleary, B.V.; Solah, V.; Gibson, T.S. Quantitative Measurement of Total Starch in Cereal Flours and Products. J. Cereal Sci. 1994, 20, 51–58. [Google Scholar] [CrossRef]
- Van Soest, P.; Robertson, J. Systems of analysis for evaluating fibrous feeds. In Standardization of Analytical Methodology for Feeds: Proceedings; IDRC: Ottawa, ON, Canada, 1979. [Google Scholar]
- Li, X.; Mettu, S.; Martin, G.J.O.; Ashokkumar, M.; Lin, C.S.K. Ultrasonic pretreatment of food waste to accelerate enzymatic hydrolysis for glucose production. Ultrason. Sonochem. 2019, 53, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, F.; Karimi, K.; Kumar, R. Sustainable biofuels and bioplastic production from the organic fraction of municipal solid waste. Waste Manag. 2020, 116, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Heidary Vinche, M.; Asachi, R.; Zamani, A.; Karimi, K. Ethanol and chitosan production from wheat hydrolysate by Mucor hiemalis. J. Chem. Technol. Biotechnol. 2013, 88, 255–260. [Google Scholar] [CrossRef]
- Sirohi, R. Sustainable utilization of food waste: Production and characterization of polyhydroxybutyrate (PHB) from damaged wheat grains. Environ. Technol. Innov. 2021, 23, 101715. [Google Scholar] [CrossRef]
- Rofeal, M.; Abdelmalek, F.; Pietrasik, J.; Steinbüchel, A. Sustainable curdlan biosynthesis by Rahnella variigena ICRI91 via alkaline hydrolysis of Musa sapientum peels and its edible, active and modified hydrogel for Quercetin controlled release. Int. J. Biol. Macromol. 2023, 225, 416–429. [Google Scholar] [CrossRef] [PubMed]
- El-malek, F.A.; Farag, A.; Omar, S.; Khairy, H. Polyhydroxyalkanoates (PHA) from Halomonas pacifica ASL10 and Halomonas salifodiane ASL11 isolated from Mariout salt lakes. Int. J. Biol. Macromol. 2020, 161, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalek, F.; Rofeal, M.; Pietrasik, J.; Steinbüchel, A. Novel Biodegradable Nanoparticulate Chain-End Functionalized Polyhydroxybutyrate–Caffeic Acid with Multifunctionalities for Active Food Coatings. ACS Sustain. Chem. Eng. 2023, 11, 7123–7135. [Google Scholar] [CrossRef]
- Savenkova, L.; Gercberga, Z.; Nikolaeva, V.; Dzene, A.; Bibers, I.; Kalnin, M. Mechanical properties and biodegradation characteristics of PHB-based films. Process Biochem. 2000, 35, 573–579. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Mo, W.; Yao, H.; Wu, Q.; Chen, J.; Chen, G.-Q. Biodegradation studies of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Polym. Degrad. Stab. 2004, 85, 815–821. [Google Scholar] [CrossRef]
- Kohan, M.; Lancoš, S.; Schnitzer, M.; Živčák, J.; Hudák, R. Analysis of PLA/PHB Biopolymer Material with Admixture of Hydroxyapatite and Tricalcium Phosphate for Clinical Use. Polymers 2022, 14, 5357. [Google Scholar] [CrossRef]
- Mills, D.K.; Jammalamadaka, U.; Tappa, K.; Weisman, J. Studies on the cytocompatibility, mechanical and antimicrobial properties of 3D printed poly(methyl methacrylate) beads. Bioact. Mater. 2018, 3, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Kanabenja, W.; Passarapark, K.; Subchokpool, T.; Nawaaukkaratharnant, N.; Román, A.J.; Osswald, T.A.; Aumnate, C.; Potiyaraj, P. 3D printing filaments from plasticized Polyhydroxybutyrate/Polylactic acid blends reinforced with hydroxyapatite. Addit. Manuf. 2022, 59, 103130. [Google Scholar] [CrossRef]
