Hydrothermal Synthesis of Functionalized Carbon Nanodots and Their Clusters as Ionic Probe for High Sensitivity and Selectivity for Sulfate Anions with Excellent Detection Level
Abstract
:1. Introduction
2. Experimental Section
2.1. Hydrothermal Synthesis of CND Samples
2.2. Photoluminescence (PL) Emission of N-Doped CND Samples
2.3. Ionic Detection by the N-Doped CND Samples
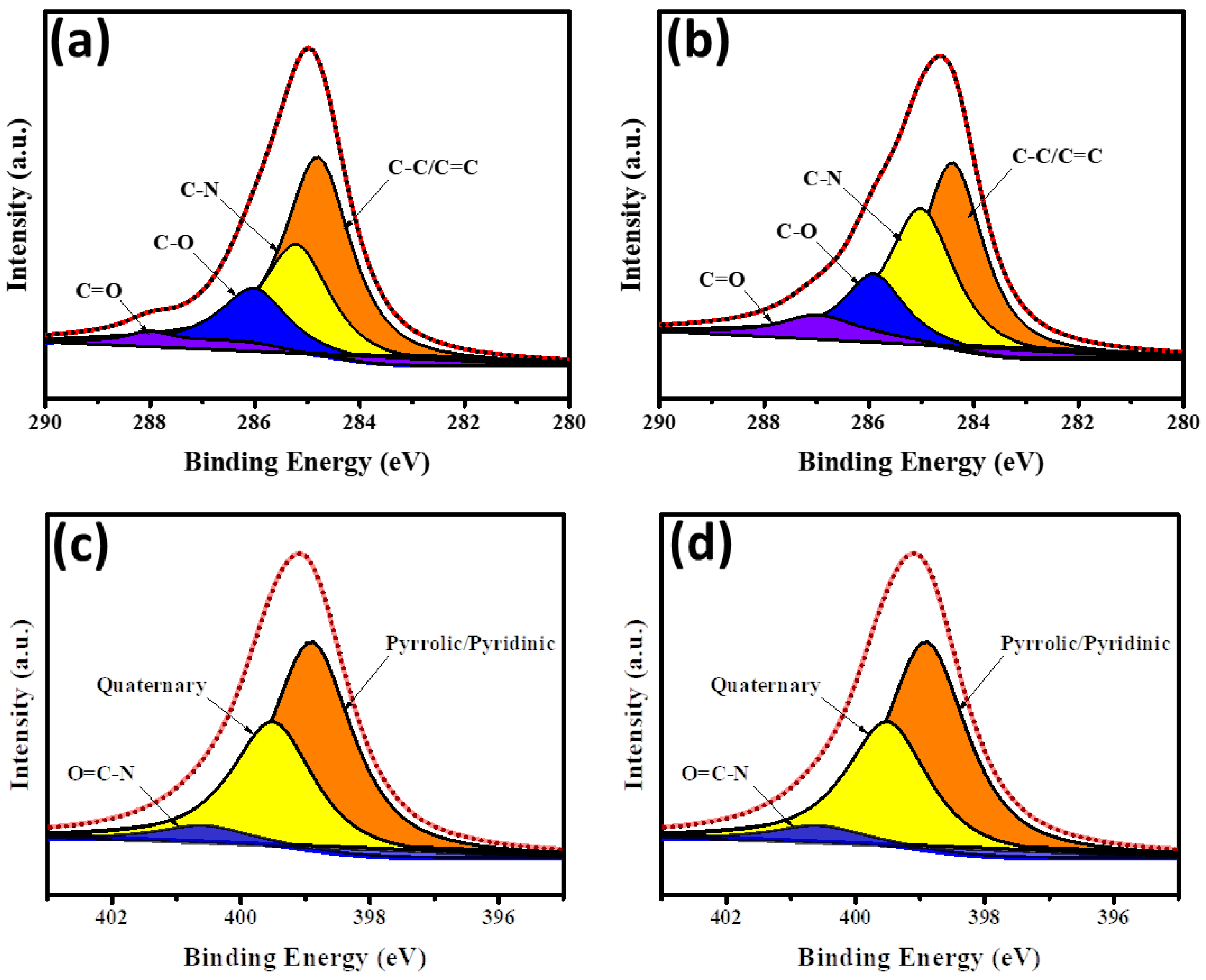
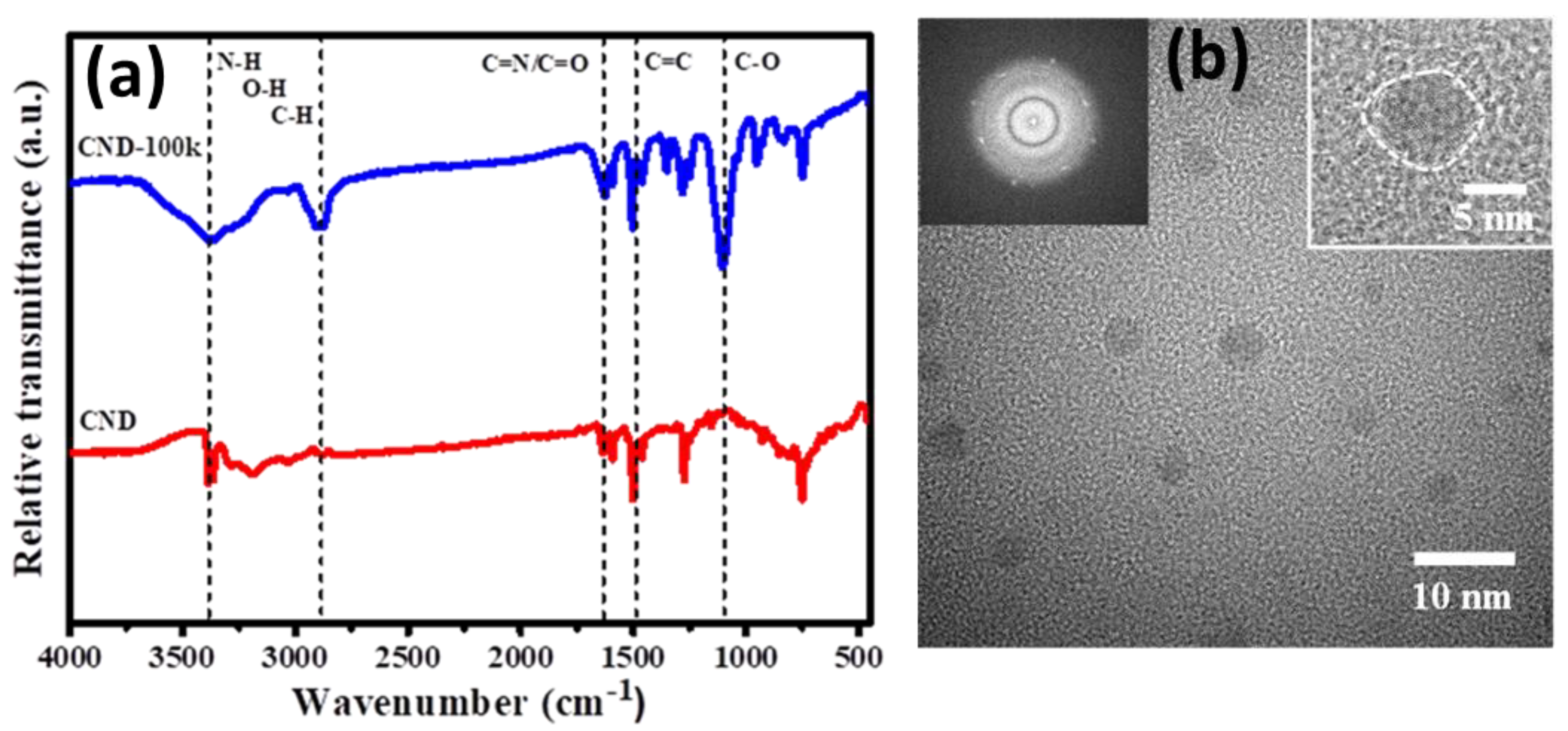
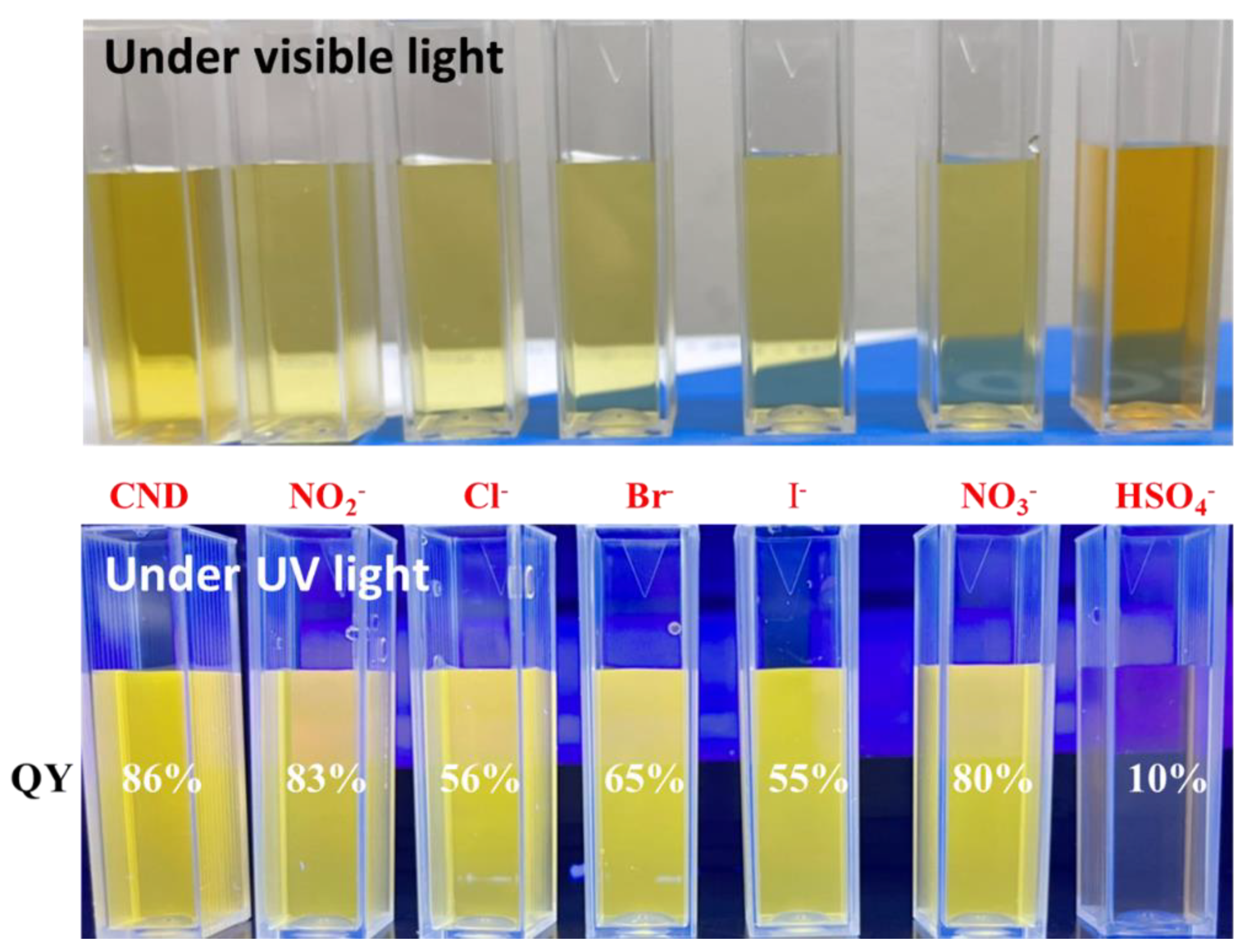
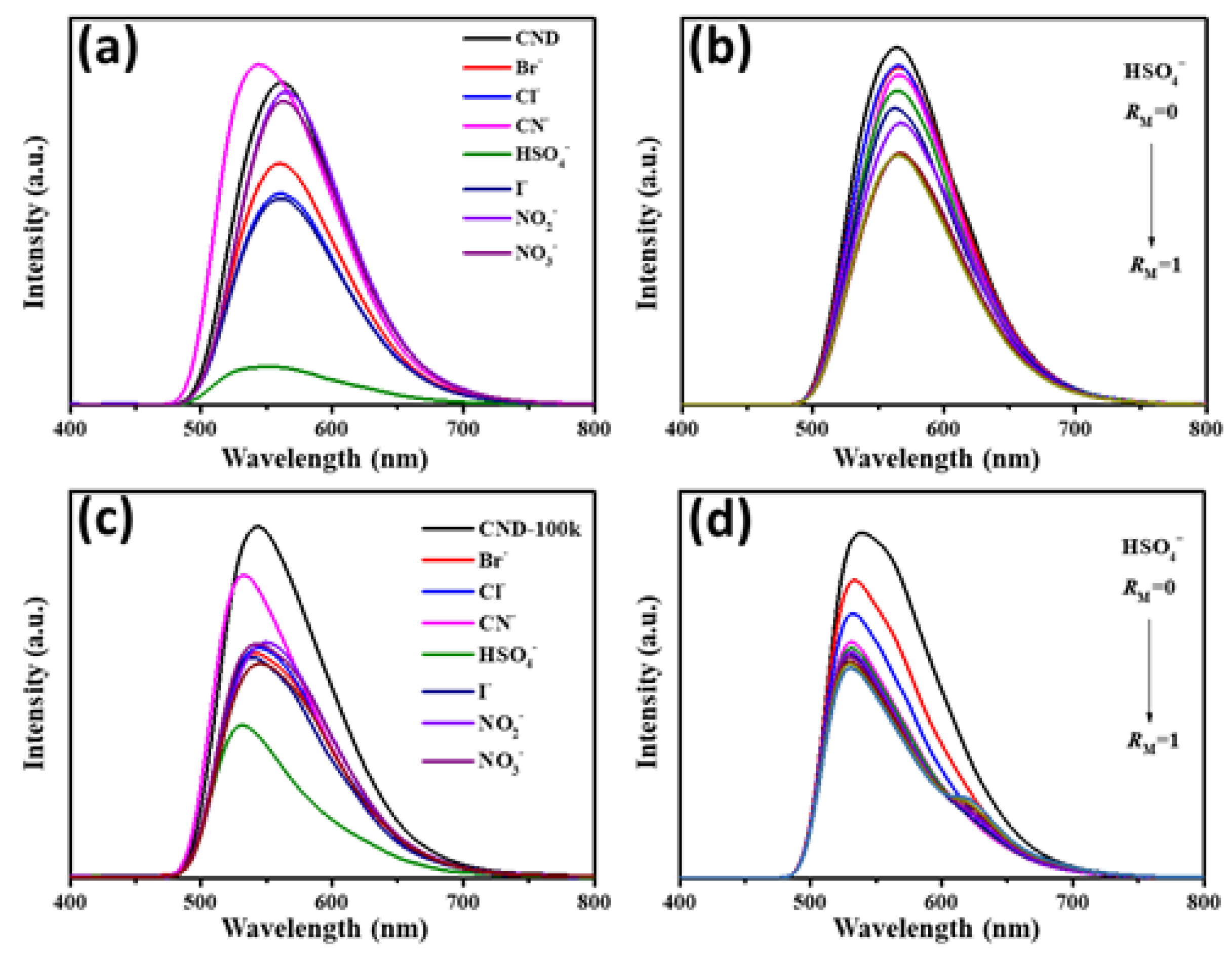
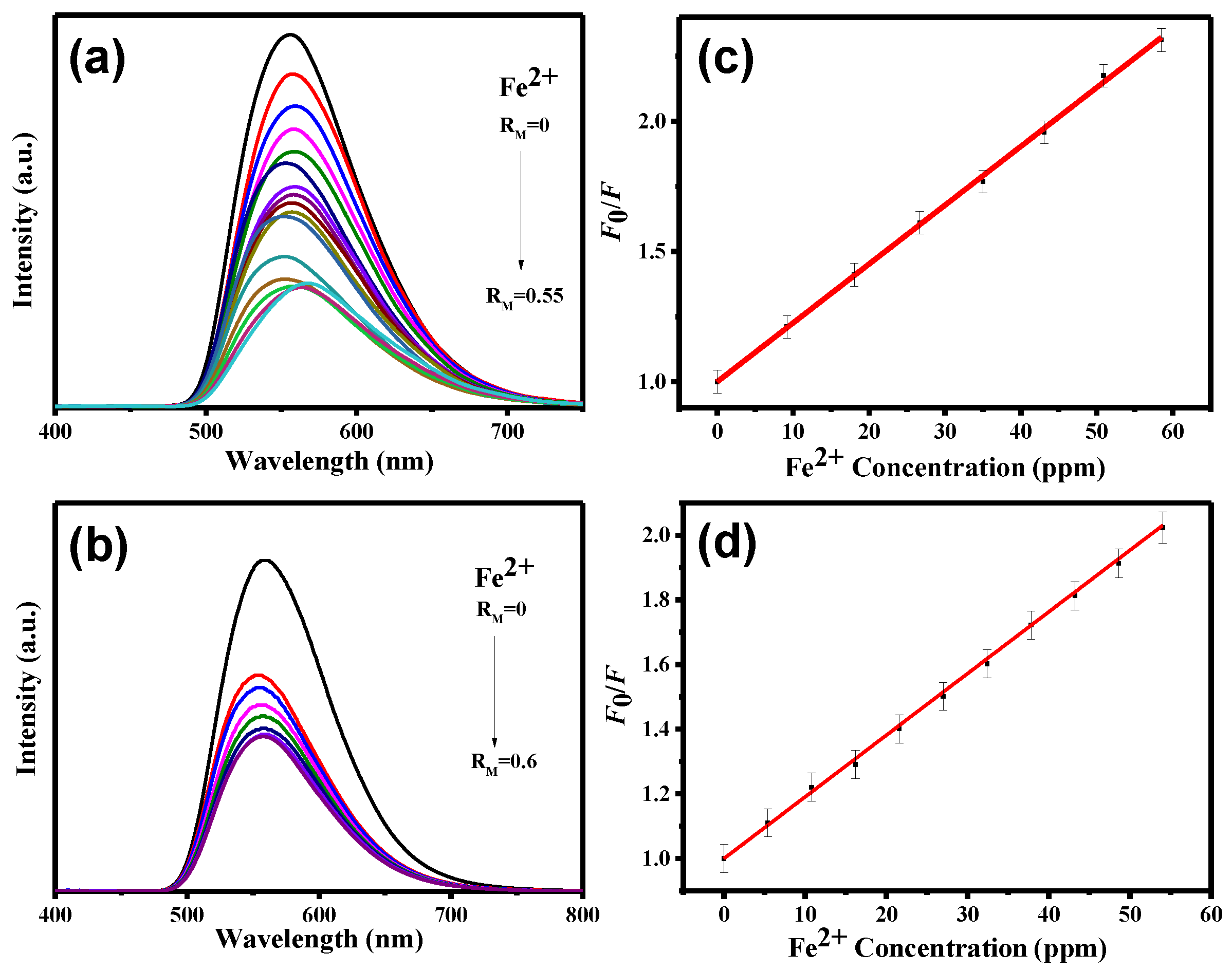
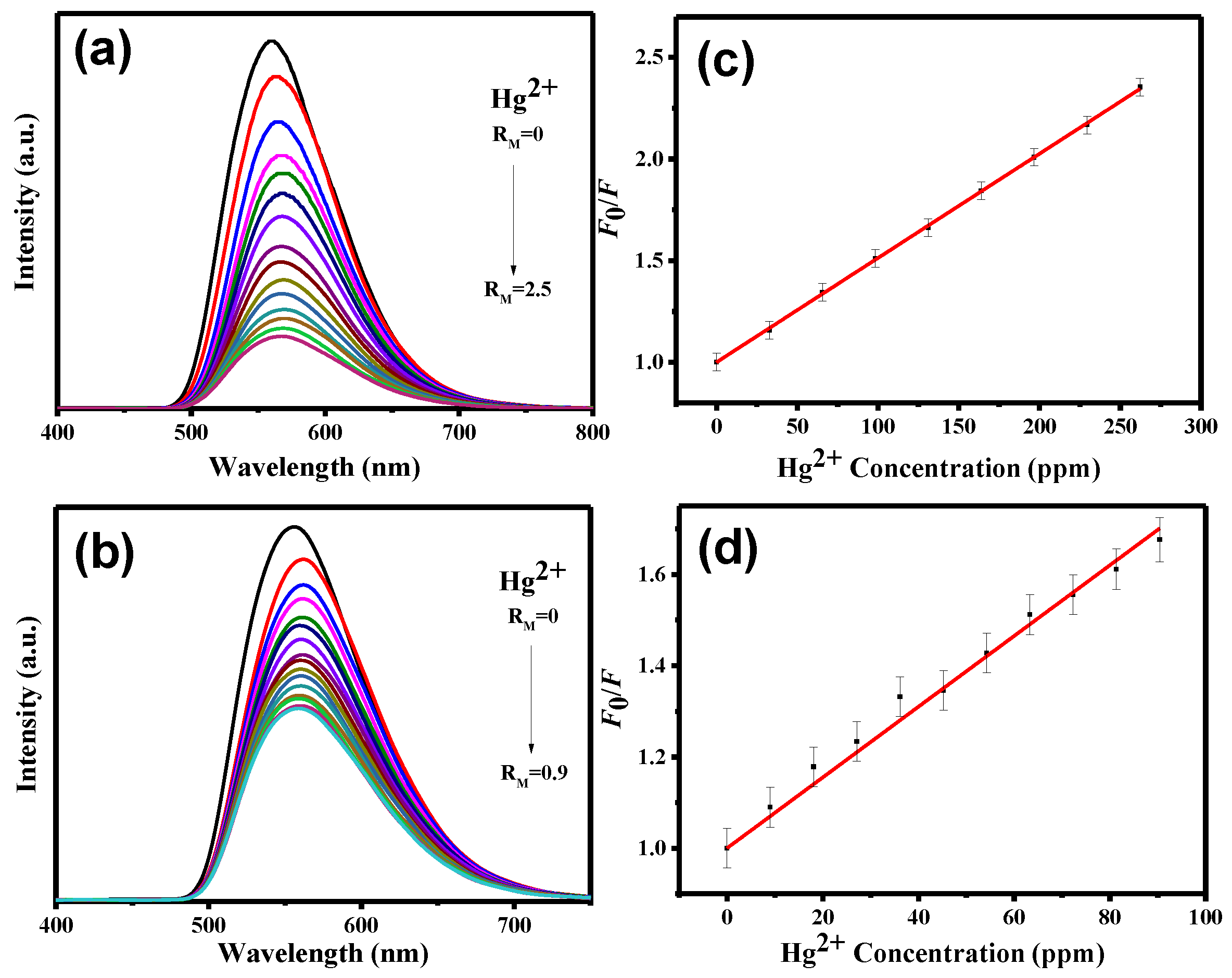
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Wu, G.; Hong, T.; Yin, Z.; Sun, D.; Abdel-Halim, E.S.; Zhu, J.-J. Graphene Quantum Dots as Fluorescence Probes for Turn-off Sensing of Melamine in the Presence of Hg2+. ACS Appl. Mater. Interfaces 2014, 6, 2858–2864. [Google Scholar]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar]
- Zhai, X.; Zhang, P.; Liu, C.; Bai, T.; Li, W.; Dai, L.; Liu, W. Highly luminescent carbon nanodots by microwave-assisted pyrolysis. Chem. Commun. 2012, 48, 7955–7957. [Google Scholar]
- Gu, S.; Hsieh, C.T.; Tsai, Y.Y.; Gandomi, A.Y.; Yeom, K.D.; Kihm, K.D.; Fu, C.C.; Juang, R.S. Sulfur and nitrogen Co-doped graphene quantum dots as a fluorescent quenching probe for highly sensitive detection toward mercury ions. ACS Appl. Nano Mater. 2019, 2, 790–798. [Google Scholar]
- Wang, Y.; Zhang, L.; Liang, R.-P.; Bai, J.-M.; Qiu, J.-D. Using Graphene Quantum Dots as Photoluminescent Probes for Protein Kinase Sensing. Anal. Chem. 2013, 85, 9148–9155. [Google Scholar]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar]
