Stretchable Gold Nanomembrane Electrode with Ionic Hydrogel Skin-Adhesive Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of SEBS-Au Electrode
2.2. Preparation of Chitosan and Alginate Hydrogel Solutions
2.3. Mechanical and Skin-Adhevise Properties of CAC Hydrogel
2.4. Electrical Properties of SEBS-Au with Hydrogels
2.5. Electrocardiogram Monitoring Using SEBS-Au with a CAC Hydrogel Layer
3. Results and Discussion
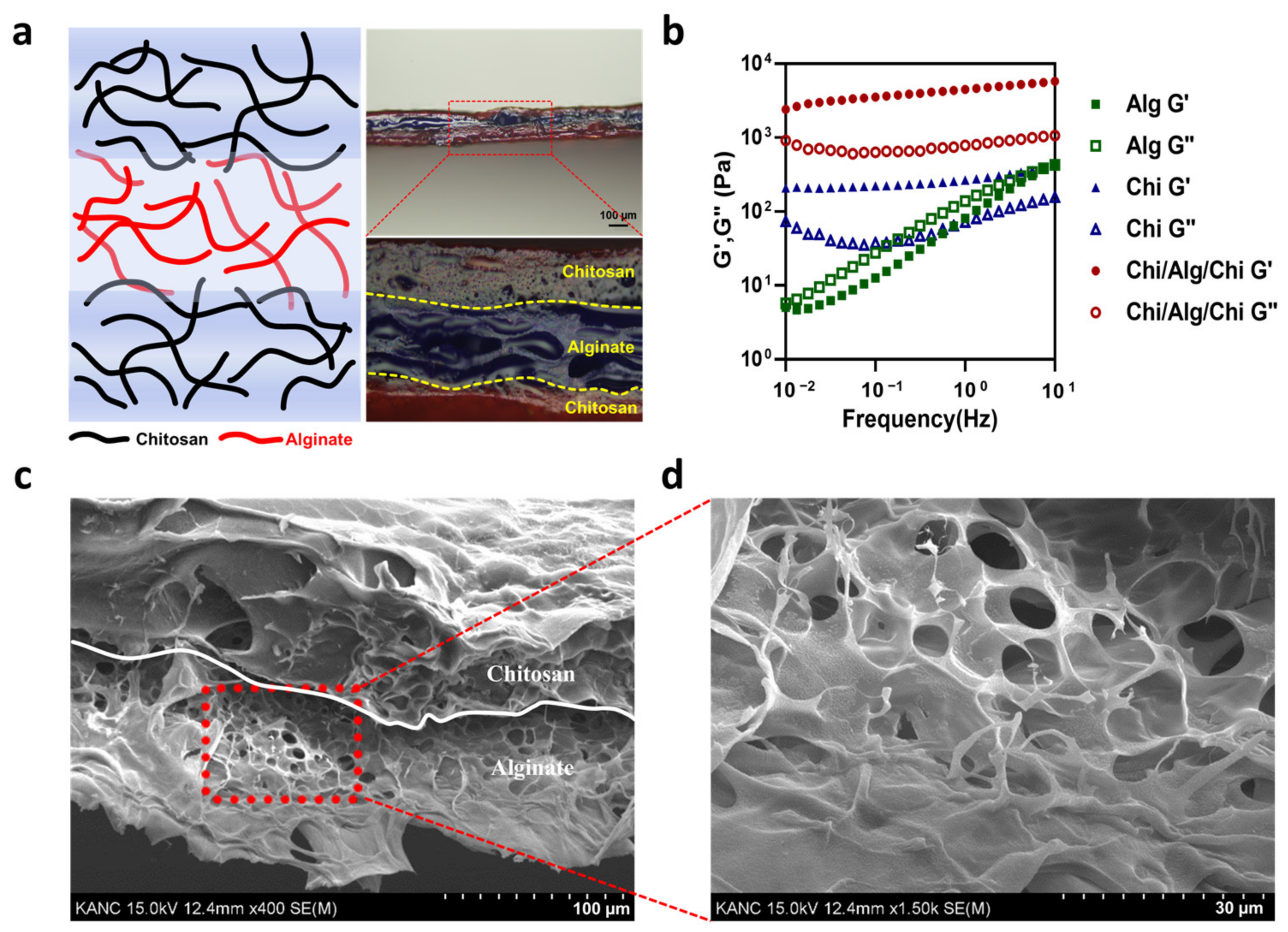
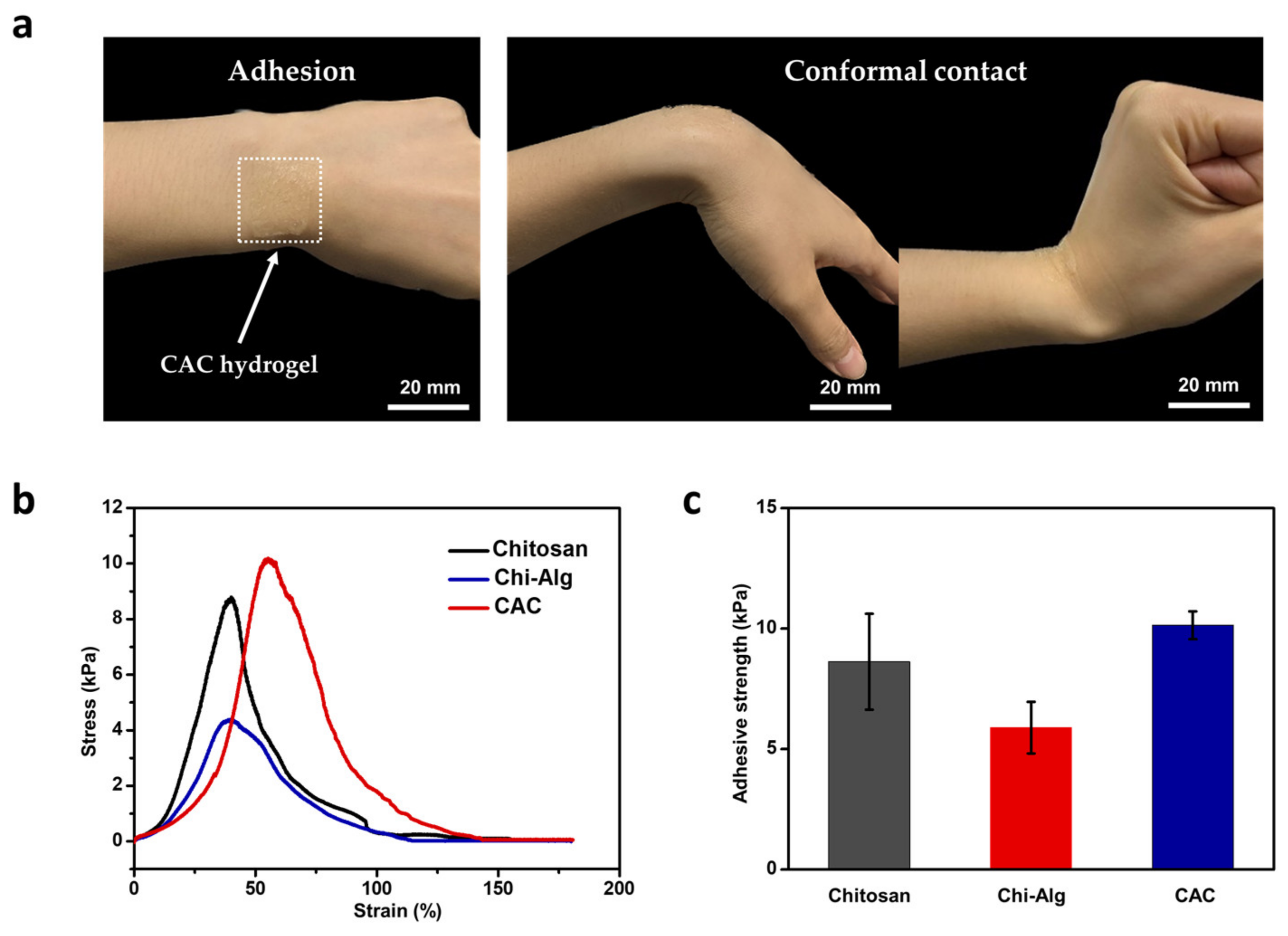
3.1. Mechanical and Skin-Adhesive Properties of CAC Hydrogel
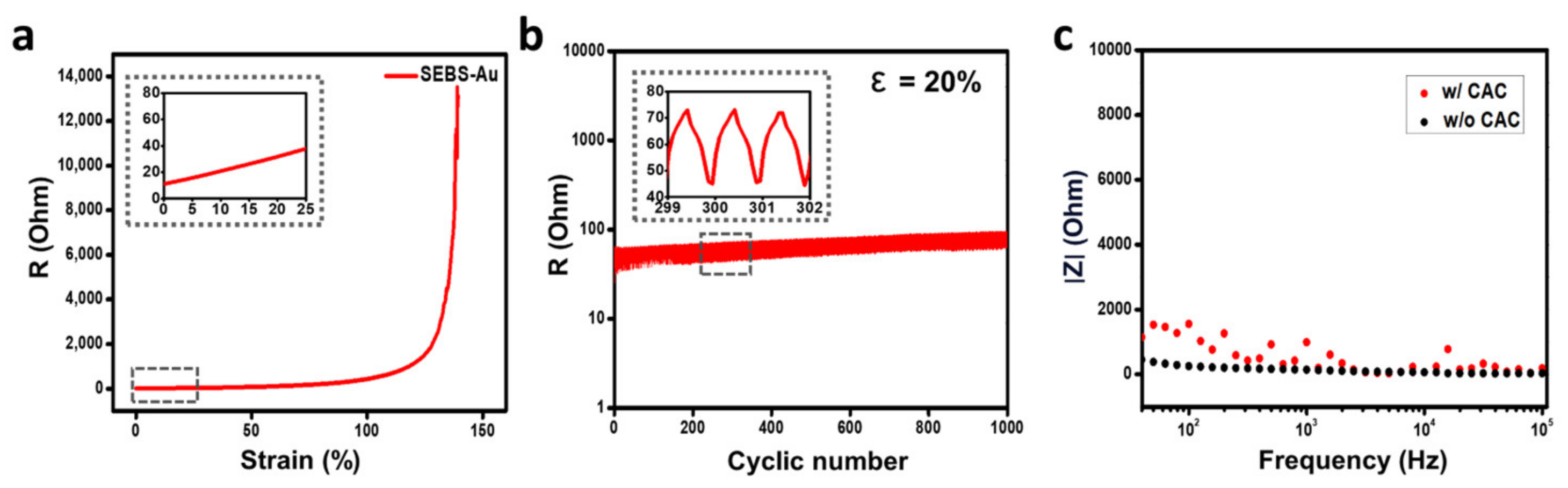
3.2. Electrical Property of SEBS-Au Electrode with CAC Hydrogel
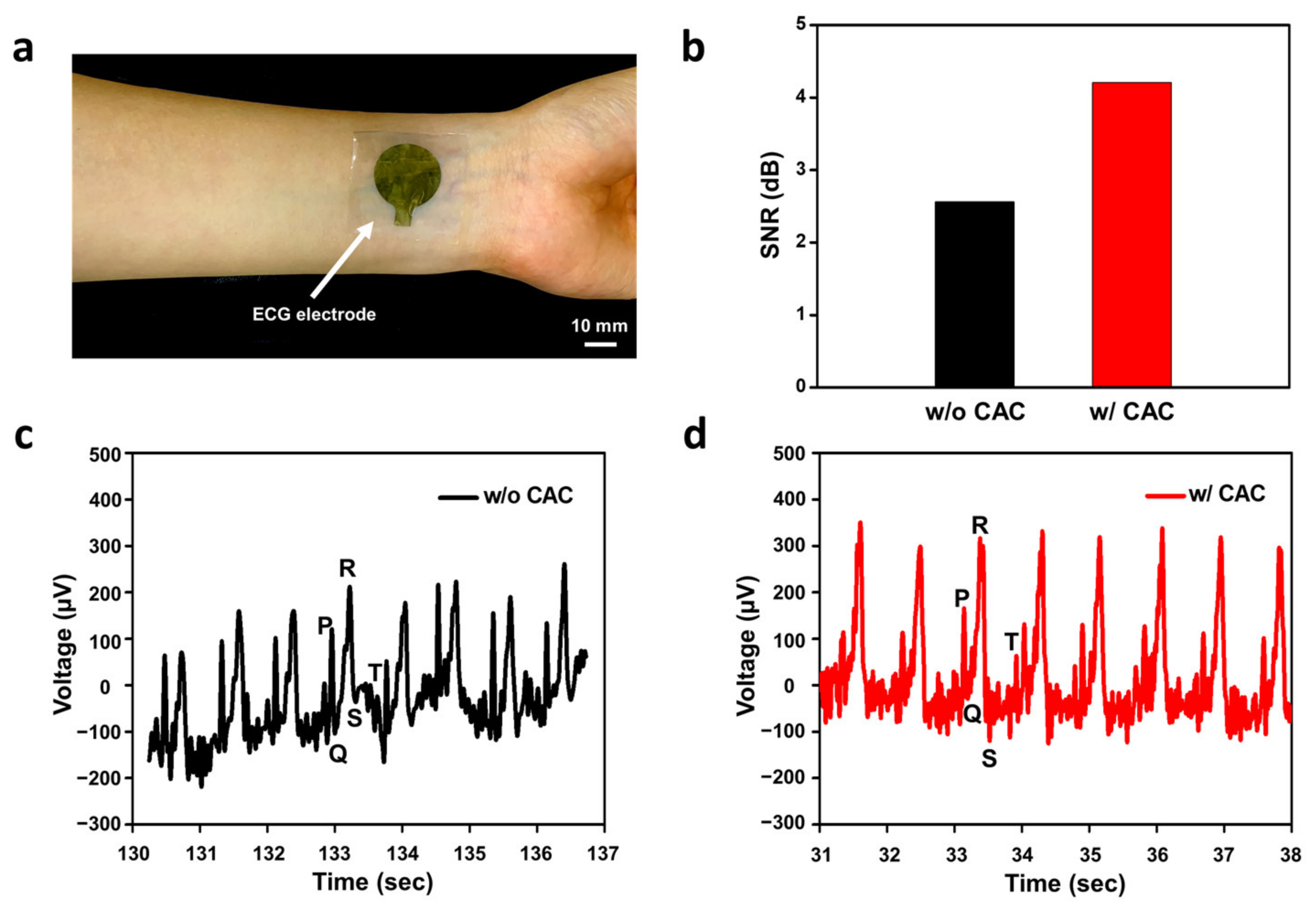
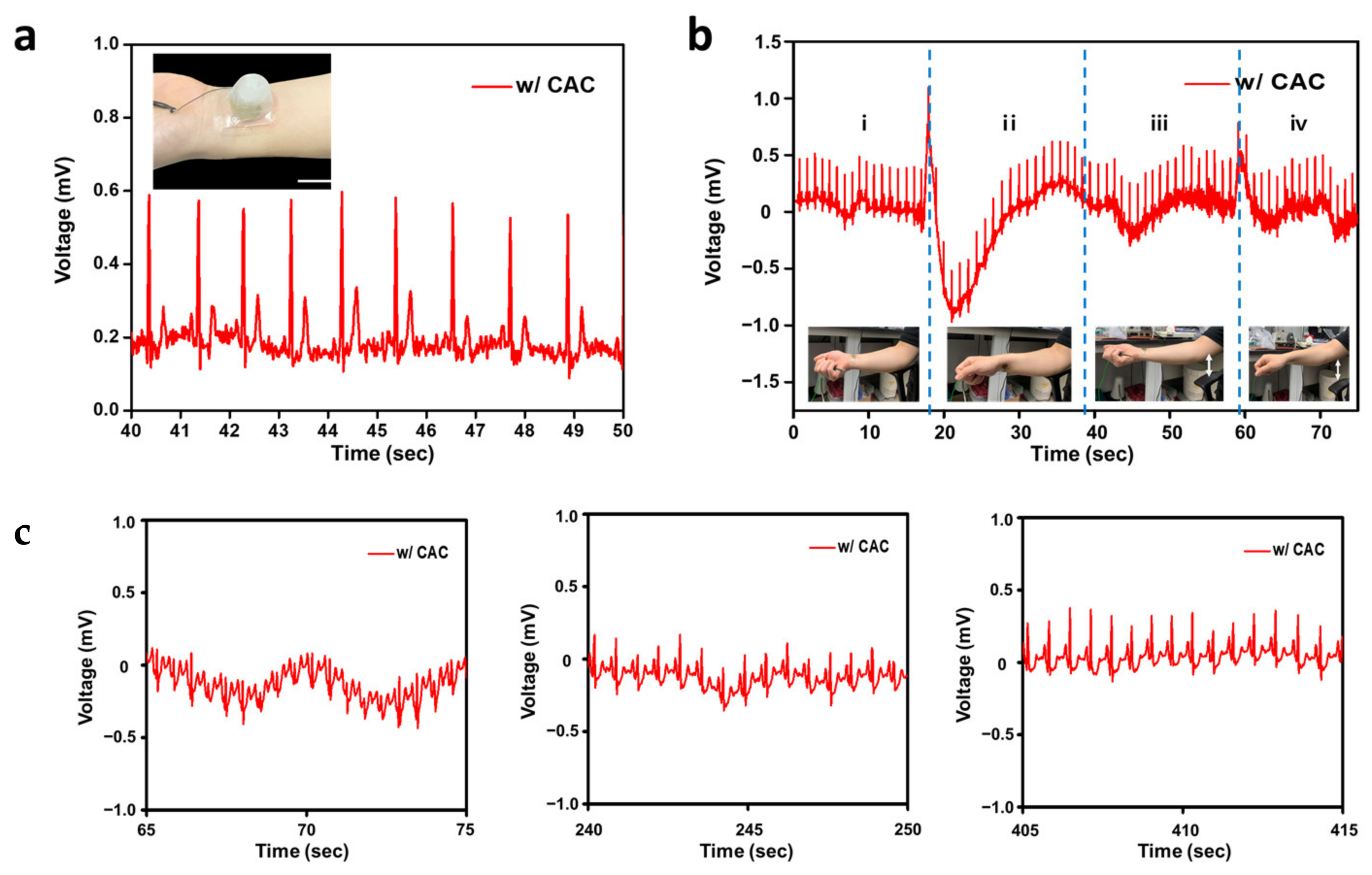
3.3. Electrocardiogram Monitoring
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Siontis, K.C.; Noseworthy, P.A.; Attia, Z.I.; Friedman, P.A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat. Rev. Cardiol. 2021, 18, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, Y.; Jia, L.; Mathewson, K.E.; Jang, K.; Kim, J.; Fu, H.; Huang, X.; Chava, P.; Wang, R.; et al. Soft microfluidic assemblies of sensors, circuits and radios for the skin. Science 2014, 344, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-K.; Son, D.; Kim, J.; Yoo, Y.J.; Lee, G.J.; Wang, L.; Choi, M.K.; Yang, J.; Lee, M.; Do, K.; et al. Wearable Force Touch Sensor Array Using a Flexible and Transparent Electrode. Adv. Funct. Mater. 2017, 27, 1605286. [Google Scholar] [CrossRef]
- Choi, H.; Kim, Y.; Kim, S.; Jung, H.; Lee, S.; Kim, K.; Han, H.-S.; Kim, J.Y.; Shin, M.; Son, D. Adhesive bioelectronics for sutureless epicardial interfacing. Nat. Electron. 2023. [Google Scholar] [CrossRef]
