Bactericidal Activity of Silver-Doped Chitosan Coatings via Electrophoretic Deposition on Ti6Al4V Additively Manufactured Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Additively Manufactured Ti6Al4V ELI Substrate
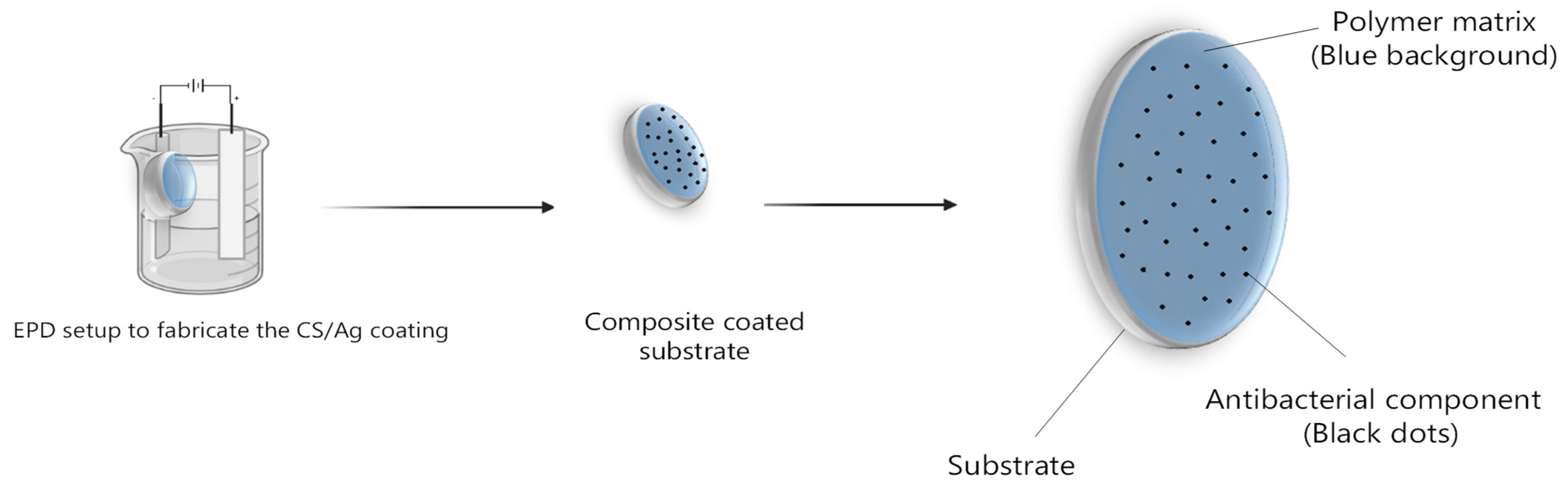
2.3. Fabrication of CS/Ag Composite Coating
2.4. Chemico-Physical Characterization of the Coating
2.4.1. Microstructural Characterization
2.4.2. Swelling Property
2.4.3. In Vitro Degradation
2.5. In Vitro Investigations
2.5.1. Cytotoxicity Tests on Extract
2.5.2. Antibacterial Investigation
2.6. Statistical Data Analysis
3. Results
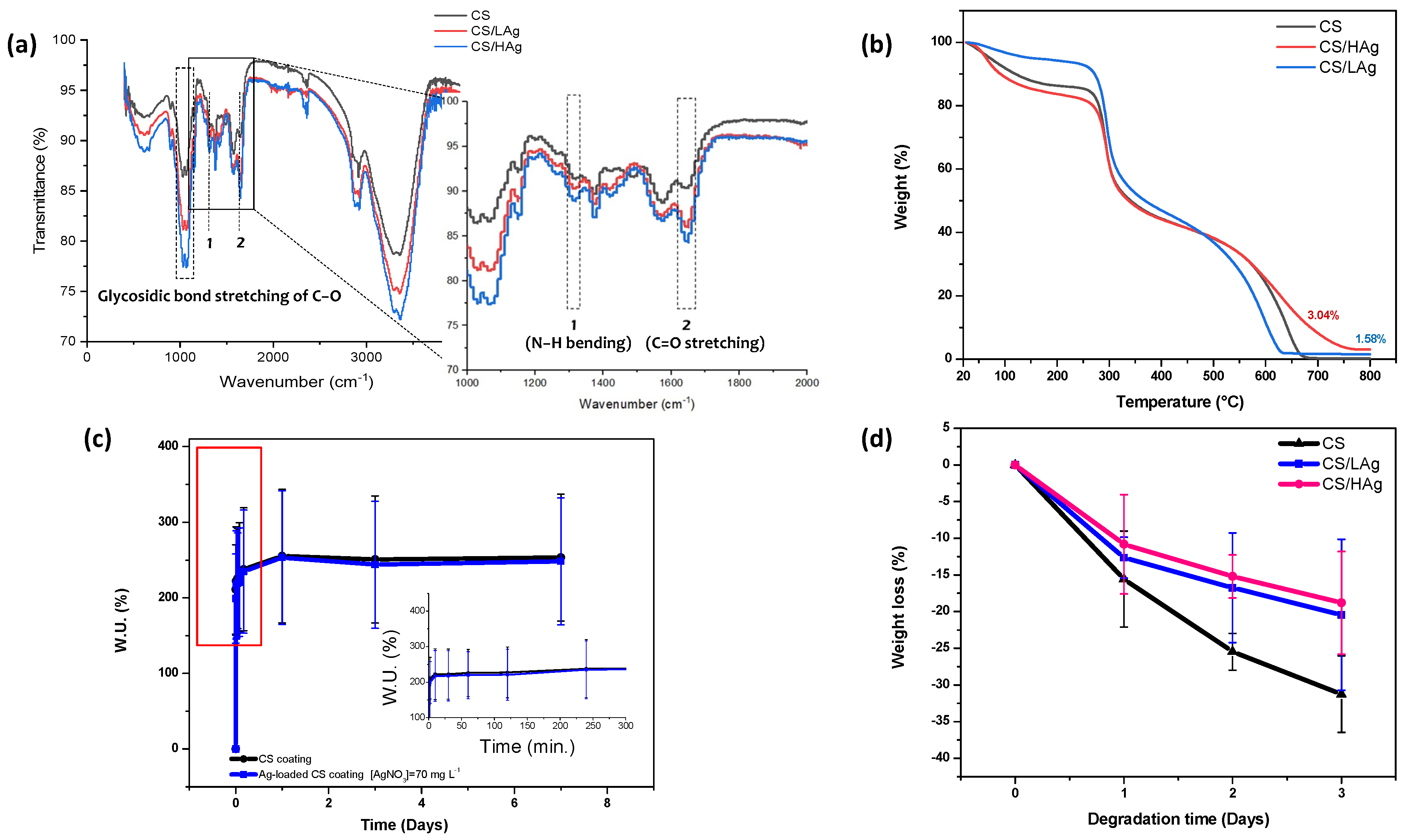
3.1. Physical and Chemical Properties of Deposited Coatings
3.1.1. Morphology of the Composite Coating Fabricated by EPD
3.1.2. Feasibility of EPD CS/Ag Composite Coating
3.1.3. Swelling
3.1.4. In Vitro Degradation
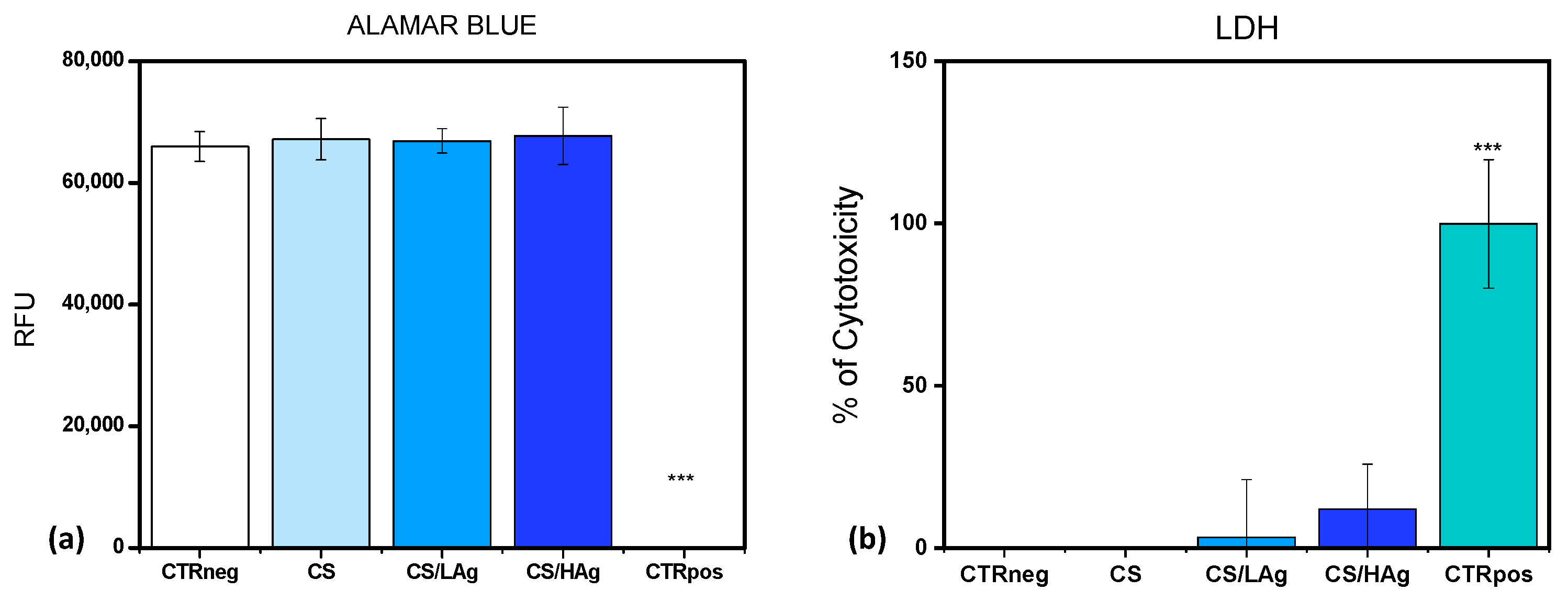
3.2. Cytotoxicity Assessment and Bactericidal Activity
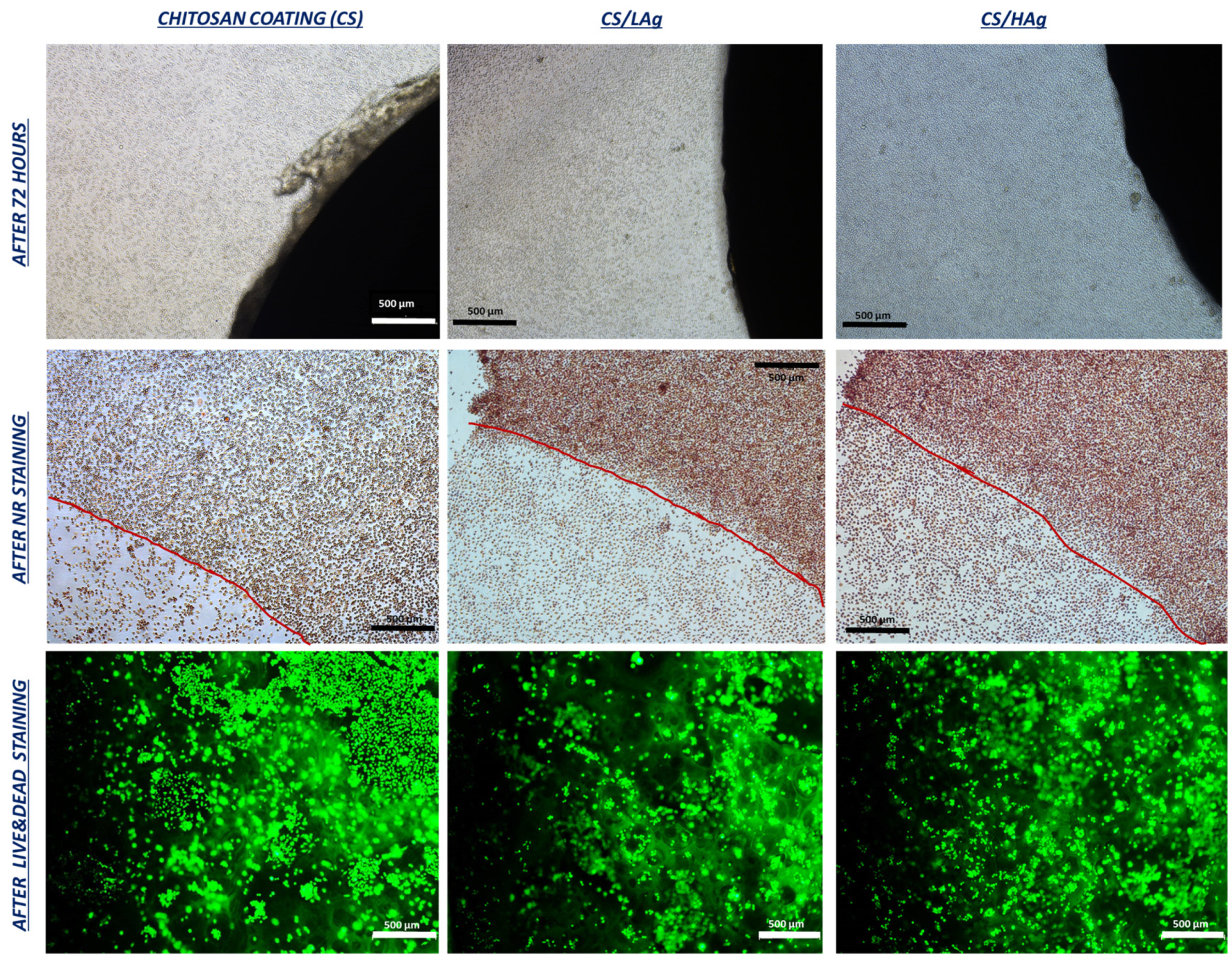
3.2.1. Cytotoxicity Test with a Direct Contact Approach
3.2.2. Antibacterial Investigation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conn, J.M.; Annest, J.L.; Ryan, G.W.; Budnitz, D.S. Non-work-related finger amputations in the United States, 2001–2002. Ann. Emerg. Med. 2005, 45, 630–635. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Swiatek, P.R.; Chung, K.C.; Ayanian, J.Z. Racial Variation in Treatment of Traumatic Finger/Thumb Amputation: A National Comparative Study of Replantation and Revision Amputation. Plast. Reconstr. Surg. 2016, 137, 576e–585e. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.B.C.; Shah, K.N.; Eltorai, A.E.M.; Got, C.C.; Daniels, A.H. Epidemiology of Finger Amputations in the United States from 1997 to 2016. J. Hand Surg. Glob. Online 2019, 1, 45–51. [Google Scholar] [CrossRef]
- Peterson, S.L.; Peterson, E.L.; Wheatley, M.J. Management of Fingertip Amputations. J. Hand Surg. Am. 2014, 39, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Manrique, O.J.; Ciudad, P.; Doscher, M.; Torto, F.L.; Liebling, R.; Galan, R. Osseointegrated finger prostheses using a tripod titanium mini-plate. Arch. Plast. Surg. 2017, 44, 150–156. [Google Scholar] [CrossRef]
