Porous Scaffolds Based on Polydopamine/Chondroitin Sulfate/Polyvinyl Alcohol Composite Hydrogels
Abstract
1. Introduction
2. Experiments
2.1. Materials
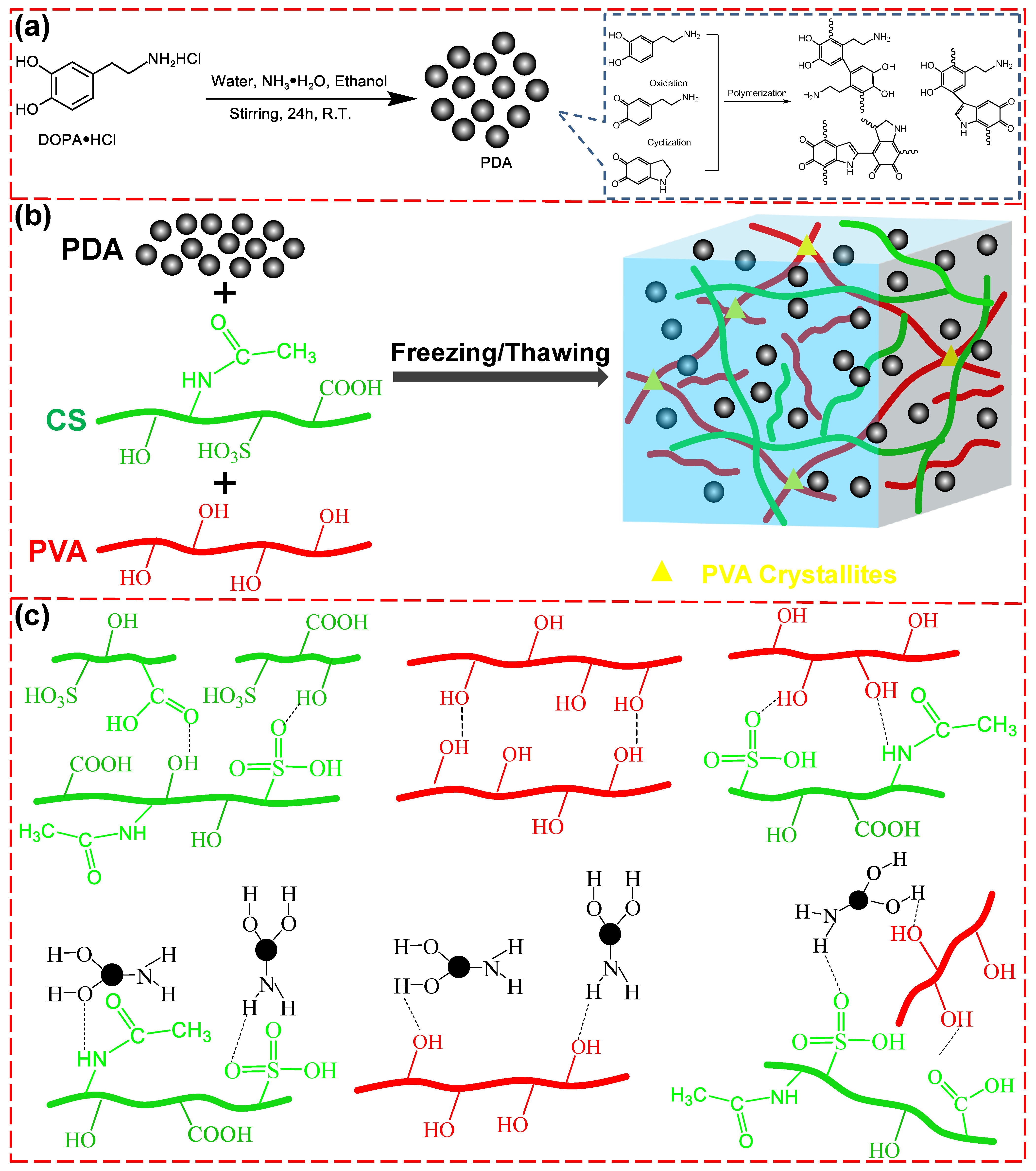
2.2. Preparation of Polydopamine (PDA)
2.3. Preparation of PDA/CS/PVA Composite Hydrogels
2.4. Characterization
2.5. Mechanical Properties
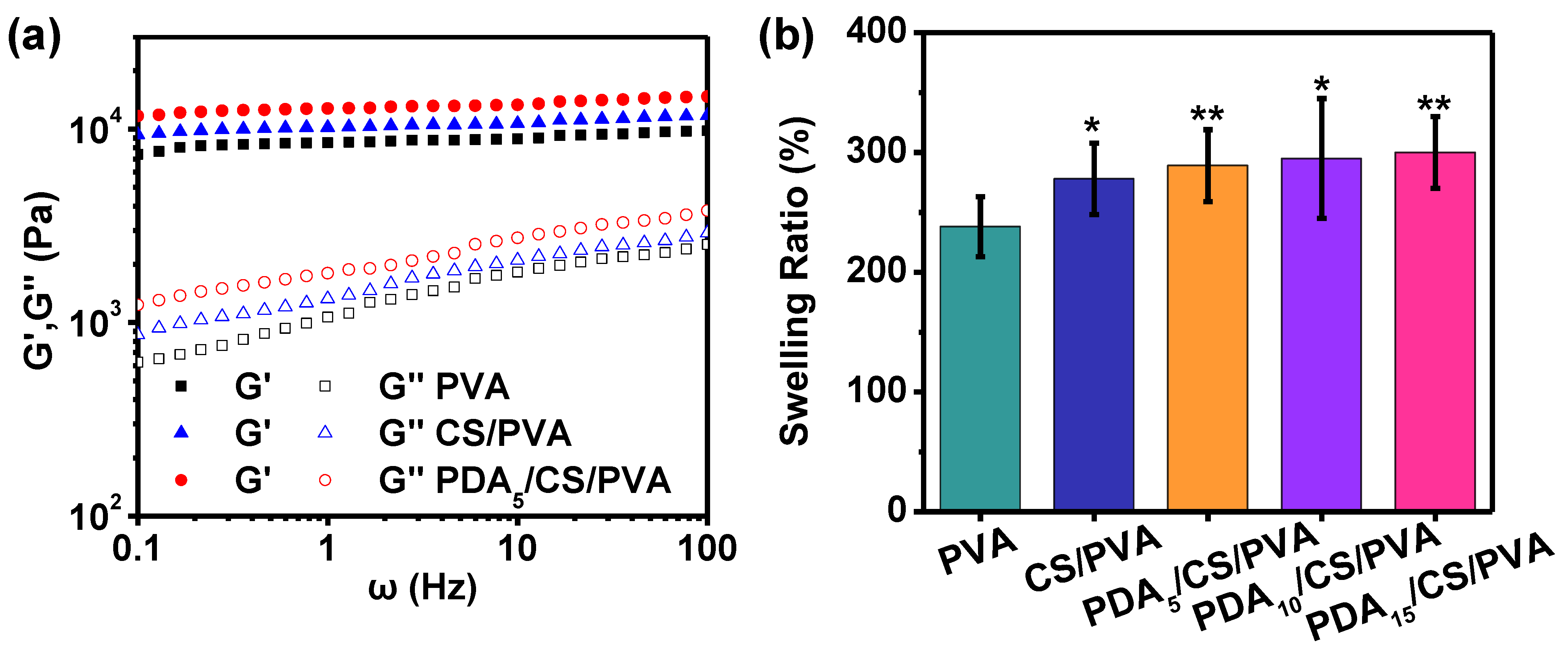
2.6. Swelling Ratio
2.7. Porosity
2.8. Mass Loss
2.9. Cytocompatibility
2.10. Statistical Analysis
3. Results
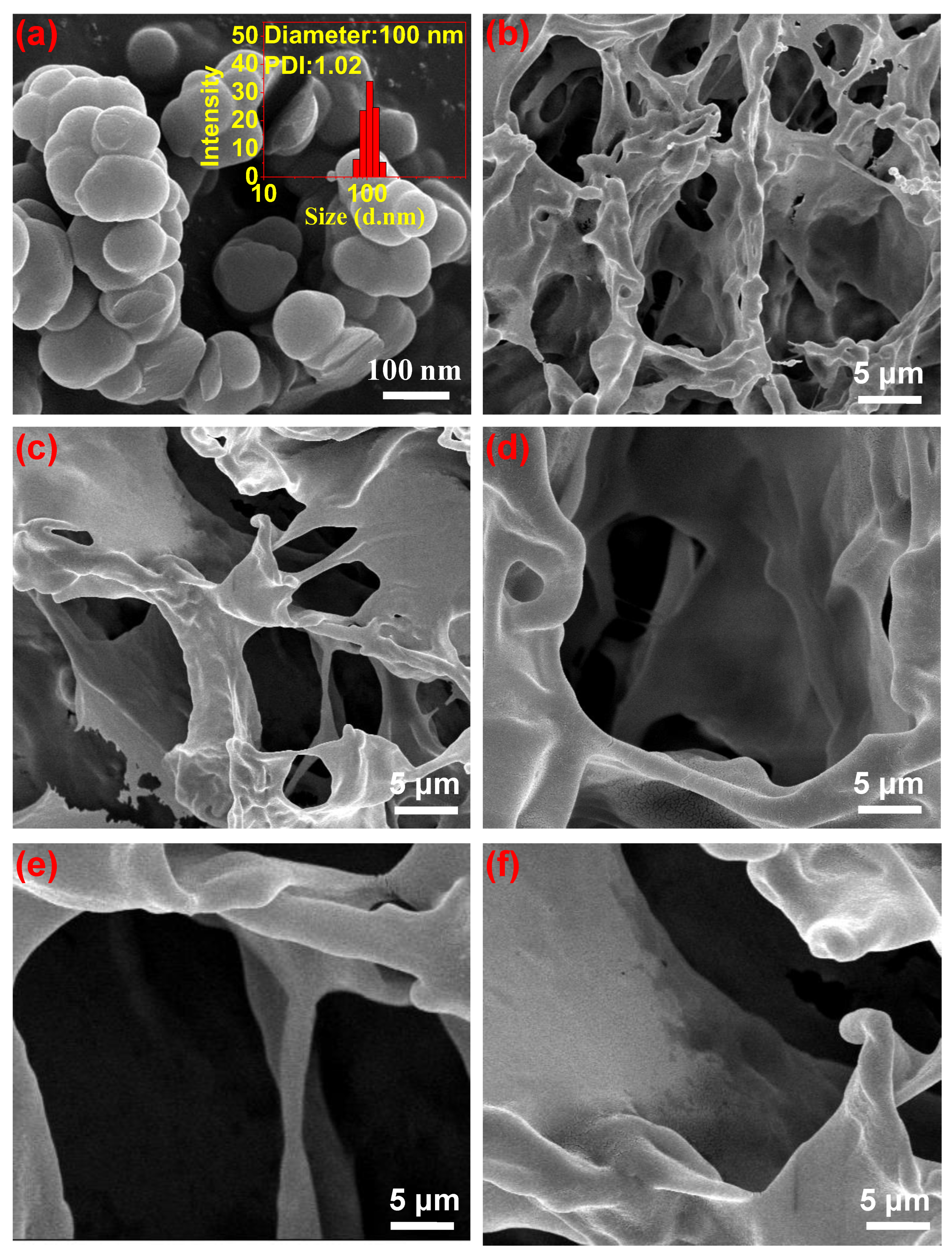
3.1. Design Concept and Characterization of PDA/CS/PVA Composite Hydrogels
3.2. Mechanical Properties
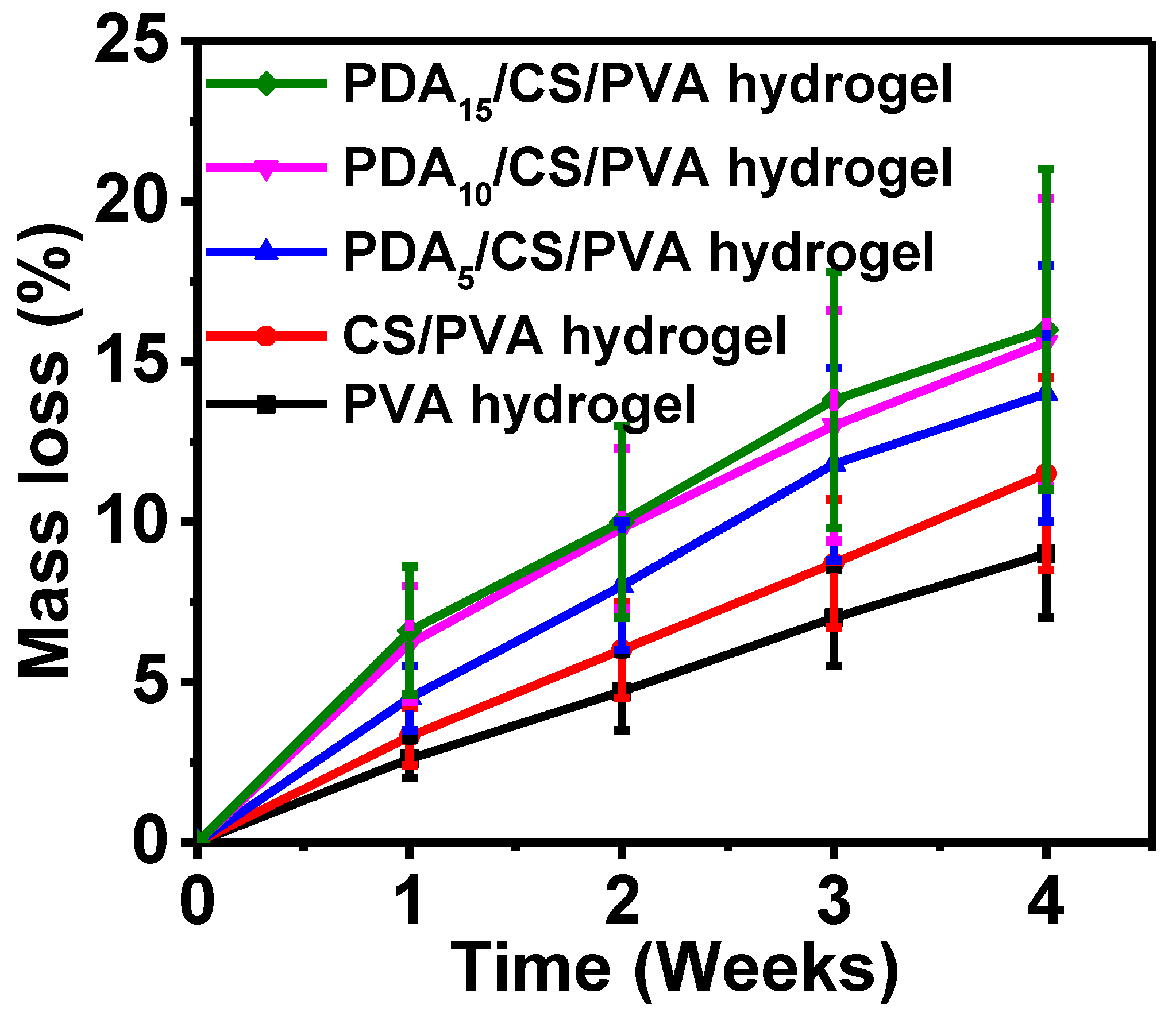
3.3. Mass Loss
3.4. Cytocompatibility
4. Discussion
5. Conclusions
- (1)
- PDA/CS/PVA hydrogel appeared in two new peaks (N 1s, S 2p)at 400.1 eV and 153.1 eV in the spectrum. The peaks of the PDA/CS/PVA hydrogel at 287.1 eV, 286.0 eV and 284.5 eV were attributed to the bonds of O=C-O/C-S, C-O/C-N and C-C, respectively. This result indicated the PDA/CS/PVA hydrogel was prepared successfully.
- (2)
- PDA/CS/PVA composite hydrogels had good porosity and mechanical properties. With the increase in PDA content, the porosity and mechanical properties of the hydrogel increased. When the PDA content was 15 mg, the composite hydrogel exhibited excellent porosity of 95.1%, compression strength of 5.2 MPa, and good lap shear strength of 21 kPa on porcine skin. In addition, the mass loss of the hydrogels was less than 20.0%. The composite hydrogel also had good cytocompatibility. PDA/CS/PVA hydrogel is cytocompatible as a starting point and it can be further investigated in tissue engineering.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Banerjee, R. Biopolymer-Based Hydrogels for Cartilage Tissue Engineering. Chem. Rev. 2011, 111, 4453–4474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Yang, P.; Duan, G.; Liu, X.; Gu, Z.; Li, Y. Polyphenol scaffolds in tissue engineering. Mater. Horiz. 2021, 8, 145–167. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Lin, Q.; Zhao, Y. Supramolecular Adhesive Hydrogels for Tissue Engineering Applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef]
