Biodegradable Nanofiber/Metal–Organic Framework/Cotton Air Filtration Membranes Enabling Simultaneous Removal of Toxic Gases and Particulate Matter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microscopic Characterization
2.3. Spectroscopic Characterization
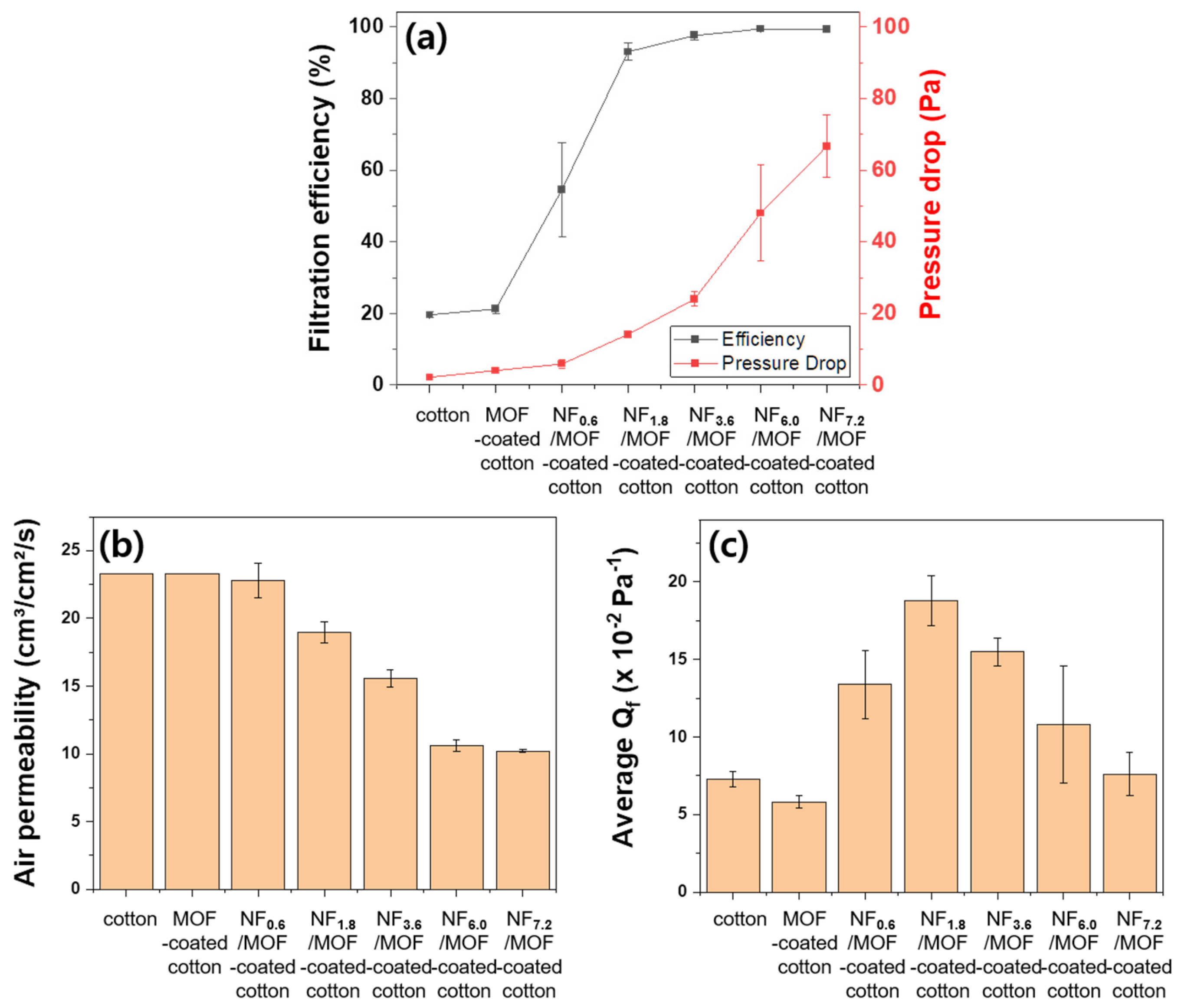
2.4. Assessment of Air Permeability
2.5. Evaluation of Gas Adsorption Capability
2.6. Assessment of Biodegradability
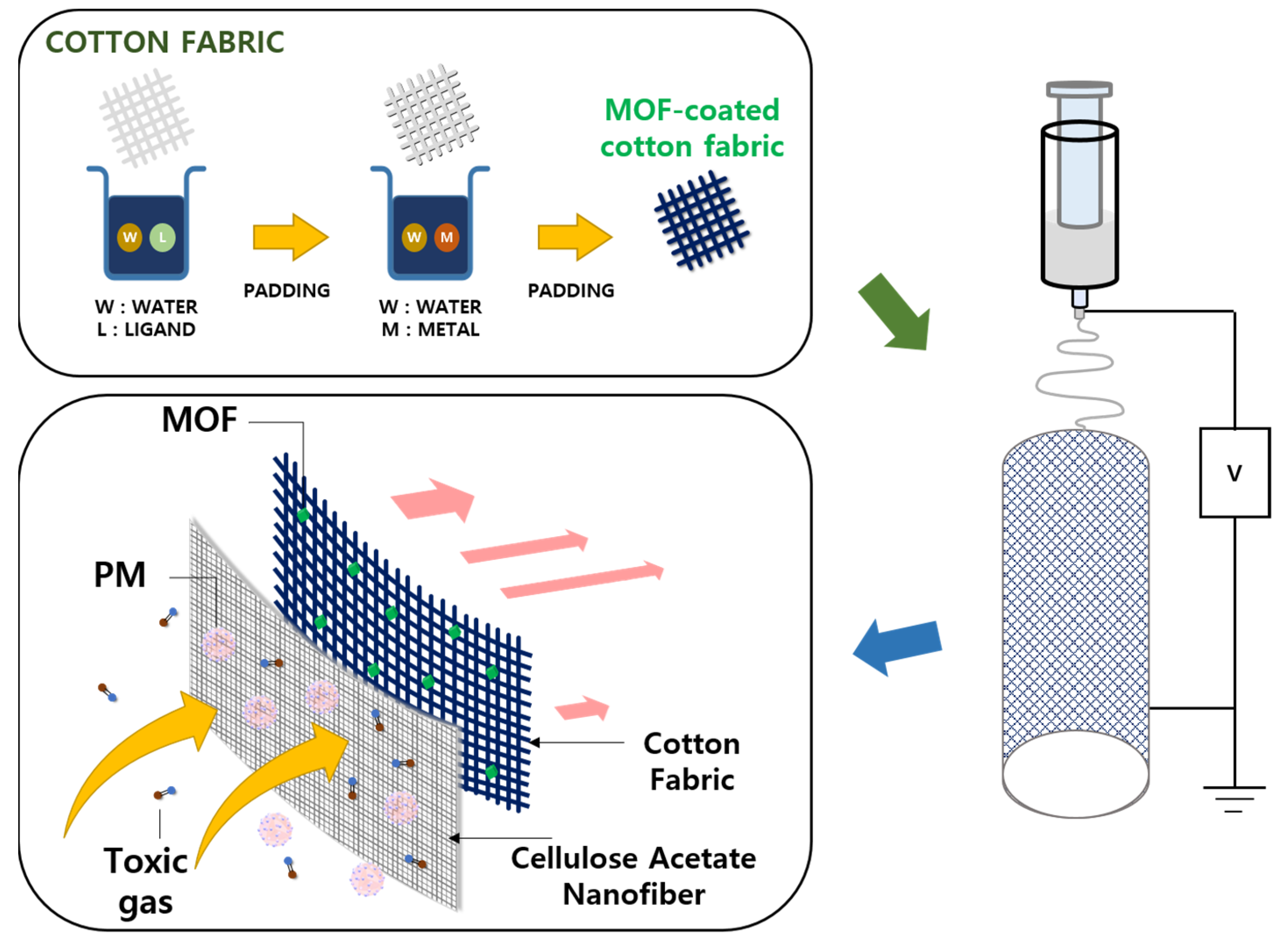
2.7. Preparation of MOF-Coated Cotton Fabric
2.8. Electrospinning of CA NFs on Cotton Fabric
3. Results and Discussion
3.1. Fabrication of MOF-Coated Cotton Fabric
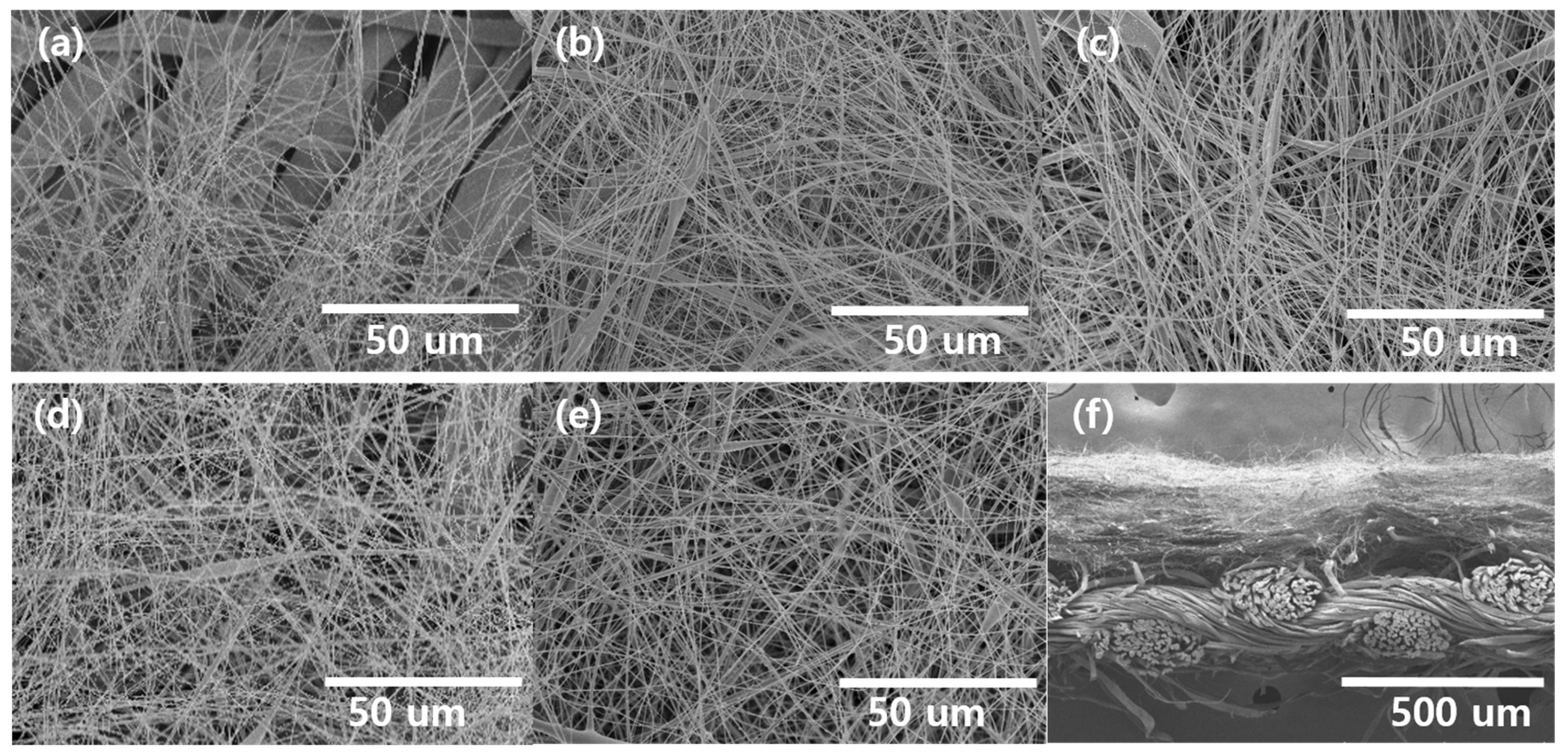
3.2. Electrospinning of CA Nanofiber on MOF-Coated Cotton Fabric
3.3. Characterization of the Fabricated Filtration Membrane
3.4. Air Filtration Performance of the Filtration Membrane
3.5. Gas Adsorption Behavior of Membrane Filter
3.6. Biodegradability of the Filtration Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, H.; Yan, W.; Elahi, E.; Cao, Y. Air pollution risks human mental health: An implication of two-stages least squares estimation of interaction effects. Environ. Sci. Pollut. Res. 2020, 27, 2036–2043. [Google Scholar] [CrossRef]
- World Health Organization. Air Pollution: The Invisible Health Threat; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Wu, S.; Deng, F.; Wei, H.; Huang, J.; Wang, X.; Hao, Y.; Zheng, C.; Qin, Y.; Lv, H.; Shima, M. Association of cardiopulmonary health effects with source-appointed ambient fine particulate in Beijing, China: A combined analysis from the Healthy Volunteer Natural Relocation (HVNR) study. Environ. Sci. Technol. 2014, 48, 3438–3448. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Kim, D.; Son, D.; Doh, S.J.; Kim, M.; Lee, H.; Yoon, K.R. Rational process design for facile fabrication of dual functional hybrid membrane of MOF and electrospun nanofiber towards high removal efficiency of PM2.5 and toxic gases. Macromol. Rapid Commun. 