- Hebert, J.S.; Rehani, M.; Stiegelmar, R. Osseointegration for Lower-Limb Amputation. JBJS Rev. 2017, 5, e10. [Google Scholar] [CrossRef]
- Al Muderis, M.; Lu, W.; Li, J.J. Osseointegrated Prosthetic Limb for the treatment of lower limb amputations. Unfallchirurg 2017, 120, 306–311. [Google Scholar] [CrossRef]
- Hoyt, B.W.; Walsh, S.A.; Forsberg, J.A. Osseointegrated prostheses for the rehabilitation of amputees (OPRA): Results and clinical perspective. Expert Rev. Med. Devices 2020, 17, 17–25. [Google Scholar] [CrossRef]
- Bregoli, C.; Biffi, C.A.; Morellato, K.; Gruppioni, E.; Primavera, M.; Rampoldi, M.; Lando, M.; Adani, R.; Tuissi, A. Osseointegrated Metallic Implants for Finger Amputees: A Review of the Literature. Orthop. Surg. 2022, 14, 1019–1033. [Google Scholar] [CrossRef]
- Bregoli, C.; Stacchiotti, F.; Fiocchi, J.; Ferrari, R.; Biffi, C.A.; Morellato, K.; Gruppioni, E.; Tuissi, A. A biomechanical study of osseointegrated patient-matched additively manufactured implant for treatment of thumb amputees. Med. Eng. Phys. 2023, 118, 104019. [Google Scholar] [CrossRef]
- Gatti, M.; Barnini, S.; Guarracino, F.; Parisio, E.M.; Spinicci, M.; Viaggi, B.; D’Arienzo, S.; Forni, S.; Galano, A.; Gemmi, F. Orthopaedic Implant-Associated Staphylococcal Infections: A Critical Reappraisal of Unmet Clinical Needs Associated with the Implementation of the Best Antibiotic Choice. Antibiotics 2022, 11, 406. [Google Scholar] [CrossRef]
- Darouiche, R.O. Treatment of Infections Associated with Surgical Implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-Joint Infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef]
- Trampuz, A.; Widmer, A.F. Electrophoretic deposition of hydroxyapatite. Curr. Opin. Infect. Dis. 2006, 19, 349–356. [Google Scholar] [CrossRef]
- Pulido, L.; Ghanem, E.; Joshi, A.; Purtill, J.J.; Parvizi, J. Periprosthetic Joint Infection: The Incidence, Timing, and Predisposing Factors. Clin. Orthop. Relat. Res. 2008, 466, 1710–1715. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; Patel, R. Infection Associated with Prosthetic Joints. N. Engl. J. Med. 2009, 361, 787–794. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Lindsay, D.; von Holy, A. Bacterial biofilms within the clinical setting: What healthcare professionals should know. J. Hosp. Infect. 2006, 64, 313–325. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef]