- Bhunia, S.K.; Saha, A.; Maity, A.R.; Ray, S.C.; Jana, N.R. Carbon Nanoparticle-based Fluorescent Bioimaging Probes. Sci. Rep. 2013, 3, srep01473. [Google Scholar]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2015, 2, 6921–6939. [Google Scholar]
- Bak, S.; Kim, D.; Lee, H. Graphene quantum dots and their possible energy applications: A review. Curr. Appl. Phys. 2016, 16, 1192–1201. [Google Scholar]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Titirici Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar]
- Yang, P.C.; Ting, Y.X.; Gu, S.; Gandomi, Y.A.; Hsieh, C.T. Hsieh Fluorescent Nitrogen-doped Carbon Nanodots Synthesized through a Hydrothermal Method with Different Isomers. J. Taiwan Inst. Chem. Eng. 2021, 123, 302–309. [Google Scholar]
- Juang, R.-S.; Hsieh, C.-T.; Kao, C.-P.; Gandomi, Y.A.; Fu, C.-C.; Liu, S.-H.; Gu, S. Highly fluorescent green and red emissions from boron-doped graphene quantum dots under blue light illumination. Carbon 2021, 176, 61–70. [Google Scholar]
- Juang, R.-S.; Fu, C.-C.; Hsieh, C.-T.; Gu, S.; Gandomi, Y.A.; Liu, S.-H. Highly luminescent aggregate-induced emission from polyethylene glycol-coated carbon quantum dot clusters under blue light illumination. J. Mater. Chem. C 2020, 8, 16569–16576. [Google Scholar]
- Bourlinos, A.B.; Stassinopoulos, A.; Anglos, D.; Zboril, R.; Georgakilas, V.; Giannelis, E.P. Photoluminescent Carbogenic Dots. Chem. Mater. 2008, 20, 4539–4541. [Google Scholar]
- Zong, J.; Zhu, Y.; Yang, X.; Shen, J.; Li, C. Synthesis of Photoluminescent Carbogenic Dots Using Mesoporous Silica Spheres as Nanoreactors. Chem. Commun. 2011, 47, 764–766. [Google Scholar]
