Drug Loaded 3D-Printed Poly(ε-Caprolactone) Scaffolds for Local Antibacterial or Anti-Inflammatory Treatment in Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Supplements and Biologicals
2.2. Manufacturing of 3D-Printed Scaffolds Loaded with CIP or DEX
2.3. Compression Test
2.4. Measurements of Specific Surface Area and Porous Characteristics
2.5. Drug Release
2.6. Cytotoxicity
2.7. Osteodifferentiation
2.8. Antibacterial Properties of PCL/CIP
2.9. Anti-Inflammatory Activity of PCL/DEX
2.10. Statistics
3. Results and Discussion
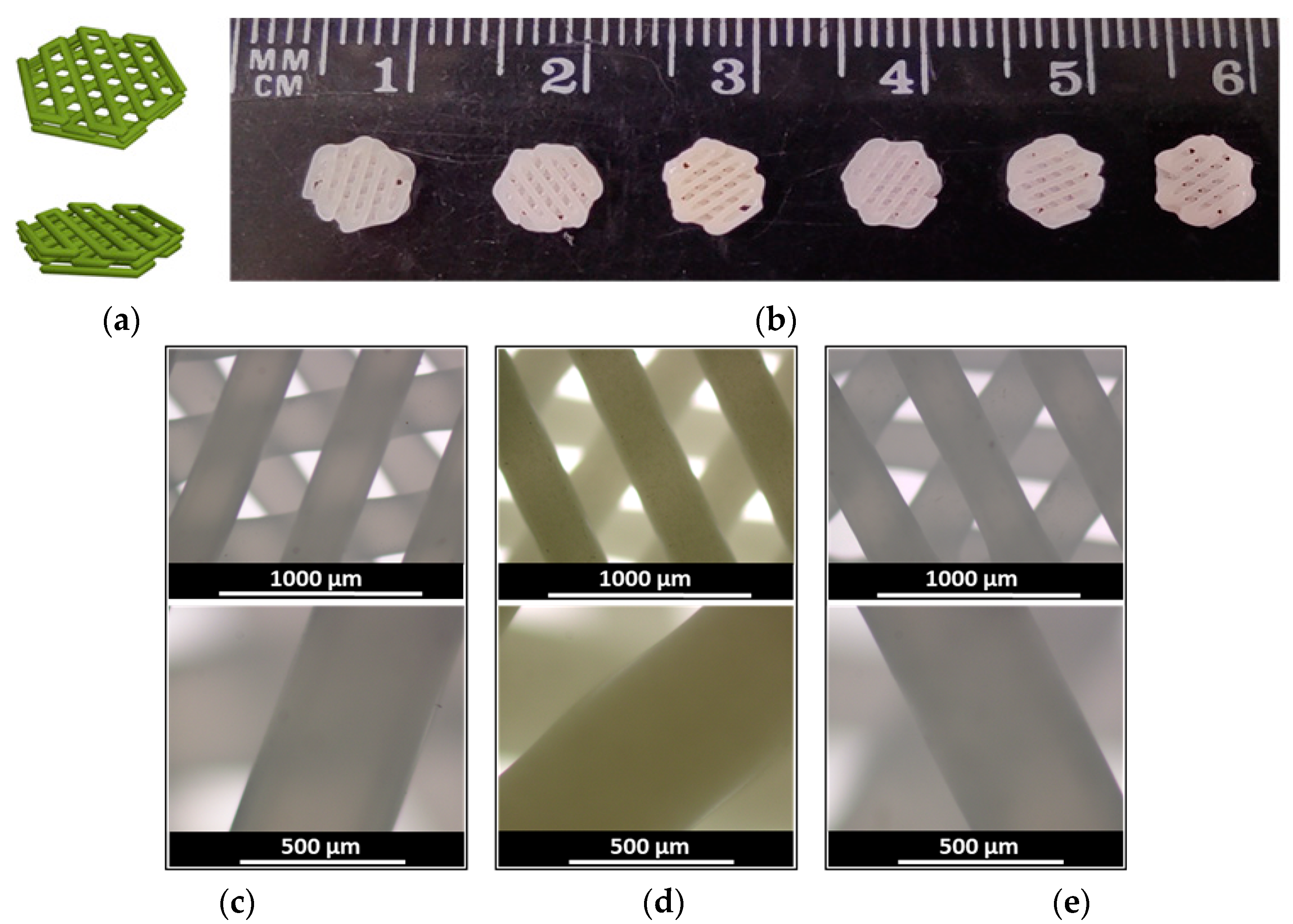
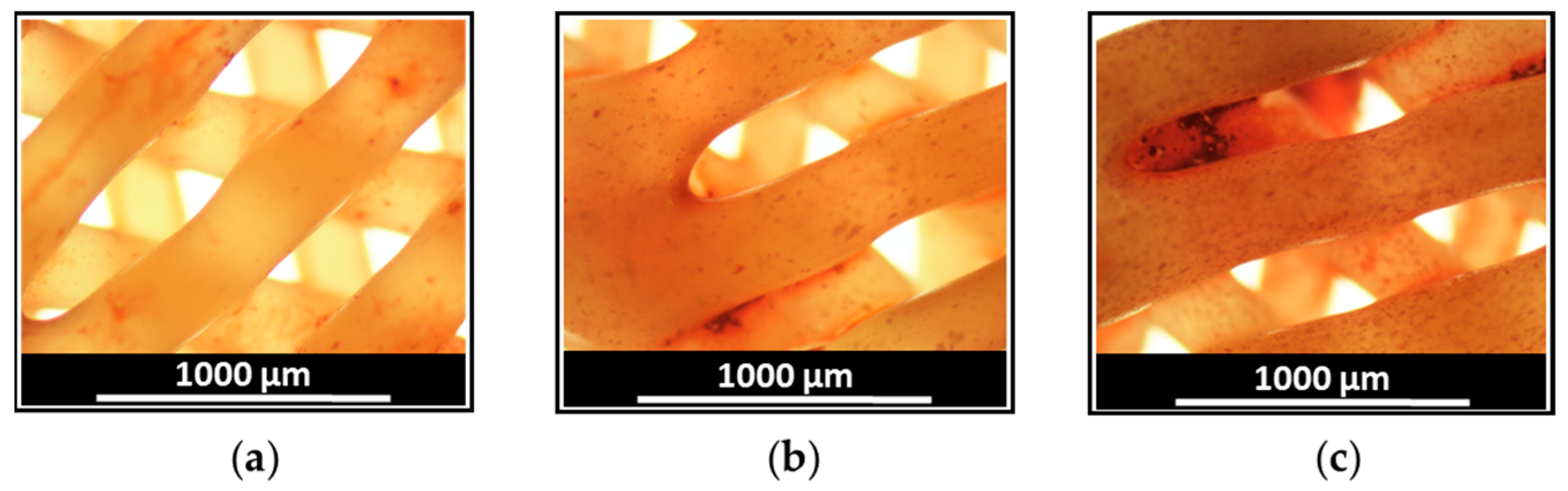
3.1. Manufacturing and Characterization of Drug-Loaded 3D-Printed Scaffolds
3.2. Drug Release Study
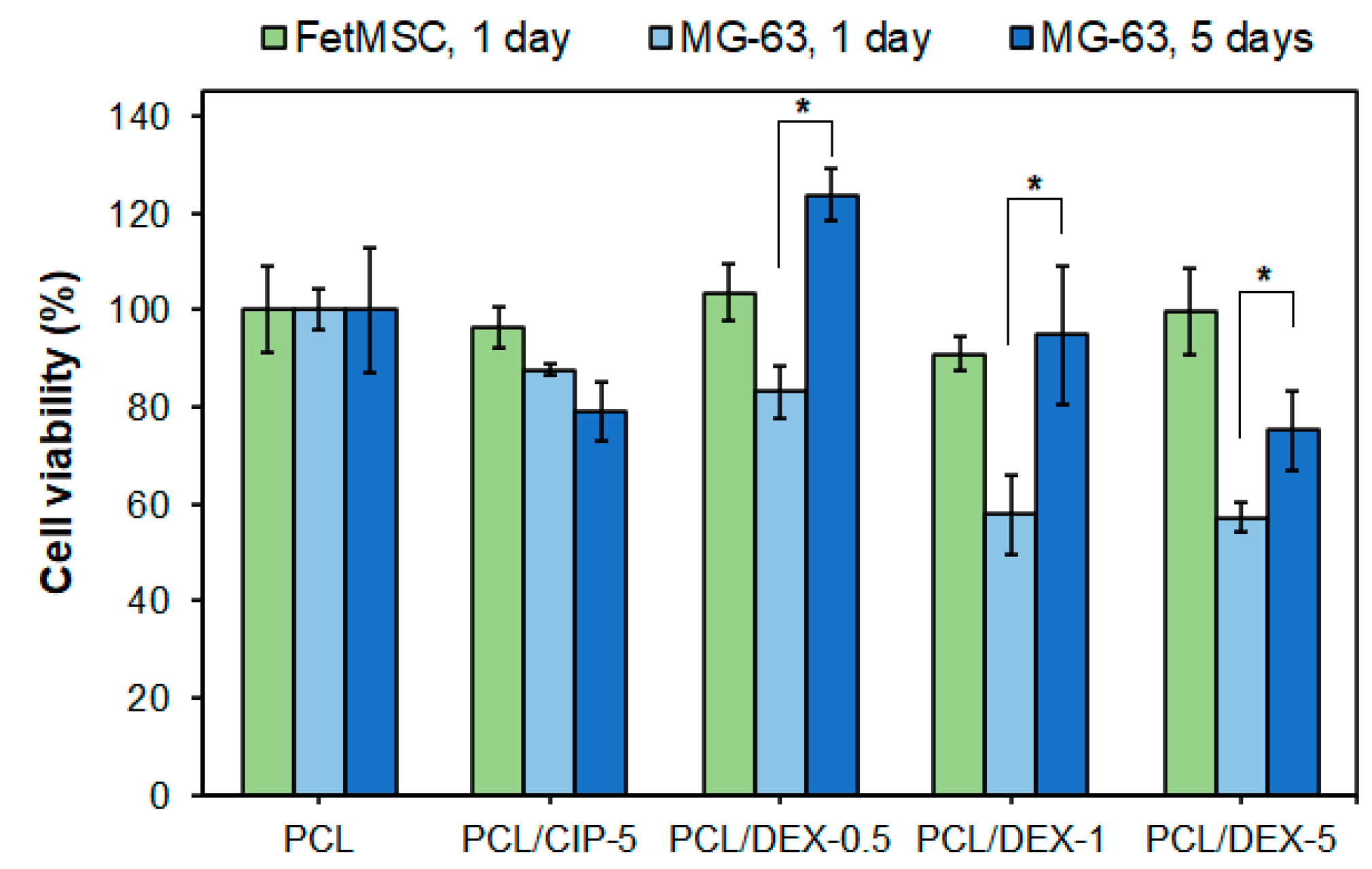
3.3. Cytotoxicity and Osteodifferentiation Study
3.4. Antibacterial Properties of PCL/CIP Scaffolds
3.5. Anti-Inflammatory Properties of PCL/DEX Scaffolds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pountos, I.; Giannoudis, P.V. Is there a role of coral bone substitutes in bone repair? Injury 2016, 47, 2606–2613. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for Bone Regeneration: From Graft to Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of scaffolds for bone-tissue regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Donzelli, E.; Salvadè, A.; Mimo, P.; Viganò, M.; Morrone, M.; Papagna, R.; Carini, F.; Zaopo, A.; Miloso, M.; Baldoni, M.; et al. Mesenchymal stem cells cultured on a collagen scaffold: In vitro osteogenic differentiation. Arch. Oral Biol. 2007, 52, 64–73. [Google Scholar] [CrossRef]
- Murizan, N.I.S.; Mustafa, N.S.; Ngadiman, N.H.A.; Yusof, N.M.; Idris, A. Review on nanocrystalline cellulose in bone tissue engineering applications. Polymers 2020, 12, 2818. [Google Scholar] [CrossRef]
- Costa-Pinto, A.R.; Correlo, V.M.; Sol, P.C.; Bhattacharya, M.; Srouji, S.; Livne, E.; Reis, R.L.; Neves, N.M. Chitosan-poly(butylene succinate) scaffolds and human bone marrow stromal cells induce bone repair in a mouse calvaria model. J. Tissue Eng. Regen. Med. 2012, 6, 21–28. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, Q.; Wootton, D.; Chiou, R.; Li, D.; Lu, B.; Lelkes, P.; Zhou, J. Mechanical study of polycaprolactone-hydroxyapatite porous scaffolds created by porogen-based solid freeform fabrication method. J. Appl. Biomater. Funct. Mater. 2014, 12, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Averianov, I.V.; Stepanova, M.A.; Gofman, I.V.; Lavrentieva, A.; Korzhikov-Vlakh, V.A.; Korzhikova-Vlakh, E.G. Osteoconductive biocompatible 3D-printed composites of poly-d,l-lactide filled with nanocrystalline cellulose modified by poly(glutamic acid). Mendeleev Commun. 2022, 32, 810–812. [Google Scholar] [CrossRef]
- Hannink, G.; Arts, J.J.C. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42, S22–S25. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, A.; Deng, A.; Yang, Y.; Gao, L.; Zhong, Z.; Yang, S. Pore architecture and cell viability on freeze dried 3D recombinant human collagen-peptide (RHC)–chitosan scaffolds. Mater. Sci. Eng. C 2015, 49, 174–182. [Google Scholar] [CrossRef]