- Jiranek, W.A.; Hanssen, A.D.; Greenwald, A.S. Antibiotic-Loaded Bone Cement for Infection Prophylaxis in Total Joint Replacement. J. Bone Jt. Surg. 2006, 88, 2487–2500. [Google Scholar] [CrossRef]
- Cui, Q.; Mihalko, W.M.; Shields, J.S.; Ries, M.; Saleh, K.J. Antibiotic-Impregnated Cement Spacers for the Treatment of Infection Associated with Total Hip or Knee Arthroplasty. JBJS 2007, 89, 871–882. [Google Scholar] [CrossRef]
- Mittal, Y.; Fehring, T.K.; Hanssen, A.; Marculescu, C.; Odum, S.M.; Osmon, D. Two-Stage Reimplantation for Periprosthetic Knee Infection Involving Resistant Organisms. J. Bone Jt. Surg. 2007, 89, 1227–1231. [Google Scholar] [CrossRef]
- Diwanji, S.R.; Kong, I.K.; Park, Y.H.; Cho, S.G.; Song, E.K.; Yoon, T.R. Two-stage reconstruction of infected hip joints. J. Arthroplast. 2008, 23, 656–661. [Google Scholar] [CrossRef]
- Chiu, F.-Y.; Lin, C.-F.J. Antibiotic-Impregnated Cement in Revision Total Knee Arthroplasty. J. Bone Jt. Surgery-American Vol. 2009, 91, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Toulson, C.; Walcott-Sapp, S.; Hur, J.; Salvati, E.; Bostrom, M.; Brause, B.; Westrich, G.H. Treatment of infected total hip arthroplasty with a 2-stage reimplantation protocol: Update on "our institution’s" experience from 1989 to 2003. J. Arthroplast. 2009, 24, 1051–1060. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Cabrini, A.; Esfahani, A.G.; Petraconi, A.; Lavorgna, M.; De Nardo, L.; Buonocore, G.G.; Andrade, R.J.E.; Cerruti, P. Progress in Organic Coatings Ultrasonic spray deposition of PEGDE-crosslinked chitosan/graphene oxide coatings for enhancing gas barrier properties of polybutylene succinate films. Prog. Org. Coat. 2023, 183, 107760. [Google Scholar] [CrossRef]
- Simchi, A.; Pishbin, F.; Boccaccini, A.R. Electrophoretic deposition of chitosan. Mater. Lett. 2009, 63, 2253–2256. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef]