- Yang, S.-T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H.; Meziani, M.J.; Liu, Y.; Qi, G.; Sun, Y.-P. Carbon Dots for Optical Imaging In Vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, Green, and Blue Luminescence by Carbon Dots: Full-Color Emission Tuning and Multicolor Cellular Imaging. Angew. Chem. 2015, 127, 5450–5453. [Google Scholar]
- Yang, P.-C.; Ting, Y.-X.; Gu, S.; Gandomi, Y.A.; Li, J.; Hsieh, C.-T. Effect of Solvent on Fluorescence Emission from Polyethylene Glycol-Coated Graphene Quantum Dots under Blue Light Illumination. Nanomaterials 2021, 11, 1383. [Google Scholar]
- Fu, C.-C.; Hsieh, C.-T.; Juang, R.-S.; Yang, J.-W.; Gu, S.; Gandomi, Y.A. Highly Efficient Carbon Quantum Dot Suspensions and Membranes for Sensitive/Selective Detection and Adsorption/Recovery of Mercury Ions from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2019, 100, 127–136. [Google Scholar]
- Huang, H.; Weng, Y.; Zheng, L.; Yao, B.; Weng, W.; Lin, X. Nitrogen-doped carbon quantum dots as fluorescent probe for “off-on” detection of mercury ions, l-cysteine and iodide ions. J. Colloid Interface Sci. 2017, 506, 373–378. [Google Scholar]
- Xia, C.; Hai, X.; Chen, X.-W.; Wang, J.-H. Simultaneously fabrication of free and solidified n, s-doped graphene quantum dots via a facile solvent-free synthesis route for fluorescent detection. Talanta 2017, 168, 269–278. [Google Scholar]
- Yang, S.; Sun, J.; Li, X.; Zhou, W.; Wang, Z.; He, P.; Ding, G.; Xie, X.; Kang, Z.; Jiang, M. Large-scale fabrication of heavy doped carbon quantum dots with tunable-photoluminescence and sensitive fluorescence detection. J. Mater. Chem. A 2014, 2, 8660–8667. [Google Scholar]