2022, 43, 2100648. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- World Health Organization. What are the WHO air quality guidelines? In Improving Health by Reducing Air Pollution; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Benson, N.U.; Fred-Ahmadu, O.H.; Bassey, D.E.; Atayero, A.A. COVID-19 pandemic and emerging plastic-based personal protective equipment waste pollution and management in Africa. J. Environ. Chem. Eng. 2021, 9, 105222. [Google Scholar] [CrossRef]
- World Health Organization. Tonnes of COVID-19 Health Care Waste Expose Urgent Need to Improve Waste Management Systems; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Venkatarajan, S.; Athijayamani, A. An overview on natural cellulose fiber reinforced polymer composites. Mater. Today Proc. 2021, 37, 3620–3624. [Google Scholar] [CrossRef]
- Luo, H.; Xie, J.; Xiong, L.; Zhu, Y.; Yang, Z.; Wan, Y. Fabrication of flexible, ultra-strong, and highly conductive bacterial cellulose-based paper by engineering dispersion of graphene nanosheets. Compos. Part B Eng. 2019, 162, 484–490. [Google Scholar] [CrossRef]
- Yao, Y.; Zeng, X.; Pan, G.; Sun, J.; Hu, J.; Huang, Y.; Sun, R.; Xu, J.-B.; Wong, C.-P. Interfacial engineering of silicon carbide nanowire/cellulose microcrystal paper toward high thermal conductivity. ACS Appl. Mater. Interfaces 2016, 8, 31248–31255. [Google Scholar] [CrossRef]
- Islam, S.U.; Ul-Islam, M.; Ahsan, H.; Ahmed, M.B.; Shehzad, A.; Fatima, A.; Sonn, J.K.; Lee, Y.S. Potential applications of bacterial cellulose and its composites for cancer treatment. Int. J. Biol. Macromol. 2021, 168, 301–309. [Google Scholar] [CrossRef]
