Babassu Coconut Fibers: Investigation of Chemical and Surface Properties (Attalea speciosa.)
Abstract
:1. Introduction
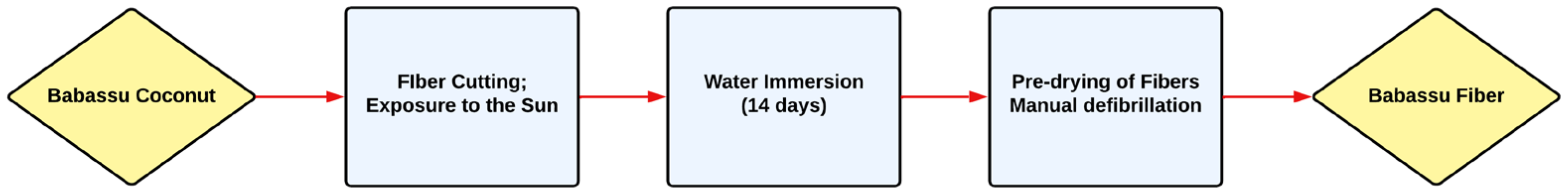
2. Materials and Methods
2.1. Materials
2.2. Characterization
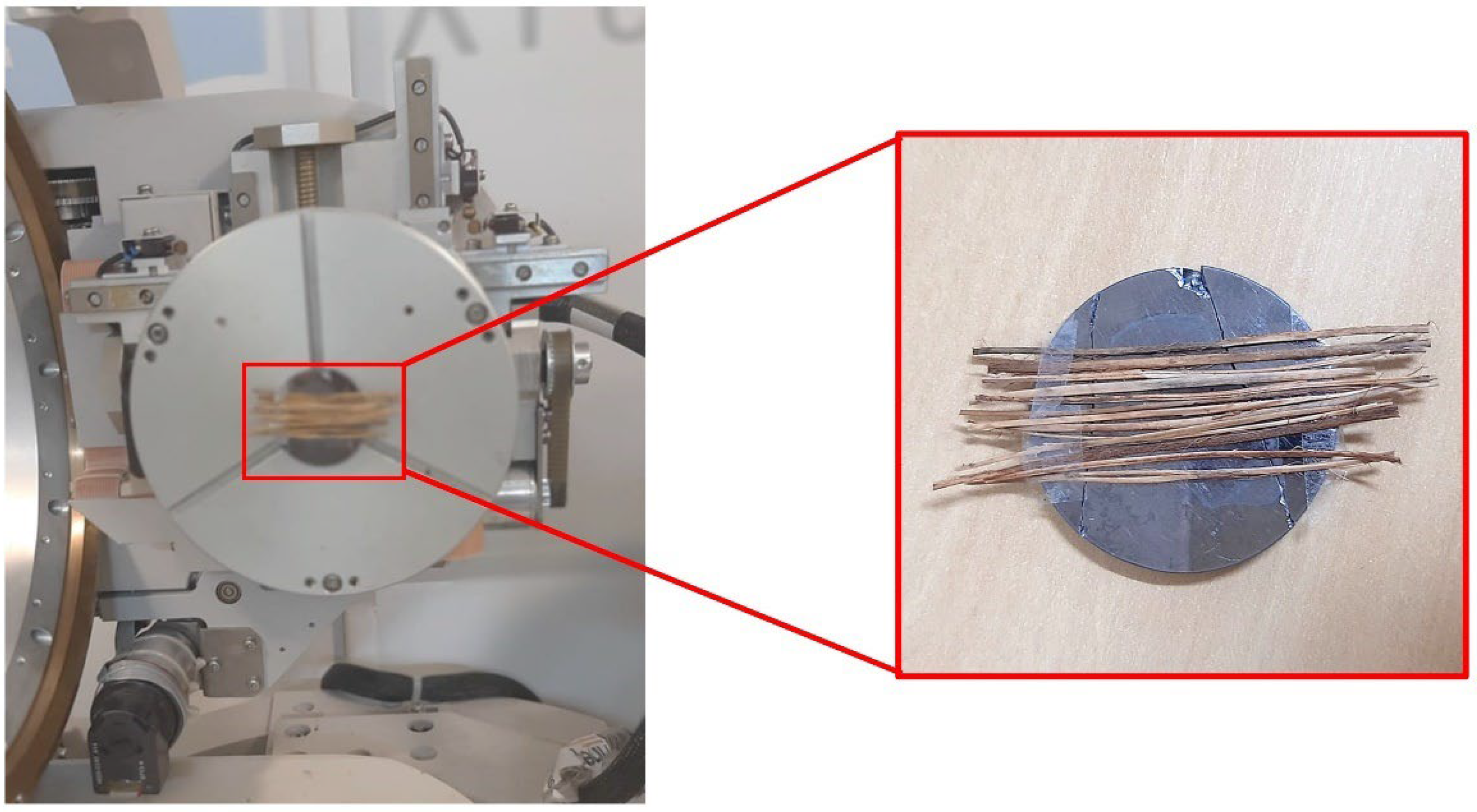
2.2.1. Diameter Measurement
2.2.2. Moisture Content
2.2.3. Determination of Chemical Composition
Determination of the Extractive Content
Determination of Ash Content
Determination of Lignin Content
Determination of Holocellulose Content
Determination of the Alphacellulose Content
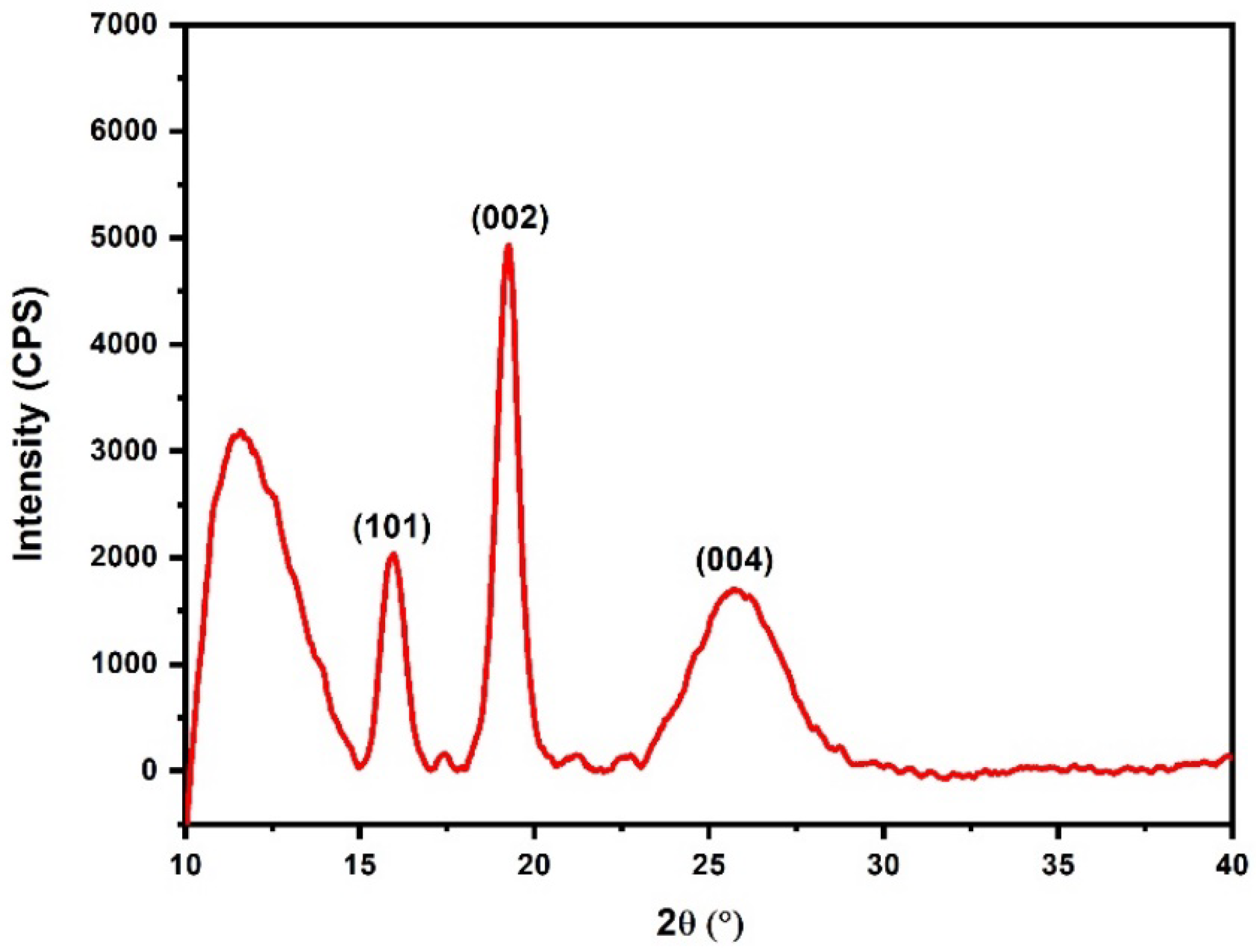
2.3. X-ray Diffraction (XRD)
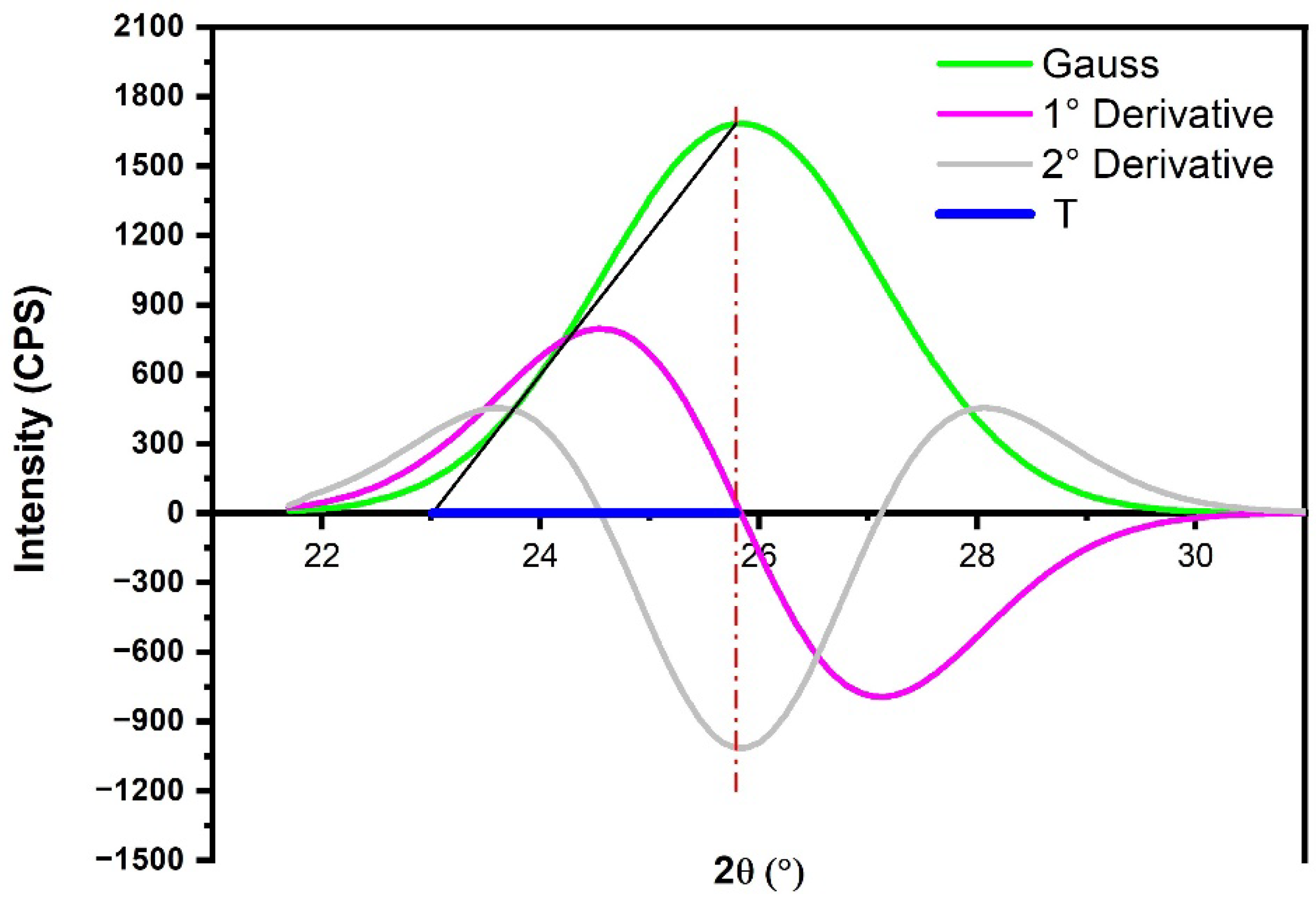
2.3.1. Crystallinity Index
2.3.2. Microfibril Angle
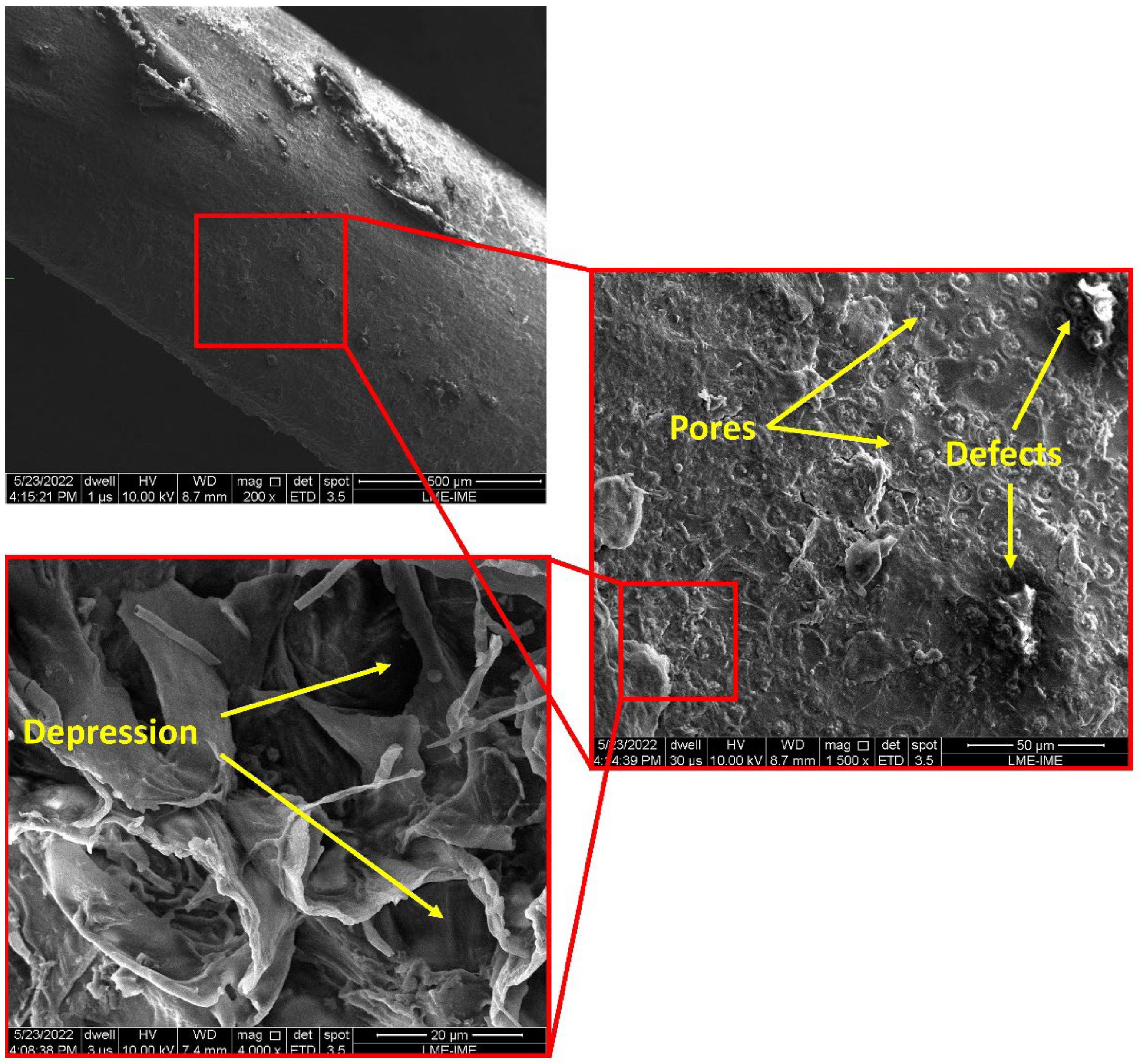
2.4. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Diameter Variation
3.2. Determination of Chemical Composition
3.3. XRD Results
3.4. Microstructural Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faruk, O.; Bledzki, A.K.; Fink, H.-P.; Sain, M. Biocomposites Reinforced with Natural Fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Louro, L.H.L.; Trindade, W.; Elias, C.N.; Ferreira, C.L.; de Sousa Lima, E.; Weber, R.P.; Miguez Suarez, J.C.; da Silva Figueiredo, A.B.-H.; Pinheiro, W.A.; et al. Natural Curaua Fiber-Reinforced Composites in Multilayered Ballistic Armor. Metall. Mater. Trans. A 2015, 46, 4567–4577. [Google Scholar] [CrossRef]
- Wambua, P.; Vangrimde, B.; Lomov, S.; Verpoest, I. The Response of Natural Fibre Composites to Ballistic Impact by Fragment Simulating Projectiles. Compos. Struct. 2007, 77, 232–240. [Google Scholar] [CrossRef]
- Da Luz, F.S.; Lima Junior, E.P.; Louro, L.H.L.; Monteiro, S.N. Ballistic Test of Multilayered Armor with Intermediate Epoxy Composite Reinforced with Jute Fabric. Mater. Res. 2015, 18, 170–177. [Google Scholar] [CrossRef]
- Dong, C. Review of Natural Fibre-Reinforced Hybrid Composites. J. Reinf. Plast. Compos. 2018, 37, 331–348. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, L.; Nie, J.; Wang, H.; Yang, D. Study on Poly(Lactic Acid)/Natural Fibers Composites. J. Appl. Polym. Sci. 2012, 125, E526–E533. [Google Scholar] [CrossRef]
- Fuqua, M.A.; Huo, S.; Ulven, C.A. Natural Fiber Reinforced Composites. Polym. Rev. 2012, 52, 259–320. [Google Scholar] [CrossRef]
