Abstract
The safety of a medicinal product is determined by its pharmacological and toxicological profile, which depends not only on the active substance’s toxicological properties, but also on the impurities it contains. Because impurities are a problem that must be considered to ensure the safety of a drug product, many studies have been conducted regarding the separation or purification of active pharmaceutical ingredients (APIs) and the determination of impurities in APIs and drug products. Several studies have applied molecularly imprinted polymers (MIPs) to separate impurities in active ingredients and as adsorbents in the sample preparation process. This review presents the design of MIPs and the methods used to synthesise MIPs to separate impurities in APIs and drug product samples, the application of MIPs to separate impurities, and a view of future studies involving MIPs to remove impurities from pharmaceutical products. Based on a comparison of the bulk and surface-imprinting polymerisation methods, the MIPs produced by the surface-imprinting polymerisation method have a higher adsorption capacity and faster adsorption kinetics than the MIPs produced by the bulk polymerisation method. However, the application of MIPs in the analysis of APIs and drug products are currently only related to organic compounds. Considering the advantages of MIPs to separate impurities, MIPs for other impurities still need to be developed, including multi-template MIPs for simultaneous separation of multiple impurities.
1. Introduction
The pharmaceutical industry aims to protect public health by ensuring that patients have access to the right medicine at the correct dose and potency and at an affordable price. Therefore, drug safety and efficacy are two major issues in drug therapy [1]. The safety of a medicinal product is determined by its pharmacological and toxicological profile, which depends not only on the active substance’s toxicological properties, but also on the impurities it contains [1]. Impurities are unwanted chemicals found in pharmaceuticals that occur during formulation or the manufacturing process, or arise from degradation of active pharmaceutical ingredients (APIs) and drug products. They are not chemicals that have been added intentionally [2,3]. According to the ICH, impurities are not active ingredients or excipients of drug products [4]. Impurities are classified as organic compounds, residual solvents, and inorganic impurities (Figure 1) [1,5]. Impurities can result from chemical changes in drug substances during drug product manufacturing and storage due to light, temperature, pH, water, and reactions with excipients [4].
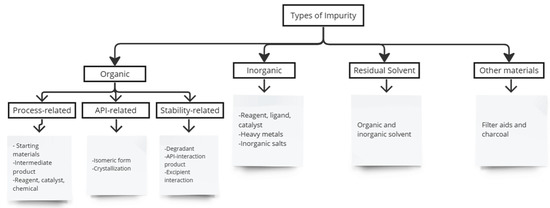
Figure 1.
The types of impurities in pharmaceutical products.
Even in small amounts, impurities can reduce the safety and effectiveness of pharmaceutical products. Therefore, API impurity profiling is becoming increasingly important, as impurities in APIs can compromise drug safety and quality [6]. In 2018, N-nitrosodimethylamine (NDMA) was found in valsartan as an impurity. In 2022, Mansouri et al. [7] evaluated the risk of cancer associated with exposure to NDMA-contaminated valsartan. They found a slightly increased risk of liver cancer and melanoma in patients exposed to NDMA via routine valsartan treatment. NDMA is produced in the process of synthesising raw materials of the sartan group. The generation of this group requires N,N-dimethylformamide as a solvent and sodium nitrite as a reagent to form the tetrazole ring [7]. In 2022, Indonesia reported a significant increase in acute kidney injury (AKI) cases in children. On 5 February 2023, over 300 cases were reported, and over half resulted in death. The cases were associated with ethylene glycol (EG) and diethylene glycol (DEG) impurities in an oral solution product [8]. DEG and EG are impurities in raw excipient materials such as glycerine and propylene glycol [9,10]. Several types of impurities in APIs are considered to be genotoxic impurities (GTIs). These compounds can cause genetic mutations, chromosomal breaks, and/or chromosomal rearrangements, resulting in cancer [11]. Therefore, it is crucial to remove impurities in pharmaceutical products.
The detection of impurities in APIs and drug products is critical to ensure the safety of a product. In this endeavour, the preparation and analytical methods are essential to determine the impurity. Table 1 shows several preparation methods and instruments that have been used to analyse impurities in APIs and drug products.

Table 1.
The methods that have been employed to analyse impurities in active pharmaceutical ingredients (APIs) and drug products.
Molecularly imprinted polymers (MIPs) have received great attention for their ability to selectively separate analytes in various samples. These synthetic polymers have a defined selectivity for a particular analyte or group of structurally related compounds, making them ideal materials for separation processes [20]. MIPs are used as an adsorbent for solid-phase extraction (SPE) [21,22], dispersive solid-phase extraction (D-SPE) [23,24], pipette solid-phase extraction (P-SPE) [25,26], and monolithic columns [27]. MIPs have also been applied to separate impurities from APIs or to purify APIs. The use of MIPs for impurity removal from APIs is quite suitable, considering that the impurity level is low and these MIPs can bind selectively and sensitively to template molecules (analytes). Until now, no review has discussed how to design MIPs for impurities and the application of MIPs to separate impurities in APIs and drug products. Hence, we discuss the application of MIPs to separate impurities in APIs and drug products and present future perspectives related to the development of MIPs to analyse impurities.
2. Design of MIPs for Pharmaceutical Impurities
Molecular imprinting is used to create specific artificial recognition sites in a polymer matrix that can bind specifically and selectively to analytes (template molecules) [28,29,30]. The components involved in generating MIPs include a template molecule, a functional monomer, a crosslinker, a porogen, and an initiator [31,32,33]. The design of the MIP-synthesis process is essential to produce an end product with good analytical performance. One of the most critical factors is to determine the best functional monomer(s) that will interact selectively with template molecules. Based on the literature, researchers have identified the functional monomers used to create MIPs for impurities with and without computer simulations. In general, when researchers have selected functional monomers without computer simulation, they have used methacrylic acid (MAA) as the functional monomer [34,35,36,37,38,39,40] because it has a carboxyl group that can act simultaneously as a hydrogen donor and acceptor. This allows strong interactions between the template molecule and the monomer via hydrogen bonding [33].