- Woo, H.C.; Yoo, D.K.; Jhung, S.H. Highly improved performance of cotton air filters in particulate matter removal by the incorporation of metal–organic frameworks with functional groups capable of large charge separation. ACS Appl. Mater. Interfaces 2020, 12, 28885–28893. [Google Scholar] [CrossRef]
- He, R.; Li, J.; Chen, M.; Zhang, S.; Cheng, Y.; Ning, X.; Wang, N. Tailoring moisture electroactive Ag/Zn@cotton coupled with electrospun PVDF/PS nanofibers for antimicrobial face masks. J. Hazard. Mater. 2022, 428, 128239. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Lu, T.; Cui, J.; Qu, Q.; Wang, Y.; Zhang, J.; Xiong, R.; Ma, W.; Huang, C. Multistructured electrospun nanofibers for air filtration: A review. ACS Appl. Mater. Interfaces 2021, 13, 23293–23313. [Google Scholar] [CrossRef]
- Wu, S.; Dong, T.; Li, Y.; Sun, M.; Qi, Y.; Liu, J.; Kuss, M.A.; Chen, S.; Duan, B. State-of-the-art review of advanced electrospun nanofiber yarn-based textiles for biomedical applications. Appl. Mater. Today 2022, 27, 101473. [Google Scholar] [CrossRef]
- An, S.; Jeon, B.; Bae, J.H.; Kim, I.S.; Paeng, K.; Kim, M.; Lee, H. Thiol-based chemistry as versatile routes for the effective functionalization of cellulose nanofibers. Carbohydr. Polym. 2019, 226, 115259. [Google Scholar] [CrossRef]
- Choi, H.Y.; Bae, J.H.; Hasegawa, Y.; An, S.; Kim, I.S.; Lee, H.; Kim, M. Thiol-functionalized cellulose nanofiber membranes for the effective adsorption of heavy metal ions in water. Carbohydr. Polym. 2020, 234, 115881. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Harris, T.D. Metal–organic framework magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef]
- Huang, D.; Xin, Q.; Ni, Y.; Shuai, Y.; Wang, S.; Li, Y.; Ye, H.; Lin, L.; Ding, X.; Zhang, Y. Synergistic effects of zeolite imidazole framework@ graphene oxide composites in humidified mixed matrix membranes on CO2 separation. RSC Adv. 2018, 8, 6099–6109. [Google Scholar] [CrossRef]