- Rohen, L.A.; Margem, F.M.; Monteiro, S.N.; Vieira, C.M.F.; Madeira de Araujo, B.; Lima, E.S. Ballistic Efficiency of an Individual Epoxy Composite Reinforced with Sisal Fibers in Multilayered Armor. Mater. Res. 2015, 18, 55–62. [Google Scholar] [CrossRef]
- Martinelli, F.R.B.; Ribeiro, F.R.C.; Marvila, M.T.; Monteiro, S.N.; da Filho, F.C.G.; de Azevedo, A.R.G. A Review of the Use of Coconut Fiber in Cement Composites. Polymers 2023, 15, 1309. [Google Scholar] [CrossRef] [PubMed]
- Da Demosthenes, L.C.C.; Nascimento, L.F.C.; Monteiro, S.N.; Costa, U.O.; da Garcia Filho, F.C.; da Luz, F.S.; Oliveira, M.S.; Ramos, F.J.H.T.V.; Pereira, A.C.; Braga, F.O. Thermal and Structural Characterization of Buriti Fibers and Their Relevance in Fabric Reinforced Composites. J. Mater. Res. Technol. 2020, 9, 115–123. [Google Scholar] [CrossRef]
- Elanchezhian, C.; Ramnath, B.V.; Ramakrishnan, G.; Rajendrakumar, M.; Naveenkumar, V.; Saravanakumar, M.K. Review on Mechanical Properties of Natural Fiber Composites. Mater. Today Proc. 2018, 5, 1785–1790. [Google Scholar] [CrossRef]
- Hibi, M.; Abe, N.; Haba, M.; Tanaka, T.; Murata, H.; Oyama, M. Phytochemical Investigation of Cyperus malaccensis subsp. Monophyllus. Planta Med. 2019, 85, 1499–1500. [Google Scholar]
- Jha, K.; Kataria, R.; Verma, J.; Pradhan, S. Potential Biodegradable Matrices and Fiber Treatment for Green Composites: A Review. AIMS Mater. Sci. 2019, 6, 119–138. [Google Scholar] [CrossRef]
- Pires, P.S.; de Borges, M.S.; Leal, J.E.C.; Pedroza, M.M.; Silva, F.L.d.N.; Grácio, H.R.; Rambo, M.C.D.; Rambo, M.K.D. Socioeconomic Analysis of Bioproducts Derived from Babassu Nut Breakers Pyrolysis in Legal Amazonia Communities. Food Sci. Technol. 2023, 43, 1–8. [Google Scholar] [CrossRef]
- Porro, R.; de Sousa, R.C. Anatomy of Babassu-Nut Value Chain for Policy Guidance in Support of Traditional Agroextractive Communities in the Mearim Valley, Maranhão, Brazil. Rev. Econ. E Sociol. Rural. 2023, 61. [Google Scholar] [CrossRef]
- Lorenzine, H. Palmeiras no Brasil; Plantarum; Nova Odessa: São Paulo, Brazil, 1996; p. 70. [Google Scholar]
- Da Silva Lima, P.N.; Ghosh, A.; Nascimento, A.; Sousa Vieira, L.H.; Costa, R.S.; Ferreira, O.P.; Abreu, G.J.P.; Fujisawa, K.; Silva-Filho, E.C.; Gomes de Souza Filho, A.; et al. Advanced Sustainable Carbon Material from Babassu Biomass and Its Adsorption Performance. J. Phys. Chem. Solids 2023, 176, 111188. [Google Scholar] [CrossRef]