- Yang, J.; Yu, H.; Wang, L.; Liu, J.; Liu, X.; Hong, Y.; Huang, Y.; Ren, S. Advances in adhesive hydrogels for tissue engineering. Eur. Polym. J. 2022, 172, 111241. [Google Scholar] [CrossRef]
- Tan, L.; Gong, M.; Zheng, F.; Zhang, B.; Yang, K. Study on compression behavior of porous magnesium used as bone tissue engineering scaffolds. Biomed. Mater. 2009, 4, 015016. [Google Scholar] [CrossRef]
- VanderStok, J.; VanderJagt, O.P.; AminYavari, S.; DeHaas, M.F.P.; Waarsing, J.H.; Jahr, H.; VanLieshout, E.M.M.; Patka, P.; Verhaar, J.A.N.; Zadpoor, A.A.; et al. Selective laser melting-produced porous titanium scaffolds regenerate bone in critical size cortical bone defects. J. Orthop. Res. 2013, 31, 792–799. [Google Scholar] [CrossRef]
- Hing, K.A.; Revell, P.A.; Smith, N.; Buckland, T. Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials 2006, 27, 5014–5026. [Google Scholar] [CrossRef]
- Yan, T.; Wu, X.; Cui, Y.; Chen, Q.; Yang, Z. Porous Calcium Sulfate/Hydroxyapatite Whiskers Scaffold for Bone Tissue Engineering. Adv. Mater. Res. 2013, 738, 38–41. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Yu, J.; Zhao, Q.; Cao, C.; Shen, J.; Shi, D. Preparation and properties of polydopamine and alginate porous complex scaffolds. Chin. J. Appl. Chem. 2018, 35, 665–673. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed]
- Peresin, M.S.; Habibi, Y.; Zoppe, J.O.; Pawlak, J.J.; Rojas, O.J. Nanofiber Composites of Polyvinyl Alcohol and Cellulose Nanocrystals: Manufacture and Characterization. Biomacromolecules 2010, 11, 674–681. [Google Scholar] [CrossRef]