- Jameh, A.A.; Mohammadi, T.; Bakhtiari, O.; Mahdyarfar, M. Synthesis and modification of Zeolitic Imidazolate Framework (ZIF-8) nanoparticles as highly efficient adsorbent for H2S and CO2 removal from natural gas. J. Environ. Chem. Eng. 2019, 7, 103058. [Google Scholar] [CrossRef]
- ASTM D737-18; Standard Test Method for Air Permeability of Textile Fabrics. ASTM: West Conshohocken, PA, USA, 2023.
- Huang, S.-H.; Chen, C.-W.; Kuo, Y.-M.; Lai, C.-Y.; McKay, R.; Chen, C.-C. Factors affecting filter penetration and quality factor of particulate respirators. Aerosol Air Qual. Res. 2013, 13, 162–171. [Google Scholar] [CrossRef]
- Pradhan, S.; Behera, S.K.; Samal, S.K.; Panda, I.; Sahu, P.K.; Priyadarshini, S. Interaction between 2-methylimidazole and 1-butanol/1-octanol: Thermophysical and computational studies. ChemistrySelect 2023, 8, e202204931. [Google Scholar] [CrossRef]
- Ramos, V.C.; Reyes, C.B.G.; García, G.M.; Quesada, M.I.S.; Barrero, F.J.M.-C.; Rábago, J.J.S.; Polo, M.S. ZIF-8 and its magnetic functionalization as vehicle for the transport and release of ciprofloxacin. Pharmaceutics 2022, 14, 2546. [Google Scholar] [CrossRef]
- Watanabe, S.; Ohsaki, S.; Hanafusa, T.; Takada, K.; Tanaka, H.; Mae, K.; Miyahara, M.T. Synthesis of zeolitic imidazolate framework-8 particles of controlled sizes, shapes, and gate adsorption characteristics using a central collision-type microreactor. Chem. Eng. J. 2017, 313, 724–733. [Google Scholar] [CrossRef]
- Lee, H.; An, S.; Kim, S.; Jeon, B.; Kim, M.; Kim, I.S. Readily functionalizable and stabilizable polymeric particles with controlled size and morphology by electrospray. Sci. Rep. 2018, 8, 15725. [Google Scholar] [CrossRef]
- Lee, H.; Woo, J.; Son, D.; Kim, M.; Choi, W.I.; Sung, D. Electrospinning/electrospray of ferrocene containing copolymers to fabricate ROS-responsive particles and fibers. Polymers 2020, 12, 2520. [Google Scholar] [CrossRef]