- Bauer, L.C.; Santos, L.S.; Sampaio, K.A.; Ferrão, S.P.B.; da Fontan, R.C.I.; Minim, L.A.; Veloso, C.M.; Bonomo, R.C.F. Physicochemical and Thermal Characterization of Babassu Oils (Orbignya phalerata Mart.) Obtained by Different Extraction Methods. Food Res. Int. 2020, 137, 109474. [Google Scholar] [CrossRef]
- Zanine, A.; De Sá, C.; Ferreira, D.; Parente, H.; Parente, M.; Santos, E.M.; Rodrigues, R.; Santos, F.N.; Lima, A.G.; Cunha, I.A.; et al. The Effect of Babassu Industry By-Products as an Alternative Feed for Dairy Cows. Agronomy 2023, 13, 491. [Google Scholar] [CrossRef]
- De Moura, C.V.R.; da Sousa, D.C.; de Moura, E.M.; de Araújo, E.C.E.; Sittolin, I.M. New Biodegradable Composites from Starch and Fibers of the Babassu Coconut. Polímeros 2021, 31. [Google Scholar] [CrossRef]
- Chaves, Y.S.; da Silveira, P.H.P.M.; de Neuba, L.M.; Junio, R.F.P.; Ribeiro, M.P.; Monteiro, S.N.; Nascimento, L.F.C. Evaluation of the Density, Mechanical, Thermal and Chemical Properties of Babassu Fibers (Attalea speciosa.) for Potential Composite Reinforcement. J. Mater. Res. Technol. 2023, 23, 2089–2100. [Google Scholar] [CrossRef]
- ASTM D3800-99; Standard Test Method for Density of High-Modulus Fibers. American Society for Testing of Mateials—ASTM International: West Conshohocken, PA, USA, 2010.
- Noryani, M.; Aida, H.J.; Nadlene, R.; Mastura, M.T.; Shaharuzaman, M.A. Correlation Study on Physical Properties and Mechanical Properties of Kenaf Fibre Composites. Mater. Today Proc. 2022, 51, 1309–1315. [Google Scholar] [CrossRef]
- Lara-Curzio, E.; Garcia, D. The Effect of Diameter Variation Along a Fiber on the Determination of Fiber Strengths and the Parameters of Their Distribution. In 25th Annual Conference on Composites, Advanced Ceramics, Materials, and Structures: A: Ceramic Engineering and Science Proceedings, Volume 22, Issue 3; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 363–370. [Google Scholar]
- Morais, J.P.S.; de Rosa, M.F.; Marconcini, J.M. Procedimento Para Análise Lignocelulósica. In Embrapa Agroindústria Tropical; Folhetos: Almelo, The Netherlands, 2011; pp. 1–54. [Google Scholar]
- Sweygers, N.; Depuydt, D.E.C.; Eyley, S.; Thielemans, W.; Mosleh, Y.; Ivens, J.; Dewil, R.; Appels, L.; Van Vuure, A.W. Prediction of the Equilibrium Moisture Content Based on the Chemical Composition and Crystallinity of Natural Fibres. Ind. Crops Prod. 2022, 186, 115187. [Google Scholar] [CrossRef]