- Chou, H.-H.; Nguyen, A.; Chortos, A.; To, J.W.F.; Lu, C.; Mei, J.; Kurowawa, T.; Bae, W.-G.; Tok, J.B.-H.; Bao, Z. A chameleon-inspired stretchable electronic skin with interactive colour changing controlled by tactile sensing. Nat. Commun. 2015, 6, 8011. [Google Scholar] [CrossRef] [PubMed]
- Son, D.; Lee, J.; Qiao, S.; Ghaffari, R.; Kim, J.; Lee, J.E.; Song, C.; Kim, S.J.; Lee, D.J.; Jun, S.W.; et al. Multifunctional wearable devices for diagnosis and therapy of movement disorders. Nat. Nanotechnol. 2014, 9, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.E.; Zhang, F.-R.; Zhang, Y.; Shi, L.-A.; Zhao, H.-Y.; Zhu, H.-W.; Jiang, H.-L.; Yu, S.-H. A stretchable electronic fabric artificial skin with pressure-, lateral strain-, and flexion-sensitive properties. Adv. Mater. 2016, 28, 722–728. [Google Scholar]
- Lee, Y.; Chung, J.W.; Lee, G.H.; Kang, H.; Kim, J.-Y.; Bae, C.; Yoo, H.; Jeong, S.; Cho, H.; Kang, S.-G.; et al. Standalone real-time health monitoring patch based on a stretchable organic optoelectronic system. Sci. Adv. 2021, 7, eabg9180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, M.; Zheng, X.; Wang, H.; Li, X.; Zhang, X.; Yang, H. Activatable Conformal Electrode Arrays for Highly Stable and Breathable 12-Lead Clinical Electrocardiographic Monitoring. Adv. Mater. Technol. 2023, 8, 2300243. [Google Scholar] [CrossRef]
- He, S.; Guo, B.; Sun, X.; Shi, M.; Zhang, H.; Yao, F.; Sun, H.; Li, J. Bio-Inspired Instant Underwater Adhesive Hydrogel Sensors. ACS Appl. Mater. Interfaces 2022, 14, 45869–45879. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Zhou, W.; Meng, S.; Zi, C.; Liu, Z.; Kong, T. Biocompatible, High-Performance, Wet-Adhesive, Stretchable All-Hydrogel Supercapacitor Implant Based on PANI@rGO/Mxenes Electrode and Hydrogel Electrolyte. Adv. Energy Mater. 2021, 11, 2101329. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.; Yu, T.; Zhang, Y.; Ye, G.; Cui, H.; He, C.; Jiang, W.; Zhai, Y.; Lu, C.; et al. Ultra-conformal skin electrodes with synergistically enhanced conductivity for long-time and low motion artifact epidermal electrophysiology. Nat. Commun. 2021, 12, 4880. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, R.A.; Jin, H.; Lee, S.; Yokota, T.; Sekino, M.; Someya, T. Self-Adhesive and Ultra-Conformable, Sub-300 nm Dry Thin-Film Electrodes for Surface Monitoring of Biopotentials. Adv. Funct. Mater. 2018, 28, 1803279. [Google Scholar] [CrossRef]
- Sim, K.; Chen, S.; Li, Z.; Rao, Z.; Liu, J.; Lu, Y.; Jang, S.; Ershad, F.; Chen, J.; Xiao, J.; et al. Three-dimensional curvy electronics created using conformal additive stamp printing. Nat. Electron. 2019, 2, 471–479. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, S.; Xiong, W.; Zhu, C.; Li, K.; Huang, Y. Self-Healing Kirigami Assembly Strategy for Conformal Electronics. Adv. Funct. Mater. 2022, 32, 2109214. [Google Scholar] [CrossRef]
- Sim, K.; Rao, Z.; Li, Y.; Yang, D.; Yu, C. Curvy surface conformal ultra-thin transfer printed Si optoelectronic penetrating microprobe arrays. NPJ Flex. Electron. 2018, 2, 2. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, L.; Rao, Z.; Dong, B.; Yin, Z.; Huang, Y. High-Performance, Micrometer Thick/Conformal, Transparent Metal-Network Electrodes for Flexible and Curved Electronic Devices. Adv. Mater. Technol. 2018, 3, 1800155. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, X.; Liu, J.; Ye, D.; Duan, Y.; Li, K.; Yin, J.; Huang, Y. Flexible Metamaterial Electronics. Adv. Mater. 2022, 34, 2200070. [Google Scholar] [CrossRef]
- Song, J.-K.; Kim, J.; Yoon, J.; Koo, J.H.; Jung, H.; Kang, K.; Sunwoo, S.-H.; Yoo, S.; Chang, H.; Jo, J.; et al. Stretchable colour-sensitive quantum dot nanocomposites for shape-tunable multiplexed phototransistor arrays. Nat. Nanotechnol. 2022, 17, 849–856. [Google Scholar] [CrossRef]
