Morphological Evolution of Hybrid Block Copolymer Particles: Toward Magnetic Responsive Particles
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Synthesis of Au NPs
2.3. Synthesis of Iron Oxide NPs
2.4. Preparation of PS-b-PB Colloidal Particles by Membrane Emulsification
2.5. Characterizations
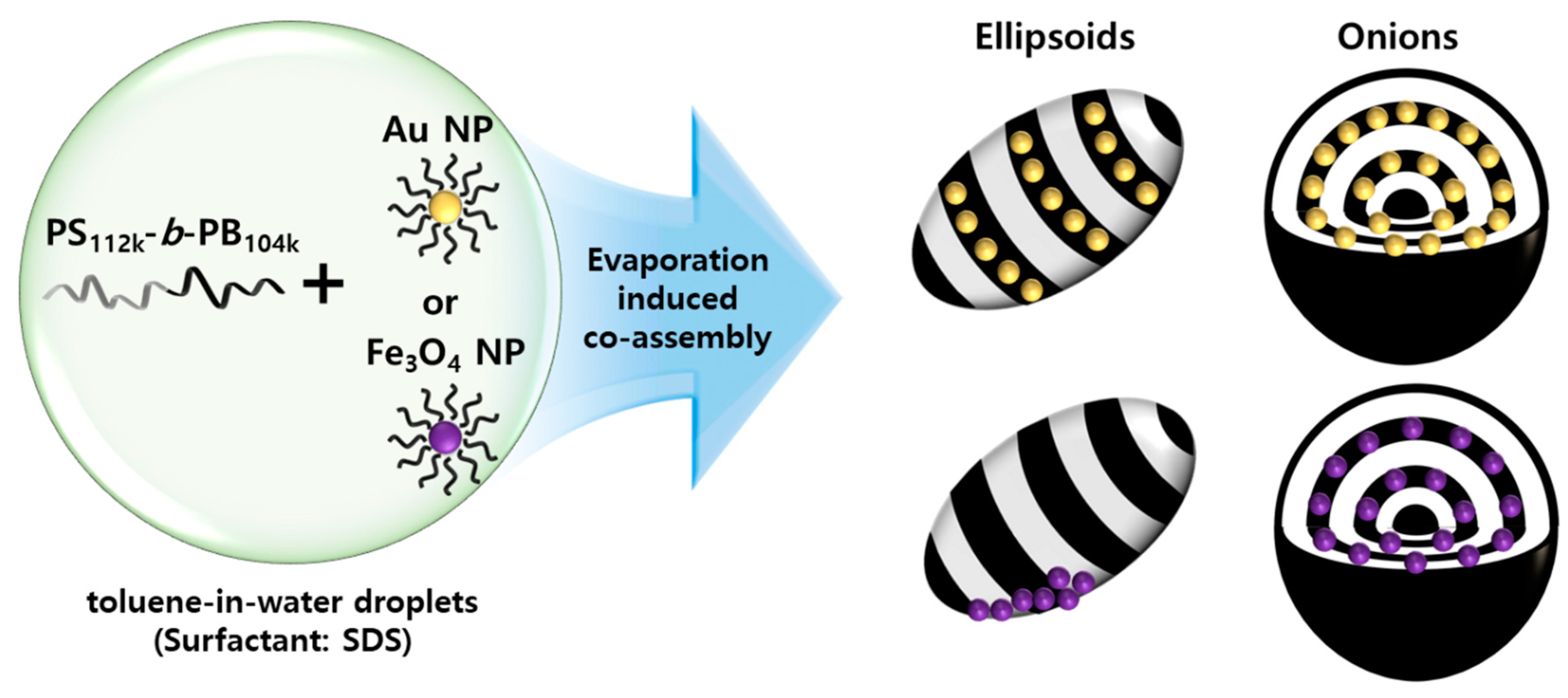
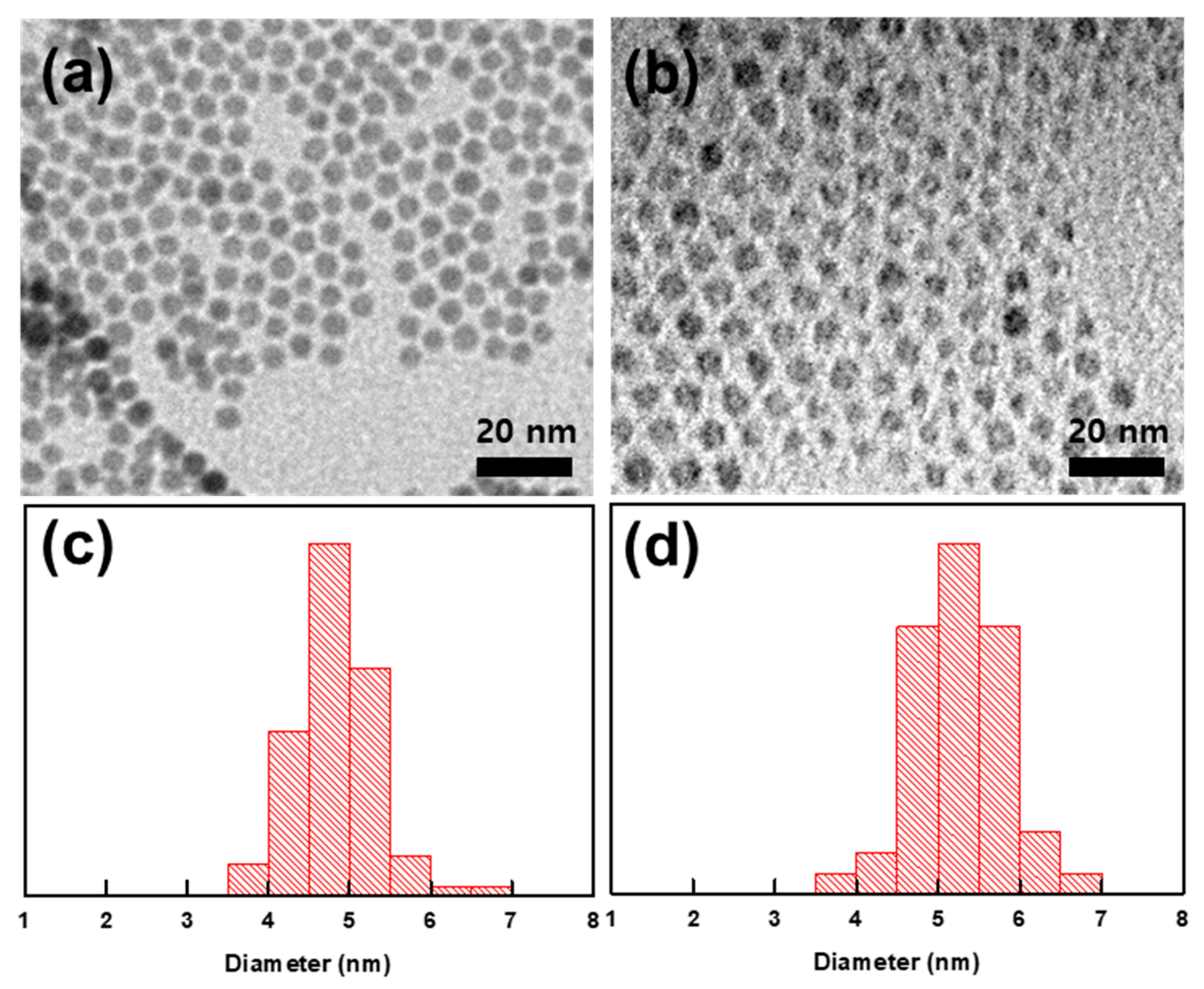
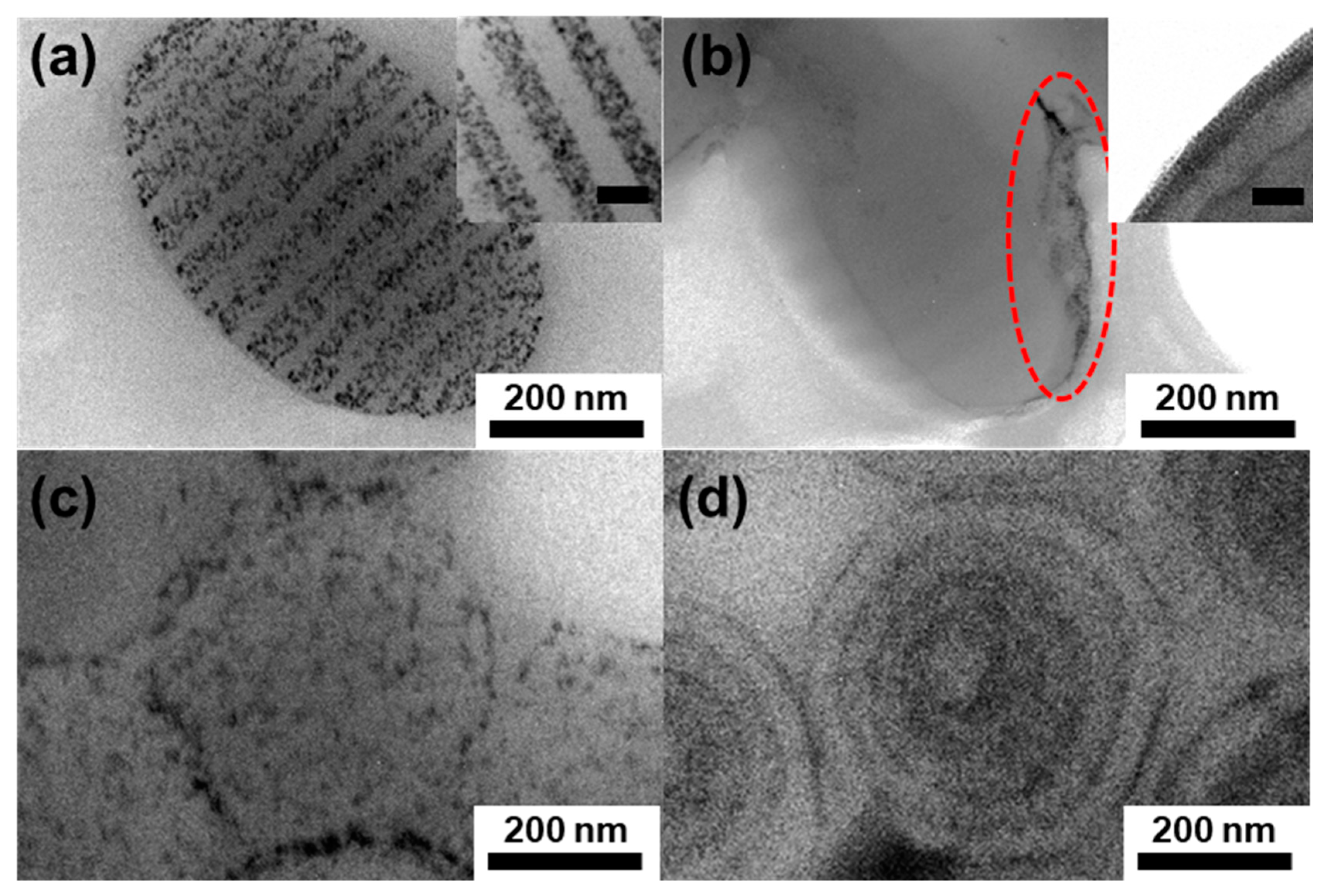
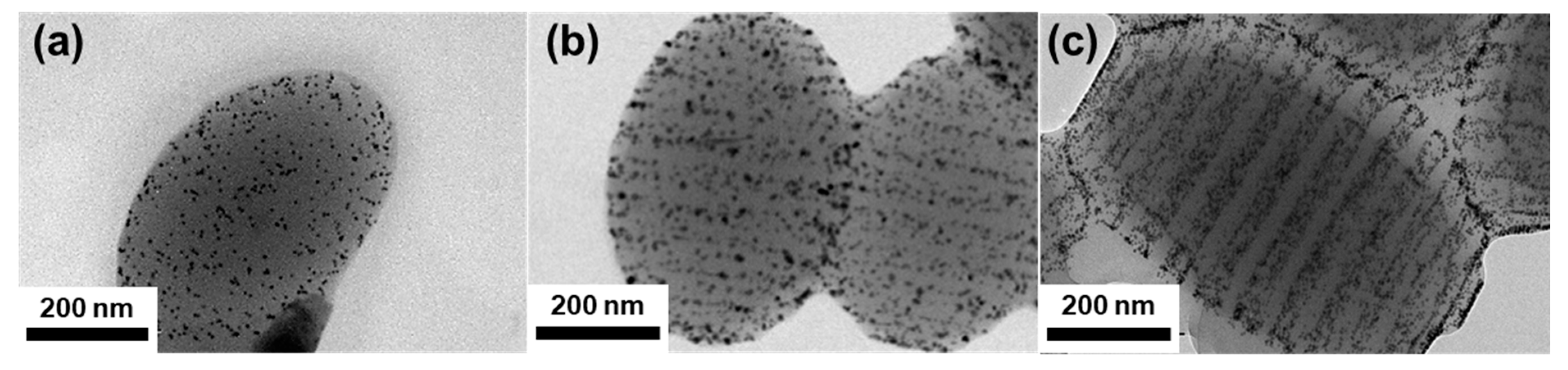
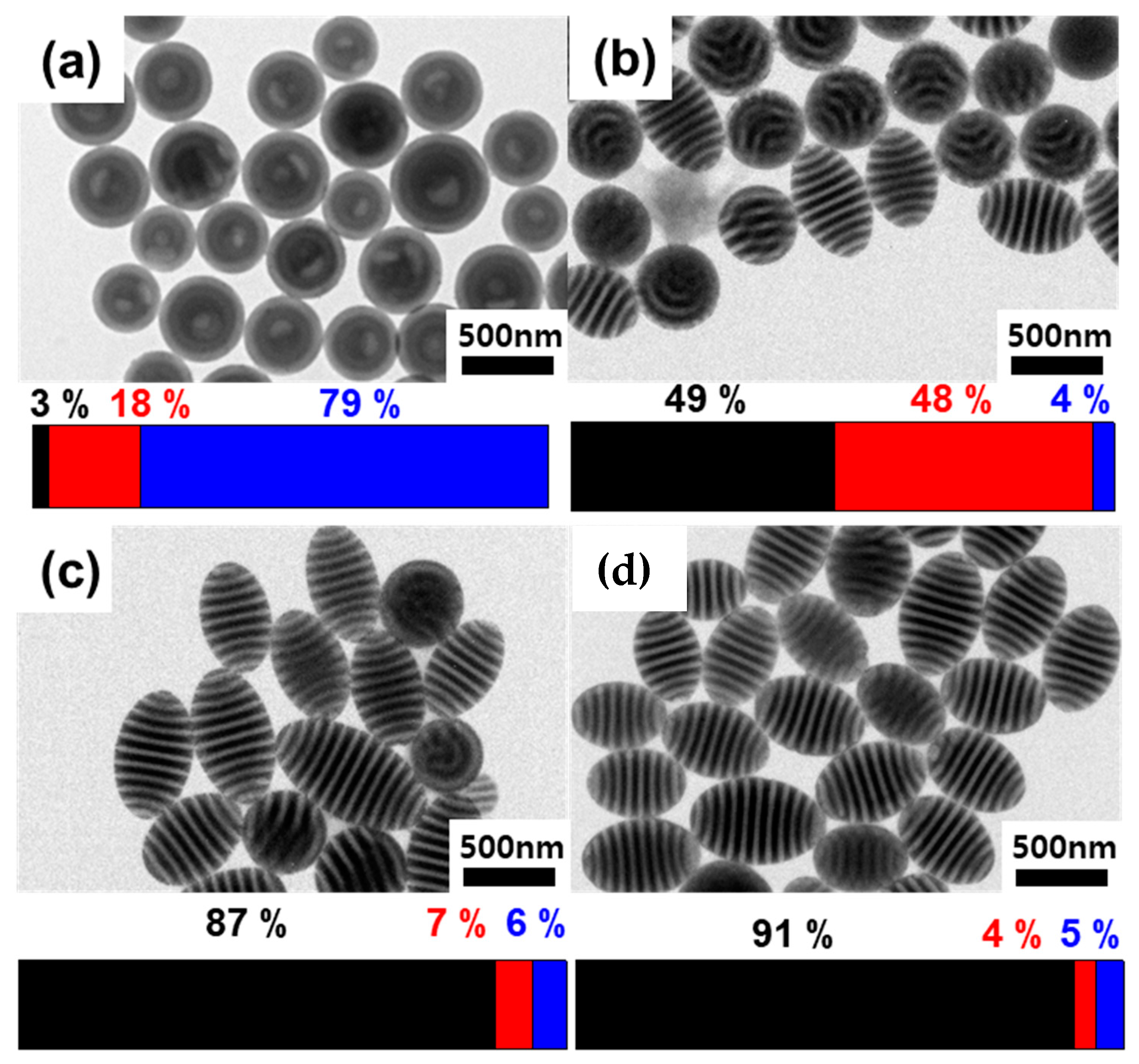
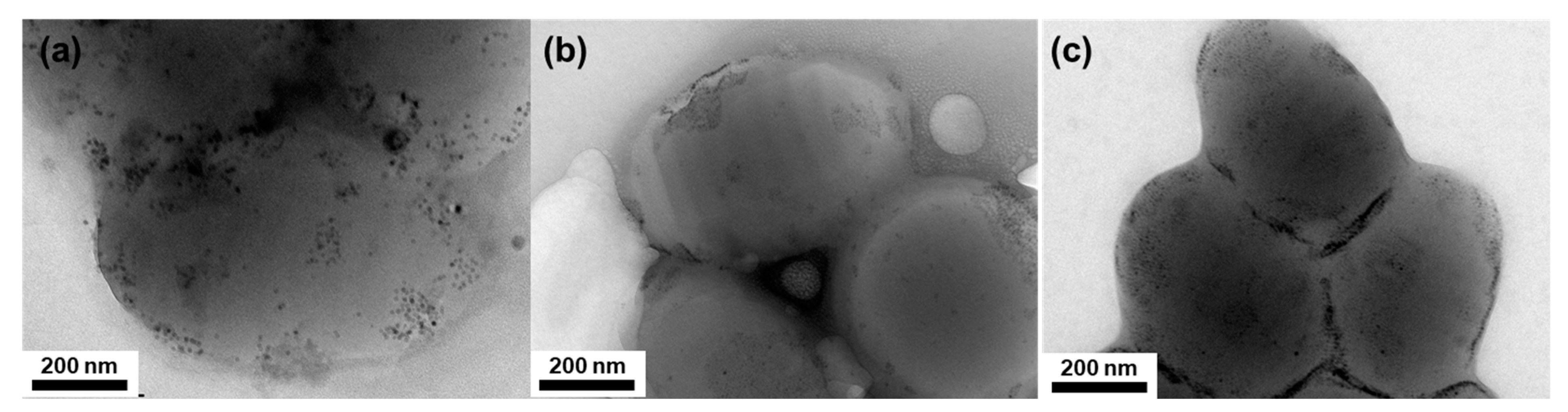
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ku, K.H.; Shin, J.M.; Yun, H.; Yi, G.-R.; Jang, S.G.; Kim, B.J. Multidimensional design of anisotropic polymer particles from solvent-evaporative emulsion. Adv. Funct. Mater. 2018, 28, 1802961. [Google Scholar] [CrossRef]
- Yan, N.; Zhu, Y.; Jiang, W. Recent progress in the self-assembly of block copolymers confined in emulsion droplets. Chem. Commun. 2018, 54, 13183–13195. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Qiang, X.; Müller, A.H.; Gröschel, A.H. Self-assembly of block copolymers into internally ordered microparticles. Prog. Polym. Sci. 2020, 102, 101211. [Google Scholar] [CrossRef]
- Ku, K.H.; Shin, J.M.; Kim, M.P.; Lee, C.H.; Seo, M.K.; Yi, G.R.; Jang, S.G.; Kim, B.J. Size-controlled nanoparticle-guided assembly of block copolymers for convex lens-shaped particles. J. Am. Chem. Soc. 2014, 136, 9982–9989. [Google Scholar] [CrossRef]
- Klinger, D.; Wang, C.X.; Connal, L.A.; Audus, D.J.; Jang, S.G.; Kraemer, S.; Killops, K.L.; Fredrickson, G.H.; Kramer, E.J.; Hawker, C.J. A facile synthesis of dynamic, shape-changing polymer particles. Angew. Chem. Int. Ed. Engl. 2014, 53, 7018–7022. [Google Scholar] [CrossRef]
- Jeon, S.-J.; Yi, G.-R.; Yang, S.-M. Cooperative Assembly of Block Copolymers with Deformable Interfaces: Toward Nanostructured Particles. Adv. Mater. 2008, 20, 4103–4108. [Google Scholar] [CrossRef]