- El-malek, F.A.; Khairy, H.; Farag, A.; Omar, S. The sustainability of microbial bioplastics, production and applications. Int. J. Biol. Macromol. 2020, 157, 319–328. [Google Scholar] [CrossRef]
- Bhangu, S.K.; Singla, R.; Colombo, E.; Ashokkumar, M.; Cavalieri, F. Sono-transformation of tannic acid into biofunctional ellagic acid micro/nanocrystals with distinct morphologies. Green Chem. 2018, 20, 816–821. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A.; Mohee, R. Ultrasound-assisted biological conversion of biomass and waste materials to biofuels: A review. Ultrason. Sonochem. 2018, 40, 298–313. [Google Scholar] [CrossRef]
- Ebrahimian, F.; Karimi, K. Efficient biohydrogen and advanced biofuel coproduction from municipal solid waste through a clean process. Bioresour. Technol. 2020, 300, 122656. [Google Scholar] [CrossRef] [PubMed]
- Dan, T.; Jing, H.; Shen, T.; Zhu, J.; Liu, Y. Performance of production of polyhydroxyalkanoates from food waste fermentation with Rhodopseudomonas palustris. Bioresour. Technol. 2023, 385, 129165. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, P.; Karimi, K.; Taherzadeh, M.J. Hydrothermal processing as pretreatment for efficient production of ethanol and biogas from municipal solid waste. Bioresour. Technol. 2018, 261, 166–175. [Google Scholar] [CrossRef]
- Sulbarán-Rangel, B.; Alarcón Aguirre, J.S.; Breton-Deval, L.; del Real-Olvera, J.; Gurubel Tun, K.J. Improvement of Anaerobic Digestion of Hydrolysed Corncob Waste by Organosolv Pretreatment for Biogas Production. Appl. Sci. 2020, 10, 2785. [Google Scholar] [CrossRef]
- Yin, J.; Wang, K.; Yang, Y.; Shen, D.; Wang, M.; Mo, H. Improving production of volatile fatty acids from food waste fermentation by hydrothermal pretreatment. Bioresour. Technol. 2014, 171, 323–329. [Google Scholar] [CrossRef]
- Rofeal, M.; Abdelmalek, F.; Steinbüchel, A. Naturally-Sourced Antibacterial Polymeric Nanomaterials with Special Reference to Modified Polymer Variants. Int. J. Mol. Sci. 2022, 23, 4101. [Google Scholar] [CrossRef] [PubMed]
- El-malek, F.A.; Rofeal, M.; Farag, A.; Omar, S.; Khairy, H. Polyhydroxyalkanoate nanoparticles produced by marine bacteria cultivated on cost effective Mediterranean algal hydrolysate media. J. Biotechnol. 2021, 328, 95–105. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.-S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Kumar, M.; Arun, S.; Upadhyaya, P.; Pugazhenthi, G. Properties of PMMA/clay nanocomposites prepared using various compatibilizers. Int. J. Mech. Mater. Eng. 2015, 10, 7. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, Y. Jointly modified mechanical properties and accelerated hydrolytic degradation of PLA by interface reinforcement of PLA-WF. J. Mech. Behav. Biomed. Mater. 2018, 88, 223–230. [Google Scholar] [CrossRef]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qin, J.; Zhang, X.; Shen, J.; Guo, S.; Sue, H.-J. Enhancing scratch damage resistance of PMMA via layer assembly with PVDF: Numerical modeling prediction and experimental verification. Polymer 2020, 194, 122382. [Google Scholar] [CrossRef]