- Bian, Y.; Wang, S.; Zhang, L.; Chen, C. Influence of fiber diameter, filter thickness, and packing density on PM2.5 removal efficiency of electrospun nanofiber air filters for indoor applications. Build. Environ. 2020, 170, 106628. [Google Scholar] [CrossRef]
- Liu, T.; Cai, C.; Ma, R.; Deng, Y.; Tu, L.; Fan, Y.; Lu, D. Super-hydrophobic cellulose nanofiber air filter with highly efficient filtration and humidity resistance. ACS Appl. Mater. Interfaces 2021, 13, 24032–24041. [Google Scholar] [CrossRef]
- Modi, A.; Jiang, Z.; Kasher, R. Hydrostable ZIF-8 layer on polyacrylonitrile membrane for efficient treatment of oilfield produced water. Chem. Eng. J. 2022, 434, 133513. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.; Zhao, L.; Cheng, F.; Chang, L.; Wu, D. Constructing ZIF-8-decorated montmorillonite composite with charge neutralization effect and pore structure optimization for enhanced Pb2+ capture from water. Chem. Eng. J. 2023, 466, 143014. [Google Scholar] [CrossRef]
- Schejn, A.; Balan, L.; Falk, V.; Aranda, L.; Medjahdi, G.; Schneider, R. Controlling ZIF-8 nano-and microcrystal formation and reactivity through zinc salt variations. CrystEngComm 2014, 16, 4493–4500. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Cabrales, L.; Abidi, N.; Manciu, F. Characterization of developing cotton fibers by confocal Raman microscopy. Fibers 2014, 2, 285–294. [Google Scholar] [CrossRef]
- Sousa-Pinto, B.; Fonte, A.P.; Lopes, A.A.; Oliveira, B.; Fonseca, J.A.; Costa-Pereira, A.; Correia, O. Face masks for community use: An awareness call to the differences in materials. Respirology 2020, 25, 894. [Google Scholar] [CrossRef]
- Binaeian, E.; El-Sayed, E.-S.M.; Matikolaei, M.K.; Yuan, D. Experimental strategies on enhancing toxic gases uptake of metal–organic frameworks. Coord. Chem. Rev. 2021, 430, 213738. [Google Scholar] [CrossRef]