- Jang, S.G.; Audus, D.J.; Klinger, D.; Krogstad, D.V.; Kim, B.J.; Cameron, A.; Kim, S.W.; Delaney, K.T.; Hur, S.M.; Killops, K.L.; et al. Striped, ellipsoidal particles by controlled assembly of diblock copolymers. J. Am. Chem. Soc. 2013, 135, 6649–6657. [Google Scholar] [CrossRef]
- Deng, R.; Liang, F.; Li, W.; Yang, Z.; Zhu, J. Reversible transformation of nanostructured polymer particles. Macromolecules 2013, 46, 7012–7017. [Google Scholar] [CrossRef]
- Ku, K.H.; Lee, Y.J.; Kim, Y.; Kim, B.J. Shape-anisotropic diblock copolymer particles from evaporative emulsions: Experiment and theory. Macromolecules 2019, 52, 1150–1157. [Google Scholar] [CrossRef]
- Ku, K.H.; Kim, Y.; Yi, G.-R.; Jung, Y.S.; Kim, B.J. Soft patchy particles of block copolymers from interface-engineered emulsions. ACS Nano 2015, 9, 11333–11341. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, H.E.; Oh, H.; Yun, H.; Lee, J.; Shin, S.; Lee, H.; Kim, B.J. Lens-shaped carbon particles with perpendicularly-oriented channels for high-performance proton exchange membrane fuel cells. ACS Nano 2022, 16, 2988–2996. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, Y.J.; Park, D.; Jeong, H.; Shin, S.; Yun, H.; Lim, J.; Han, J.; Kim, E.J.; Jeon, S.S.; et al. Highly durable fuel cell catalysts using crosslinkable block copolymer-based carbon supports with ultralow Pt loadings. Energy Environ. Sci. 2020, 13, 4921–4929. [Google Scholar] [CrossRef]
- Wang, M.; Mao, X.; Liu, J.; Deng, B.; Deng, S.; Jin, S.; Li, W.; Gong, J.; Deng, R.; Zhu, J. A versatile 3D-confined self-assembly strategy for anisotropic and ordered mesoporous carbon microparticles. Adv. Sci. 2022, 9, e2202394. [Google Scholar] [CrossRef] [PubMed]