- Yang, C.; Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 2018, 3, 125–142. [Google Scholar] [CrossRef]
- Hu, B.; Owh, C.; Chee, P.L.; Leow, W.R.; Liu, X.; Wu, Y.-L.; Guo, P.; Loh, X.J.; Chen, X. Supramolecular hydrogels for antimicrobial therapy. Chem. Soc. Rev. 2018, 47, 6917–6929. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fang, Y.; He, H.; Zhang, L.; Li, C.; Ouyang, J. Wearable Stretchable Dry and Self-Adhesive Strain Sensors with Conformal Contact to Skin for High-Quality Motion Monitoring. Adv. Funct. Mater. 2021, 31, 2007495. [Google Scholar] [CrossRef]
- Yuk, H.; Wu, J.; Zhao, X. Hydrogel interfaces for merging humans and machines. Nat. Rev. Mater. 2022, 7, 935–952. [Google Scholar] [CrossRef]
- Kim, S.; Choi, H.; Son, D.; Shin, M. Conductive and Adhesive Granular Alginate Hydrogels for On-Tissue Writable Bioelectronics. Gels 2023, 9, 167. [Google Scholar] [CrossRef]
- Jin, S.; Kim, Y.; Son, D.; Shin, M. Tissue Adhesive, Conductive, and Injectable Cellulose Hydrogel Ink for On-Skin Direct Writing of Electronics. Gels 2022, 8, 336. [Google Scholar] [CrossRef]
- Kim, Y.; Song, J.; An, S.; Shin, M.; Son, D. Soft Liquid Metal-Based Conducting Composite with Robust Electrical Durability for a Wearable Electrocardiogram Sensor. Polymers 2022, 14, 3409. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Progress. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, superstiff, and conductive alginate hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef]
- Baspinar, Y.; Borchert, H.-H. Penetration and release studies of positively and negatively charged nanoemulsions—Is there a benefit of the positive charge? Int. J. Pharm. 2012, 430, 247–252. [Google Scholar] [CrossRef]