- Lin, L.; Rong, M.; Luo, F.; Chen, D.; Wang, Y.; Chen, X. Luminescent graphene quantum dots as new fluorescent materials for environmental and biological applications. TrAC Trends Anal. Chem. 2014, 54, 83–102. [Google Scholar]
- Qin, J.; Yang, X.; Shen, C.; Chang, Y.; Deng, Y.; Zhang, Z.; Liu, H.; Lv, C.; Li, Y.; Zhang, C.; et al. Carbon nanodot-based humidity sensor for self-powered respiratory monitoring. Nano Energy 2022, 101, 107549. [Google Scholar]
- Kishioka, A.; Matsushita, Y.; Miyake, M. Detection of interfering ions using ion flux phenomena in flow through CI-ISEs with ion exchange membranes. Anal. Chem. 2023, 95, 7584–7593. [Google Scholar]
- Zhu, Z.; Ma, J.; Wang, Z.; Mu, C.; Fan, Z.; Du, L.; Bai, Y.; Fan, L.; Yan, H.; Phillips, D.L.; et al. Efficiency Enhancement of Perovskite Solar Cells through Fast Electron Extraction: The Role of Graphene Quantum Dots. J. Am. Chem. Soc. 2014, 136, 3760–3763. [Google Scholar]
- Tang, L.; Ji, R.; Li, X.; Bai, G.; Liu, C.P.; Hao, J.; Lin, J.; Jiang, H.; Teng, K.S.; Yang, Z.; et al. Deep Ultraviolet to Near-Infrared Emission and Photoresponse in Layered N-Doped Graphene Quantum Dots. ACS Nano 2014, 8, 6312–6320. [Google Scholar]
- Chen, C.-M.; Zang, Q.; Zhao, X.-C.; Zang, B.; Kong, Q.-Q.; Yang, M.-G.; Yang, Q.-H.; Wang, M.-Z.; Yang, Y.-G.; Schlögl, R.; et al. Hierarchically aminated graphene honeycombs for electrochemical capacitive energy storage. J. Mater. Chem. 2012, 22, 14076–14084. [Google Scholar]
- Dai, Y.; Long, H.; Wang, X.; Wang, Y.; Gu, Q.; Jiang, W.; Wang, Y.; Li, C.; Zeng, T.H.; Sun, Y.; et al. Doping: Versatile graphene quantum dots with tunable nitrogen doping. Part. Part. Syst. Charact. 2014, 31, 509. [Google Scholar]