Computational simulations can assist in selecting functional monomers by evaluating hydrogen interactions between template molecules and functional monomers [29]. Apart from playing a role in determining the type of functional monomer, computational simulations also play a role in determining the best monomer ratio in less time than conducting trials in the laboratory (Table 2) [41]. For example, Viveiros et al. [42] used the SYBYL™ 7.6 software for the entire computational process to identify the best functional monomer and composition for an acetamide MIP. They entered all of the tested monomer structures using Gasteiger–Hückel calculus and refined the design with molecular mechanics methods by applying energy minimization with the MAXIMIN2 command. They screened individual functional monomers of the library against the template using the LEAPFROG™ algorithm, which allows energy-based evaluation of binding values for ligand structures [42]. They ran the program for 60,000 iterations and scored the binding energies of template–monomer interactions; the highest binding score corresponds to the best combination [43]. Based on the computer simulations, they selected monomers with the highest binding score and used them in a simulated molecular dynamics or annealing process to study their interaction with acetamide as a template molecule in the presence of carbon dioxide (CO2) as a porogen. They also obtained the template-to-monomer ratios from this computer simulation. They selected itaconic acid with a binding score of −33.31 kcal/mol and 2-hydroxyethyl methacrylate with a binding score of −15.71 kcal/mol as the optimal monomers to interact with acetamide [42]. Based on the binding score, itaconic acid had a stronger affinity to acetamide than 2-hydroxyethyl methacrylate. Based on the experiment in the laboratory, acetamide–itaconic acid MIPs had a 2.3-fold higher affinity towards 2-hydroxyethyl methacrylate than acetamide-2-hydroxyethyl methacrylate MIPs. These results were consistent with the computational results [42].

Table 2.
Comparison of static binding capacities in standard acetamide solutions (250 ppm) for molecularly imprinted templates designed with and without computer simulations.
Although MIPs can be designed without computer simulations, this approach is desirable when designing MIPs. The advantages of computational simulations are the reduction in time when determining which monomer has good binding affinity to the template molecules and better cost-effectiveness.
A computational study can be applied to select a dummy template [45], which resembles the target molecule in structure, shape, size, and function, and serves as a template for imprinting. Using a dummy template avoids template leaks that can lead to analysis errors [46]. Fu et al. [45] performed a computational study to select the dummy template for separating the GTIs. They evaluated 2,6-dichloroaniline, p-toluidine, and aniline as dummy templates. The template molecule and functional monomer complex were constructed to evaluate the interaction strength between aromatic amines and functional monomers at the molecular level [45]. They optimized the most stable template–monomer complex first, calculating its interaction energy, ∆E, with the equation: ∆E = E(template−monomer) − E(template) − E(monomer) [45,47]. In this study, the authors used MAA as a functional monomer. ∆E for 2,6-dichloroaniline, p-toluidine, and aniline was −9.60, −8.11, and −7.88 kcal/mol, respectively. Based on the results, the authors chose aniline as a dummy template because it had the lowest binding energy with the MAA, indicating that would have the most potent effect on MAA and is the most stable compound [45].
In another study, researchers prepared theoretical MIPs, using S-pramipexole as a model drug and its structural analogue, S-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole, as the template. The authors constructed theoretical polymeric models based on different functional monomers and ethylene glycol dimethacrylate as a crosslinker [48]. Computer modelling provides one way to study the adsorption process. This involves several steps: first, remove the template molecule from the computational model cavity. Second, insert the analyte and the solvent into the model. Third, run a computer simulation. Fourth, calculate the binding energy using the equation: ΔEB = Esystem − Eanalyte − Ecavity. Esystem refers to the potential energy of the cavity with bound analyte in the solvent, Eanalyte refers to the potential energy of the analyte, and Ecavity refers to the potential energy of the cavity without analyte in the solvent [48]. The authors used S-pramipexol as an analyte and included it in various MIP models to confirm that the adsorption capacity results aligned with previous experimental results [49]. The interactions between S-pramipexol in the MIP cavities constructed with itaconic acid as a functional monomer had the lowest ΔEB of −114.75 kcal/mol [48]. The result correlated well with the experimental adsorption capacity [49]. The authors also conducted theoretical analysis on the selectivity of the MIP system towards a particular group of compounds, known as the S-pramipexole impurities and degradants. The template and model drug had the lowest ΔEB values of −358.40 kcal/mol and −339.51 kcal/mol, respectively. However, the analysed impurities had higher ΔEB values [48].
3. Methods Used to Synthesize MIPs for Pharmaceutical Impurities
Several synthesis methods have been developed to obtain MIPs with good performance. Based on the literature, bulk polymerization, surface polymerization, and supercritical fluid (SCF) technology have been used to synthesize MIP to separate impurities in APIs and drug preparations. In addition, particular strategies such as dummy templates have been applied to obtain MIPs.
3.1. Bulk Polymerization
Bulk polymerization is a conventional method often used to prepare MIPs because it is simple and inexpensive. The template molecules, functional monomers, crosslinkers, and initiators are mixed in a porogen solvent. Polymerization is initiated by light or thermal irradiation. A block polymer is obtained with this method. Therefore, grounding, crushing, and sieving are required after polymerization (Figure 2). Table 3 lists the MIPs that have been synthesized using bulk polymerization and then applied to separate pharmaceutical impurities.
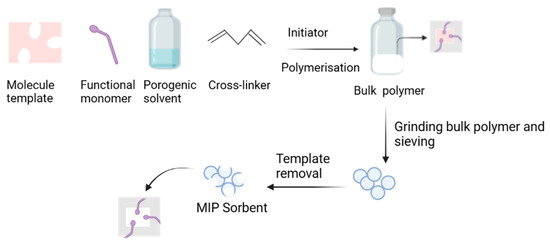
Figure 2.
The bulk polymerization method to obtain molecularly imprinted polymers.