- Shi, X.; Wu, H.; Yan, H.; Wang, Y.; Wang, Z.; Zhang, P. Electroactive Nanocomposite Porous Scaffolds of PAPn/op-HA/PLGA Enhance Osteogenesis in Vivo. ACS Appl. Bio Mater. 2019, 2, 1464–1476. [Google Scholar] [CrossRef]
- Jiang, C.P.; Chen, Y.Y.; Hsieh, M.F. Biofabrication and in vitro study of hydroxyapatite/mPEG–PCL–mPEG scaffolds for bone tissue engineering using air pressure-aided deposition technology. Mater. Sci. Eng. C 2013, 33, 680–690. [Google Scholar] [CrossRef]
- Branciforti, M.C.; Bellani, C.F.; Morelli, C.L.; Ferrand, A.; Benkirane-Jessel, N.; Bretas, R.E.S. Poly (Butylene adipate-co-terephthalate) and poly (Ɛ-caprolactone) and their bionanocomposites with cellulose nanocrystals: Thermo-mechanical properties and cell viability study. J. Renew. Mater. 2019, 7, 269–277. [Google Scholar] [CrossRef]
- Prasadh, S.; Suresh, S.; Wong, R. Osteogenic Potential of Graphene in Bone Tissue Engineering Scaffolds. Materials 2018, 11, 1430. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Zhao, Q.; Dai, L.; Li, Y.; Jiang, T. Fabrication of polylactic acid/hydroxyapatite/graphene oxide composite and their thermal stability, hydrophobic and mechanical properties. J. Asian Ceram. Soc. 2017, 5, 160–168. [Google Scholar] [CrossRef]
- Pavia, F.C.; La Carrubba, V.; Piccarolo, S.; Brucato, V. Polymeric scaffolds prepared via thermally induced phase separation: Tuning of structure and morphology. J. Biomed. Mater. Res. Part A 2008, 86A, 459–466. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Teoh, J.E.M.; Suntornnond, R.; Chua, C.K. Design and 3D Printing of Scaffolds and Tissues. Engineering 2015, 1, 261–268. [Google Scholar] [CrossRef]
- Stepanova, M.; Eremin, A.; Averianov, I.; Gofman, I.; Lavrentieva, A.; Korzhikov-Vlakh, V.; Korzhikova-Vlakh, E. Comparison of Supermacroporous Polyester Matrices Fabricated by Thermally Induced Phase Separation and 3D Printing Techniques. Key Eng. Mater. 2019, 822, 277–283. [Google Scholar] [CrossRef]
- Vakharia, V.S.; Kuentz, L.; Salem, A.; Halbig, M.C.; Salem, J.A.; Singh, M. Additive Manufacturing and Characterization of Metal Particulate Reinforced Polylactic Acid (PLA) Polymer Composites. Polymers 2021, 13, 3545. [Google Scholar] [CrossRef]
- Arefin, A.M.E.; Khatri, N.R.; Kulkarni, N.; Egan, P.F. Polymer 3D Printing Review: Materials, Process, and Design Strategies for Medical Applications. Polymers 2021, 13, 1499. [Google Scholar] [CrossRef]
- Bakhshandeh, S.; Amin Yavari, S. Electrophoretic deposition: A versatile tool against biomaterial associated infections. J. Mater. Chem. B 2018, 6, 1128–1148. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, Z.; Jiang, X.; Yarmolenko, M.A.; Rogachev, A.A.; Rogachev, A.V. Antibacterial coatings based on polycaprolactone and polyurethane with prolonged release of ciprofloxacin. Surf. Coat. Technol. 2021, 405, 126584. [Google Scholar] [CrossRef]