- Busto, M.D.; Meza, V.; Ortega, N.; Perez-Mateos, M. Immobilization of naringinase from Aspergillus niger CECT 2088 in poly(vinyl alcohol) cryogels for the debittering of juices. Food Chem. 2007, 104, 1177–1182. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, X.; Wang, Y.; Xu, H.; Chen, X. Performance and characterization of irradiated poly(vinyl alcohol)/polyvinylpyrrolidone composite hydrogels used as cartilages replacement. J. Appl. Polym. Sci. 2009, 113, 736–741. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Chen, Y.; Han, X.; Jiang, F. Cellulose Nanofibrils Enhanced, Strong, Stretchable, Freezing-Tolerant Ionic Conductive Organohydrogel for Multi-Functional Sensors. Adv. Funct. Mater. 2020, 30, 2003430. [Google Scholar] [CrossRef]
- Wen, J.; Tang, J.; Ning, H.; Hu, N.; Zhu, Y.; Gong, Y.; Xu, C.; Zhao, Q.; Jiang, X.; Hu, X.; et al. Multifunctional Ionic Skin with Sensing, UV-Filtering, Water-Retaining, and Anti-Freezing Capabilities. Adv. Funct. Mater. 2021, 31, 2011176. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, J.; Yu, H.-Y.; Li, Y. Fabricating robust soft-hard network of self-healable polyvinyl alcohol composite films with functionalized cellulose nanocrystals. Compos. Sci. Technol. 2020, 194, 108165. [Google Scholar] [CrossRef]
- Ding, B.; Kimura, E.; Sato, T.; Fujita, S.; Shiratori, S. Fabrication of blend biodegradable nanofibrous nonwoven mats via multi-jet electrospinning. Polymer 2004, 45, 1895–1902. [Google Scholar] [CrossRef]
- Jeun, J.-P.; Jeon, Y.-K.; Nho, Y.-C.; Kang, P.-H. Effects of gamma irradiation on the thermal and mechanical properties of chitosan/PVA nanofibrous mats. J. Ind. Eng. Chem. 2009, 15, 430–433. [Google Scholar] [CrossRef]
- Ding, B.; Kim, H.-Y.; Lee, S.-C.; Shao, C.-L.; Lee, D.-R.; Park, S.-J.; Kwag, G.-B.; Choi, K.-J. Preparation and characterization of a nanoscale poly(vinyl alcohol) fiber aggregate produced by an electrospinning method. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1261–1268. [Google Scholar] [CrossRef]