Székely et al. [50] designed MIPs using bulk polymerization to remove GTIs such as acetamide and aryl sulfonate from APIs. Acetamide is a pharmaceutical impurity that is potentially genotoxic because it can interact with DNA. Acetamide is typically present in the final stages of API manufacturing [50]. There are many sources of genotoxic arylsulfonate contamination in APIs, one of which is esterification between p-toluene-sulphonic acid (TsOH) and residual solvents such as methanol, which produces the genotoxic byproduct methyl tosylate (MeTs) [51]. The best-performing MIP for separating acetamide was synthesized using MAA as a functional monomer and toluene as a porogen solvent. Toluene can improve the analytical performance of MIPs because it is a nonpolar solvent that encourages polymerization by forming complexes between templates and monomers [51]. The MIP was applied as an SPE adsorbent. The authors used a mixture of acetamide and Etodolac in acetonitrile as a model sample solution (load sample). The MIP could bind 100% acetamide in the load step while the non-molecularly imprinted template (NIP) bound 77% of the acetamide [51]. The MIP for the removal of aryl sulfonate was synthesized using MeTs as the template molecule, 1-(4-vinylphenyl)-3-(3,5bis(trifluoromethyl)phenyl)urea (U) as a functional monomer, and two different crosslinkers, ethylene glycol dimethacrylate (EGDMA) or divinylbenzene, in chloroform with two ratios of T/U/EGDMA 0.1/0.1/2 (MIP 1) and 1/0.1/2 (MIP 2) or T/U/DVB 0.1/0.1/2 (MIP 3) and 1/0.1/2 (MIP 4). The authors evaluated the binding affinity of the MIPs using a solution of MeTs and halobetasol propionate (the API). MIP 1 and MIP 2 were better able to bind specifically to MeTS than the API. Compared with the NIP, MIP 1 and MIP 2 had a high imprinting factor (IF) [51].
The addition of base to the pre-polymerization solution could increase the adsorption capacity when MAA is used as a functional monomer. Székely et al. [39] added 1,2,2,6,6-pentamethylpiperidine (PMP) base to a pre-polymerization solution containing MAA as the functional monomer and 1,3-diisopropyl urea (IPU) as the template to produce MIP to remove IPU from Keppra (Kp), mometasone furoate (Meta), and roxithromycin (Roxi) as APIs. PMP converts MAA to MAA carboxylate (carboxylate anion) that can bind with two donors of NHs of IPU in a syn arrangement; an interaction that is stronger than neutral free acid. The MIPs were synthesized with two formulas, without PMP (MIP 1) and with PMP (MIP 2). The degree of IPU binding with MIP 2 was higher than with MIP 1 (80% and 55%, respectively) [39].
Using a dummy template is one strategy to develop a MIP. In one study, the researchers used a dummy template to avoid template leakage that could reduce the accuracy of the analysis [52]. The most commonly used dummy templates are structural analogues of the analyte [53] or isotope-labelled template [54]. Aniline was used as a dummy template to prepare a dummy MIP that could pretreat a sample containing aromatic amine GTIs. The aniline–MAA–EGDMA molar ratio of 1:4:8 produced a dummy MIP with the highest capacity to adsorb aniline (Q = 8.6 mg/g) and good blotting effect (IF = 1.3) [45]. The dummy MIP could simultaneously extract p-toluidine, p-acetotoluidide, and 2,6-dichloroaniline. The authors applied the dummy MIP as an SPE sorbent to remove 5 ppm 2,6-dichloroaniline from a diclofenac sodium sample and 10 ppm p-toluidine from a torasemide sample. After extraction, the solutions did not contain 2,6-dichloroaniline or p-toluidine, indicating that the dummy MIP could be used for quality control of the drug [45].


Table 3.
Molecularly imprinted polymers (MIPs) that have been synthesized using the bulk polymerization method.
Table 3.
Molecularly imprinted polymers (MIPs) that have been synthesized using the bulk polymerization method.
| Sample | Impurity | Type of Impurity | Template | Binding Capacity | Imprinting Factor | Ref. |
|---|---|---|---|---|---|---|
| Mometasone furoate (APIs) | 4-Dimethylamino pyridine | Organic (genotoxic impurity) from API post-reaction stream | 4-Dimethylamino pyridine | 5.03 mg/g | NM | [37] |
| Diclofenac sodium and torasemide | 2,6-Dichloroaniline | Organic (genotoxic impurities) from synthesis, storage, or transportation of APIs | Aniline (dummy template) | 4.08 mg/g | NM for 2,6-dichloroaniline or p-toluidine Aniline: 1.3 | [45] |
| p-Toluidine | ±6 mg/g | |||||
| Keppra (Kp), mometasone furoate (Meta), and roxithromycin (Roxi) as APIs | 1,3-Diisopropylurea | Organic (genotoxic impurity) from API post-reaction stream | 1,3-Diisopropylurea | NM, but 80% binding for MIP synthesized when base was added | NM | [39] |
| Diphenhydramine hydrochloride | Benzhydrol | Organic (genotoxic impurity) from intermediate of pharmaceuticals | Benzhydrol | 98.3 µmol/g | NM | [55] |
| Fluvoxamine maleate hydrochloride (APIs) | ((2RS)-2-[[2-[[[(1E)-5-methoxy- 1-[4(trifluoromethyl) phenyl] pentylidene]amino] oxy]ethyl]amino] butanedioic acid | Organic | ((2RS)-2-[[2-[[[(1E)-5-methoxy- 1-[4(trifluoromethyl) phenyl] pentylidene]amino] oxy]ethyl]amino] butanedioic acid | 100 µg/mg | NM | [34] |
NM, not mentioned in the article.
3.2. Surface-Imprinting Polymerization
Surface-imprinting polymerization has been developed to overcome the drawbacks of the conventional bulk and precipitation MIP-synthesis methods [56]. The imprinted materials were thick and needed large amounts of solvent to remove the molecule template [57]. In this method, molecule imprinting occurs on the surface of the solid matrix where recognition sites are distributed on the layer. The advantages of this method are the ability to reduce ‘embedding’ incidents, to promote efficient mass transfer, and to enhance the adsorption capacity [58]. The solid matrix commonly used in this method is silica nanoparticles and Fe3O4 (the magnetic component). In general, surface-imprinting polymerization occurs via three steps: (i) synthesis of the solid matrix, (ii) modification of the solid matrix, and (iii) surface molecular imprinting. The latter step begins by forming a monomer–template molecular complex under certain conditions. Then, polymerization occurs on the surface of the solid matrix with an initiator and crosslinker. After polymerization, the template molecules are removed to form cavities identical to those of the template molecule (Figure 3) [58]. Several studies have been carried out to synthesize MIPs to separate impurities using the surface polymerization method (Table 4).
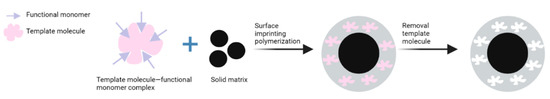
Figure 3.