- Cometta, S.; Jones, R.T.; Juárez-Saldivar, A.; Donose, B.C.; Yasir, M.; Bock, N.; Dargaville, T.R.; Bertling, K.; Brünig, M.; Rakić, A.D.; et al. Melimine-Modified 3D-Printed Polycaprolactone Scaffolds for the Prevention of Biofilm-Related Biomaterial Infections. ACS Nano 2022, 16, 16497–16512. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.; Hills, T.; Santillo, M.; Gilchrist, M. Extended stability of antimicrobial agents in administration devices. J. Antimicrob. Chemother. 2017, 72, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Iga, C.; Agata, T.; Marcin, Ł.; Natalia, F.; Justyna, K.-L. Ciprofloxacin-Modified Degradable Hybrid Polyurethane-Polylactide Porous Scaffolds Developed for Potential Use as an Antibacterial Scaffold for Regeneration of Skin. Polymers 2020, 12, 171. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, F.; Huang, Y.; Fang, Y.; Shen, M.; Zhu, M.; Shi, X. Encapsulation of Amoxicillin within Laponite-Doped Poly(lactic-co-glycolic acid) Nanofibers: Preparation, Characterization, and Antibacterial Activity. ACS Appl. Mater. Interfaces 2012, 4, 6393–6401. [Google Scholar] [CrossRef] [PubMed]
- Kalwar, K.; Zhang, X.; Bhutto, M.A.; Dali, L.; Shan, D. Incorporation of ciprofloxacin/laponite in polycaprolactone electrospun nanofibers: Drug release and antibacterial studies. Mater. Res. Express 2017, 4, 125401. [Google Scholar] [CrossRef]
- Puga, A.M.; Rey-Rico, A.; Magariños, B.; Alvarez-Lorenzo, C.; Concheiro, A. Hot melt poly-ε-caprolactone/poloxamine implantable matrices for sustained delivery of ciprofloxacin. Acta Biomater. 2012, 8, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, N.M.; Rajendran, N. Biodegradation and cytotoxicity of ciprofloxacin-loaded hydroxyapatite-polycaprolactone nanocomposite film for sustainable bone implants. Int. J. Nanomed. 2015, 10, 119–127. [Google Scholar] [CrossRef]
- Goimil, L.; Jaeger, P.; Ardao, I.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; García-González, C.A. Preparation and stability of dexamethasone-loaded polymeric scaffolds for bone regeneration processed by compressed CO2 foaming. J. CO2 Util. 2018, 24, 89–98. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Nagarajan, S.; Belaid, H.; Farha, C.; Iatsunskyi, I.; Coy, E.; Soussan, L.; Huon, V.; Bares, J.; Belkacemi, K.; et al. Fabrication of 3D printed antimicrobial polycaprolactone scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111525. [Google Scholar] [CrossRef]
- Zhou, Z.; Yao, Q.; Li, L.; Zhang, X.; Wei, B.; Yuan, L.; Wang, L. Antimicrobial Activity of 3D-Printed Poly(ε-Caprolactone) (PCL) Composite Scaffolds Presenting Vancomycin-Loaded Polylactic Acid-Glycolic Acid (PLGA) Microspheres. Med. Sci. Monit. 2018, 24, 6934–6945. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, J.S.; Eom, M.R.; Jo, H.H.; Kwon, I.K.; Kwon, S.K.; Park, S.A. Dexamethasone loaded bilayered 3D tubular scaffold reduces restenosis at the anastomotic site of tracheal replacement: In vitro and in vivo assessments. Nanoscale 2020, 12, 4846–4858. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wu, F.; Gao, H.; Cui, K.; Xian, M.; Zhong, J.; Tian, Y.; Fan, S.; Wu, G. A Dexamethasone-Eluting Porous Scaffold for Bone Regeneration Fabricated by Selective Laser Sintering. ACS Appl. Bio Mater. 2020, 3, 8739–8747. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, M.; Seifi, S.; Akbari, A.; Mohammadi, K.; Shamloo, A.; Movahhedy, M.R. Melt electrowriting of PLA, PCL, and composite PLA/PCL scaffolds for tissue engineering application. Sci. Rep. 2022, 12, 19935. [Google Scholar] [CrossRef] [PubMed]
- Baptista, C.; Azagury, A.; Shin, H.; Baker, C.M.; Ly, E.; Lee, R.; Mathiowitz, E. The effect of temperature and pressure on polycaprolactone morphology. Polymer 2020, 191, 122227. [Google Scholar] [CrossRef]
- Stepanova, M.; Averianov, I.; Serdobintsev, M.; Gofman, I.; Blum, N.; Semenova, N.; Nashchekina, Y.; Vinogradova, T.; Korzhikov-Vlakh, V.; Karttunen, M.; et al. PGlu-Modified Nanocrystalline Cellulose Improves Mechanical Properties, Biocompatibility, and Mineralization of Polyester-Based Composites. Materials 2019, 12, 3435. [Google Scholar] [CrossRef]
- Stepanova, M.; Dobrodumov, A.; Averianov, I.; Gofman, I.; Nashchekina, J.; Guryanov, I.; Klyukin, I.; Zhdanov, A.; Korzhikova-Vlakh, E.; Zhizhin, K. Design, Fabrication and Characterization of Biodegradable Composites Containing Closo-Borates as Potential Materials for Boron Neutron Capture Therapy. Polymers 2022, 14, 3864. [Google Scholar] [CrossRef]
- Nikiforov, A.; Panina, N.; Blinou, D.; Gurzhiy, V.; Nashchekina, J.; Korzhikova-Vlakh, E.; Eremin, A.; Stepanova, M. Ring-Opening Polymerization of rac-Lactide Catalyzed by Octahedral Nickel Carboxylate Complexes. Catalysts 2023, 13, 304. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Golovkin, A.S.; Kudryavtsev, I.V.; Prikhodko, S.S.; Trulioff, A.S.; Bokatyi, A.N.; Poshina, D.N.; Raik, S.V.; Skorik, Y.A. Mucoadhesive cholesterol-chitosan self-assembled particles for topical ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2020, 158, 811–818. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug dissolution. Int. J. Pharm. 2013, 453, 12–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Mindukshev, I.V.; Kudryavtsev, I.V.; Serebriakova, M.K.; Trulioff, A.S.; Gambaryan, S.P.; Sudnitsyna, J.S.; Avdonin, P.V.; Jenkins, R.O.; Goncharov, N.V. Flow cytometry and light-scattering techniques in evaluation of nutraceuticals. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 379–393. [Google Scholar]
- Dubashynskaya, N.V.; Bokatyi, A.N.; Golovkin, A.S.; Kudryavtsev, I.V.; Serebryakova, M.K.; Trulioff, A.S.; Dubrovskii, Y.A.; Skorik, Y.A. Synthesis and Characterization of Novel Succinyl Chitosan-Dexamethasone Conjugates for Potential Intravitreal Dexamethasone Delivery. Int. J. Mol. Sci. 2021, 22, 10960. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Villanueva, J.; Bravo-Osuna, I.; Herrero-Vanrell, R.; Molina Martínez, I.T.; Guzmán Navarro, M. Optimising the controlled release of dexamethasone from a new generation of PLGA-based microspheres intended for intravitreal administration. Eur. J. Pharm. Sci. 2016, 92, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Averianov, I.; Stepanova, M.; Solomakha, O.; Gofman, I.; Serdobintsev, M.; Blum, N.; Kaftuirev, A.; Baulin, I.; Nashchekina, J.; Lavrentieva, A.; et al. 3D-Printed composite scaffolds based on poly(ε-caprolactone) filled with poly(glutamic acid)-modified cellulose nanocrystals for improved bone tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2422–2437. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, D.; Gładysz, A.; Pitucha, M.; Kamiński, D.M.; Barańska, A.; Drop, B. Spectroscopic Study of the Molecular Structure of the New Hybrid with a Potential Two-Way Antibacterial Effect. Molecules 2021, 26, 1442. [Google Scholar] [CrossRef]
- Nugrahani, I.; Tjengal, B.; Gusdinar, T.; Horikawa, A.; Uekusa, H. A Comprehensive Study of a New 1.75 Hydrate of Ciprofloxacin Salicylate: SCXRD Structure Determination, Solid Characterization, Water Stability, Solubility, and Dissolution Study. Crystals 2020, 10, 349. [Google Scholar] [CrossRef]
- Uhljar, L.É.; Kan, S.Y.; Radacsi, N.; Koutsos, V.; Szabó-Révész, P.; Ambrus, R. In Vitro Drug Release, Permeability, and Structural Test of Ciprofloxacin-Loaded Nanofibers. Pharmaceutics 2021, 13, 556. [Google Scholar] [CrossRef]
- Lin, M.; Liu, Y.; Gao, J.; Wang, D.; Xia, D.; Liang, C.; Li, N.; Xu, R. Synergistic Effect of Co-Delivering Ciprofloxacin and Tetracycline Hydrochloride for Promoted Wound Healing by Utilizing Coaxial PCL/Gelatin Nanofiber Membrane. Int. J. Mol. Sci. 2022, 23, 1895. [Google Scholar] [CrossRef]
- Sau, S.; Agarwalla, P.; Mukherjee, S.; Bag, I.; Sreedhar, B.; Pal-Bhadra, M.; Patra, C.R.; Banerjee, R. Cancer cell-selective promoter recognition accompanies antitumor effect by glucocorticoid receptor-targeted gold nanoparticle. Nanoscale 2014, 6, 6745. [Google Scholar] [CrossRef]
- Permatasari, D.A.I.; Kurniasri, N.; Mahardika, M.P. Qualitative and Quantitative Analysis of Dexamethasone in Rheumatic Pain Herbal Medicine Using Thin-Layer Chromatography (TLC)—Densitometry. J. Fundam. Appl. Pharm. Sci. 2021, 2, 10–22. [Google Scholar] [CrossRef]
- Ghorbani, F.; Zamanian, A. An efficient functionalization of dexamethasone-loaded polymeric scaffold with [3-(2,3-epoxypropoxy)-propyl]-trimethoxysilane coupling agent for bone regeneration: Synthesis, characterization, and in vitro evaluation. J. Bioact. Compat. Polym. 2020, 35, 139–159. [Google Scholar] [CrossRef]
- Long, J.; Nand, A.V.; Bunt, C.; Seyfoddin, A. Controlled release of dexamethasone from poly(vinyl alcohol) hydrogel. Pharm. Dev. Technol. 2019, 24, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Z.-C.; Yu, S.-H.; Chao, A.-C.; Dong, G.-C. Preparation and characterization of dexamethasone-immobilized chitosan scaffold. J. Biosci. Bioeng. 2012, 113, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Hart, N.H.; Nimphius, S.; Rantalainen, T.; Ireland, A.; Siafarikas, A.; Newton, R.U. Mechanical basis of bone strength: Influence of bone material, bone structure and muscle action. J. Musculoskelet. Neuronal Interact. 2017, 17, 114–139. [Google Scholar] [PubMed]