- De Araújo, A.A.S.; Mercuri, L.P.; Seixas, S.R.S.; Storpirtis, S.; Matos, J.D.R. Determinação Dos Teores de Umidade e Cinzas de Amostras Comerciais de Guaraná Utilizando Métodos Convencionais e Análise Térmica. Rev. Bras. Ciências Farm. 2006, 42, 269–277. [Google Scholar] [CrossRef]
- Yong, T.L.-K.; Matsumura, Y. Reaction Kinetics of the Lignin Conversion in Supercritical Water. Ind. Eng. Chem. Res. 2012, 51, 11975–11988. [Google Scholar] [CrossRef]
- Pérez, J.; Muñoz-Dorado, J.; de la Rubia, T.; Martínez, J. Biodegradation and Biological Treatments of Cellulose, Hemicellulose and Lignin: An Overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef]
- Abe, K.; Iwamoto, S.; Yano, H. Obtaining Cellulose Nanofibers with a Uniform Width of 15 Nm from Wood. Biomacromolecules 2007, 8, 3276–3278. [Google Scholar] [CrossRef]
- Gouveia, E.R.; do Nascimento, R.T.; Souto-Maior, A.M.; de Rocha, G.J.M. Validação de Metodologia Para a Caracterização Química de Bagaço de Cana-de-Açúcar. Quim. Nova 2009, 32, 1500–1503. [Google Scholar] [CrossRef]
- Martin, A.R.; Martins, M.A.; Mattoso, L.H.C.; Silva, O.R.R.F. Caracterização Química e Estrutural de Fibra de Sisal Da Variedade Agave Sisalana. Polímeros 2009, 19, 40–46. [Google Scholar] [CrossRef]
- Vilardell, A.M.; Yadroitsev, I.; Yadroitsava, I.; Albu, M.; Takata, N.; Kobashi, M.; Krakhmalev, P.; Kouprianoff, D.; Kothleitner, G.; Plessis, A. du Manufacturing and Characterization of In-Situ Alloyed Ti6Al4V(ELI)-3 at.% Cu by Laser Powder Bed Fusion. Addit. Manuf. 2020, 36, 101436. [Google Scholar] [CrossRef]
- Holm, T.P.; Knopp, M.M.; Löbmann, K.; Berthelsen, R. Microwave Induced in Situ Amorphisation Facilitated by Crystalline Hydrates. Eur. J. Pharm. Sci. 2021, 163, 105858. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Then, Y.Y.; Ibrahim, N.A.; Zainuddin, N.; Ariffin, H.; Wan Yunus, W.M.Z.; Chieng, B.W. Surface Modifications of Oil Palm Mesocarp Fiber by Superheated Steam, Alkali, and Superheated Steam-Alkali for Biocomposite Applications. Bioresources 2014, 9, 7467–7483. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of Cellulose and Preparation of Nanocellulose from Sisal Fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Mwaikambo, L.Y.; Ansell, M.P. Chemical Modification of Hemp, Sisal, Jute, and Kapok Fibers by Alkalization. J. Appl. Polym. Sci. 2002, 84, 2222–2234. [Google Scholar] [CrossRef]