- Moriceau, G.; Kilchoer, C.; Djeghdi, K.; Weder, C.; Steiner, U.; Wilts, B.D.; Gunkel, I. Photonic particles made by the confined self-assembly of a supramolecular comb-like block copolymer. Macromol. Rapid Commun. 2021, 42, e2100522. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, H.; Xu, J.; Hwang, M.S.; Tian, D.; Wang, K.; Zhang, L.; Liao, Y.; Park, H.G.; Yi, G.R.; et al. Responsive block copolymer photonic microspheres. Adv. Mater. 2018, 30, e1707344. [Google Scholar] [CrossRef]
- Yang, Y.; Kang, T.H.; Wang, K.; Ren, M.; Chen, S.; Xiong, B.; Xu, J.; Zhang, L.; Yi, G.R.; Zhu, J. Tunable photonic microspheres of comb-like supramolecules. Small 2020, 16, e2001315. [Google Scholar] [CrossRef]
- Kim, J.; Yun, H.; Lee, Y.J.; Lee, J.; Kim, S.H.; Ku, K.H.; Kim, B.J. Photoswitchable surfactant-driven reversible shape- and color-changing block copolymer particles. J. Am. Chem. Soc. 2021, 143, 13333–13341. [Google Scholar] [CrossRef]
- Lee, J.; Ku, K.H.; Park, C.H.; Lee, Y.J.; Yun, H.; Kim, B.J. Shape and color switchable block copolymer particles by temperature and pH dual responses. ACS Nano 2019, 13, 4230–4237. [Google Scholar] [CrossRef]
- Hu, D.; Chang, X.; Xu, Y.; Yu, Q.; Zhu, Y. Light-enabled reversible shape transformation of block copolymer particles. ACS Macro Lett. 2021, 10, 914–920. [Google Scholar] [CrossRef]
- Deng, R.; Xu, J.; Yi, G.R.; Kim, J.W.; Zhu, J. Responsive colloidal polymer particles with ordered mesostructures. Adv. Func. Mater. 2020, 31, 2008169. [Google Scholar] [CrossRef]
- Lee, D.; Kim, J.; Ku, K.H.; Li, S.; Shin, J.J.; Kim, B.J. Poly(vinylpyridine)-containing block copolymers for smart, multicompartment particles. Polym. Chem. 2022, 13, 2570–2588. [Google Scholar] [CrossRef]
- Ku, K.H.; Yang, H.; Jang, S.G.; Bang, J.; Kim, B.J. Tailoring block copolymer and polymer blend morphology using nanoparticle surfactants. J. Polym. Sci. A Polym. Chem. 2016, 54, 228–237. [Google Scholar] [CrossRef]