- Varoni, E.M.; Altomare, L.; Cochis, A.; GhalayaniEsfahani, A.; Cigada, A.; Rimondini, L.; De Nardo, L. Hierarchic micro-patterned porous scaffolds via electrochemical replica-deposition enhance neo-vascularization. Biomed. Mater. 2016, 11, 025018. [Google Scholar] [CrossRef]
- Gritsch, L.; Lovell, C.; Goldmann, W.H.; Boccaccini, A.R. Fabrication and characterization of copper(II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr. Polym. 2018, 179, 370–378. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Fan, L.; Liu, H.; Hu, Y. Chitosan- metal complexes as antimicrobial agent: Synthesis, characterization and Structure-activity study. Polym. Bull. 2005, 55, 105–113. [Google Scholar] [CrossRef]
- Xue, B.; Li, Q.; Wang, L.; Deng, M.; Zhou, H.; Li, N.; Tan, M.; Hao, D.; Du, H.; Wang, Q. Ferric-ellagate complex: A promising multifunctional photocatalyst. Chemosphere 2023, 332, 138829. [Google Scholar] [CrossRef]
- Mouriño, V.; Cattalini, J.P.; Boccaccini, A.R. Metallic ions as therapeutic agents in tissue engineering scaffolds: An overview of their biological applications and strategies for new developments. J. R. Soc. Interface 2012, 9, 401–419. [Google Scholar] [CrossRef]
- Hoffman, R.K.; Surkiewicz, B.F.; Chambers, L.A.; Phillips, C.R. Bactericidal Action of Movidyn. Ind. Eng. Chem. 1953, 45, 2571–2573. [Google Scholar] [CrossRef]
- Fong, J.; Wood, F. Nanocrystalline silver dressings in wound management: A review. Int. J. Nanomed. 2006, 1, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver enhances antibiotic activity against gram-negative bacteria. Sci. Transl. Med. 2013, 5, 190ra81. [Google Scholar] [CrossRef]
- Patton, M.Q. Kinetic studies of the interaction between silver ion and deoxyribonucleic acid. Chem. Lett. 1980, 9, 373–376. [Google Scholar]
- Kim, H.S.; Lee, S.H.; Eun, C.J.; Yoo, J.; Seo, Y.S. Dispersion of chitosan nanoparticles stable over a wide pH range by adsorption of polyglycerol monostearate. Nanomater. Nanotechnol. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Won, H.I.; Nersisyan, H.; Won, C.W.; Lee, J.-M.; Hwang, J.-S. Preparation of porous silver particles using ammonium formate and its formation mechanism. Chem. Eng. J. 2010, 156, 459–464. [Google Scholar] [CrossRef]