- Schädli, G.; Vetsch, J.; Baumann, R.; de Leeuw, A.; Wehrle, E.; Rubert, M.; Müller, R. Time-lapsed imaging of nanocomposite scaffolds reveals increased bone formation in dynamic compression bioreactors. Commun. Biol. 2021, 4, 110. [Google Scholar] [CrossRef]
- Ma, J.; Lin, L.; Zuo, Y.; Zou, Q.; Ren, X.; Li, J.; Li, Y. Modification of 3D printed PCL scaffolds by PVAc and HA to enhance cytocompatibility and osteogenesis. RSC Adv. 2019, 9, 5338–5346. [Google Scholar] [CrossRef]
- Goldstein, S.A. The mechanical properties of trabecular bone: Dependence on anatomic location and function. J. Biomech. 1987, 20, 1055–1061. [Google Scholar] [CrossRef]
- Costa, P.F.; Puga, A.M.; Díaz-Gomez, L.; Concheiro, A.; Busch, D.H.; Alvarez-Lorenzo, C. Additive manufacturing of scaffolds with dexamethasone controlled release for enhanced bone regeneration. Int. J. Pharm. 2015, 496, 541–550. [Google Scholar] [CrossRef]
- Kumar, A.; Chauhan, S. Pancreatic lipase inhibitors: The road voyaged and successes. Life Sci. 2021, 271, 119115. [Google Scholar] [CrossRef]
- Ries, W.L. Osteogenic periosteum esterase activity: A comparative morphological and cytochemical study of bone cells in situ on rat proximal tibiae and in smears. J. Histochem. Cytochem. 1984, 32, 55–62. [Google Scholar] [CrossRef]
- Mäkinen, T.J.; Veiranto, M.; Lankinen, P.; Moritz, N.; Jalava, J.; Törmälä, P.; Aro, H.T. In vitro and in vivo release of ciprofloxacin from osteoconductive bone defect filler. J. Antimicrob. Chemother. 2005, 56, 1063–1068. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Activity of Antimicrobial Peptides and Ciprofloxacin against Pseudomonas aeruginosa Biofilms. Molecules 2020, 25, 3843. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, S.; Li, X.; He, X.; Jian, L. Development of in vitro resistance to fluoroquinolones in Pseudomonas aeruginosa. Antimicrob. Resist. Infect. Control 2020, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Abdella, S.; Afinjuomo, F.; Song, Y.; Upton, R.; Garg, S. 3D printed bilayer mucoadhesive buccal film of estradiol: Impact of design on film properties, release kinetics and predicted in vivo performance. Int. J. Pharm. 2022, 628, 122324. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef]
- Hashmat, D.; Shoaib, M.H.; Ali, F.R.; Siddiqui, F. Lornoxicam controlled release transdermal gel patch: Design, characterization and optimization using co-solvents as penetration enhancers. PLoS ONE 2020, 15, e0228908. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Farshadi, M.; Johari, B.; Erfani Ezadyar, E.; Gholipourmalekabadi, M.; Azami, M.; Madanchi, H.; Haramshahi, S.M.A.; Yari, A.; Karimizade, A.; Nekouian, R.; et al. Nanocomposite scaffold seeded with mesenchymal stem cells for bone repair. Cell Biol. Int. 2019, 43, 1379–1392. [Google Scholar] [CrossRef]