- Cave, I.D. Theory of X-Ray Measurement of Microfibril Angle in Wood. Wood Sci. Technol. 1997, 31, 143–152. [Google Scholar] [CrossRef]
- De Neuba, L.M.; Junio, R.F.P.; Souza, A.T.; Ribeiro, M.P.; da Silveira, P.H.P.M.; da Silva, T.T.; Pereira, A.C.; Monteiro, S.N. Mechanical Properties, Critical Length, and Interfacial Strength of Seven-Islands-Sedge Fibers (Cyperus malaccensis) for Possible Epoxy Matrix Reinforcement. Polymers 2022, 14, 3807. [Google Scholar] [CrossRef]
- Hamad, S.F.; Stehling, N.; Holland, C.; Foreman, J.P.; Rodenburg, C. Low-Voltage SEM of Natural Plant Fibers: Microstructure Properties (Surface and Cross-Section) and Their Link to the Tensile Properties. Procedia Eng. 2017, 200, 295–302. [Google Scholar] [CrossRef]
- Dinu, L.-D.; Iordache, O.; Vamanu, E. Scanning Electron Microscopy Study on the Biodeterioration of Natural Fiber Materials Compared to Disposable Hygiene and Sanitary Products. Fermentation 2022, 8, 287. [Google Scholar] [CrossRef]
- Da Batista, M.S.; Teixeira, L.A.; de Louly, A.S.; Silva, S.O.; da Luz, S.M. Fatigue Damage Propagation and Creep Behavior on Sisal/Epoxy Composites. Polímeros 2022, 32, e2022008. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Satyanarayana, K.G.; Lopes, F.P.D. High Strength Natural Fibers for Improved Polymer Matrix Composites. Mater. Sci. Forum 2010, 638–642, 961–966. [Google Scholar] [CrossRef]
- Satyanarayana, K.G.; Ravikumar, K.K.; Sukumaran, K.; Mukherjee, P.S.; Pillai, S.G.K.; Kulkarni, A.G. Structure and Properties of Some Vegetable Fibres. J. Mater. Sci. 1986, 21, 57–63. [Google Scholar] [CrossRef]
- De Furtado, J.B.M.; Furtado Filho, P.A.; Pereira Oliveira, T.; De Sousa Caetano, M.R.; De Souza Araújo, I.M.; Figueiredo, F.C.; Dos Santos Júnior, J.R. Caracterização Química Da Fibra Do Caule Da Palmeira de Babaçu Natural e Após Tratamento. Rev. Eng. Pesqui. Apl. 2020, 5, 56–64. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Reihmane, M.P.; Gassan, J. Properties and Modification Methods for Vegetable Fibers for Natural Fiber Composites. J. Appl. Polym. Sci. 1996, 59, 1329. [Google Scholar] [CrossRef]
- Ashori, A.; Bahreini, Z. Evaluation of Calotropis Gigantea as a Promising Raw Material for Fiber-Reinforced Composite. J. Compos. Mater. 2009, 43, 1297–1304. [Google Scholar] [CrossRef]