- Barman, S.; Barman, M.S. Facile bulk production of highly blue fluorescent graphitic carbon nitride quantum dots and their application as highly selective and sensitive sensors for the detection of mercuric and iodide ions in aqueous media. J. Mater. Chem. 2012, 22, 21832–21837. [Google Scholar]
- Wang, A.; Wang, C.; Fu, L.; Wong-Ng, W.; Lan, Y. Recent Advances of Graphitic Carbon Nitride-Based Structures and Applications in Catalyst, Sensing, Imaging, and LEDs. Nano-Micro Lett. 2017, 9, 47. [Google Scholar]
- Chen, K.; Chai, Z.; Li, C.; Shi, L.; Liu, M.; Xie, Q.; Zhang, Y.; Xu, D.; Manivannan, A.; Liu, Z. Catalyst-Free Growth of Three-Dimensional Graphene Flakes and Graphene/g-C3N4 Composite for Hydrocarbon Oxidation. ACS Nano 2016, 10, 3665–3673. [Google Scholar]
- Li, J.; Jiao, Y.; Feng, L.; Zhong, Y.; Zuo, G.; Xie, A.; Dong, W. Highly N,P-doped carbon dots: Rational design, photoluminescence and cellular imaging. Microchim. Acta 2017, 184, 2933–2940. [Google Scholar]
- Yan, S.; Yang, S.; He, L.; Ye, C.; Song, X.; Liao, F. Quantum size effect of poly(o-phenylenediamine) quantum dots: From controllable fabrication to tunable photoluminescence properties. Synth. Met. 2014, 198, 142–149. [Google Scholar]
- Russo, P.; Hu, A.; Compagnini, G.; Duley, W.W.; Zhou, N.Y. Femtosecond laser ablation of highly oriented pyrolytic graphite: A green route for large-scale production of porous graphene and graphene quantum dots. Nanoscale 2014, 6, 2381–2389. [Google Scholar]
- Thangavel, S.; Ramaraj, R. Electroless synthesis of multi branched gold nanostructures encapsulated by poly(o-phenylenediamine) in Nafion. J. Colloid Interface Sci. 2011, 355, 293–299. [Google Scholar]