- Han, L.; Wang, M.; Li, P.; Gan, D.; Yan, L.; Xu, J.; Wang, K.; Fang, L.; Chan, C.W.; Zhang, H.; et al. Mussel-Inspired Tissue-Adhesive Hydrogel Based on the Polydopamine–Chondroitin Sulfate Complex for Growth-Factor-Free Cartilage Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 28015–28026. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Lee, E.A.; An, Y.-H.; Kim, S.L.; Lee, S.S.; Yu, S.J.; Jang, H.L.; Nam, K.T.; Im, S.G.; Hwang, N.S. Chondroitin Sulfate-Based Biomineralizing Surface Hydrogels for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2017, 9, 21639–21650. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Sun, D.; Liu, Y.; Liu, Y.; Wang, Y.; Liu, C.; Wu, H.; Lv, Y.; Ren, Y.; et al. Development of a Biomimetic Chondroitin Sulfate-modified Hydrogel to Enhance the Metastasis of Tumor Cells. Sci. Rep. 2016, 6, 29858. [Google Scholar] [CrossRef]
- Ma, J.; He, Y.; Zeng, G.; Li, F.; Li, Y.; Xiao, J.; Yang, S. Bio-inspired method to fabricate poly-dopamine/reduced graphene oxide composite membranes for dyes and heavy metalion removal. Polym. Adv. Technol. 2018, 29, 941–950. [Google Scholar] [CrossRef]
- Cheng, W.; Zeng, X.; Chen, H.; Li, Z.; Zeng, W.; Mei, L.; Zhao, Y. Versatile Polydopamine Platforms: Synthesis and Promising Applications for Surface Modification and Advanced Nanomedicine. ACS Nano 2019, 13, 8537–8565. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, X.; Yang, P.; Liang, G.; Yang, Y.; Gu, Z.; Li, Y. Regulating the absorption spectrum of polydopamine. Sci. Adv. 2020, 6, eabb4696. [Google Scholar] [CrossRef]
- Jin, Z.; Yang, L.; Shi, S.; Wang, T.; Duan, G.; Liu, X.; Li, Y. Flexible Polydopamine Bioelectronics. Adv. Funct. Mater. 2021, 31, 2103391. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, M.; Wang, Y.; Zhang, W.; Zhang, M.; Xiao, H.; Huang, L.; Chen, L.; Ouyang, X.; Zeng, H.; et al. Mussel-inspired cellulose-based adhesive with biocompatibility and strong mechanical strength via metal coordination. Int. J. Biol. Macromol. 2020, 144, 127–134. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, K.; Zhang, Y.; Jiang, Y.; Lu, X.; Fang, L.; Gan, D.; Lv, C.; Zhang, H.; Qu, S. Protein-Affinitive Polydopamine Nanoparticles as an Efficient Surface Modification Strategy for Versatile Porous Scaffolds Enhancing Tissue Regeneration. Part. Part. Syst. Charact. 