The surface molecular imprinting process.

Table 4.
Molecularly imprinted polymers (MIPs) that have been synthesized using surface polymerization.
Hashemi-Moghaddam and Abbasi [35] synthesized a surface molecularly imprinted on silica nanoparticles to remove the p-nitrophenol (4-NP) from paracetamol. 4-NP is used as an intermediate in manufacturing pharmaceuticals such as analgesics/antipyretics It is an impurity in medicinal substances that causes carcinogenic risks to humans [59]. Silica nanoparticles were obtained by hydrolysis of tetraethyl orthosilicate (TEOS); it was functionalized using 3-(methacryloxy)propyltrimethoxysilane (MPTS) to obtain the vinyl end groups. MAA was used as a functional monomer for surface molecular imprinting. The maximum adsorption capacity was 72 × 10−3 mmol/L. The MIP (5 mg) could adsorb 85% of 4-NP at 10 ppm, while 5 mg of the NIP adsorbed 4% of 4-NP at the same concentration. These findings indicate that the MIP has a recognition site that provides better adsorption than the NIP. The selectivity factor is a ratio of the distribution factor of the analyte (molecule template) with a similar compound. In this study, the selectivity factor of the MIP between 4-NP and paracetamol was 18.48. Meanwhile, the selectivity factor of NIP was 2.66. Hence, the MIP selectively bound 4-NP [35].
A magnetic component (Fe3O4) is a popular solid matrix used to synthesize MIP. The MIPs synthesized using a solid magnetic matrix are called magnetic molecularly imprinted polymers (MMIPs). These MMIPs can be directly applied to the sample solution, and an external magnet can be used for the separation process [60]. MMIPs for the removal of sulphanilamide has been synthesized using Fe3O4 as a solid magnetic matrix. Then, the surface of Fe3O4 was functionalized with SiO2 and 3-methacryloxypropyl trimethoxy-silane (MPTS) (Fe3O4@SiO2@MPTS). Sulphanilamide is a major degradation product of sulphacetamide preparations; it is formed when exposed to light, extreme temperatures, or long storage [61,62]. In one study, researchers used sulphanilamide as a template molecule, MAA as a functional monomer, EGDMA as a crosslinker, and 2,2-azobisisobutyronitrile (AIBN) as a radical initiator in a mixture of acetonitrile and toluene (60:40, v/v) as a porogen [36]. The adsorption capacity of the MMIP was 114.2 µmol/g. The authors applied it to separate sulphanilamide after spiking 10 mL of eye drop solution with 10 mL of 0.1 mmol−1 sulphanilamide, then diluting the solution to 50 mL with water. They adjusted the pH to 6.0 and then mixed 10 mL of this solution with 0.1 g of the MMIP for 30 min. They injected the supernatant into a high-performance liquid chromatography column. The sulphanilamide peak intensity decreased after purification while the sulfacetamide (the API) peak intensity did not decrease, indicating that the synthesized MMIP had good selectivity [36].
Luo et al. [38] confirmed that surface polymerization could overcome the drawback of bulk polymerization. They synthesized a MIP with surface polymerization (S-MIP) using SiO2 modified by 3-aminopropyl triethoxysilane (SiO2-APTES) as a solid matrix. They used penicilloic acid as a template, MAA as a functional monomer, and EGDMA as a crosslinker. They also synthesized a MIP with bulk polymerization (B-MIP) using the same conditions but without a solid matrix (SiO2-APTES) [38]. Based on the adsorption isotherm, the saturated adsorption capacity of S-MIP was 22.67 mg/g; much higher than the B-MIP (10.31 mg/g). The IFs for S-MIP and B-MIP were 6.3 and 2.2, respectively. The S-MIP showed better analytical performance than the B-MIP [38]. The S-MIP reached adsorption equilibrium (45 min) faster than B-MIP (90 min). The template required longer to reach adsorption equilibrium with the B-MIP due to embedded active sites [38]. In the S-MIP, most of the template binding sites were located on the surface of the polymer, enhancing the molecular recognition ability between the polymer and the target compound and improving the mass transfer kinetics of the S-MIP [63].
3.3. SCF Technology
Based on environmental regulations and safety hazards, the pharmaceutical industry is trying to reduce the use of organic solvents [64]. SCFs are one alternative to replace hazardous organic solvents with environmentally friendly approaches. SCFs are highly compressible gases: they exceed a liquid’s critical temperature and pressure but are below the pressure required to condense from the liquid to the solid state [65]. SCFs have many advantages as green solvents, such as being non-toxic, chemically inert, non-flammable, of a high purity, low-cost, and easily removed [66]. In addition, SCFs have been applied as a solvent for molecularly imprinted technologies because they can dissolve the majority of monomers [44].
Polymerization is carried out in a stainless steel high-pressure reactor. In one study, the authors introduced the template molecule, functional monomer, crosslinker, and initiator into the reactor immersed in a thermostat water bath. They added CO2 up to 21 MPa and performed polymerization for 24 h with stirring. The next step was desorption of the template molecule using supercritical CO2 or a co-solvent to obtain the specific binding sites [44]. Figure 4 illustrates the synthesis of MIPs using supercritical CO2. The studies that have applied supercritical CO2 to synthesize MIPs are summarized in Table 5.
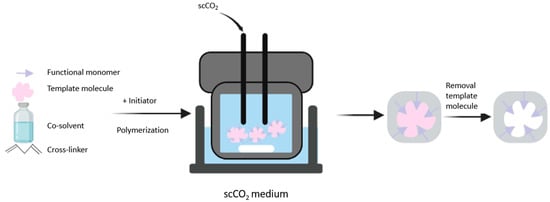
Figure 4.
Schematic of the synthesis of molecularly imprinted polymers using supercritical carbon dioxide fluid.

Table 5.
Synthesis of molecularly imprinted polymers using supercritical carbon dioxide as a solvent.