- Marro, D.; Guy, R.H.; Delgado-Charro, M.B. Characterization of the iontophoretic permselectivity properties of human and pig skin. J. Control. Release 2001, 70, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Ilyina, A.V.; Tikhonov, V.E.; Albulov, A.I.; Varlamov, V.P. Enzymic preparation of acid-free-water-soluble chitosan. Process Biochem. 2000, 35, 563–568. [Google Scholar] [CrossRef]
- Giuseppe, M.D.; Law, N.; Webb, B.; Macrae, R.A.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical behaviour of alginate-gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wu, P. A highly transparent and ultra-stretchable conductor with stable conductivity during large deformation. Nat. Commun. 2019, 10, 3429. [Google Scholar] [CrossRef]
- Qi, D.; Zhang, K.; Tian, G.; Jiang, B.; Huang, Y. Stretchable Electronics Based on PDMS Substrates. Adv. Mater. 2021, 33, 2003155. [Google Scholar] [CrossRef]
- Hwang, I.; Kin, H.N.; Seong, M.; Lee, S.-H.; Kang, M.; Yi, H.; Bae, W.G.; Kwak, M.K.; Jeong, H.E. Multifunctional Smart Skin Adhesive Patches for Advanced Health Care. Adv. Healthc. Mater. 2018, 7, 1800275. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Khutoryanskiy, V.V.; Charalampopoulos, D. Layer-by-layer coating of alginate matrices with chitosan–alginate for the improved survival and targeted delivery of probiotic bacteria after oral administration. J. Mater. Chem. B 2013, 1, 52–60. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Caseli, L.; Oliveira, O.N., Jr. Chitosan in Nanostructured Thin Films. Biomacromolecules 2010, 11, 1897–1908. [Google Scholar] [CrossRef]
- Burnette, R.R.; Ongpipattanakul, B. Characterization of the Permselective Properties of Excised Human Skin During Iontophoresis. J. Pharm. Sci. 1987, 76, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Prolongo, S.G.; Urena, A. Effect of surface pre-treatment on the adhesive strength of epoxy–aluminium joints. Int. J. Adhes. Adhes. 2009, 29, 23–31. [Google Scholar] [CrossRef]