2016, 33, 89–100. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.; Ryu, J.H.; Lee, H. Chitosan-catechol: A polymer with long-lasting mucoadhesive properties. Biomaterials 2015, 52, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Xie, J.; Zheng, Y.; Wei, D.; Yao, D.; Zhang, J.; Zhang, T. Antibacterial, Self-Adhesive, Recyclable, and Tough Conductive Composite Hydrogels for Ultrasensitive Strain Sensing. ACS Appl. Mater. Interfaces 2020, 12, 22225–22236. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Bian, S.; Wei, J.; Xiao, H.; Zhang, M.; Liu, K.; Huang, L.; Chen, L.; Ni, Y.; Wu, H. Plant-inspired conductive adhesive organohydrogel with extreme environmental tolerance as a wearable dressing for multifunctional sensors. Colloids Surf. B Biointerfaces 2022, 215, 112509. [Google Scholar] [CrossRef]
- Meredith, H.J.; Jenkins, C.L.; Wilker, J.J. Enhancing the Adhesion of a Biomimetic Polymer Yields Performance Rivaling Commercial Glues. Adv. Funct. Mater. 2014, 24, 3259–3267. [Google Scholar] [CrossRef]
- Panchireddy, S.; Grignard, B.; Thomassin, J.-M.; Jerome, C.; Detrembleur, C. Catechol Containing Polyhydroxyurethanes as High-Performance Coatings and Adhesives. ACS Sustain. Chem. Eng. 2018, 6, 14936–14944. [Google Scholar] [CrossRef]
- Zhao, M.; Tang, Z.; Zhang, X.; Li, Z.; Xiao, H.; Zhang, M.; Liu, K.; Ni, Y.; Huang, L.; Chen, L.; et al. A self-healing, stretchable, and conductive Poly(N-vinylpyrrolidone)/gallic acid composite hydrogel formed via hydrogen bonding for wearable electronic sensors. Compos. Sci. Technol. 2020, 198, 108294. [Google Scholar] [CrossRef]
- Hsu, Y.Y.; Gresser, J.D.; Trantolo, D.J.; Lyons, C.M.; Gangadharam, P.R.; Wise, D.L. Effect of polymer foam morphology and density on kinetics of in vitro controlled release of isoniazid from compressed foam matrices. J. Biomed. Mater. Res. 1997, 35, 107–116. [Google Scholar] [CrossRef]