- Gutiérrez-Prieto, S.J.; Perdomo-Lara, S.J.; Diaz-Peraza, J.M.; Sequeda-Castañeda, L.G. Analysis of In Vitro Osteoblast Culture on Scaffolds for Future Bone Regeneration Purposes in Dentistry. Adv. Pharmacol. Sci. 2019, 2019, 5420752. [Google Scholar] [CrossRef]
- Setiawati, R.; Rahardjo, P. Bone Development and Growth. In Osteogenesis and Bone Regeneration; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Zhu, C.; Yao, C.; Zhu, L.; She, C.; Zhou, X. Dexamethasone-induced cytotoxicity in human osteoblasts is associated with circular RNA HIPK3 downregulation. Biochem. Biophys. Res. Commun. 2019, 516, 645–652. [Google Scholar] [CrossRef]
- Sato, A.Y.; Tu, X.; McAndrews, K.A.; Plotkin, L.I.; Bellido, T. Prevention of glucocorticoid induced-apoptosis of osteoblasts and osteocytes by protecting against endoplasmic reticulum (ER) stress in vitro and in vivo in female mice. Bone 2015, 73, 60–68. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, T.; Song, K.; Fan, X.; Ma, X.; Cui, Z. Adipose-derived stem cell: A better stem cell than BMSC. Cell Biochem. Funct. 2008, 26, 664–675. [Google Scholar] [CrossRef]
- Wlodarski, K.H.; Reddi, A.H. Alkaline phosphatase as a marker of osteoinductive cells. Calcif. Tissue Int. 1986, 39, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, B.; Liu, X.; Pan, Z.; Liu, C.; Ma, H.; Liu, X.; Liu, L.; Jiang, C. The dosage effects of dexamethasone on osteogenic activity andbiocompatibility of poly(lactic-co-glycolic acid)/hydroxyapatite nanofibers. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [PubMed]
- Prié, H.; Meyssonnier, V.; Kerroumi, Y.; Heym, B.; Lidove, O.; Marmor, S.; Zeller, V. Pseudomonas aeruginosa prosthetic joint-infection outcomes: Prospective, observational study on 43 patients. Front. Med. 2022, 9, 1039596. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa Biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef]
- Jang, D.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Sivaraman, S.; Deshpande, C.G.; Ranganathan, R.; Huang, X.; Jajeh, A.; O’brien, T.; Huang, R.-W.; Gregory, S.A.; Venugopal, P.; Preisler, H.D. Tumor necrosis factor modulates CD 20 expression on cells from chronic lymphocytic leukemia: A new role for TNF alpha? Microsc. Res. Technol. 2000, 50, 251–257. [Google Scholar] [CrossRef]
- Gaede, K.I.; Fitschen, J.; Ernst, M.; Martinet, N.; Schlaak, M.; Müller-Quernheim, J. Expression of tumour necrosis factor receptors (CD120a and CD120b) on bronchoalveolar cells. Cytokine 1999, 11, 611–616. [Google Scholar] [CrossRef]
- Portillo, J.C.; Feliciano, L.M.; Okenka, G.; Heinzel, F.; Subauste, M.C.; Subauste, C.S. CD40 and tumour necrosis factor-α co-operate to up-regulate inducuble nitric oxide synthase expression in macrophages. Immunology 2012, 135, 140–150. [Google Scholar] [CrossRef]
- Ranheim, E.A.; Kipps, T.J. Tumor Necrosis Factor-α Facilitates Induction of CD80 (B7-1) and CD54 on Human B Cells by Activated T Cells: Complex Regulation by IL-4, IL-10, and CD40L. Cell. Immunol. 1995, 161, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Dubashynskaya, N.V.; Bokatyi, A.N.; Skorik, Y.A. Dexamethasone Conjugates: Synthetic Approaches and Medical Prospects. Biomedicines 2021, 9, 341. [Google Scholar] [CrossRef] [PubMed]