- Kwak, M.K.; Jeong, H.-E.; Suh, K.Y. Rational Design and Enhanced Biocompatibility of a Dry Adhesive Medical Skin Patch. Adv. Mater. 2011, 23, 3949–3953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.; Zhao, B.; Pinnaratip, R.; et al. Catechol-functionalized hydrogels: Biomimetic design, adhesion mechanism, and biomedical applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef]
- Nam, S.; Mooney, D. Polymeric Tissue Adhesives. Chem. Rev. 2021, 121, 11336–11384. [Google Scholar]
- Jung, E.C.; Maibach, H.I. Animal models for percutaneous absorption. In Topical Drug Bioavailability, Bioequivalence, and Penetration; Springer: New York, NY, USA, 2014; pp. 21–40. [Google Scholar]
- Li, Y.; Tanaka, T. Phase transitions of gels. Annu. Rev. Mater. Sci. 1992, 22, 243–277. [Google Scholar] [CrossRef]
- Khan, Y.A.; Ozaltin, K.; Bernal-Ballen, A.; Martino, A.D. Chitosan-alginate hydrogels for simultaneous and sustained releases of ciprofloxacin, amoxicillin and vancomycin for combination therapy. J. Drug Deliv. Sci. Technol. 2021, 61, 102126. [Google Scholar] [CrossRef]
- Dechiraju, H.; Jia, M.; Luo, L.; Rolandi, M. Ion-Conducting Hydrogels and Their Applications in Bioelectronics. Adv. Sustain. Syst. 2022, 6, 2100173. [Google Scholar] [CrossRef]
- Nagamine, K.; Chihara, S.; Kai, H.; Kaji, H.; Nishizawa, M. Totally shape-conformable electrode/hydrogel composite for on-skin electrophysiological measurements. Sens. Actuators B 2016, 237, 49–53. [Google Scholar] [CrossRef]
- Wirthl, D.; Pichler, R.; Drack, M.; Kettlguber, G.; Moser, R.; Gerstmayr, R.; Hartmann, F.; Bradt, E.; Kaltseis, R.; Siket, C.M.; et al. Instant tough bonding of hydrogels for soft machines and electronics. Sci. Adv. 2017, 3, e1700053. [Google Scholar] [CrossRef]