- Qiu, H.; Yang, J.; Kodali, P.; Koh, J.; Ameer, G.A. A citricacid-based hydroxyapatite composite for orthopedic implants. Biomaterials 2006, 27, 5845–5854. [Google Scholar] [CrossRef] [PubMed]
- Fullenkamp, D.E.; Rivera, J.G.; Gong, Y.-K.; Lau, K.H.A.; He, L.; Varshney, R.; Messersmith, P.B. Mussel-inspired silver-releasing antibacterial hydrogels. Biomaterials 2012, 33, 3783–3791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; He, J.; Bai, Y.; Zeng, H. Mussel-inspired adhesive and conductive hydrogel with tunable mechanical properties for wearable strain sensors. J. Colloid Interface Sci. 2021, 585, 420–432. [Google Scholar] [CrossRef]
- Chai, F.; Abdelkarim, M.; Laurent, T.; Tabary, N.; Degoutin, S.; Simon, N.; Peters, F.; Blanchemain, N.; Martel, B.; Hildebrand, H.F. Poly-cyclodextrin functionalized porous bioceramics for local chemotherapy and anticancer bone reconstruction. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2014, 102, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Scully, A.; Young, D.A.; Kim, S.; Tsao, H.; Sen, M.; Yang, Y. Enhanced mechanical performance and biological evaluation of a PLGA coated β-TCP composite scaffold for load-bearing applications. Eur. Polym. J. 2011, 47, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ma, S.; Yang, Z.; Zhou, W.; Du, Z.; Huang, J.; Yi, H.; Wang, C. Facile fabrication of poly(L-lacticacid) microsphere-incorporated calcium alginate/hydroxyapatite porous scaffolds based on Pickering emulsion templates. Colloids Surf. B Biointerfaces 2016, 140, 382–391. [Google Scholar] [CrossRef]
- Kundu, B.; Kundu, S.C. Silk sericin/polyacrylamide in situ forming hydrogels for dermal reconstruction. Biomaterials 2012, 33, 7456–7467. [Google Scholar] [CrossRef]
- Cao, B.; Yin, J.; Yan, S.; Cui, L.; Chen, X.; Xie, Y. Porous Scaffolds Based on Cross-Linking of Poly(L-glutamicacid). Macromol. Biosci. 2011, 11, 427–434. [Google Scholar] [CrossRef]