- Ku, K.H.; Yang, H.; Shin, J.M.; Kim, B.J. Aspect ratio effect of nanorod surfactants on the shape and internal morphology of block copolymer particles. J. Polym. Sci. A Polym. Chem. 2015, 53, 188–192. [Google Scholar] [CrossRef]
- Xu, M.; Ku, K.H.; Lee, Y.J.; Kim, T.; Shin, J.J.; Kim, E.J.; Choi, S.-H.; Yun, H.; Kim, B.J. Effect of polymer ligand conformation on the self-assembly of block copolymers and polymer-grafted nanoparticles within an evaporative emulsion. Macromolecules 2021, 54, 3084–3092. [Google Scholar] [CrossRef]
- Xu, M.; Ku, K.H.; Lee, Y.J.; Shin, J.J.; Kim, E.J.; Jang, S.G.; Yun, H.; Kim, B.J. Entropy-driven assembly of nanoparticles within emulsion-evaporative block copolymer particles: Crusted, seeded, and alternate-layered onions. Chem. Mater. 2020, 32, 7036–7043. [Google Scholar] [CrossRef]
- Ku, K.H.; Ryu, J.H.; Kim, J.; Yun, H.; Nam, C.; Shin, J.M.; Kim, Y.; Jang, S.G.; Lee, W.B.; Kim, B.J. Mechanistic study on the shape transition of block copolymer particles driven by length-controlled nanorod surfactants. Chem. Mater. 2018, 30, 8669–8678. [Google Scholar] [CrossRef]
- Mei, S.; Qi, H.; Zhou, T.; Li, C.Y. Precisely assembled cyclic gold nanoparticle frames by 2D polymer single-crystal templating. Angew. Chem. Int. Ed. 2017, 56, 13645–13649. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Yang, J.; Kang, K.; Kim, D.C.; Choi, C.; Park, C.; Kim, S.J.; Chae, S.I.; Kim, T.H.; Kim, J.H.; et al. Wearable red-green-blue quantum dot light-emitting diode array using high-resolution intaglio transfer printing. Nat. Commun. 2015, 6, 7149. [Google Scholar] [CrossRef]
- Pyun, J. Nanocomposite materials from functional polymers and magnetic colloids. Polym. Rev. 2007, 47, 231–263. [Google Scholar] [CrossRef]