- Ashori, A. Pulp and Paper from Kenaf Bast Fibers. Fiber Polym. 2006, 7, 26–29. [Google Scholar] [CrossRef]
- Moshi, A.A.M.; Ravindran, D.; Bharathi, S.R.S.; Indran, S.; Saravanakumar, S.S.; Liu, Y. Characterization of a New Cellulosic Natural Fiber Extracted from the Root of Ficus Religiosa Tree. Int. J. Biol. Macromol. 2020, 142, 212–221. [Google Scholar] [CrossRef]
- Balaji, A.N.; Nagarajan, K.J. Characterization of Alkali Treated and Untreated New Cellulosic Fiber from Saharan Aloe Vera Cactus Leaves. Carbohydr. Polym. 2017, 174, 200–208. [Google Scholar] [CrossRef]
- Palanisamy, S.; Kalimuthu, M.; Palaniappan, M.; Alavudeen, A.; Rajini, N.; Santulli, C. Morphological Characterization of Soapbark Fibers. J. Mater. Sci. Res. Rev. 2021, 8, 19–26. [Google Scholar]
- Monteiro, S.N.; Lopes, F.P.D.; Barbosa, A.P.; Bevitori, A.B.; Da Silva, I.L.A.; Costa, L.L. Da Natural Lignocellulosic Fibers as Engineering Materials—An Overview. Metall. Mater. Trans. A 2011, 42, 2963–2974. [Google Scholar] [CrossRef]
- Saheb, D.N.; Jog, J.P. Natural Fiber Polymer Composites: A Review. Adv. Polym. Technol. 1999, 18, 351–363. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Hinrichsen, G. Biofibres, Biodegradable Polymers and Biocomposites: An Overview. Macromol. Mater. Eng. 2000, 276–277, 1–24. [Google Scholar] [CrossRef]
- Feldman, D. Wood—Chemistry, Ultrastructure, Reactions, by D. Fengel and G. Wegener, Walter de Gruyter, Berlin and New York, 1984, 613 Pp. Price: 245 DM. J. Polym. Sci. Polym. Lett. Ed. 1985, 23, 601–602. [Google Scholar] [CrossRef]
- Spinacé, M.A.S.; Lambert, C.S.; Fermoselli, K.K.G.; De Paoli, M.-A. Characterization of Lignocellulosic Curaua Fibres. Carbohydr. Polym. 2009, 77, 47–53. [Google Scholar] [CrossRef]
- Sumesh, K.R.; Kanthavel, K.; Kavimani, V. Peanut Oil Cake-Derived Cellulose Fiber: Extraction, Application of Mechanical and Thermal Properties in Pineapple/Flax Natural Fiber Composites. Int. J. Biol. Macromol. 2020, 150, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Vijay, R.; Vinod, A.; Lenin Singaravelu, D.; Sanjay, M.R.; Siengchin, S. Characterization of Chemical Treated and Untreated Natural Fibers from Pennisetum orientale Grass—A Potential Reinforcement for Lightweight Polymeric Applications. Int. J. Lightweight Mater. Manuf. 2021, 4, 43–49. [Google Scholar] [CrossRef]