- Muthirulan, P.; Rajendran, N. Poly(o-phenylenediamine) coatings onmild steel: Electrosynthesis, characterization and its corrosion protection ability in acid medium. Surf. Coat. Technol. 2012, 206, 2072–2078. [Google Scholar]
- Chan, H.; Ng, S.; Hor, T.; Sun, J.; Tan, K.; Tan, B. Poly(m-phenylenediamine): Synthesis and characterization by X-ray photoelectron spectroscopy. Eur. Polym. J. 1991, 27, 1303–1308. [Google Scholar]
- Manivel, P.; Sathiyanarahyanan, S.; Venkatachari, G. Synthesis of poly(p-phenylenediamine) and its corrosion inhibition effect on iron in 1 M HCl. J. Appl. Polym. Sci. 2008, 110, 2807–2814. [Google Scholar]
- Tian, L.; Yang, S.; Yang, Y.; Li, J.; Deng, Y.; Tian, S.; He, P.; Ding, G.; Xie, X.; Wang, Z. Green, simple and large scale synthesis of N-doped graphene quantum dots with uniform edge groups by electrochemical bottom-up synthesis. RSC Adv. 2016, 6, 82648–82653. [Google Scholar]
- Gu, S.; Hsieh, C.-T.; Gandomi, Y.A.; Chang, J.-K.; Li, J.; Li, J.; Zhang, H.; Guo, Q.; Lau, K.C.; Pandey, R. Microwave growth and tunable photoluminescence of nitrogen-doped graphene and carbon nitride quantum dots. J. Mater. Chem. C 2019, 7, 5468–5476. [Google Scholar]
- Bian, S.; Shen, C.; Qian, Y.; Liu, J.; Xi, F.; Dong, X. Facile synthesis of sulfur-doped graphene quantum dots as fluorescent sensing probes for Ag+ ions detection. Sens. Actuators B Chem. 2017, 242, 231–237. [Google Scholar]
- Li, M.; Cushing, S.K.; Wang, Q.; Shi, X.; Hornak, L.A.; Hong, Z.; Wu, N. Size-Dependent Energy Transfer between CdSe/ZnS Quantum Dots and Gold Nanoparticles. J. Phys. Chem. Lett. 2011, 2, 2125–2129. [Google Scholar]