- Corr, S.A.; Byrne, S.J.; Tekoriute, R.; Meledandri, C.J.; Brougham, D.F.; Lynch, M.; Kerskens, C.; O’Dwyer, L.; Gun’ko, Y.K. Linear assemblies of magnetic nanoparticles as MRI contrast agents. J. Am. Chem. Soc. 2008, 130, 4214–4215. [Google Scholar] [CrossRef]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Tan, X.; Dewapriya, P.; Prasad, P.; Chang, Y.; Huang, X.; Wang, Y.; Gong, X.; Hopkins, T.E.; Fu, C.; Thomas, K.V.; et al. Efficient removal of perfluorinated chemicals from contaminated water sources using magnetic fluorinated polymer sorbents. Angew. Chem. Int. Ed. 2022, 61, e202213071. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ku, K.H.; Vijayamohanan, H.; Kim, B.J.; Swager, T.M. Switchable full-color reflective photonic ellipsoidal particles. J. Am. Chem. Soc. 2020, 142, 10424–10430. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Cho, J.; Kim, H.; Lim, M.; Jo, H.; Kim, H.; Min, S.-J.; Rhee, H.; Kim, J.W. Janus colloid surfactant catalysts for in situ organic reactions in Pickering emulsion microreactors. Green. Chem. 2018, 20, 2840–2844. [Google Scholar] [CrossRef]
- Kim, H.; Cho, J.; Cho, J.; Park, B.J.; Kim, J.W. Magnetic-patchy Janus colloid surfactants for reversible recovery of Pickering emulsions. ACS Appl. Mater. Interfaces 2018, 10, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.H.; Lee, Y.J.; Yi, G.-R.; Jang, S.G.; Schmidt, B.V.K.J.; Liao, K.; Klinger, D.; Hawker, C.J.; Kim, B.J. Shape-tunable biphasic Janus particles as pH-responsive switchable surfactants. Macromolecules 2017, 50, 9276–9285. [Google Scholar] [CrossRef]
- Hou, Z.; Ren, M.; Wang, K.; Yang, Y.; Xu, J.; Zhu, J. Deformable block copolymer microparticles by controllable localization of pH-responsive nanoparticles. Macromolecules 2019, 53, 473–481. [Google Scholar] [CrossRef]
- Yabu, H.; Kanahara, M.; Shimomura, M.; Arita, T.; Harano, K.; Nakamura, E.; Higuchi, T.; Jinnai, H. Polymer Janus particles containing block-copolymer stabilized magnetic nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 3262–3266. [Google Scholar] [CrossRef]