- Lu, S.; Tang, Z.; Li, W.; Ouyang, X.; Cao, S.; Chen, L.; Huang, L.; Wu, H.; Ni, Y. Diallyl dimethyl ammonium chloride-grafted cellulose filter membrane via ATRP for selective removal of anionic dye. Cellulose 2018, 25, 7261–7275. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Peng, X.-F.; Turng, L.-S. Biocompatible, self-healing, highly stretchable polyacrylic acid/reduced graphene oxide nanocomposite hydrogel sensors via mussel-inspired chemistry. Carbon 2018, 136, 63–72. [Google Scholar] [CrossRef]
- Vogt, A.D.; Han, T.; Beebe, T.P. Adsorption of 11-Mercaptoundecanoic Acid on Ni(111) and Its Interaction with Probe Molecules. Langmuir 1997, 13, 3397–3403. [Google Scholar] [CrossRef]
- Guo, T.; Gu, L.; Zhang, Y.; Chen, H.; Jiang, B.; Zhao, H.; Jin, Y.; Xiao, H. Bioinspired self-assembled films of carboxymethyl cellulose–dopamine/montmorillonite. J. Mater. Chem. A 2019, 7, 14033–14041. [Google Scholar] [CrossRef]
- Li, W.; Lu, S.; Zhao, M.; Lin, X.; Zhang, M.; Xiao, H.; Liu, K.; Huang, L.; Chen, L.; Ouyang, X.; et al. Self-Healing Cellulose Nanocrystals-Containing Gels via Reshuffling of Thiuram Disulfide Bonds. Polymers 2018, 10, 1392. [Google Scholar] [CrossRef]
- Tang, Z.; Miao, Y.; Zhao, J.; Xiao, H.; Zhang, M.; Liu, K.; Zhang, X.; Huang, L.; Chen, L.; Wu, H. Mussel-inspired biocompatible polydopamine/carboxymethyl cellulose/polyacrylic acid adhesive hydrogels with UV-shielding capacity. Cellulose 2021, 28, 1527–1540. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, H.; Konst, S.; Sarmiento, R.; Rajachar, R.; Lee, B.P. Injectable Dopamine-Modified Poly(ethylene glycol) Nanocomposite Hydrogel with Enhanced Adhesive Property and Bioactivity. ACS Appl. Mater. Interfaces 2014, 6, 16982–16992. [Google Scholar] [CrossRef] [PubMed]



| Samples | PVA (g) | CS (mg) | PDA (mg) | Water (g) |
|---|---|---|---|---|
| PVA Hydrogel | 2 | 0 | 0 | 18 |
| CS/PVA Hydrogel | 2 | 100 | 0 | 18 |
| PDA5/CS/PVA Hydrogel | 2 | 100 | 5 | 18 |
| PDA10/CS/PVA Hydrogel | 2 | 100 | 10 | 18 |
| PDA15/CS/PVA Hydrogel | 2 | 100 | 15 | 18 |
| Samples | ε1/% | ε2/% | ε3/% | /% |
|---|---|---|---|---|
| PVA Hydrogel | 58.5 | 59.7 | 60.6 | 59.6 |