- Ma, P.; Hristova-Bogaerds, D.G.; Lemstra, P.J.; Zhang, Y.; Wang, S. Toughening of PHBV/PBS and PHB/PBS Blends via In situ Compatibilization Using Dicumyl Peroxide as a Free-Radical Grafting Initiator. Macromol. Mater. Eng. 2012, 297, 402–410. [Google Scholar] [CrossRef]
- Srimalanon, P.; Prapagdee, B.; Markpin, T.; Sombatsompop, N. Effects of DCP as a free radical producer and HPQM as a biocide on the mechanical properties and antibacterial performance of in situ compatibilized PBS/PLA blends. Polym. Test. 2018, 67, 331–341. [Google Scholar] [CrossRef]
- Gu, J.; Xu, H.; Wu, C. The Effect of PP and Peroxide on the Properties and Morphology of HDPE and HDPE/PP Blends. Adv. Polym. Technol. 2013, 32. [Google Scholar] [CrossRef]
- Semba, T.; Kitagawa, K.; Ishiaku, U.S.; Kotaki, M.; Hamada, H. Effect of compounding procedure on mechanical properties and dispersed phase morphology of poly(lactic acid)/polycaprolactone blends containing peroxide. J. Appl. Polym. Sci. 2007, 103, 1066–1074. [Google Scholar] [CrossRef]
- Rytlewski, P.; Żenkiewicz, M.; Malinowski, R. Influence of Dicumyl Peroxide Content on Thermal and Mechanical Properties of Polylactide. Int. Polym. Process. 2011, 26, 580–586. [Google Scholar] [CrossRef]
- Wei, L.; McDonald, A.G.; Stark, N.M. Grafting of Bacterial Polyhydroxybutyrate (PHB) onto Cellulose via In Situ Reactive Extrusion with Dicumyl Peroxide. Biomacromolecules 2015, 16, 1040–1049. [Google Scholar] [CrossRef]
- Dhar, P.; Tarafder, D.; Kumar, A.; Katiyar, V. Thermally recyclable polylactic acid/cellulose nanocrystal films through reactive extrusion process. Polymer 2016, 87, 268–282. [Google Scholar] [CrossRef]
- Pradhan, S.; Dikshit, P.K.; Moholkar, V.S. Production, ultrasonic extraction, and characterization of poly (3-hydroxybutyrate) (PHB) using Bacillus megaterium and Cupriavidus necator. Polym. Adv. Technol. 2018, 29, 2392–2400. [Google Scholar] [CrossRef]
- Nikolaidis, A.K.; Achilias, D.S. Thermal Degradation Kinetics and Viscoelastic Behavior of Poly(Methyl Methacrylate)/Organomodified Montmorillonite Nanocomposites Prepared via In Situ Bulk Radical Polymerization. Polymers 2018, 10, 491. [Google Scholar] [CrossRef]
- Teoh, E.L.; Mariatti, M.; Chow, W.S. Thermal and Flame Resistant Properties of Poly (Lactic Acid)/Poly (Methyl Methacrylate) Blends Containing Halogen-free Flame Retardant. Procedia Chem. 2016, 19, 795–802. [Google Scholar] [CrossRef]
- Yang, S.-l.; Wu, Z.-H.; Yang, W.; Yang, M.-B. Thermal and mechanical properties of chemical crosslinked polylactide (PLA). Polym. Test. 2008, 27, 957–963. [Google Scholar] [CrossRef]
- Danko, M.; Mosnáčková, K.; Vykydalová, A.; Kleinová, A.; Puškárová, A.; Pangallo, D.; Bujdoš, M.; Mosnáček, J. Properties and Degradation Performances of Biodegradable Poly(lactic acid)/Poly(3-hydroxybutyrate) Blends and Keratin Composites. Polymers 2021, 13, 2693. [Google Scholar] [CrossRef]