- Bregoli, C.; Fiocchi, J.; Biffi, C.A.; Tuissi, A. Additively manufactured medical bone screws: An initial study to investigate the impact of lattice-based Voronoi structure on implant primary stability. Rapid Prototyp. 2023. [Google Scholar] [CrossRef]
- Renzo, D.A.; Sgambitterra, E.; Magarò, P.; Furgiuele, F.; Maletta, C.; Biffi, C.A.; Fiocchi, J.; Tuissi, A. Multiaxial fatigue behavior of additively manufactured Ti6Al4V alloy: Axial–torsional proportional loads. Mater. Des. Process. Commun. 2021, 3, e190. [Google Scholar] [CrossRef]
- Esfahani, A.G.; Altomare, L.; Varoni, E.M.; Bertoldi, S.; Farè, S.; De Nardo, L. Electrophoretic bottom up design of chitosan patches for topical drug delivery. J. Mater. Sci. Mater. Med. 2019, 30, 40. [Google Scholar] [CrossRef]
- Isfahani, A.G.; Ghorbani, M. Electrophoretic Deposition of Ni/SiO2 Nanocomposite Coating: Fabrication Process and Tribological and Corrosion Properties. J. Nano Res. 2013, 26, 45–51. [Google Scholar] [CrossRef]
- Ghalayani Esfahani, A.; Lazazzera, B.; Draghi, L.; Farè, S.; Chiesa, R.; De Nardo, L.; Billi, F. Bactericidal activity of Gallium-doped chitosan coatings against staphylococcal infection. J. Appl. Microbiol. 2018, 126, 87–101. [Google Scholar] [CrossRef]
- Ghalayani Esfahani, A.; Lazazzera, B.; Draghi, L.; Farè, S.; Chiesa, R.; De Nardo, L.; Billi, F. Micro-Structured Patches for Dermal Regeneration Obtained via Electrophoretic Replica Deposition. Appl. Sci. 2020, 10, 5010. [Google Scholar] [CrossRef]
- Ghalayani Esfahani, A.; Oleimanzade, M.; Campiglio, C.E.; Federici, A.; Altomare, L.; Draghi, L.; Boccaccini, A.R.; De Nardo, L. Hierarchical microchannel architecture in chitosan/bioactive glass scaffolds via electrophoretic deposition positive-replica. J. Biomed. Mater. Res. Part A 2019, 107, 1455–1465. [Google Scholar] [CrossRef]
- Brouwer, J.; van Leeuwen-Herberts, T.; de Ruit, M.O. Determination of lysozyme in serum, urine, cerebrospinal fluid and feces by enzyme immunoassay. Clin. Chim. Acta 1984, 142, 21–30. [Google Scholar] [CrossRef]