| CS/PVA Hydrogel | 79.9 | 77.8 | 80.7 | 79.4 |
| PDA5/CS/PVA Hydrogel | 80.2 | 81.6 | 81.0 | 80.9 |
| PDA10/CS/PVA Hydrogel | 85.1 | 83.3 | 85.7 | 84.7 |
| PDA15/CS/PVA Hydrogel | 94.7 | 95.8 | 94.9 | 95.1 |
| Samples | Bonding Energy (eV) | Assignment |
|---|---|---|
| PVA hydrogels | O 1s | 532.1 eV |
| C 1s | 285.1 eV | |
| C-O | 286.0 eV | |
| C-C | 284.5 eV | |
| CS/PVA hydrogels | O 1s | 532.1 eV |
| C 1s | 285.1 eV | |
| N 1s | 400.1 eV | |
| S 2p | 153.1 eV | |
| O=C-O/C-S | 287.1 eV | |
| C-N/C-O | 286.0 eV | |
| C-C | 284.5 eV | |
| PDA/CS/PVA hydrogels | O 1s | 532.1 eV |
| C 1s | 285.1 eV | |
| N 1s | 400.1 eV | |
| S 2p | 153.1 eV | |
| O=C-O/C-S | 287.1 eV | |
| C-O/C-N | 286.0 eV | |
| C-C | 284.5 eV |
| Scaffold | Pore Size (μm) | Porosity | Biocompatiblity | References |
|---|---|---|---|---|
| Titanium | 490 | 68%, 88% | Bioinert | [9] |
| β-Tricalcium phosphate | 350–500 | 81% | Bioactive | [45] |
| Poly(L-lacticacid) | >100 | 90% | Biocompatible | [46] |
| Polyacrylamide | 23–52 | 98% | Biocompatible | [47] |
| Poly(L-glutamic acid) | 20–50 | 95% | - | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Yu, M.; Mondal, A.K.; Lin, X. Porous Scaffolds Based on Polydopamine/Chondroitin Sulfate/Polyvinyl Alcohol Composite Hydrogels. Polymers 2023, 15, 271. https://doi.org/10.3390/polym15020271
Tang Z, Yu M, Mondal AK, Lin X. Porous Scaffolds Based on Polydopamine/Chondroitin Sulfate/Polyvinyl Alcohol Composite Hydrogels. Polymers. 2023; 15(2):271. https://doi.org/10.3390/polym15020271
Chicago/Turabian StyleTang, Zuwu, Meiqiong Yu, Ajoy Kanti Mondal, and Xinxing Lin. 2023. "Porous Scaffolds Based on Polydopamine/Chondroitin Sulfate/Polyvinyl Alcohol Composite Hydrogels" Polymers 15, no. 2: 271. https://doi.org/10.3390/polym15020271
APA StyleTang, Z., Yu, M., Mondal, A. K., & Lin, X. (2023). Porous Scaffolds Based on Polydopamine/Chondroitin Sulfate/Polyvinyl Alcohol Composite Hydrogels. Polymers, 15(2), 271. https://doi.org/10.3390/polym15020271