- Shin, J.M.; Kim, Y.; Yun, H.; Yi, G.R.; Kim, B.J. Morphological evolution of block copolymer particles: Effect of solvent evaporation rate on particle shape and morphology. ACS Nano 2017, 11, 2133–2142. [Google Scholar] [CrossRef]
- Peng, S.; Lee, Y.; Wang, C.; Yin, H.; Dai, S.; Sun, S. A facile synthesis of monodisperse Au nanoparticles and their catalysis of CO oxidation. Nano Res. 2008, 1, 229–234. [Google Scholar] [CrossRef]
- Yun, H.; Kim, J.; Paik, T.; Meng, L.; Jo, P.S.; Kikkawa, J.M.; Kagan, C.R.; Allen, M.G.; Murray, C.B. Alternate current magnetic property characterization of nonstoichiometric zinc ferrite nanocrystals for inductor fabrication via a solution based process. J. Appl. Phys. 2016, 119, 113901. [Google Scholar] [CrossRef]
- Shin, J.M.; Kim, Y.; Ku, K.H.; Lee, Y.J.; Kim, E.J.; Yi, G.-R.; Kim, B.J. Aspect ratio-controlled synthesis of uniform colloidal block copolymer ellipsoids from evaporative emulsions. Chem. Mater. 2018, 30, 6277–6288. [Google Scholar] [CrossRef]
- Shin, J.M.; Kim, M.P.; Yang, H.; Ku, K.H.; Jang, S.G.; Youm, K.H.; Yi, G.-R.; Kim, B.J. Monodisperse nanostructured spheres of block copolymers and nanoparticles via cross-flow membrane emulsification. Chem. Mater. 2015, 27, 6314–6321. [Google Scholar] [CrossRef]
- Kao, J.; Thorkelsson, K.; Bai, P.; Rancatore, B.J.; Xu, T. Toward functional nanocomposites: Taking the best of nanoparticles, polymers, and small molecules. Chem. Soc. Rev. 2013, 42, 2654–2678. [Google Scholar] [CrossRef] [PubMed]
- Balazs Anna, C.; Emrick, T.; Russell Thomas, P. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.J.; Kim, B.J.; Kramer, E.J.; Pine, D.J. Control of nanoparticle location in block copolymers. J. Am. Chem. Soc. 2005, 127, 5036–5037. [Google Scholar] [CrossRef]
- Kim, B.J.; Bang, J.; Hawker, C.J.; Kramer, E.J. Effect of areal chain density on the location of polymer-modified gold nanoparticles in a block copolymer template. Macromolecules 2006, 39, 4108. [Google Scholar] [CrossRef]