- Liang, Z.; Kang, M.; Payne, G.F.; Wang, X.; Sun, R.-C. Probing Energy and Electron Transfer Mechanisms in Fluorescence Quenching of Biomass Carbon Quantum Dots. ACS Appl. Mater. Interfaces 2016, 8, 17478–17488. [Google Scholar]
- David, A.A.; Terry, P. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin. Biochem. Rev. 2008, 29, 49–52. [Google Scholar]
- Wang, B.; Zhuo, S.; Chen, L.; Zhang, Y. Fluorescent graphene quantum dot nanoprobes for the sensitive and selective detection of mercury ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 131, 384–387. [Google Scholar]
- Guo, Y.; Wang, Z.; Shao, H.; Jiang, X. Hydrothermal synthesis of highly fluorescent carbon nanoparticles from sodium citrate and their use for the detection of mercury ions. Carbon 2013, 52, 583–589. [Google Scholar]
- Kumar, V.; Vaid, K.; Sarawagi, N.; Dhiman, J. Influence of Fe(III) on the Fluorescence of Lysozyme: A Facile and Direct Method for Sensitive and Selective Sensing of Fe(III). J. Fluoresc. 2021, 31, 1815–1821. [Google Scholar]
- Apilux, A.; Siangproh, W.; Praphairaksit, N.; Chailapakul, O. Simple and rapid colorimetric detection of Hg(II) by a paper-based device using silver nanoplates. Talanta 2012, 97, 388–394. [Google Scholar]
- Sun, Y.; Xu, G.; Wang, Y.; Zhang, Y.; Xia, L. Fe2+-induced surface plasmon-catalyzed reduction reaction for the detection of Fe2+. Plasmonics 2022, 5, 5–15. [Google Scholar]
- Bian, S.; Shen, C.; Hua, H.; Zhou, L.; Zhu, H.; Xi, F.; Liu, J.; Dong, X. One-pot synthesis of sulfur-doped graphene quantum dots as a novel fluorescent probe for highly selective and sensitive detection of lead(ii). RSC Adv. 2016, 6, 69977–69983. [Google Scholar]