Viveiros et al. [44] synthesized MIPs to purify acetamide from an API. The authors used MAA and methacrylamide (MAM) as functional monomers. They performed four different polymerizations: (i) MAA with 0.5 mL acetonitrile as a co-solvent (MIP 1), (ii) MAA without a co-solvent (MIP 2), (iii) MAM with 0.5 mL acetonitrile as a co-solvent (MIP 3), and (iv) MAM without a co-solvent (MIP 4). Based on the static binding analysis, MIP 3 had a higher adsorption ability of acetamide at 250 ppm and an IF of 1.31. The adsorption capacity (Qmax) based on the Langmuir isotherm of MIP 3 was 2.99 mmol/g. The MAM and acetamide interactions were stronger than the MAA and acetamide interactions. The structural similarities between MAM and the acetamide also lead to a higher affinity for it than for MAA [44].
Viveiros et al. [44] synthesized two kinds of MIPs for selective removal of acetamide in APIs using benzamide (BENZ) and pivalamide (PIV) as dummy templates (MIP 1 and MIP 2, respectively). BENZ is a planar-shaped template molecule, while PIV is a three-dimensional analogue template molecule. They used supercritical CO2 for the synthesis. MIP 1 and MIP 2 were free-flowing, dry, ready-to-use, and homogenous powders. The advantages of using supercritical CO2 compared with an organic solvent were the absence of residual solvent and insufficient grinding and sieving [63]. In addition, MIP 2 showed a higher adsorption capacity of all amide-based compounds in the static binding study (acetamide, BNZ, and PIV) than MIP 1. This is because MIP 2 has a three-dimensional cavity that is more accessible than MIP 1 (which has a planar cavity). In a dynamic study using an mixed solution (acetamide, BNZ, and PIV), MIP 2 could remove 32% more acetamide than MIP 1, making it potentially applicable for the removal of amide-based genotoxins from crude pharmaceutical mixtures [67].
Based on Table 3, Table 4 and Table 5, organic compounds have commonly been used to develop MIPs that recognize pharmaceutical impurities. These impurities are usually generated during API synthesis or are due to product degradation. However, MIPs for impurities have not been developed for other types of impurities such as heavy metals, inorganic salts, reagents, and residual solvents. Ionic MIPs could be developed to separate heavy metal impurities in APIs. There may be problems with the development of MIPs for these impurities because an API could contain more than one type of impurity, so the development of these MIPs could take more time. This potential disadvantage could be overcome by using multi-template molecularly imprinted polymers (MT-MIPs). This simple and reliable approach can be used to efficiently remove and enrich multiple analytes simultaneously in a single process [68,69]. In addition, factors that can become obstacles in the MIPs development process are related to impurity raw materials used as templates, such as unavailable, toxic, expensive, and unstable raw materials for the synthesis process. This problem might be overcome by using a dummy template, as Fu et al. [45] did.
The advantages and disadvantages of the polymerization methods used to synthesize MIPs to separate impurities are listed in Table 6.

Table 6.
The advantages and disadvantages of the polymerization methods used to synthesize molecularly imprinted polymers (MIPs) to separate impurities.
4. Conclusions
MIPs can be used to separate impurities in APIs and drug products. In addition, they can be applied in the preparation process to determine the levels of impurities in APIs or drug products. Computer simulations are a good choice to guide the selection of functional monomers and to determine the template-to-monomer ratio. This approach produces MIPs with better performance in a shorter amount of time. The most common methods used in MIP synthesis for impurities are bulk polymerization, surface-imprinting polymerization, and SCF technology. Based on the comparison of the bulk and surface-imprinting polymerization, the MIPs produced by the latter method have a higher adsorption capacity and faster adsorption. Overall, the application of MIPs to analyse APIs and drug products as well as adsorbents for purification, is still relatively low, considering that MIPs have the advantage of separating impurities to increase separation efficiency selectively. Future research involving the use of MIPs to separate and analyse impurities in pharmaceutical products should focus on the following:
- Develop MIPs for other types of impurities. Ionic MIPs can be developed to detect and separate heavy metals in pharmaceutical products.
- Compare the analytical performance of MIPs obtained using SCF technology with those obtained using other methods. In addition, compare the costs required for each technique to determine cost-effectiveness and analytical performance.
- Develop MT-MIPs to separate multiple impurities simultaneously and to reduce the time required for analysis.
Author Contributions
Conceptualization, A.N.H. and I.S.; methodology, A.N.H. and I.S.; writing—original draft preparation, I.S.; writing—review and editing, A.N.H.; visualization, I.S.; funding acquisition, A.N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Academic Leadership Grant of Universitas Padjadjaran Grant no. 1549/UN6.3.1/PT.00/2023.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Acknowledgments
We thank the Directorate of Research and Community Engagement Universitas Padjadjaran for APC Funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kątny, M.; Frankowski, M. Impurities in Drug Products and Active Pharmaceutical Ingredients. Crit. Rev. Anal. Chem. 2017, 47, 187–193. [Google Scholar] [CrossRef]
- Patole, S.; Gosar, A.; Shaikh, T. Impurities Characterization in Pharmaceuticals: A Review. Ijppr. Hum. 2019, 14, 1170–1177. [Google Scholar]
- Maggio, R.M.; Calvo, N.L.; Vignaduzzo, S.E.; Kaufman, T.S. Pharmaceutical Impurities and Degradation Products: Uses and Applications of NMR Techniques. J. Pharm. Biomed. Anal. 2014, 101, 102–122. [Google Scholar] [CrossRef]
- ICH Q3B (R2) on Impurities: Impurities in New Drug Products; EMEA European Medicines Agency: Amsterdam, The Netherlands, 2006.
- Parmar, I.; Rathod, H.; Siddique, S. A Review: Recent Trends in Analytical Techniques for Characterization and Structure Elucidation of Impurities in the Drug Substances. Indian J. Pharm. Sci. 2021, 83, 402–415. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.; Afreen, S.; Pratap Singh, D. A Review on Pharmaceutical Impurities and Their Importance. World J. Pharm. Pharm. Sci. 2017, 6, 1337–1354. [Google Scholar]
- Mansouri, I.; Botton, J.; Semenzato, L.; Haddy, N.; Zureik, M. N-Nitrosodimethylamine-Contaminated Valsartan and Risk of Cancer: A Nationwide Study of 1.4 Million Valsartan Users. J. Am. Heart Assoc. 2022, 11, e8067. [Google Scholar] [CrossRef]
- WHO. Investigation of Acute Kidney Injury in Children in Indonesia: Results and Regulatory Actions. Available online: https://www.who.int/indonesia/news/detail/01-03-2023-investigation-of-acute-kidney-injury-in-children-in-indonesia--results-and-regulatory-actions (accessed on 5 June 2023).