- Kim, S.H.; Jung, S.; Yoon, I.S.; Lee, C.; Oh, Y.; Hong, J.-M. Ultrastretchable Conductor Fabricated on Skin-Like Hydrogel–Elastomer Hybrid Substrates for Skin Electronics. Adv. Mater. 2018, 30, 1800109. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Lv, J.; Lee, P.S. Natural Polymer in Soft Electronics: Opportunities, Challenges, and Future Prospects. Adv. Mater. 2022, 34, 2105020. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, S.-H.; Han, S.I.; Joo, H.; Cha, G.D.; Kim, D.; Choi, S.H.; Hyeon, T.; Kim, D.-H. Advances in Soft Bioelectronics for Brain Research and Clinical Neuroengineering. Matter 2020, 3, 1923–1947. [Google Scholar] [CrossRef]
- Yokota, T.; Zalar, P.; Kaltenbrunner, M.; Jinno, H.; Matsuhisa, N.; Kitanosako, H.; Tachibana, Y.; Yukita, W.; Koizumi, M.; Someya, T. Ultraflexible organic photonic skin. Sci. Adv. 2016, 2, e1501856. [Google Scholar] [CrossRef] [PubMed]
- Son, D.; Kang, J.; Vardoulis, O.; Kim, Y.; Matsuhisa, N.; Oh, J.Y.; To, J.W.F.; Mun, J.; Katsumata, T.; Liu, Y.; et al. An integrated self-healable electronic skin system fabricated via dynamic reconstruction of a nanostructured conducting network. Nat. Nanotech. 2018, 13, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef]
- Kim, S.H.; Seo, H.; Kang, J.; Hong, J.; Seong, D.; Kim, H.-J.; Kim, J.; Mun, J.; Youn, I.; Kim, J.; et al. An Ultrastretchable and Self-Healable Nanocomposite Conductor Enabled by Autonomously Percolative Electrical Pathways. ACS Nano 2019, 13, 6531–6539. [Google Scholar] [CrossRef]
- Serhani, M.A.; Kassabi, H.T.E.; Ismail, H.; Navaz, A.N. ECG Monitoring Systems: Review, Architecture, Processes, and Key Challenges. Sensors 2020, 20, 1796. [Google Scholar] [CrossRef]