- Saravanakumar, S.S.; Kumaravel, A.; Nagarajan, T.; Moorthy, I.G. Investigation of Physico-Chemical Properties of Alkali-Treated Prosopis juliflora Fibers. Int. J. Polym. Anal. Charact. 2014, 19, 309–317. [Google Scholar] [CrossRef]
- Boudjellal, A.; Trache, D.; Khimeche, K.; Hafsaoui, S.L.; Razali, M.S. Preparation and Characterization of Graphene Oxide-Based Natural Hybrids Containing Alfa Fibers or Microcrystalline Cellulose. J. Nat. Fibers 2022, 19, 5321–5332. [Google Scholar] [CrossRef]
- Okafor, C.E.; Kebodi, L.C.; Ihueze, C.C.; Rangappa, S.M.; Siengchin, S.; Okonkwo, U.C. Development of Dioscorea Alata Stem Fibers as Eco-Friendly Reinforcement for Composite Materials. J. King Saud. Univ. Eng. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Saraiva Rodrigues, S.C.; da Silva, A.S.; de Carvalho, L.H.; Alves, T.S.; Barbosa, R. Morphological, Structural, Thermal Properties of a Native Starch Obtained from Babassu Mesocarp for Food Packaging Application. J. Mater. Res. Technol. 2020, 9, 15670–15678. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Tapia-Blácido, D.R. Isolation and Characterization of Starch from Babassu Mesocarp. Food Hydrocoll. 2016, 55, 47–55. [Google Scholar] [CrossRef]
- Reis, R.H.M.; Nunes, L.F.; Oliveira, M.S.; de Junior, V.F.V.; Filho, F.D.C.G.; Pinheiro, M.A.; Candido, V.S.; Monteiro, S.N. Guaruman Fiber: Another Possible Reinforcement in Composites. J. Mater. Res. Technol. 2020, 9, 622–628. [Google Scholar] [CrossRef]
- Kretschmann, D.E.; Alden, H.A.; Verrill, S. Variations of Microfibril Angle in Loblolly Pine: Comparison of Iodine Crystallization and X-ray Diffraction Techniques. Microfibril Angle in Wood; University of Canterbury: Christchurch, New Zealand, 1998; pp. 157–176. [Google Scholar]
- Gherardi Hein, P.R.; Tarcísio Lima, J. Relationships between Microfibril Angle, Modulus of Elasticity and Compressive Strength in Eucalyptus Wood. Maderas. Cienc. Tecnol. 2012, 14, 267–274. [Google Scholar] [CrossRef]
- Mahdi, E.; Ochoa, D.R.H.; Vaziri, A.; Dean, A.; Kucukvar, M. Khalasa Date Palm Leaf Fiber as a Potential Reinforcement for Polymeric Composite Materials. Compos. Struct. 2021, 256, 113501. [Google Scholar] [CrossRef]
- Pinheiro, M.A.; Ribeiro, M.M.; Rosa, D.L.S.; da Nascimento, D.C.B.; da Silva, A.C.R.; dos Reis, M.A.L.; Monteiro, S.N.; Candido, V.S. Periquiteira (Cochlospermum orinocense): A Promising Amazon Fiber for Application in Composite Materials. Polymers 2023, 15, 2120. [Google Scholar] [CrossRef] [PubMed]