- Ghanem, M.P.H. Detection of Diethylene Glycol in Glycerin and Propylene Glycol by Using High Performance Thin Layer Chromatography HPTLC. IOSR J. Pharm. 2011, 1, 29–34. [Google Scholar] [CrossRef]
- Baranwal, M.; Kaur, A.; Kumar, R. Challenges in Utilizing Diethylene Glycol and Ethylene Glycol as Excipient: A Thorough Overview. Pharmaspire 2023, 15, 8–15. [Google Scholar] [CrossRef]
- Wood, C. In-Process Control Testing, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Kalauz, A.; Kapui, I. Determination of Potentially Genotoxic Impurities in Crotamiton Active Pharmaceutical Ingredient by Gas Chromatography. J. Pharm. Biomed. Anal. 2022, 210, 114544. [Google Scholar] [CrossRef]
- Jireš, J.; Gibala, P.; Kalášek, S.; Douša, M.; Doubský, J. The Determination of Two Analogues of 4-(Azidomethyl)-1,1′-Biphenyl as Potential Genotoxic Impurities in the Active Pharmaceutical Ingredient of Several Sartans Containing a Tetrazole Group. J. Pharm. Biomed. Anal. 2021, 205, 114300. [Google Scholar] [CrossRef]
- Generalova, Y.; Sipkina, N.; Alekseeva, G. Determination of Related Impurities in a New Active Pharmaceutical Ingredient—Sodium 4,4′-(Propanediamido)Dibenzoate. Microchem. J. 2021, 168, 106498. [Google Scholar] [CrossRef]
- Vogel, M.; Norwig, J. Analysis of Genotoxic N-Nitrosamines in Active Pharmaceutical Ingredients and Market Authorized Products in Low Abundance by Means of Liquid Chromatography—Tandem Mass Spectrometry. SSRN Electron. J. 2022, 219, 114910. [Google Scholar] [CrossRef]
- Wohlfart, J.; Scherf-Clavel, O.; Kinzig, M.; Sörgel, F.; Holzgrabe, U. The Nitrosamine Contamination of Drugs, Part 3: Quantification of 4-Methyl-1-Nitrosopiperazine in Rifampicin Capsules by LC-MS/HRMS. J. Pharm. Biomed. Anal. 2021, 203, 114205. [Google Scholar] [CrossRef]
- Matmour, D.; Bouaffad, A.; Merad, Y.; Ziani, N.H. From the Limit Test for Trace Elements Control to the Elemental Impurities Analysis by Inductively Coupled Plasma Optical Emission Spectrometry: Application on Six Samples of Metronidazole API. J. Trace Elem. Miner. 2022, 2, 100017. [Google Scholar] [CrossRef]
- Lim, H.H.; Oh, Y.S.; Shin, H.S. Determination of N-Nitrosodimethylamine and N-Nitrosomethylethylamine in Drug Substances and Products of Sartans, Metformin and Ranitidine by Precipitation and Solid Phase Extraction and Gas Chromatography—Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2020, 189, 113460. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Z.; Yang, X.; Jin, Y.; Liu, X.; Wang, C.; Zhang, Z. Determination of N-Nitrosodimethylamine in Ranitidine Dosage Forms by ESI-LC-MS/MS.; Applications for Routine Laboratory Testing. Iran. J. Pharm. Res. 2021, 20, 255. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzaman, S.; Nasir, N.M.; Faudzi, S.M.M.; Yahaya, N.; Hanapi, N.S.M.; Ibrahim, W.N.W. Solid-Phase Extraction of Active Compounds from Natural Products by Molecularly Imprinted Polymers: Synthesis and Extraction Parameters. Polymers 2021, 13, 3780. [Google Scholar] [CrossRef] [PubMed]
- Lucci, P.; David, S.; Conchione, C.; Milani, A.; Moret, S.; Pacetti, D.; Conte, L. Molecularly Imprinted Polymer as Selective Sorbent for the Extraction of Zearalenone in Edible Vegetable Oils. Foods 2020, 9, 1439. [Google Scholar] [CrossRef]
- Fu, Y.; Pessagno, F.; Manesiotis, P.; Borrull, F.; Fontanals, N.; Maria Marcé, R. Preparation and Evaluation of Molecularly Imprinted Polymers as Selective SPE Sorbents for the Determination of Cathinones in River Water. Microchem. J. 2022, 175, 107100. [Google Scholar] [CrossRef]
- Dil, E.A.; Doustimotlagh, A.H.; Javadian, H.; Asfaram, A.; Ghaedi, M. Nano-Sized Fe3O4@SiO2-Molecular Imprinted Polymer as a Sorbent for Dispersive Solid-Phase Microextraction of Melatonin in the Methanolic Extract of Portulaca Oleracea, Biological, and Water Samples. Talanta 2021, 221, 121620. [Google Scholar] [CrossRef]
- Bashir, K.; Luo, Z.; Chen, G.; Shu, H.; Cui, X.; Li, W.; Lu, W.; Fu, Q. Development of Surface Molecularly Imprinted Polymers as Dispersive Solid Phase Extraction Coupled with HPLC Method for the Removal and Detection of Griseofulvin in Surface Water. Int. J. Environ. Res. Public Health 2020, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.T.M.; de Oliveira, H.L.; Silva, C.F.; Fonseca, M.C.; Pereira, T.F.D.; Nascimento, C.S.; de Figueiredo, E.C.; Borges, K.B. Efficient Molecularly Imprinted Polymer as a Pipette-Tip Solid-Phase Sorbent for Determination of Carvedilol Enantiomers in Human Urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1061, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.R.; Ghaedi, M. Synthesis of Chitosan Based Molecularly Imprinted Polymer for Pipette-Tip Solid Phase Extraction of Rhodamine B from Chili Powder Samples. Int. J. Biol. Macromol. 2019, 139, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Hasanah, A.N.; Maelaningsih, F.S.; Apriliandi, F.; Sabarudin, A. Synthesis and Characterisation of a Monolithic Imprinted Column Using a Methacrylic Acid Monomer with Porogen Propanol for Atenolol Analysis. J. Anal. Methods Chem. 2020, 2020, 3027618. [Google Scholar] [CrossRef]