- Park, J.; Seong, D.; Park, Y.J.; Park, S.H.; Jung, H.; Kim, Y.; Bacc, H.W.; Shin, M.; Lee, S.; Lee, M.; et al. Reversible electrical percolation in a stretchable and self-healable silver-gradient nanocomposite bilayer. Nat. Commun. 2022, 13, 5233. [Google Scholar] [CrossRef]
- Dargusch, M.; Liu, W.-D.; Chen, Z.-G. Thermoelectric Generators: Alternative Power Supply for Wearable Electrocardiographic Systems. Adv. Sci. 2020, 7, 2001362. [Google Scholar] [CrossRef]
- Zhang, L.; Kumar, K.S.; He, H.; Cai, C.J.; He, X.; Gao, H.; Yue, S.; Li, C.; Seet, R.C.-S.; Ren, H.; et al. Fully organic compliant dry electrodes self-adhesive to skin for long-term motion-robust epidermal biopotential monitoring. Nat. Commun. 2020, 11, 4683. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-E. Surface electrocardiography of supraventricular tachycardia—differential diagnosis. Int. J. Arrhythmia 2011, 12, 23–31. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Jang, J.; Lee, J.; Shin, M.; Lee, J.S.; Son, D. Stretchable Gold Nanomembrane Electrode with Ionic Hydrogel Skin-Adhesive Properties. Polymers 2023, 15, 3852. https://doi.org/10.3390/polym15183852
Lee H, Jang J, Lee J, Shin M, Lee JS, Son D. Stretchable Gold Nanomembrane Electrode with Ionic Hydrogel Skin-Adhesive Properties. Polymers. 2023; 15(18):3852. https://doi.org/10.3390/polym15183852
Chicago/Turabian StyleLee, Hyelim, Jaepyo Jang, Jaebeom Lee, Mikyung Shin, Jung Seung Lee, and Donghee Son. 2023. "Stretchable Gold Nanomembrane Electrode with Ionic Hydrogel Skin-Adhesive Properties" Polymers 15, no. 18: 3852. https://doi.org/10.3390/polym15183852
APA StyleLee, H., Jang, J., Lee, J., Shin, M., Lee, J. S., & Son, D. (2023). Stretchable Gold Nanomembrane Electrode with Ionic Hydrogel Skin-Adhesive Properties. Polymers, 15(18), 3852. https://doi.org/10.3390/polym15183852