- Rana, A.; Biswas, S. Electrophilicity modulated targeted luminescence of MOF-coated cotton composite for dual analyte detection in aqueous medium. Inorg. Chem. Front. 2023, 10, 2742–2753. [Google Scholar] [CrossRef]
- ASTM D6400-04; Standard Specification for Compostable Plastics. ASTM: West Conshohocken, PA, USA, 1999.
- Zambrano, M.C.; Pawlak, J.J.; Venditti, R.A. Effects of chemical and morphological structure on biodegradability of fibers, fabrics, and other polymeric materials. BioResources 2020, 15, 9786. [Google Scholar] [CrossRef]
- Li, L.; Frey, M.; Browning, K.J. Biodegradability study on cotton and polyester fabrics. J. Eng. Fibers Fabr. 2010, 5, 155892501000500406. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, S.; Kim, D.; Lee, H.; Kim, Y.; Lee, Y.; Kim, M.; Lee, H.; Lee, H. Biodegradable Nanofiber/Metal–Organic Framework/Cotton Air Filtration Membranes Enabling Simultaneous Removal of Toxic Gases and Particulate Matter. Polymers 2023, 15, 3965. https://doi.org/10.3390/polym15193965
Ryu S, Kim D, Lee H, Kim Y, Lee Y, Kim M, Lee H, Lee H. Biodegradable Nanofiber/Metal–Organic Framework/Cotton Air Filtration Membranes Enabling Simultaneous Removal of Toxic Gases and Particulate Matter. Polymers. 2023; 15(19):3965. https://doi.org/10.3390/polym15193965
Chicago/Turabian StyleRyu, Sujin, Doyeon Kim, Hyewon Lee, Yoonjin Kim, Youngbok Lee, Myungwoong Kim, Heedong Lee, and Hoik Lee. 2023. "Biodegradable Nanofiber/Metal–Organic Framework/Cotton Air Filtration Membranes Enabling Simultaneous Removal of Toxic Gases and Particulate Matter" Polymers 15, no. 19: 3965. https://doi.org/10.3390/polym15193965
APA StyleRyu, S., Kim, D., Lee, H., Kim, Y., Lee, Y., Kim, M., Lee, H., & Lee, H. (2023). Biodegradable Nanofiber/Metal–Organic Framework/Cotton Air Filtration Membranes Enabling Simultaneous Removal of Toxic Gases and Particulate Matter. Polymers, 15(19), 3965. https://doi.org/10.3390/polym15193965