- Porstmann, B.; Jung, K.; Schmechta, H.; Evers, U.; Pergande, M.; Porstmann, T.; Kramm, H.J.; Krause, H. Measurement of lysozyme in human body fluids: Comparison of various enzyme immunoassay techniques and their diagnostic application. Clin. Biochem. 1989, 22, 349–355. [Google Scholar] [CrossRef] [PubMed]
- UNI EN ISO 10993:5-2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Cárdenas, G.; Miranda, S.P. FTIR and TGA studies of chitosan composite films. J. Chil. Chem. Soc. 2004, 49, 291–295. [Google Scholar] [CrossRef]
- Kittur, F.S.; Prashanth, K.V.H.; Sankar, K.U.; Tharanathan, R.N. Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Polym. 2002, 49, 185–193. [Google Scholar] [CrossRef]
- Aimin, C.; Chunlin, H.; Juliang, B.; Tinyin, Z.; Zhichao, D. Antibiotic Loaded Chitosan Bar: An In Vitro, In Vivo Study of a Possible Treatment for Osteomyelitis. Clin. Orthop. Relat. Res. 1999, 366, 239–247. Available online: https://journals.lww.com/clinorthop/Fulltext/1999/09000/Antibiotic_Loaded_Chitosan_Bar__An_In_Vitro,_In.31.aspx (accessed on 12 December 2022). [CrossRef]
- Valour, F.; Trouillet-Assant, S.; Rasigade, J.P.; Lustig, S.; Chanard, E.; Meugnier, H.; Tigaud, S.; Vandenesch, F.; Etienne, J.; Ferry, T.; et al. Staphylococcus epidermidis in Orthopedic Device Infections: The Role of Bacterial Internalization in Human Osteoblasts and Biofilm Formation. PLoS ONE 2013, 8, e67240. [Google Scholar] [CrossRef]
- Huq, A.; Parvez, A.K.; Balusamy, S.R. Chitosan-Coated Polymeric Silver and Gold Nanoparticles: Biosynthesis, Characterization and Potential Antibacterial Applications: A Review. Polymers 2022, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Lanzi, O. Effect of Pore Structure on Current and Potential Distributions in a Porous Electrode. J. Electrochem. Soc. 1990, 137, 585. [Google Scholar] [CrossRef]
- Zhitomirsky, I.; Gal-or, L. Electrophoretic deposition of hydroxyapatite. J. Mater. Sci. Mater. Med. 1997, 8, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Pangburn, S.; Trescony, P.; Heller, J. Lysozyme degradation of partially deacetylated chitin, its films and hydrogels. Biomaterials 1982, 3, 105–108. [Google Scholar] [CrossRef]