- Kharangarh, P.R.; Umapathy, S.; Singh, G. Thermal Effect of Sulfur Doping for Luminescent Graphene Quantum Dots. ECS J. Solid State Sci. Technol. 2018, 7, M29–M34. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.-C.; Panda, P.K.; Li, C.-H.; Ting, Y.-X.; Ashraf Gandomi, Y.; Hsieh, C.-T. Hydrothermal Synthesis of Functionalized Carbon Nanodots and Their Clusters as Ionic Probe for High Sensitivity and Selectivity for Sulfate Anions with Excellent Detection Level. Polymers 2023, 15, 2655. https://doi.org/10.3390/polym15122655
Yang P-C, Panda PK, Li C-H, Ting Y-X, Ashraf Gandomi Y, Hsieh C-T. Hydrothermal Synthesis of Functionalized Carbon Nanodots and Their Clusters as Ionic Probe for High Sensitivity and Selectivity for Sulfate Anions with Excellent Detection Level. Polymers. 2023; 15(12):2655. https://doi.org/10.3390/polym15122655
Chicago/Turabian StyleYang, Po-Chih, Pradeep Kumar Panda, Cheng-Han Li, Yu-Xuan Ting, Yasser Ashraf Gandomi, and Chien-Te Hsieh. 2023. "Hydrothermal Synthesis of Functionalized Carbon Nanodots and Their Clusters as Ionic Probe for High Sensitivity and Selectivity for Sulfate Anions with Excellent Detection Level" Polymers 15, no. 12: 2655. https://doi.org/10.3390/polym15122655
APA StyleYang, P.-C., Panda, P. K., Li, C.-H., Ting, Y.-X., Ashraf Gandomi, Y., & Hsieh, C.-T. (2023). Hydrothermal Synthesis of Functionalized Carbon Nanodots and Their Clusters as Ionic Probe for High Sensitivity and Selectivity for Sulfate Anions with Excellent Detection Level. Polymers, 15(12), 2655. https://doi.org/10.3390/polym15122655