- Ertürk, G.; Mattiasson, B. Molecular Imprinting Techniques Used for the Preparation of Biosensors. Sensors 2017, 17, 288. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Susanti, I.; Mutakin, M. An Update on the Use of Molecularly Imprinted Polymers in Beta-Blocker Drug Analysis as a Selective Separation Method in Biological and Environmental Analysis. Molecules 2022, 27, 2880. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Kılıç, S.; Esen, C.; Denizli, A. Molecularly Imprinted Polymer-Based Sensors for Protein Detection. Polymers 2023, 15, 629. [Google Scholar] [CrossRef]
- Bakhtiar, S.; Bhawani, S.A.; Shafqat, S.R. Synthesis and Characterization of Molecular Imprinting Polymer for the Removal of 2-Phenylphenol from Spiked Blood Serum and River Water. Chem. Biol. Technol. Agric. 2019, 6, 15. [Google Scholar] [CrossRef]
- Sajini, T.; Mathew, B. A Brief Overview of Molecularly Imprinted Polymers: Highlighting Computational Design, Nano and Photo-Responsive Imprinting. Talanta Open 2021, 4, 100072. [Google Scholar] [CrossRef]
- Susanti, I.; Safitri, N.; Pratiwi, R.; Hasanah, A.N. Synthesis of Molecular Imprinted Polymer Salbutamol using Methacrylic Acid Monomer and Trimethyl Propane Trimethacrylate (TRIM) as a Cross-Linker through Suspension Polymerization. Int. J. Appl. Pharm. 2022, 14, 32–39. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Shakeri, M. Removal of Potentioally Genotoxic Impurity from Fluroxamine Maleate Crude Drug by Molecularly Imprinted Polymer. Korean J. Chem. Eng. 2014, 31, 1898–1902. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Abbasi, F. Synthesis of Molecularly Imprinted Polymers Coated on Silica Nanoparticles for Removal of P-Nitrophenol from Crude Pharmaceuticals. Pharm. Chem. J. 2015, 49, 280–286. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Shabestani-Trojeni, M. Application of a Magnetic Molecularly Imprinted Polymer for the Removal of Sulfanilamide as Major Impurity in Eye Drops (Sulfacetamide). Pharm. Chem. J. 2020, 54, 977–983. [Google Scholar] [CrossRef]
- Esteves, T.; Viveiros, R.; Bandarra, J.; Heggie, W.; Casimiro, T.; Ferreira, F.C. Molecularly Imprinted Polymer Strategies for Removal of a Genotoxic Impurity, 4-Dimethylaminopyridine, from an Active Pharmaceutical Ingredient Post-Reaction Stream. Sep. Purif. Technol. 2016, 163, 206–214. [Google Scholar] [CrossRef]
- Luo, Z.; Zeng, A.; Zheng, P.; Guo, P.; Du, W.; Du, K.; Fu, Q. Preparation of Surface Molecularly Imprinted Polymers as the Solid-Phase Extraction Sorbents for the Specific Recognition of Penicilloic Acid in Penicillin. Anal. Meth. 2014, 6, 7865–7874. [Google Scholar] [CrossRef]
- Székely, G.; Bandarra, J.; Heggie, W.; Ferreira, F.C.; Sellergren, B. Design, Preparation and Characterization of Novel Molecularly Imprinted Polymers for Removal of Potentially Genotoxic 1,3-Diisopropylurea from API Solutions. Sep. Purif. Technol. 2012, 86, 190–198. [Google Scholar] [CrossRef]
- Balamurugan, K.; Prakasam, T.; Srinivasan, K.R. Determination of Enantiomeric Impurity of D-Pseudoephedrine Determination of Enantiomeric Impurity of D- Pseudoephedrine Using Mip Column. Indian J. Pharm. 2015, 4, 13–19. [Google Scholar]
- Abdel Ghani, N.T.; Mohamed El Nashar, R.; Abdel-Haleem, F.M.; Madbouly, A. Computational Design, Synthesis and Application of a New Selective Molecularly Imprinted Polymer for Electrochemical Detection. Electroanalysis 2016, 28, 1530–1538. [Google Scholar] [CrossRef]
- Viveiros, R.; Karim, K.; Piletsky, S.A.; Heggie, W.; Casimiro, T. Development of a Molecularly Imprinted Polymer for a Pharmaceutical Impurity in Supercritical CO2: Rational Design Using Computational Approach. J. Clean. Prod. 2017, 168, 1025–1031. [Google Scholar] [CrossRef]
- Guerreiro, A.; Soares, A.; Piletska, E.; Mattiasson, B.; Piletsky, S. Preliminary Evaluation of New Polymer Matrix for Solid-Phase Extraction of Nonylphenol from Water Samples. Anal. Chim. Acta 2008, 612, 99–104. [Google Scholar] [CrossRef]
- Viveiros, R.; Lopes, M.I.; Heggie, W.; Casimiro, T. Green Approach on the Development of Lock-and-Key Polymers for API Purification. Chem. Eng. J. 2017, 308, 229–239. [Google Scholar] [CrossRef]
- Fu, X.; Wang, X.; Xia, Z.; Huang, Y. Preparation of Dummy Molecularly Imprinted Polymers for Selective Extraction of Aromatic Amine Genotoxic Impurities. J. Chromatogr. A 2022, 1685, 463617. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, W.; Tan, L.; Wang, J.; Li, H.; Wang, J. Separation and Detection of Trace Atrazine from Seawater Using Dummy-Template Molecularly Imprinted Solid-Phase Extraction Followed by High-Performance Liquid Chromatography. Mar. Pollut. Bull. 2019, 149, 110502. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Liu, J.; Tang, S.; Wang, Y.; Wang, Y.; Jin, R. Optimization of Enrofloxacin-Imprinted Polymers by Computer-Aided Design. J. Mol. Model. 2015, 21, 290. [Google Scholar] [CrossRef]
- Sobiech, M.; Giebułtowicz, J.; Woźnica, M.; Jaworski, I.; Luliński, P. Theoretical and Experimental Model of Molecularly Imprinted Polymer Surface Microenvironment for Selective Stationary Phase—Exemplary of S-Pramipexole for Potential Pharmaceutical Analysis. Microchem. J. 2022, 182, 107875. [Google Scholar] [CrossRef]