- Lee, K.Y.; Ha, W.S.; Park, W.H. Blood compatibility and biodegradability of partially N-acylated chitosan derivatives. Biomaterials 1995, 16, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Tomihata, K.; Ikada, Y. In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials 1997, 18, 567–575. [Google Scholar] [CrossRef]
- Vårum, K.M.; Myhr, M.M.; Hjerde, R.J.N.; Smidsrød, O. In vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydr. Res. 1997, 299, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Nordtveit, R.J.; Vårum, K.M.; Smidsrød, O. Degradation of fully water-soluble, partially N-acetylated chitosans with lysozyme. Carbohydr. Polym. 1994, 23, 253–260. [Google Scholar] [CrossRef]
- Zilberman, M.; Elsner, J.J. Antibiotic-eluting medical devices for various applications. J. Control. Release 2008, 130, 202–215. [Google Scholar] [CrossRef]
- Simchi, A.; Tamjid, E.; Pishbin, F.; Boccaccini, A.R. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomedicine 2011, 7, 22–39. [Google Scholar] [CrossRef]
- Ordikhani, F.; Tamjid, E.; Simchi, A. Characterization and antibacterial performance of electrodeposited chitosan-vancomycin composite coatings for prevention of implant-associated infections. Mater. Sci. Eng. C 2014, 41, 240–248. [Google Scholar] [CrossRef]
- Bernthal, N.M.; Stavrakis, A.I.; Billi, F.; Cho, J.S.; Kremen, T.J.; Simon, S.I.; Cheung, A.L.; Finerman, G.A.; Lieberman, J.R.; Adams, J.S.; et al. A Mouse Model of Post-Arthroplasty Staphylococcus aureus Joint Infection to Evaluate In Vivo the Efficacy of Antimicrobial Implant Coatings. PLoS ONE 2010, 5, e12580. [Google Scholar] [CrossRef]
- Zou, X.; Zhao, X.; Ye, L.; Wang, Q.; Li, H. Preparation and drug release behavior of pH-responsive bovine serum albumin-loaded chitosan microspheres. J. Ind. Eng. Chem. 2015, 21, 1389–1397. [Google Scholar] [CrossRef]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Yoo, M.K.; Park, I.Y.; Kim, B.C.; Cho, C.S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Polinarski, M.A.; Beal, A.L.; Silva, F.E.; Bernardi-Wenzel, J.; Burin, G.R.; de Muniz, G.I.; Alves, H.J. New Perspectives of Using Chitosan, Silver, and Chitosan–Silver Nanoparticles against Multidrug-Resistant Bacteria. Part. Part. Syst. Charact. 2021, 38, 1–18. [Google Scholar] [CrossRef]