- Cho, Y.-M.; Kim, J.-H.; Choi, J.-H.; Kim, J.-C.; Cho, S.-M.; Park, S.-W.; Kwak, H.W.; Choi, I.-G. Physicochemical characteristics of lignin-g-PMMA/PLA blend via atom transfer radical polymerization depending on the structural difference of organosolv lignin. Int. J. Biol. Macromol. 2023, 226, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Effect of active phenolic acids on properties of PLA-PHBV blend films. Food Packag. Shelf Life 2022, 33, 100894. [Google Scholar] [CrossRef]
- Brtnicky, M.; Pecina, V.; Holatko, J.; Hammerschmiedt, T.; Mustafa, A.; Kintl, A.; Fojt, J.; Baltazar, T.; Kucerik, J. Effect of biodegradable poly-3-hydroxybutyrate amendment on the soil biochemical properties and fertility under varying sand loads. Chem. Biol. Technol. Agric. 2022, 9, 75. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Bhat, D.K. Enhancement and investigation of biodegradability of poly (methyl methacrylate) and poly (vinyl chloride) by blending with biodegradable polymer. Polym. Bull. 2022, 80, 5623–5639. [Google Scholar] [CrossRef]



| Film Thickness (mm) | TS (MPa) | EB (%) | |
|---|---|---|---|
| PHB | 0.24 ± 0.12 a | 30.2 ± 0.06 a | 7.6 ± 0.01 a |
| PMMA | 0.25 ± 0.23 a | 59.4 ± 0.1 b | 5.5 ±0.02 b |
| PHB/PMMA (1:1) | 0.24 ± 0.16 a | 39.2 ± 0.11 c | 8.2 ± 0.07 c |
| PHB/PMMA (2:1) | 0.24 ± 0.07 a | 41.7 ± 0.06 d | 9.4 ± 0.02 c |
| PHB/PMMA (3:1) | 0.26 ± 0.04 b | 42.8 ± 0.12 d | 6.3 ± 0.04 d |
| PHB/PMMA (1:2) | 0.27 ± 0.09 b | 63.4 ± 0.06 e | 9.8 ± 0.11 e |
| PHB/PMMA (1:3) | 0.25 ± 0.06 c | 66.2 ± 0.06 e | 8.9 ± 0.01 d |
| PHB/PMMA (1:2 w/w) | |||
|---|---|---|---|
| DCP Concentration (wt%) | Film Thickness (mm) | TS (MPa) | EB (%) |
| 0.1 | 0.27 ± 0.12 a | 67.8 ± 0.12 a | 9.9 ± 0.04 a |
| 0.2 | 0.26 ± 0.07 a | 73.1 ± 0.01 a | 10.2 ± 0.17 a |
| 0.3 | 0.25 ± 0.13 a | 78.6 ± 0.06 b | 10.9 ± 0.12 b |
| 0.4 | 0.27 ± 0.03 b | 72.8 ± 0.02 c | 8.2 ± 0.1 c |
| 0.5 | 0.26 ± 0.05 b | 66.4 ± 0.1 d | 7.3 ± 0.03 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rofeal, M.; Abdelmalek, F.; Pietrasik, J. Sustainable Polyhydroxyalkanoate Production from Food Waste via Bacillus mycoides ICRI89: Enhanced 3D Printing with Poly (Methyl Methacrylate) Blend. Polymers 2023, 15, 4173. https://doi.org/10.3390/polym15204173
Rofeal M, Abdelmalek F, Pietrasik J. Sustainable Polyhydroxyalkanoate Production from Food Waste via Bacillus mycoides ICRI89: Enhanced 3D Printing with Poly (Methyl Methacrylate) Blend. Polymers. 2023; 15(20):4173. https://doi.org/10.3390/polym15204173
Chicago/Turabian StyleRofeal, Marian, Fady Abdelmalek, and Joanna Pietrasik. 2023. "Sustainable Polyhydroxyalkanoate Production from Food Waste via Bacillus mycoides ICRI89: Enhanced 3D Printing with Poly (Methyl Methacrylate) Blend" Polymers 15, no. 20: 4173. https://doi.org/10.3390/polym15204173
APA StyleRofeal, M., Abdelmalek, F., & Pietrasik, J. (2023). Sustainable Polyhydroxyalkanoate Production from Food Waste via Bacillus mycoides ICRI89: Enhanced 3D Printing with Poly (Methyl Methacrylate) Blend. Polymers, 15(20), 4173. https://doi.org/10.3390/polym15204173