- Kao, J.; Bai, P.; Lucas, J.M.; Alivisatos, A.P.; Xu, T. Size-dependent assemblies of nanoparticle mixtures in thin films. J. Am. Chem. Soc. 2013, 135, 1680–1683. [Google Scholar] [CrossRef]
- Bockstaller, M.R.; Lapetnikov, Y.; Margel, S.; Thomas, E.L. Size-selective organization of enthalpic compatibilized nanocrystals in ternary block copolymer/particle mixtures. J. Am. Chem. Soc. 2003, 125, 5276–5277. [Google Scholar] [CrossRef]
- Thorkelsson, K.; Mastroianni, A.J.; Ercius, P.; Xu, T. Direct nanorod assembly using block copolymer-based supramolecules. Nano Lett. 2012, 12, 498–504. [Google Scholar] [CrossRef]
- Chiu, J.J.; Kim, B.J.; Yi, G.-R.; Bang, J.; Kramer, E.J.; Pine, D.J. Distribution of nanoparticles in lamellar domains of block copolymers. Macromolecules 2007, 40, 3361–3365. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook, 4th ed.; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Wu, S. Polymer Interface and Adhesion; Marcel Dekker: New York, NY, USA, 1982. [Google Scholar]
- Ye, X.; Zhu, C.; Ercius, P.; Raja, S.N.; He, B.; Jones, M.R.; Hauwiller, M.R.; Liu, Y.; Xu, T.; Alivisatos, A.P. Structural diversity in binary superlattices self-assembled from polymer-grafted nanocrystals. Nat. Commun. 2015, 6, 10052. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, H.; Wu, S.; Huang, G.; Xing, W.; Tang, M.; Fu, Q. Influence of magnetic nanoparticle size on the particle dispersion and phase separation in an ABA triblock copolymer. J. Phys. Chem. B 2014, 118, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.B.; Ginzburg, V.V.; Matsen, M.W.; Balazs, A.C. Predicting the mesophases of copolymer-nanoparticle composites. Science 2001, 292, 2469–2472. [Google Scholar] [CrossRef] [PubMed]