- Woźnica, M.; Luliński, P. Design of Selective Molecularly Imprinted Sorbent for the Optimized Solid-Phase Extraction of S-Pramipexole from the Model Multicomponent Sample of Human Urine. J. Sep. Sci. 2019, 42, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Dow, L.K.; Hansen, M.M.; Pack, B.W.; Page, T.J.; Baertschi, S.W. The Assessment of Impurities for Genotoxic Potential and Subsequent Control in Drug Substance and Drug Product. J. Pharm. Sci. 2013, 102, 1404–1418. [Google Scholar] [CrossRef]
- Székely, G.; Fritz, E.; Bandarra, J.; Heggie, W.; Sellergren, B. Removal of Potentially Genotoxic Acetamide and Arylsulfonate Impurities from Crude Drugs by Molecular Imprinting. J. Chromatogr. A 2012, 1240, 52–58. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Huang, C.; Jiao, Y.; Chen, J. A Phenolphthalein-Dummy Template Molecularly Imprinted Polymer for Highly Selective Extraction and Clean-up of Bisphenol A in Complex Biological, Environmental and Food Samples. Polymers 2018, 10, 1150. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, J.; Li, Y.; Jin, J.; Zhang, B.; Shah, S.M.; Wang, X.; Chen, J. Highly Selective Dummy Molecularly Imprinted Polymer as a Solid-Phase Extraction Sorbent for Five Bisphenols in Tap and River Water. J. Chromatogr. A 2014, 1343, 33–41. [Google Scholar] [CrossRef]
- Sambe, H.; Hoshina, K.; Hosoya, K.; Haginaka, J. Direct Injection Analysis of Bisphenol A in Serum by Combination of Isotope Imprinting with Liquid Chromatography-Mass Spectrometry. Analyst 2005, 130, 38–40. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Alaeian, M.R. Synthesis of Molecularly Imprinted Polymer for Removal of Effective Impurity (Benzhydrol) from Diphenhydramine Hydrochloride Drug. J. Chin. Chem. Soc. 2014, 61, 643–648. [Google Scholar] [CrossRef]
- Susanti, I.; Hasanah, A.N. How to Develop Molecularly Imprinted Mesoporous Silica for Selective Recognition of Analytes in Pharmaceutical, Environmental, and Food Samples. Polym. Adv. Technol. 2021, 32, 1965–1980. [Google Scholar] [CrossRef]
- He, J.X.; Pan, H.Y.; Xu, L.; Tang, R.Y. Application of Molecularly Imprinted Polymers for the Separation and Detection of Aflatoxin. J. Chem. Res. 2020, 45, 400–410. [Google Scholar] [CrossRef]
- Dong, C.; Shi, H.; Han, Y.; Yang, Y.; Wang, R.; Men, J. Molecularly Imprinted Polymers by the Surface Imprinting Technique. Eur. Polym. J. 2021, 145, 110231. [Google Scholar] [CrossRef]
- Eichenbaum, G.; Johnson, M.; Kirkland, D.; O’Neill, P.; Stellar, S.; Bielawne, J.; DeWire, R.; Areia, D.; Bryant, S.; Weiner, S.; et al. Assessment of the Genotoxic and Carcinogenic Risks of P-Nitrophenol When It Is Present as an Impurity in a Drug Product. Regul. Toxicol. Pharmacol. 2009, 55, 33–42. [Google Scholar] [CrossRef]
- Susanti, I.; Holik, H.A. Review: Application of Magnetic Solid-Phase Extraction (Mspe) in Various Types of Samples. Int. J. Appl. Pharm. 2021, 13, 59–68. [Google Scholar] [CrossRef]
- Wojtowicz, E.J. A Column Chromatographic Method for the Determination of Sulfanilamide in Pharmaceutical Preparations Containing Sulfacetamide or Its Sodium Salt. J. Pharm. Sci. 1970, 59, 240–241. [Google Scholar] [CrossRef]
- Ahmed, S.; Anwar, N.; Sheraz, M.A.; Ahmad, I. Validation of a Stability-Indicating Spectrometric Method for the Determination of Sulfacetamide Sodium in Pure form and Ophthalmic Preparations. J. Pharm. Bioallied Sci. 2017, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Peng, K.; Su, Y.; Song, X.; Qiu, J.; Xiong, R.; He, L. Preparation of Surface Molecularly Imprinted Polymer and Its Application for the Selective Extraction of Teicoplanin from Water. RSC Adv. 2021, 11, 13615–13623. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Casimiro, T.; Aguiar-Ricardo, A.; Simplício, A.L.; Duarte, C.M.M. Supercritical Fluid Polymerisation and Impregnation of Molecularly Imprinted Polymers for Drug Delivery. J. Supercrit. Fluids 2006, 39, 102–106. [Google Scholar] [CrossRef]
- Furtado, A.I.; Viveiros, R.; Casimiro, T. MIP Synthesis and Processing Using Supercritical Fluids. In Molecularly Imprinted Polymers: Methods in Molecular Biology; Martín-Esteban, A., Ed.; Humana Press: Totowa, NJ, USA, 2021; p. 20. [Google Scholar]
- Boyère, C.; Jérôme, C.; Debuigne, A. Input of Supercritical Carbon Dioxide to Polymer Synthesis: An Overview. Eur. Polym. J. 2014, 61, 45–63. [Google Scholar] [CrossRef]
- Viveiros, R.; Bonifácio, V.D.B.; Heggie, W.; Casimiro, T. Green Development of Polymeric Dummy Artificial Receptors with Affinity for Amide-Based Pharmaceutical Impurities. ACS Sustain. Chem. Eng. 2019, 7, 15445–15451. [Google Scholar] [CrossRef]
- Sun, C.; Wang, J.; Huang, J.; Yao, D.; Wang, C.Z.; Zhang, L.; Hou, S.; Chen, L.; Yuan, C.S. The Multi-Template Molecularly Imprinted Polymer Based on SBA-15 for Selective Separation and Determination of Panax Notoginseng Saponins Simultaneously in Biological Samples. Polymers 2017, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zeng, G.; Ma, X. Multi-Templates Surface Molecularly Imprinted Polymer for Rapid Separation and Analysis of Quinolones in Water. Environ. Sci. Pollut. Res. 2020, 27, 7177–7187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).