| Diameter Intervals (mm) | Average Density (g/cm3) | Ultimate Tensile Strength (MPa) | Elongation (%) | Elastic Modulus (GPa) |
|---|---|---|---|---|
| 0.18–0.23 | 0.79 ± 0.03 | 100.76 ± 10.18 | 1.59 ± 0.16 | 6.33 ± 0.64 |
| 0.23–0.29 | 0.70 ± 0.04 | 76.41 ± 7.72 | 2.98 ± 0.30 | 2.56 ± 0.26 |
| 0.29–0.34 | 0.66 ± 0.03 | 47.61 ± 5.32 | 2.05 ± 0.20 | 2.32 ± 0.23 |
| 0.34–0.40 | 0.53 ± 0.04 | 31.18 ± 3.15 | 1.18 ± 0.11 | 2.87 ± 0.29 |
| 0.40–0.45 | 0.42 ± 0.03 | 24.97 ± 2.52 | 1.94 ± 0.20 | 1.94 ± 0.13 |
| 0.45–0.47 | 0.27 ± 0.01 | 17.96 ± 0.61 | 1.56 ± 0.15 | 1.15 ± 0.11 |
| Fiber | MC% | EC% | ASC% | LC% | HC% | HEC% * | AC% | Ref. |
|---|---|---|---|---|---|---|---|---|
| Babassu | 7.05 | 15.72 | 2.43 | 28.53 | 70.31 | 32.34 | 37.97 | p.a * |
| Babassu | - | 8.50 | - | 21.9 | 74.50 | 8.90 | 65.50 | [46] |
| Jute | 12.6 | 0.50 | - | 12.00 | 74.60 | 13.60 | 61.00 | [47] |
| Mudar | - | - | 2.50 | 18.00 | 76.00 | - | 57.00 | [48] |
| Kenaf | - | - | 2.20–6.00 | 14.00–17.00 | 76.00–77.00 | - | 45.00–46.00 | [49] |
| Ficus | 9.33 | - | 3.96 | 10.13 | - | 13.86 | 55.38 | [50] |
| Cactus | 5.80 | - | - | 13.70 | - | 8.20 | 67.40 | [51] |
| Soapbark | 12.0 | - | 5.00 | 18.00 | - | 20.00 | 37.00 | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves, Y.S.; da Silveira, P.H.P.M.; Monteiro, S.N.; Nascimento, L.F.C. Babassu Coconut Fibers: Investigation of Chemical and Surface Properties (Attalea speciosa.). Polymers 2023, 15, 3863. https://doi.org/10.3390/polym15193863
Chaves YS, da Silveira PHPM, Monteiro SN, Nascimento LFC. Babassu Coconut Fibers: Investigation of Chemical and Surface Properties (Attalea speciosa.). Polymers. 2023; 15(19):3863. https://doi.org/10.3390/polym15193863
Chicago/Turabian StyleChaves, Yago Soares, Pedro Henrique Poubel Mendonça da Silveira, Sergio Neves Monteiro, and Lucio Fabio Cassiano Nascimento. 2023. "Babassu Coconut Fibers: Investigation of Chemical and Surface Properties (Attalea speciosa.)" Polymers 15, no. 19: 3863. https://doi.org/10.3390/polym15193863
APA StyleChaves, Y. S., da Silveira, P. H. P. M., Monteiro, S. N., & Nascimento, L. F. C. (2023). Babassu Coconut Fibers: Investigation of Chemical and Surface Properties (Attalea speciosa.). Polymers, 15(19), 3863. https://doi.org/10.3390/polym15193863