| Model | Equation |
|---|---|
| Zero-order | F = k0 × t |
| First-order | F = 100 × [1 − Exp(−k1 × t)] |
| Higuchi | F = kH × t0.5 |
| Korsmeyer-Peppas | F = kKP × tn |
| Hixon-Crowell | F = 100 × [1 − (1 − kHC × t)3] |
| Hopfenberg | F = 100 × [1 − (1 − kHB × t)n] |
| Weibull | F = 100 × {1 − Exp[−((t − Ti)β)/α]} |
| Makoid-Banakar | F = kMB × tn × Exp(−k × t) |
| Peppas-Sahlin | F = k1 × tm + k2 × t(2 × m) |
| Sample | Pore Volume (m3/g) | Average Pore Size (nm) | Specific Surface Area (m2/g) |
|---|---|---|---|
| PCL | 0.002 | 3.32 ± 0.19 | 1.78 ± 0.10 |
| PCL/CIP-1 | 0.005 | 3.17 ± 0.13 | 4.98 ± 0.20 |
| PCL/CIP-5 | 0.001 | 4.54 ± 0.26 | 0.59 ± 0.03 |
| PCL/DEX-0.5 | 0.001 | 3.16 ± 0.17 | 0.95 ± 0.05 |
| PCL/DEX-1 | 0.001 | 4.54 ± 0.20 | 0.72 ± 0.03 |
| PCL/DEX-5 | <0.001 | 4.50 ± 0.38 | 0.17 ± 0.01 |
| Series | Cell Viability (%) | |
|---|---|---|
| Without TNFα Treatment | With TNFα Treatment | |
| Negative control | 94.4 ± 0.2 | 94.1 ± 0.4 |
| PCL | 95.3 ± 0.4 | 90.8 ± 0.8 *,** |
| PCL/DEX-1 | 63.2 ± 1.9 * | 57.9 ± 4.3 * |
| Series | CD54 Expression (MFI) | |
|---|---|---|
| Without TNFα Treatment | With TNFα Treatment | |
| Negative control | 0.77 ± 0.09 | 5.40 ± 1.01 |
| PCL | 0.98 ± 0.10 | 2.30 ± 0.23 * |
| PCL/DEX-1 | 0.96 ± 0.04 * | 1.35 ± 0.08 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, M.; Averianov, I.; Gofman, I.; Shevchenko, N.; Rubinstein, A.; Egorova, T.; Trulioff, A.; Nashchekina, Y.; Kudryavtsev, I.; Demyanova, E.; et al. Drug Loaded 3D-Printed Poly(ε-Caprolactone) Scaffolds for Local Antibacterial or Anti-Inflammatory Treatment in Bone Regeneration. Polymers 2023, 15, 3957. https://doi.org/10.3390/polym15193957
Stepanova M, Averianov I, Gofman I, Shevchenko N, Rubinstein A, Egorova T, Trulioff A, Nashchekina Y, Kudryavtsev I, Demyanova E, et al. Drug Loaded 3D-Printed Poly(ε-Caprolactone) Scaffolds for Local Antibacterial or Anti-Inflammatory Treatment in Bone Regeneration. Polymers. 2023; 15(19):3957. https://doi.org/10.3390/polym15193957
Chicago/Turabian StyleStepanova, Mariia, Ilia Averianov, Iosif Gofman, Natalia Shevchenko, Artem Rubinstein, Tatiana Egorova, Andrey Trulioff, Yulia Nashchekina, Igor Kudryavtsev, Elena Demyanova, and et al. 2023. "Drug Loaded 3D-Printed Poly(ε-Caprolactone) Scaffolds for Local Antibacterial or Anti-Inflammatory Treatment in Bone Regeneration" Polymers 15, no. 19: 3957. https://doi.org/10.3390/polym15193957
APA StyleStepanova, M., Averianov, I., Gofman, I., Shevchenko, N., Rubinstein, A., Egorova, T., Trulioff, A., Nashchekina, Y., Kudryavtsev, I., Demyanova, E., Korzhikova-Vlakh, E., & Korzhikov-Vlakh, V. (2023). Drug Loaded 3D-Printed Poly(ε-Caprolactone) Scaffolds for Local Antibacterial or Anti-Inflammatory Treatment in Bone Regeneration. Polymers, 15(19), 3957. https://doi.org/10.3390/polym15193957