| Process Parameters | Value |
|---|---|
| Power (W) | 200 |
| Exposure time (µs) | 50 |
| Layer thickness (µm) | 30 |
| Point distance (µm) | 75 |
| Hatch distance (µm) | 65 |
| Atmosphere | argon |
| Strategy | Meander |
| Sample | Weight (%) | ||||
|---|---|---|---|---|---|
| C | O | Al | Ti | Ag | |
| CS/LAg | 40.7 | 38.28 | 0.84 | 18.91 | 1.27 |
| CS/HAg | 38.5 | 38.52 | 0.88 | 19.99 | 2.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghalayani Esfahani, A.; Sartori, M.; Bregoli, C.; Fiocchi, J.; Biffi, C.A.; Tuissi, A.; Giavaresi, G.; Presentato, A.; Alduina, R.; De Luca, A.; et al. Bactericidal Activity of Silver-Doped Chitosan Coatings via Electrophoretic Deposition on Ti6Al4V Additively Manufactured Substrates. Polymers 2023, 15, 4130. https://doi.org/10.3390/polym15204130
Ghalayani Esfahani A, Sartori M, Bregoli C, Fiocchi J, Biffi CA, Tuissi A, Giavaresi G, Presentato A, Alduina R, De Luca A, et al. Bactericidal Activity of Silver-Doped Chitosan Coatings via Electrophoretic Deposition on Ti6Al4V Additively Manufactured Substrates. Polymers. 2023; 15(20):4130. https://doi.org/10.3390/polym15204130
Chicago/Turabian StyleGhalayani Esfahani, Arash, Maria Sartori, Chiara Bregoli, Jacopo Fiocchi, Carlo Alberto Biffi, Ausonio Tuissi, Gianluca Giavaresi, Alessandro Presentato, Rosa Alduina, Angela De Luca, and et al. 2023. "Bactericidal Activity of Silver-Doped Chitosan Coatings via Electrophoretic Deposition on Ti6Al4V Additively Manufactured Substrates" Polymers 15, no. 20: 4130. https://doi.org/10.3390/polym15204130
APA StyleGhalayani Esfahani, A., Sartori, M., Bregoli, C., Fiocchi, J., Biffi, C. A., Tuissi, A., Giavaresi, G., Presentato, A., Alduina, R., De Luca, A., Cabrini, A., De Capitani, C., Fini, M., Gruppioni, E., Lavorgna, M., & Ronca, A. (2023). Bactericidal Activity of Silver-Doped Chitosan Coatings via Electrophoretic Deposition on Ti6Al4V Additively Manufactured Substrates. Polymers, 15(20), 4130. https://doi.org/10.3390/polym15204130