- Keng, P.Y.; Shim, I.; Korth, B.D.; Douglas, J.F.; Pyun, J. Synthesis and self-assembly of polymer-coated ferromagnetic nanoparticles. ACS Nano 2007, 1, 279–292. [Google Scholar] [CrossRef]
- Kralj, S.; Makovec, D. Magnetic assembly of superparamagnetic iron oxide nanoparticle clusters into nanochains and nanobundles. ACS Nano 2015, 9, 9700–9707. [Google Scholar] [CrossRef]
- Lo, C.-T.; Chao, C.-J. Synthesis and characterization of magnetic nanoparticle/block copolymer composites. Langmuir 2009, 25, 12865–12869. [Google Scholar] [CrossRef]
- Park, M.J.; Char, K.; Park, J.; Hyeon, T. Effect of the casting solvent on the morphology of poly(styrene-b-isoprene) diblock copolymer/magnetic nanoparticle mixtures. Langmuir 2007, 22, 1375. [Google Scholar] [CrossRef]
- Turturro, A.; Gattiglia, E.; Vacca, P.; Viola, G.T. Free surface morphology of block copolymers: 1. styrene-butadiene diblock copolymers. Polymer 1995, 36, 3987–3996. [Google Scholar] [CrossRef]
- Kalra, V.; Lee, J.; Lee, J.H.; Lee, S.G.; Marquez, M.; Wiesner, U.; Joo, Y.L. Controlling nanoparticle location via confined assembly in electrospun block copolymer nanofibers. Small 2008, 4, 2067–2073. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.J. Morphological Evolution of Hybrid Block Copolymer Particles: Toward Magnetic Responsive Particles. Polymers 2023, 15, 3689. https://doi.org/10.3390/polym15183689
Shin JJ. Morphological Evolution of Hybrid Block Copolymer Particles: Toward Magnetic Responsive Particles. Polymers. 2023; 15(18):3689. https://doi.org/10.3390/polym15183689
Chicago/Turabian StyleShin, Jaeman J. 2023. "Morphological Evolution of Hybrid Block Copolymer Particles: Toward Magnetic Responsive Particles" Polymers 15, no. 18: 3689. https://doi.org/10.3390/polym15183689
APA StyleShin, J. J. (2023). Morphological Evolution of Hybrid Block Copolymer Particles: Toward Magnetic Responsive Particles. Polymers, 15(18), 3689. https://doi.org/10.3390/polym15183689







